Abstract
Heavy metal resistance genes (MRGs) and antibiotic resistance genes (ARGs) in bacteria can respond to the inducement of heavy metals. However, the co-occurrence of MRGs and ARGs in the long-term heavy metal contaminated area is still poorly understood. Here, we investigated the relationship between the abundance of soil bacteria MRGs, ARGs and heavy metal pollution in a copper tailing dam area of northern China. We found that arsC and ereA genes coding for resistance mechanisms to arsenic and to macrolides, respectively, are the most abundant MRG and ARG in the study area. The abundance of MRGs is positively correlated with cadmium (Cd) concentration, and this indicates the importance of Cd in the selection of MRGs. The network analysis results show that sulII and MRGs co-occur and copB occur with ARGs, which suggests that MRGs and ARGs can be co-selected in the soil contaminated by heavy metal. The network analysis also reveals the co-occurrence of Cd and MRGs, and thus heavy metal with a high ‘toxic-response’ factor can be used as the indicator of MRGs. This study improves the understanding of the relationship between bacterial resistance and multi-metal contamination, and underlies the exploration of the adaptive mechanism of microbes in the multi-metal contaminated environment.
Keywords: metal resistance genes, antibiotic resistance genes, mobile genetic element, co-occurrence, metal pollution, risk index, copper tailing
Introduction
The rapid industrialization has led to a series of ecological and environmental problems (Sawut et al., 2018). One of the most urgent issues is heavy metal contamination that is harmful to both ecosystem functions and human health. Heavy metals are widely distributed in almost all types of soils (Zhao et al., 2012), sediment (Gati et al., 2016), and water bodies (Tang et al., 2014). Some heavy metals are essential micronutrients for several cellular functions and components of biological macromolecules [for example, zinc (Zn) is an important component of DNA-polymerases] (Seiler and Berendonk, 2012), but can be toxic when accumulated to a certain concentration (for example, the excessive uptake of Zn leads to zinc-induced copper deficiency) (Fosmire, 1990; Zhao et al., 2012). In recent years, industrial (e.g., mining) and agricultural (e.g., land application of metal contained fertilizers) activities contribute to heavy metal accumulation (Charlesworth et al., 2011; Chen et al., 2015; Li L.G. et al., 2017).
Environmental compartments are subject to anthropogenic pressures (Berendonk et al., 2015). As a vital component of terrestrial ecosystems, soil microbes play a significant role in the material and energy cycle. However, microbial communities are highly sensitive to environmental changes (Kandeler et al., 2000; Blakely et al., 2002; Kelly et al., 2003; Epelde et al., 2015). The excessive heavy metals found in the soil may impose selection pressures on soil microbes (Berendonk et al., 2015) and even change the diversity of soil microbial communities (Kandeler et al., 1996, 2000; Kelly et al., 2003; Epelde et al., 2015). To cope with these situations, an effective strategy for microorganisms is to evolve a system based on biochemical and genetically encoded mechanisms (Aka and Babalola, 2017). This has been found in many bacteria strains isolated from different heavy metal polluted scenarios (Zafar et al., 2007; Sabry et al., 2010; Rehman and Anjum, 2011; Muñoz et al., 2012). Bacteria can thus be used for the remediation of heavy metal polluted areas (Aka and Babalola, 2017; Chen et al., 2018). Nevertheless, present efforts of heavy metal resistance are mainly centered on single isolated strains (Zafar et al., 2007; Sabry et al., 2010; Rehman and Anjum, 2011; Muñoz et al., 2012). A better understanding of the distribution of resistance in heavy metal contaminated area, especially the long-term polluted sites, is critical to optimize such remediation schemes.
Antibiotic resistant genes (ARGs) have been reported as a new pollutant by the World Health Organization because of their emerging prevalence and wide distribution (World Health Organization [WHO], 2014). This is a threat to public health (Rodriguez-Mozaz et al., 2015). The increasing ARGs have been recognized as a consequence of the massive use of antibiotics in therapeutics and agriculture (Levy and Marshall, 2004; Huerta et al., 2013). However, more evidence shows that the dissemination of ARGs can also be influenced by heavy metal contamination (Pruden et al., 2006; Knapp et al., 2017; Tan et al., 2018). As early as in the 1970s, it was found that heavy metal resistance and antibiotic resistance can be selected simultaneously in the heavy metal contaminant ecosystem (Timoney et al., 1978). These phenomena can be interpreted on the molecular level as co-selection (two or more genetically linked resistance genes) or cross-selection (single genetic element provides tolerance to more than one antimicrobial agents) (Baker-Austin et al., 2006; Seiler and Berendonk, 2012). The co-selection of heavy metal resistance genes (MRGs) and ARGs have been reported in agriculture (Hu et al., 2016), animal husbandry (Zhu et al., 2013), wastewater treatment system (Cesare et al., 2016), and sediment (Wright et al., 2010). However, bacterial communities are shaped by a complex array of evolutionary, ecological, and environmental factors (Berendonk et al., 2015). Patterns of MRGs and ARGs in the area of long-term heavy metal contamination are still poorly studied.
Zhongtiaoshan Copper Mine is the largest non-coal underground mine in northern China (Liu et al., 2018). A huge tailing dam was built as a result of the long-term mining processes. The body of the dam was formed by large amounts of waste residue containing multiple heavy metals. As a result, the surrounding area of the dam is contaminated by heavy metals through wind or seep water.
The purpose of this study is to assess the heavy metal and antibiotic resistance in the downstream region of the tailing dam from the following aspects: (1) what the heavy metal contamination level is in the region, (2) what the main MRGs types are and how they are distributed in this region, and (3) what the co-occurrence patterns of MRGs and ARGs are in this region. The objective of this study is to reveal the occurrence of MRGs and ARGs and the relationships between heavy metal and MRGs/ARGs in this copper tailing dam area.
Materials and Methods
Soil Sampling
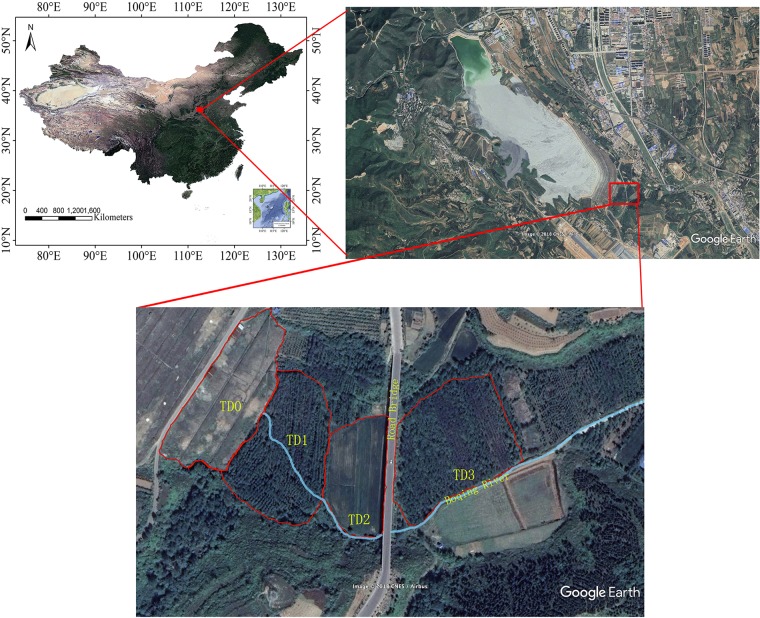
The study area (35°15′N, 111°39′E) is located in a copper tailing dam area in the southern part of Shanxi Province, China. The climate is monsoonal climate, with average annual temperature 14°C, mean annual rainfall 780 mm and annual frost-free period more than 200 days (Liu et al., 2018). The tailing dam has been used since 1972 (Figure 1). At the bottom of the tailing dam, a stream forms due to the seeping water. Four sampling sites TD0, TD1, TD2, and TD3 were selected along the stream in this study. TD0 site is on the top of the tailing dam and is covered by herbs. TD1 site is at the bottom of the dam and has been associated with poplar plantation of about 15 years. TD2 site, adjacent to TD1 site, is farmland. TD3 site is the most far away from the dam and has been associated with poplar plantation of about 10 years.
FIGURE 1.
Locations of sampling sites around the Shibahe tailings dam area in Shanxi Province, China.
Sampling was carried out in June 2016, just after the wheat harvest. The upper 20 cm of soil was collected from three random locations at the TD0 site and nine random locations at each of the other three sites. All of the soil samples were sieved, thoroughly homogenized, and divided into two portions. One portion was stored at −80°C for molecular analysis, and the other was air-dried for chemical analysis. Soil pH in ddH2O was measured at a soil:solution ratio of 1:2.5 (w:v). Soil total carbon (TC), total nitrogen (TN), and total sulfur (TS) were determined by vario macro cube elemental analyzer (vario macro cube, elementar, Germany) (Table 1).
TABLE 1.
Soil physicochemical characteristics and heavy metal content (mg kg–1 dry soil) at each sampling site.
| Sampling site | TD0 | TD1 | TD2 | TD3 | Background value |
| pH | 8.00 ± 0.02ab | 7.76 ± 0.20c | 8.04 ± 0.21a | 7.80 ± 0.11bc | – |
| TN (g/kg) | 0.58 ± 0.03c | 0.89 ± 0.16b | 1.12 ± 0.06a | 0.89 ± 0.07b | – |
| TC (g/kg) | 12.28 ± 0.26c | 16.20 ± 1.27a | 13.95 ± 0.46b | 15.86 ± 1.52a | – |
| TS (g/kg) | 1.51 ± 0.71a | 0.73 ± 0.10b | 0.59 ± 0.02b | 0.68 ± 0.11b | – |
| As | 6.94 ± 2.42b | 12.28 ± 4.32a | 7.15 ± 3.44b | 10.52 ± 3.96ab | 9.1 |
| Cd | 0.17 ± 0.08c | 1.25 ± 0.66a | 0.54 ± 0.34bc | 0.76 ± 0.25ab | 0.1 |
| Cr | 47.24 ± 20.04b | 381.91 ± 176.75a | 371.90 ± 169.15a | 339.05 ± 174.71a | 55.3 |
| Cu | 338.00 ± 184.21a | 323.28 ± 157.59a | 122.10 ± 36.15b | 157.91 ± 36.37b | 22.9 |
| Ni | 34.17 ± 7.63a | 79.51 ± 55.13a | 77.89 ± 44.53a | 101.79 ± 67.16a | 29.9 |
| Pb | 756.76 ± 221.91a | 1227.24 ± 360.65a | 983.42 ± 292.03a | 1098.53 ± 349.17a | 14.7 |
| Zn | 33.43 ± 18.91b | 77.17 ± 36.37a | 89.23 ± 23.84a | 64.67 ± 16.73ab | 63.5 |
Data are means ± standard deviation. The different letters indicate that the means are significantly different among soils (P < 0.05) with Duncan test. TN, TC, and TS represent soil total nitrogen, soil total carbon and soil total sulfur, respectively.
Heavy Metal Content Analysis
Soil samples were digested using the HNO3- HF-H2O2 method (Raven and Loeppert, 1997) in a microwave digesting apparatus (CEM MARs XPRSS, United States). The digested samples were diluted with deionized water to 50 mL. The concentrations of heavy metals (As, Cr, Cd, Cu, Ni, Pb, and Zn) in the solutions were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, iCAP 6000, Thermo Fisher, United Kingdom). A mixed standard solution including all the heavy metals and reagent blanks were carried out through digestion and analyzed as part of the quality control protocol. Results were adopted when the measured concentrations in the reference materials were within one standard deviation of the certified values (Li et al., 2012).
Ecology Risk Analysis
The potential ecological risk index (RI) is used to characterize the toxicity level of heavy metals. RI is the potential ecological risk caused by overall contamination (Hakanson, 1980), which covers a variety of research domains (e.g., biological toxicology, environmental chemistry, and ecology) (Zhai et al., 2014). RI can be used to evaluate the comprehensive ecological risks caused by a single pollutant and the overall risk from various pollutants. The assessment methods of RI are shown as follows:
Where is the single heavy metal pollution index, is the concentration of individual heavy metal in samples, is the reference value of heavy metal and usually uses the soil background value, is the monomial potential ecological risk factor of individual heavy metal, and is the heavy metal toxic response factor. The response values for each heavy metal are in the order of Zn = 1 < Cr = 2 < Cu = Pb = Ni = 5 < As = 10 < Cd = 30 (Hakanson, 1980).
DNA Extraction and Quantitative Real Time PCR
Total genomic DNA was extracted from 0.25 g fresh soils using the Qiagen DNeasy PowerSoil Kit (Qiagen, Carlsbad, CA, United States) according to the manufacturer’s instructions. DNA concentration and purity were assessed by Infinite 200 PRO (TECAN, Sweden) and the DNA concentration of each sample was adjusted to yield a concentration of 10 ng μL–1. DNA extractions were stored at −20°C for further analysis.
The heavy MRGs were selected according to the findings of Li L.G. et al. (2017) that 9 MRGs types have distinct preference for ARG types as their closest genetic neighbor, and we also selected to test the antibiotic resistance genes that are widely detected in many long-term contaminated fields in China (Hu et al., 2016, 2017). Here, we quantified the absolute abundances of 13 MRGs, 28 ARGs, and 2 mobile genes elements (MGEs) through quantitative real-time PCR (qPCR). The MRGs include 5 copper resistance genes (copA, copB, pcoA, pcoC, and pcoD), 3 multiple heavy MRGs (czcA, czcC, and czcD), 2 arsenic resistance genes (arsB and arsC) and 3 resistance genes (nccA, pbrT, and chrB to nickel, lead, chromium, respectively). The ARGs include 15 tetracycline resistance genes (tetA, tetC, tetE, tetK, tetL, tetA/P, tetG, tetM, tetO, tetQ, tetS, tetT, tetW, tetB/P, and tetX), 4 β-lactam resistance genes (blaCTX–M, blaTEM, blaSHV, and blaampC), 3 sulfonamide resistance genes (sulI, sulII, and sulIII), 3 quinolone resistance genes (qnrA, qnrB, and qnrS), and 3 macrolides resistance genes (ereA, ereB, and mphA). The MGEs include an integrase gene of class 1 integrons (intI1) and a transposon-transposase gene (tnpA).
The qPCR analysis was conducted using the iCycler iQ5 thermocycler (Bio-Rad, Hercules, CA, United States). Each sample was amplified in triplicate with 10 μL reaction mixture that consisted of 5 μL of SYBR Premix Ex TaqTM (Takara Bio, Inc., Shiga, Japan), 0.5 μL of each primer (10 mM), 3 μL of diluted template DNA, and microbial DNA-free water. The primers and thermal cycling conditions of MRGs, ARGs and MGEs are described in Supplementary Table S1. A negative control was included in each run. Melting curve analysis was performed at the end of each qPCR run to check the specificity of the amplicons.
Data Analysis
The data were square root- or log-transformed to improve normality and reduce heteroscedasticity. MRGs and ARGs data were converted to relative abundance (normalized per 16S rRNA genes) because the bacterial population size has not been taken into account for the non-normalized data. The 16S abundance rRNA genes were used to assess the size of the bacterial population. Normalizing MRGs or ARGs count to 16S rRNA genes presents an approximate proportion of bacteria that carry the corresponding genes.
One-way analysis of variance (ANOVA) was used to assess the differences of heavy metals, RI, 16S rRNA genes, MRGs, ARGs and MGEs among the study sites. Spearman linear correlation analysis was conducted to determine whether there were significant correlations between heavy metals, RI, MRGs, ARGs, and MGEs. Network analysis was used to explore the co-occurrence patterns of RI, MRGs, and ARGs.
ANOVA and Spearman linear correlation analysis were conducted in SPSS version 13.0 and R software version 3.4.4. Network analysis was performed and visualized with Gephi version 0.9.2.
Results
Physicochemical Characteristics and Risk Assessment
Soil total carbon (TC) and total nitrogen (TN) at the TD0 site are significantly lower than those at the other three sites, while the TS shows the opposite. Soil pH is significantly higher at the TD2 site than at the TD1 and TD3 sites (Table 1). There is no difference in the concentrations of Ni and Pb among the four sites; however, the concentrations of other metals (As, Cd, Cr, Cu, and Zn) are significantly different among the four sites (P < 0.05, Table 1). The concentrations of all the metals measured are highest at the TD1 site except the concentrations of Cu, Ni, and Zn that are highest in TD0, TD3, and TD2, respectively. The analysis of the overall risk shows that RI is significantly higher at the TD1 site than at the other three sites (P < 0.05, Table 2), while the TD0 site represents the lowest potential ecological risk of all the sampling sites.
TABLE 2.
Potential ecology risk index (RI) at each sampling site.
| Sampling site | TD0 | TD1 | TD2 | TD3 | Contribute to RI |
| RI-As | 7.63 ± 2.66b | 13.5 ± 4.74a | 7.86 ± 3.79b | 11.56 ± 4.35ab | 1.63% |
| RI-Cd | 49.00 ± 23.73c | 367.91 ± 192.79a | 159.13 ± 100.72bc | 223.98 ± 73.14ab | 32.08% |
| RI-Cr | 1.71 ± 0.72b | 13.81 ± 6.39a | 13.45 ± 6.12a | 12.26 ± 6.32a | 1.65% |
| RI-Cu | 73.80 ± 40.22a | 70.59 ± 34.41a | 26.66 ± 7.89b | 34.48 ± 7.94b | 8.24% |
| RI-Ni | 2.29 ± 0.51a | 5.32 ± 3.69a | 5.21 ± 2.98a | 6.81 ± 4.49a | 0.79% |
| RI-Pb | 257.40 ± 75.48a | 417.43 ± 122.67a | 334.50 ± 99.33a | 373.65 ± 118.77a | 55.45% |
| RI-Zn | 0.53 ± 0.30b | 1.22 ± 0.57a | 1.41 ± 0.38a | 1.02 ± 0.26ab | 0.17% |
| RI | 392.35 ± 37.34c | 889.78 ± 278.7a | 548.21 ± 119.19bc | 663.76 ± 148.39b | – |
Data are means ± standard deviation. The different letters indicate that the means are significantly different among soils (P < 0.05) with Duncan test. Contribute to RI means the percentage of the single metal ecological risk to the overall risk.
Absolute Abundance of 16S rRNA Genes, MRGs, ARGs, and MGEs
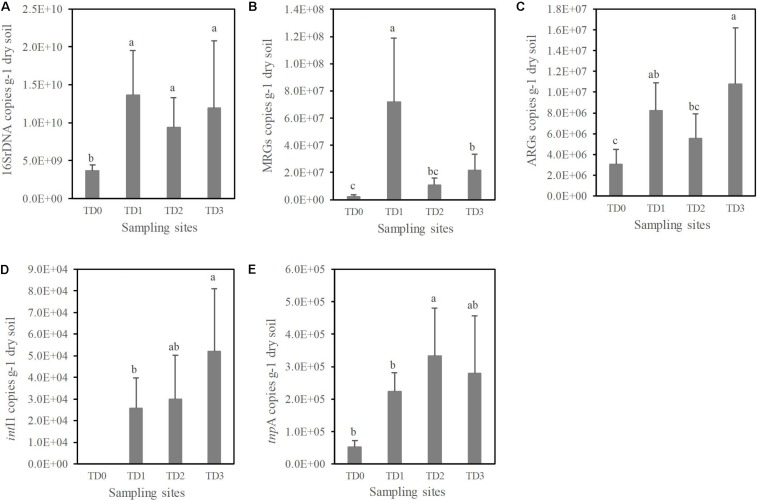
Quantitative PCR was performed to analyze the abundances of total bacteria (by targeting the bacterial 16S rRNA genes), MRGs, ARGs, and MGEs. The abundance of 16S rRNA genes at the TD0 site is significantly lower than at the other sites (P < 0.01, Figure 2A). A total of 10 MRGs, 17 ARGs, and 2 MGEs (out of 13 MRGs, 28 ARGs, and 2 MGEs targeted) are detected in this study. The lowest abundances of MRGs and ARGs are found at the TD0 site (Figures 2B,C). The highest abundance of MRGs is found at the TD1 site, while the highest abundance of ARGs is found at the TD3 site. There is a significant difference for MGEs among all the sampling sites (P < 0.05). The MGEs shows the lowest level at the TD0 site, and it has not been detected for the intI1 gene at the TD0 site (Figures 2D,E).
FIGURE 2.
The abundances of 16S rDNA (A), total detected metal resistance genes (MRGs) (B), total detected antibiotic resistance genes (ARGs) (C) and mobile gene elements (intI1 and tnpA) (D,E) at each sampling site. TD0 site is on the tailing dam and covered with annual herbs. TD1 site with about 15 years of poplar plantation is at the bottom of the dam. TD2 site is farmland and adjacent to TD1 site. TD3 site with about 10 years of poplar plantation is the farthest away from the dam. Columns show the means of replicates within the sampling sites, and vertical bars show standard error. Different letters indicate that the means are significantly different among sampling sites with Duncan test (P < 0.05).
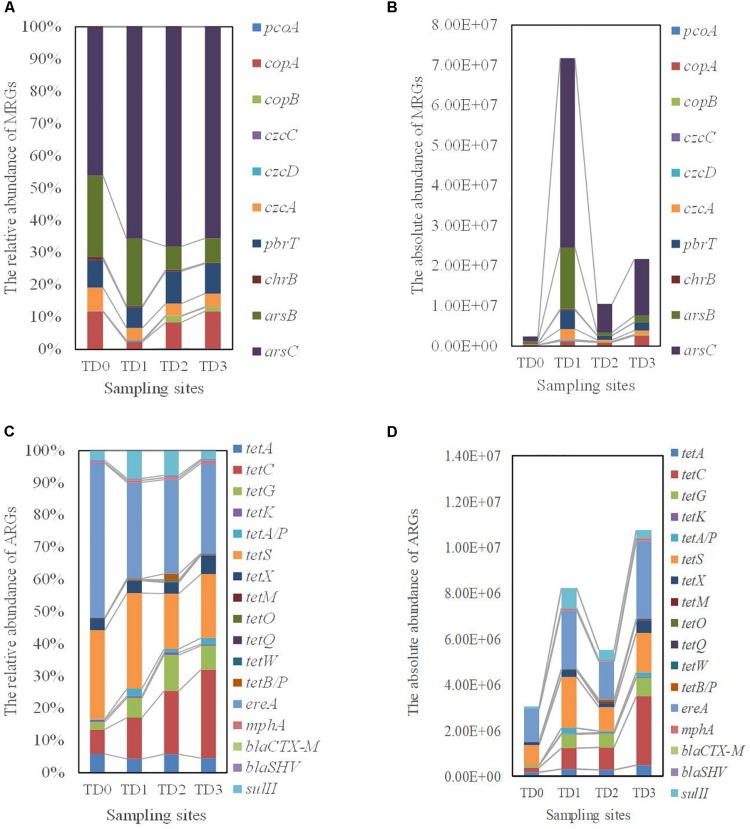
The Distribution Patterns of MRGs
Of all the tested MRGs genes, arsB, arsC, pbrT, czcA, and copA genes are major components at all the sampling sites. In particular, the arsC gene (coding for resistance mechanism to arsenic) accounts for nearly 46.13, 65.65, 68.48, and 65.52% of the total MRGs at the TD0, TD1, TD2, and TD3 site, respectively (Figure 3A). copB genes have not been detected at the TD0 and TD1 sites, and czcC genes have only been detected at the TD1 site. In accordance with the sampling along the seeping stream, an increasing trend of all the copper resistance genes (pcoA, copA, and copB) is observed, while all the other resistance genes is found at the highest abundance at the TD1 site and there are significant differences in these genes among all the sampling sites (P < 0.01) (Figure 3B and Supplementary Table S2).
FIGURE 3.
The distribution of Metal resistance genes (MRGs) and antibiotic resistance genes (ARGs) detected at each sampling site. TD0 site is on the tailing dam and covered with annual herbs. TD1 site with about 15 years of poplar plantation is at the bottom of the dam. TD2 site is farmland and adjacent to TD1 site. TD3 site with about 10 years of poplar plantation is the farthest away from the dam. (A) The distribution of relative abundance of MRGs. (B) The distribution of absolute abundance of MRGs. (C) The distribution of relative abundance of ARGs. (D) The distribution of absolute abundance of ARGs.
The Distribution Patterns of ARGs
The main genes of ARGs detected in this study are five tetracyclines resistance genes (tetA, tetC, tetG, tetS, and tetX), one sulfonamides resistance gene (sulII), and one macrolides resistances gene (ereA). They represent 98.08, 95.21, 94.07, and 95.66% of the total ARGs at the TD0, TD1, TD2, and TD3 sites, respectively (Figure 3C). The ereA gene has the largest distribution at each site, and it contributes to 48.04, 29.83, 29.22, and 28.00% of the total ARGs at each site, respectively (Figure 3C). When all ARGs are concerned, there are significant differences in tetC, tetG, tetA/P, tetX, tetM, tetO, tetQ, tetW, tetB/P, mphA, and sulII genes among the sampling sites (P < 0.05, Supplementary Table S3).
The ARGs patterns are more complex than MRGs. ARGs patterns can be classified as the following aspects: (1) there is no difference in the relative abundance of tetA, tetK, tetS, ereA, blaCTX–M, and blaSHV genes among the sampling sites, and the highest abundance of these genes is found at the TD0 site apart from tetK and blaCTX–M genes. (2) Similar to the majority of MRGs, the sulII gene is significantly more abundant at the TD1 site than at the other three sites. (3) The tetO, tetW, and tetB/P genes, which are not detected at the TD0 site, are significantly more abundant at the TD2 site than at the TD1 and TD3 sites. (4) There are significant differences in the relative abundance of the tetC, tetG, tetA/P, tetX, tetM, tetQ, and mphA genes among the sampling sites, with the highest abundance of those genes at the TD3 site (Figure 3D).
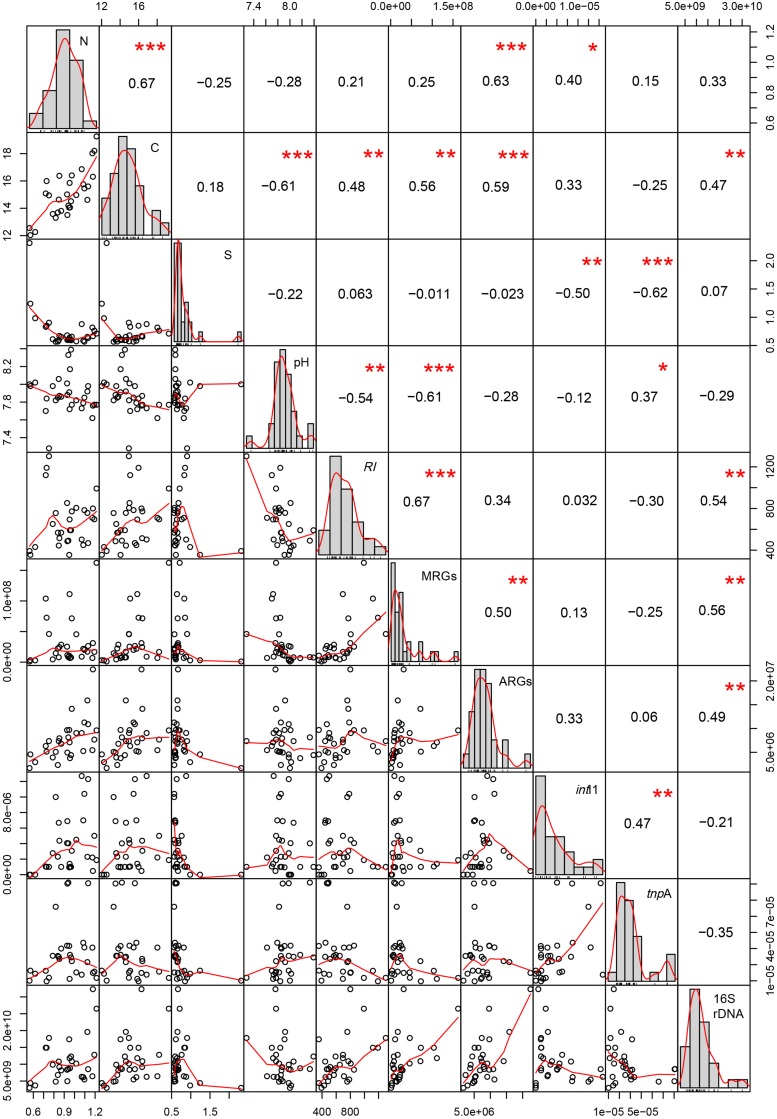
Correlations Between the RI, MRGs, ARGs, and MGEs
The potential RI is positively correlated with soil TC, the abundance of MRGs, and 16S rRNA genes (P < 0.01), and is negatively correlated with soil pH (P < 0.01, Figure 4). The abundance of MRGs is strongly positively correlated with TC, the abundance of ARGs and 16S rRNA genes (P < 0.01), and is negatively correlated with soil pH (P < 0.01). The abundance of ARGs is correlated with soil TN, TC, and the abundance of 16S rRNA genes. The abundance of intI1 gene is positively correlated with the abundance of the tnpA gene (P < 0.01) and TN (P < 0.05), and negatively correlated with soil TS. The abundance of the tnpA gene is positively correlated with soil pH (P < 0.05) and negatively correlated with TS (P < 0.01). The abundance of MGEs has no significant correlation with 16S rRNA genes (P > 0.05).
FIGURE 4.
Correlations between soil physicochemical characteristics, RI, MRGs, ARGs, MGEs and 16S rDNA. ∗∗∗ Means that correlation is significant at the 0.001 level (two-tailed). ∗∗ Means that correlation is significant at the 0.01 level (two-tailed). ∗ Means that correlation is significant at the 0.05 level (two-tailed).
Co-occurrence Patterns of MRGs and ARGs
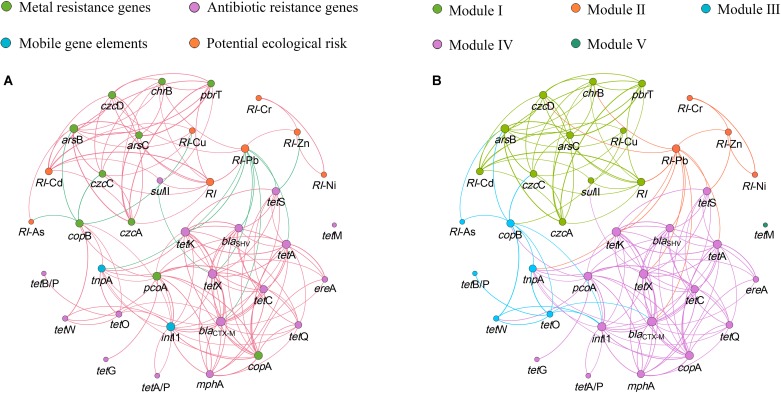
Network analysis is conducted to explore the co-occurrence patterns among the relative abundance of MRGs, ARGs, and MGEs based on significant correlation analysis by Spearman correlation (P < 0.05). The resultant network consists of 37 nodes (8 RIs, 10 MRGs, 17 ARGs, and 2 MGEs) and 144 edges, and can be clearly separated into five modules (Figure 5). Genes in the same module may co-occur under the same environmental pressure. The majority of MRGs and ARGs coexist within their internal genes, while the copA and pcoA may co-exist with many ARGs, and sulII may co-exist with many MRGs. Genes co-existence with the MGEs indicate the mobility of these genes in the same module. The most densely connected node in each module is defined as ‘hubs,’ for example, in module III, blactx–M may be the hub. The hubs can act as indicators to represent the quantity of other co-occurring genes in the same module.
FIGURE 5.
The network analysis showing the co-occurrence patterns among the detected MRGs, ARGs, MGEs and RI. (A) The nodes with different colors represent MRGs, ARGs, MGEs and RI, respectively. The red edges correspond to positive correlations, and the blue edges correspond to negative correlations. (B) The nodes with different colors represent different modularity classes, and the colors of edges correspond to the different modules. A connection stands for a strong (ρ > 0.6) and significant (P < 0.05) correlation. The size of each node is proportional to the number of connections.
Discussion
Heavy Metal Contamination and Bacterial Abundance
The RI results of heavy metals in the study area show that the TD1 site is suffering more ecological risk than the other three sites, and the TD0 site is at the lowest ecological risk level (Table 2). This distribution pattern results mainly from the accumulations of As, Cd, Cr, and Pb at the TD1 site (Table 1). This is probably related with the unique terrain conditions of this sampling site and the migration of heavy metals. There are two main sources of heavy metals for the TD1 site, of which one is the dissolved load with the leachate from the tailing dam (TD0) and the other is the sediment transported with the wash load (Merrington and Alloway, 1994). A similar accumulation pattern was observed in a similar area (Merrington and Alloway, 1994). The migration of metals in a dissoluble state in such an area can be achieved through the oxidation of metal sulfides, which aggravates the process of leaching when the solubility of heavy metal is increased in the form of metal sulfates (Simón et al., 2001).
The TS concentration is significantly higher at the TD0 site than at the other three sites (Table 1), and the soil at the TD0 site is alkalescence with the pH 8.00 ± 0.02 (Table 1). The high concentration of sulfur and the alkaline conditions indicate that the heavy metals exist in the form of metal sulfides at the TD0 site, which limits the migration of heavy metals to some extent. This interpretation is also supported by the significantly negative correlation between pH and RI (Figure 4). Thus, the transport of heavy metals from the TD0 site to the TD1 site is mainly the result of wash load. Evidence also shows that afforestation could effectively inhibit the oxidation of metal sulfides in the mining wasteland and reduce the production of acid wastewater and the release of heavy metals (Néel et al., 2003; Changul et al., 2010; Yang et al., 2010). After the accumulation of wash load, heavy metals can be fixed in the soil of the TD1 site under the influence of vegetation.
Although the highest heavy metal content is observed at the TD1 site, the TD1 site has the highest abundance of bacteria indicated by the abundance of the 16S rRNA genes (Figure 2A). Heavy metal contamination modifies the microbial diversity and biomass, because bacteria community is sensitive to heavy metals (Giller et al., 1998; Azarbad et al., 2015). Most studies have shown that high contamination of heavy metals can significantly decrease the soil bacterial biomass (Fließbach et al., 1994; Chander et al., 1995; Stefanowicz et al., 2010), but these studies focused on the short-term impacts of toxic pollutants. However, under a long-term polluted circumstance, Bourceret et al. (2015) found high richness and diversity of the bacterial community, and this is because edaphic parameters (nutrients like soil organic matter, soil available phosphorous, etc.) are increased simultaneously over the time of remediation. In this study, we also found a significant correlation between the soil bacterial abundance and TC (Figure 4).
Heavy Metal and Metal Resistance
Heavy metals can form complex compounds, of which some are important for micronutrient physiological activities in all living microorganisms (Nies, 1999). However, most complex compounds are toxic at high concentrations (Nies, 1999). It is critical for microbial communities to develop the resistant systems (specific or unspecific) under long-term heavy metal stresses (Nies, 1999; Hemme et al., 2010; Chen et al., 2018). Such systems with the genetic basis of MRGs are inducible under adverse circumstances (Nies and Silver, 1995). The MRGs at the TD1 site are more abundant than at the other three sites (Figure 2B); this difference is also revealed by RI at the four sampling sites. This suggests that high RI would induce more bacterial resistance in the long-term contaminated area, which is also supported by the significant correlation between the abundance of MRGs and RI (P < 0.001) (Figure 4).
Soil copper in the form of divalent copper is dominant in our study site; both copA and pcoA function by the way of oxidizing Cu+ to Cu2+ for the purpose of detoxifying (Rensing and Grass, 2003). Therefore, both pcoA and copA genes are not significantly correlated with soil Cu contents (Supplementary Table S4). This relationship was also observed in sediment (Roosa et al., 2014). The copB gene in TD2 and TD3 is associated with low Cu concentration (Table 1 and Supplementary Table S2), indicating that copB system may be functioned in low Cu circumstance.
Among these MRGs at all the sampling sites, arsB and arsC genes are the main components and they have the similar distribution pattern with arsenic and RI (Tables 1, 2, Figure 3A, and Supplementary Table S2). Arsenic is positively correlated with the abundances of arsB and arsC genes (Supplementary Table S4). The significantly positive correlation between arsenic and ars gene was also found in the batch growth experiments (Poirel et al., 2013). Arsenic can induce the expression of ars genes (Tauriainen et al., 1997; Lópezmaury et al., 2003; Ordóñez et al., 2005), and ars genes may be suitable for exploring the microbial response to arsenic stress.
There are significant correlations between Cd and its target resistant genes (czcA, czcC, czcD). Meanwhile, Cd is also significantly correlated with pbrT, chrB, arsB, and arsC, respectively (P < 0.05) (Supplementary Table S4). This can be explained by the fact that different resistance genes may be located on the same unit of selection such as the same plasmid, transposon, or genome (Monchy et al., 2007). Van Houdt et al. (2009) reported that both Pb and Zn resistance increased because the transposon named Tn6048 proliferated under the Pb contaminated environment. Cd with a ‘toxic-response’ factor equal to 30 and Pb with a ‘toxic-response’ factor equal to 5 (Hakanson, 1980) contribute 32.08 and 55.45% to RI, respectively (Table 2). However, the abundance of MRGs is strongly correlated with Cd rather than Pb (Supplementary Table S4), and the network analysis reveals that MRGs and RI-Cd, not RI-Pb, were found in Module I (Figure 5). This indicates that heavy metal with high ‘toxic-response’ factor may be an indicator of the MRGs in the heavy metal contaminated area.
Heavy Metal and the Co-occurrence of MRGs and ARGs
There are 17 ARGs detected in the copper tailing dam area (Supplementary Table S3), and ARGs exist in many metal contaminated circumstances (Hu et al., 2016, 2017). Correlations exist between metals and ARGs, but the trends are diversified (Knapp et al., 2017). In this study, significant correlations are observed between specific ARGs and heavy metals (Figure 5 and Supplementary Table S5). For example, Zn is significantly correlated with sulII. Ji et al. (2012) reported that Zn was positively correlated with sulI and sulIII, rather than sulII in agriculture soil and manure samples. However, sulI and sulIII are not detected in this study. The different samples may account for the distinct results. Previous studies have reported that Zn directly triggers the selection of tetracycline resistance genes including tetA, tetC, and tetG, genes functioning as an efflux pump system (Yamaguchi et al., 1990; Palm et al., 2008). We find that Zn is significantly correlated with tetW and tetB/P, genes encoding ribosomal protection proteins, which are detected in metal polluted areas (Berg et al., 2010; Knapp et al., 2011, 2017). This indicates that Zn may induce both the efflux pump system and ribosomal protection proteins.
The network analysis reveals strong correlation between sulII and MRGs in Module I. pcoA and most of ARGs are detected in Module III (Figure 5), suggesting the possibility of the co-occurrence of MRGs and ARGs in soil microbe. The co-occurrence of MRGs and ARGs is verified by an IncA/C plasmid carrying mer operon (coding for resistance mechanism to mercury) and ARGs including sulII harbored in the Aeromonas salmonicida sub sp. salmonicida strains isolated from aquaculture facilities (McIntosh et al., 2008). IntI1 and tnpA are in the different modules, and show different correlations with each specific MRGs and ARGs (Figure 5). The similar correlations between MGEs and ARGs were observed in greenhouse soils with long-term dairy cattle and chicken manure (Li J. et al., 2017). Previous studies reported that the same ARGs or MRGs could be located in the same or different conjugative MGEs (Gillings et al., 2008; Herrick et al., 2014). Future studies are necessary to carry out detailed investigations of the horizontal transfer mechanism of MRGs and ARGs.
Conclusion
This study selected a long-term heavy metal contaminated area, the Shibahe copper tailing dam area in Mountain Zhongtiaoshan of northern China, analyzed the levels of the contamination of multiple metals, and explored the relationship between soil microbial resistance and multi-metal contamination. Based on in situ monitoring, RI is adopted to evaluate the level of contamination. The abundance of soil bacteria is high at the high-RI site, and this is probably attributed to high soil nutrient and strong bacterial resistance. Both MRGs and ARGs exist in the multi-metal polluted soil. Of the MRGs, arsC coding for resistance mechanism to arsenic is at the highest abundance; ereA, which belongs to a deactivate system against macrolides, is the most abundant among the ARGs. The abundance of MRGs is positively correlated with Cd concentration, indicating that Cd plays a key role in the selection of MRGs. For ARGs, the abundances of tetW and tetB/P are significantly correlated with Zn concentration, which indicates that Zn may induce the antibiotic resistance of ribosome protection in such a long-term copper tailing dam area. The network analysis results show that sulII and MRGs co-occur and copB occur with ARGs, and this suggests that MRGs and ARGs can be co-selected in the heavy metal contaminated soil. The network analysis also reveals the co-occurrence of Cd and MRGs, and accordingly heavy metal with a high ‘toxic-response’ factor can be used to indicate the occurrence of MRGs. In all, this study highlights the necessity to consider the contamination of multi heavy metals when assessing microbial resistance, and further improves the understanding of the relationship between bacterial resistance and multi-metal contamination. Meanwhile, our findings are alarming for the future evaluation of public health risk associated with heavy metal-induced selection of ARGs in multi-metal polluted environments.
Author Contributions
JC wrote the main manuscript and prepared the figures. JL helped with the sampling design and prepared the data analytical methods. WS helped in the field work, sampling, and soil parameter achieving. HZ prepared the assessment method. YL was responsible for project administration and funding acquisition. HZ and YL provided many suggestions for the experimental design and implementation. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Zhengjun Feng, Shuang Liu, and Chao Su for their helpful discussions and the laboratory team of the Institute of Loess Plateau for their technical assistance.
Footnotes
Funding. This work was financially supported by the National Natural Science Foundation of China (Grant Number: 41771548), the Key R&D Program of Shanxi Province (201803D31024), and the innovation projects of Shanxi Provincial Education Department of China (2018), sponsored by “1331 Project” Soil Pollution Ecological Restoration Group of Shanxi Province, China (Grant Number: 20181401).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01916/full#supplementary-material
References
- Aka R. J. N., Babalola O. O. (2017). Identification and characterization of Cr-, Cd-, and Ni-tolerant bacteria isolated from mine tailings. Bioremediat. J. 21 1–19. 10.1080/10889868.2017.1282933 [DOI] [Google Scholar]
- Azarbad H., Niklińska M., Laskowski R., van Straalen N. M., van Gestel C. A., Zhou J., et al. (2015). Microbial community composition and functions are resilient to metal pollution along two forest soil gradients. FEMS Microbiol. Ecol. 91 1–11. 10.1093/femsec/fiu003 [DOI] [PubMed] [Google Scholar]
- Baker-Austin C., Wright M. S., Stepanauskas R., Mcarthur J. V. (2006). Co-selection of antibiotic and metal resistance. Trends Microbiol. 14 176–182. 10.1016/j.tim.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Berendonk T. U., Manaia C. M., Merlin C., Fatta-Kassinos D., Cytryn E., Walsh F., et al. (2015). Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 13 310–317. 10.1038/nrmicro3439 [DOI] [PubMed] [Google Scholar]
- Berg J., Tom-Petersen A., Nybroe O. (2010). Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett. Appl. Microbiol. 40 146–151. 10.1111/j.1472-765x.2004.01650.x [DOI] [PubMed] [Google Scholar]
- Blakely J. K., Neher D. A., Spongberg A. L. (2002). Soil invertebrate and microbial communities, and decomposition as indicators of polycyclic aromatic hydrocarbon contamination. Appl. Soil Ecol. 21 71–88. 10.1016/s0929-1393(02)00023-9 [DOI] [Google Scholar]
- Bourceret A., Cébron A., Tisserant E., Poupin P., Bauda P., Beguiristain T., et al. (2015). The bacterial and fungal diversity of an aged PAH- and heavy metal-contaminated soil is affected by plant cover and edaphic parameters. Microb. Ecol. 71 711–724. 10.1007/s00248-015-0682-8 [DOI] [PubMed] [Google Scholar]
- Cesare A. D., AndreaDi Eckert E. M., D’Urso S., Bertoni R., Gillan D. C., Wattiez R., et al. (2016). Co-occurrence of integrase 1, antibiotic and heavy metal resistance genes in municipal wastewater treatment plants. Water Res. 94 208–214. 10.1016/j.watres.2016.02.049 [DOI] [PubMed] [Google Scholar]
- Chander K., Brookes P. C., Harding S. A. (1995). Microbial biomass dynamics following addition of metal-enriched sewage sludges to a sandy loam. Soil Biol. Biochem. 27 1409–1421. 10.1016/0038-0717(95)00074-o [DOI] [Google Scholar]
- Changul C., Sutthirat C., Padmanahban G., Tongcumpou C. (2010). Chemical characteristics and acid drainage assessment of mine tailings from Akara Gold mine in Thailand. Environ. Earth Sci. 60 1583–1595. 10.1007/s12665-009-0293-0 [DOI] [Google Scholar]
- Charlesworth S., Miguel E. D., Ordóñez A. (2011). A review of the distribution of particulate trace elements in urban terrestrial environments and its application to considerations of risk. Environ. Geochem. Health 33 103–123. 10.1007/s10653-010-9325-7 [DOI] [PubMed] [Google Scholar]
- Chen H., Teng Y., Lu S., Wang Y., Wang J. (2015). Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 51 143–153. 10.1016/j.scitotenv.2015.01.025 [DOI] [PubMed] [Google Scholar]
- Chen Y., Jiang Y., Huang H., Mou L., Ru J., Zhao J., et al. (2018). Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 637–638 1400–1412. 10.1016/j.scitotenv.2018.05.109 [DOI] [PubMed] [Google Scholar]
- Epelde L., Lanzén A., Blanco F., Urich T., Garbisu C. (2015). Adaptation of soil microbial community structure and function to chronic metal contamination at an abandoned Pb-Zn mine. FEMS Microbiol. Ecol. 91 1–11. 10.1093/femsec/fiu007 [DOI] [PubMed] [Google Scholar]
- Fließbach A., Martens R., Reber H. H. (1994). Soil microbial biomass and microbial activity in soils treated with heavy metal contaminated sewage sludge. Soil Biol. Biochem. 26 1201–1205. 10.1016/0038-0717(94)90144-9 [DOI] [Google Scholar]
- Fosmire G. J. (1990). Zinc toxicity. Am. J. Clin. Nutr. 51 225–227. [DOI] [PubMed] [Google Scholar]
- Gati G., Pop C., Brudaşcă F., Gurzãu A. E., Spînu M. (2016). The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 25 688–696. 10.1007/s10646-016-1627-9 [DOI] [PubMed] [Google Scholar]
- Giller K. E., Witter E., Mcgrath S. P. (1998). Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol. Biochem. 30 1389–1414. 10.1016/j.envint.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Gillings M., Boucher Y., Labbate M., Holmes A., Krishnan S., Holley M., et al. (2008). The evolution of class 1 integrons and the rise of antibiotic resistance. J. Bacteriol. 190 5095–5100. 10.1128/JB.00152-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakanson L. (1980). An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 14 975–1001. 10.1016/0043-1354(80)90143-8 [DOI] [Google Scholar]
- Hemme C. L., Deng Y., Gentry T. J., Fields M. W., Wu L., Barua S., et al. (2010). Metagenomic insights into evolution of heavy metal-contaminated groundwater microbial community. ISME J. Emultidiscip. J. Microb. Ecol. 4 660–672. 10.1038/ismej.2009.154 [DOI] [PubMed] [Google Scholar]
- Herrick J. B., Haynes R., Heringa S., Brooks J. M., Sobota L. T. (2014). Coselection for resistance to multiple late-generation human therapeutic antibiotics encoded on tetracycline resistance plasmids captured from uncultivated stream and soil bacteria. J. Appl. Microbiol. 117 380–389. 10.1111/jam.12538 [DOI] [PubMed] [Google Scholar]
- Hu H., Wang J. T., Jing L., Shi X., Ma Y., Chen D., et al. (2017). Long-term nickel contamination increases the occurrence of antibiotic resistance genes in agricultural soils. Environ. Sci. Technol. 51 790–800. 10.1021/acs.est.6b03383 [DOI] [PubMed] [Google Scholar]
- Hu H. W., Wang J. T., Li J., Li J. J., Ma Y. B., Chen D., et al. (2016). Field-based evidence for copper contamination induced changes of antibiotic resistance in agricultural soils. Environ. Microbiol. 18 3896–3909. 10.1111/1462-2920.13370 [DOI] [PubMed] [Google Scholar]
- Huerta B., Marti E., Gros M., López P., Pompêo M., Armengol J., et al. (2013). Exploring the links between antibiotic occurrence, antibiotic resistance, and bacterial communities in water supply reservoirs. Sci. Total Environ. 456-457 161–170. 10.1016/j.scitotenv.2013.03.071 [DOI] [PubMed] [Google Scholar]
- Ji X., Shen Q., Liu F., Ma J., Xu G., Wang Y., et al. (2012). Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. J. Hazard. Mater. 235-236 178–185. 10.1016/j.jhazmat.2012.07.040 [DOI] [PubMed] [Google Scholar]
- Kandeler E., Tscherko D., Bruce K. D., Stemmer M., Hobbs P. J., Bardgett R. D., et al. (2000). Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol. Fertil. Soils 32 390–400. 10.1007/s003740000268 16478493 [DOI] [Google Scholar]
- Kandeler F., Kampichler C., Horak O. (1996). Influence of heavy metals on the functional diversity of soil microbial communities. Biol. Fertil. Soils 23 299–306. 10.1007/bf00335958 [DOI] [Google Scholar]
- Kelly J. J., Häggblom M. M., Tate R. L. (2003). Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter as indicated by analysis of microbial community phospholipid fatty acid profiles. Biol. Fertil. Soils 38 65–71. 10.1007/s00374-003-0642-1 [DOI] [Google Scholar]
- Knapp C. W., Callan A. C., Aitken B., Shearn R., Koenders A., Hinwood A. (2017). Relationship between antibiotic resistance genes and metals in residential soil samples from Western Australia. Environ. Sci. Pollut. Res. Int. 24 2484–2494. 10.1007/s11356-016-7997-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C. W., Mccluskey S. M., Singh B. K., Campbell C. D., Hudson G., Graham D. W. (2011). Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived scottish soils. PLoS One 6:e27300. 10.1371/journal.pone.0027300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Marshall B. (2004). Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. Suppl. 10(12 Suppl.), S122–S129. [DOI] [PubMed] [Google Scholar]
- Li J., Xin Z., Zhang Y., Chen J., Yan J., Li H., et al. (2017). Long-term manure application increased the levels of antibiotics and antibiotic resistance genes in a greenhouse soil. Appl. Soil Ecol. 121 193–200. 10.1016/j.apsoil.2017.10.007 [DOI] [Google Scholar]
- Li L. G., Xia Y., Zhang T. (2017). Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection. ISME J. 11 651–662. 10.1038/ismej.2016.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Chen Y., Fu H., Cui Z., Shi L., Wang L., et al. (2012). Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazard. Mater. 227-228 148–154. 10.1016/j.jhazmat.2012.05.023 [DOI] [PubMed] [Google Scholar]
- Liu J., Li C., Jing J., Zhao P., Luo Z., Cao M., et al. (2018). Ecological patterns and adaptability of bacterial communities in alkaline copper mine drainage. Water Res. 133 99–109. 10.1016/j.watres.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Lópezmaury L., Florencio F. J., Reyes J. C. (2003). Arsenic Sensing and resistance system in the Cyanobacterium Synechocystis sp. Strain PCC 6803. J. Bacteriol. 185 5363–5371. 10.1128/jb.185.18.5363-5371.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D., Cunningham M. B., Fekete F., Parry E., Clark S., Zalinger Z., et al. (2008). Transferable, multiple antibiotic and mercury resistance in Atlantic Canadian isolates of Aeromonas salmonicida subsp. salmonicida is associated with carriage of an IncA/C plasmid similar to the Salmonella enterica plasmid pSN254. J. Antimicrob. Chemother. 61 1221–1228. 10.1093/jac/dkn123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrington G., Alloway B. J. (1994). The transfer and fate of Cd, Cu, Pb and Zn from two historic metalliferous mine sites in the U.K. Appl. Geochem. 9 677–687. 10.1016/0883-2927(94)90027-2 [DOI] [Google Scholar]
- Monchy S., Benotmane M. A., Janssen P., Vallaeys T., Taghavi S., Van D. D. L., et al. (2007). Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J. Bacteriol. 189 7417–7425. 10.1128/jb.00375-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz A. J., Ruiz E., Abriouel H., Gálvez A., Ezzouhri L., Lairini K., et al. (2012). Heavy metal tolerance of microorganisms isolated from wastewaters: identification and evaluation of its potential for biosorption. Chem. Eng. J. 210 325–332. 10.1016/j.cej.2012.09.007 [DOI] [Google Scholar]
- Néel C., Bril H., Courtin-Nomade A., Dutreuil J. P. (2003). Factors affecting natural development of soil on 35-year-old sulphide-rich mine tailings. Geoderma 111 1–20. 10.1016/s0016-7061(02)00237-9 [DOI] [Google Scholar]
- Nies D. H. (1999). Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51 730–750. 10.1007/s002530051457 [DOI] [PubMed] [Google Scholar]
- Nies D. H., Silver S. (1995). Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14 186–199. 10.1007/bf01569902 [DOI] [PubMed] [Google Scholar]
- Ordóñez E., Letek M., Valbuena N., Gil J. A., Mateos L. M. (2005). Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032. Appl. Environ. Microbiol. 71 6206–6215. 10.1128/aem.71.10.6206-6215.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm G. J., Lederer T., Orth P., Saenger W., Takahashi M., Hillen W., et al. (2008). Specific binding of divalent metal ions to tetracycline and to the Tet repressor/tetracycline complex. JBIC J. Biol. Inorg. Chem. 13 1097–1110. 10.1007/s00775-008-0395-2 [DOI] [PubMed] [Google Scholar]
- Poirel J., Joulian C., Leyval C., Billard P. (2013). Arsenite-induced changes in abundance and expression of arsenite transporter and arsenite oxidase genes of a soil microbial community. Res. Microbiol. 164 457–465. 10.1016/j.resmic.2013.01.012 [DOI] [PubMed] [Google Scholar]
- Pruden A., Pei R., Storteboom H., Carlson K. H. (2006). Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environ. Sci. Technol. 40 7445–7450. 10.1021/es060413l [DOI] [PubMed] [Google Scholar]
- Raven K. P., Loeppert R. H. (1997). Trace element composition of fertilizers and soil amendments. J. Environ. Qual. 26 2947–2971. [Google Scholar]
- Rehman A., Anjum M. S. (2011). Multiple metal tolerance and biosorption of cadmium by Candida tropicalis isolated from industrial effluents: glutathione as detoxifying agent. Environ. Monit. Assess. 174 585–595. 10.1007/s10661-010-1480-x [DOI] [PubMed] [Google Scholar]
- Rensing C., Grass G. (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27 197–213. 10.1016/s0168-6445(03)00049-4 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mozaz S., Chamorro S., Marti E., Huerta B., Gros M., Sànchez-Melsió A., et al. (2015). Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 69 234–242. 10.1016/j.watres.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Roosa S., Wattiez R., Prygiel E., Lesven L., Billon G., Gillan D. C. (2014). Bacterial metal resistance genes and metal bioavailability in contaminated sediments. Environ. Pollut. 189 143–151. 10.1016/j.envpol.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Sabry S. A., Ghozlan H. A., Abou-zeid D. M. (2010). Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. J. Appl. Microbiol. 82 245–252. 10.1111/j.1365-2672.1997.tb02858.x [DOI] [PubMed] [Google Scholar]
- Sawut R., Kasim N., Maihemuti B., Hu L., Abliz A., Abdujappar A., et al. (2018). Pollution characteristics and health risk assessment of heavy metals in the vegetable bases of northwest China. Sci. Total Environ. 642 864–878. 10.1016/j.scitotenv.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Seiler C., Berendonk T. U. (2012). Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 3:399. 10.3389/fmicb.2012.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón M., MartıìN F., Ortiz I., GarcıìA I., Fernández J., Fernández E., et al. (2001). Soil pollution by oxidation of tailings from toxic spill of a pyrite mine. Sci. Total Environ. 279 63–74. 10.1016/s0048-9697(01)00726-4 [DOI] [PubMed] [Google Scholar]
- Stefanowicz A. M., Nikliñska M., Laskowski R. (2010). Metals affect soil bacterial and fungal functional diversity differently. Environ. Toxicol. Chem. 27 591–598. [DOI] [PubMed] [Google Scholar]
- Tan L., Li L., Ashbolt N., Wang X., Cui Y., Zhu X., et al. (2018). Arctic antibiotic resistance gene contamination, a result of anthropogenic activities and natural origin. Sci. Total Environ. 621 1176–1184. 10.1016/j.scitotenv.2017.10.110 [DOI] [PubMed] [Google Scholar]
- Tang W., Shan B., Zhang H., Zhang W., Zhao Y., Ding Y., et al. (2014). Heavy metal contamination in the surface sediments of representative limnetic ecosystems in Eastern China. Sci. Rep. 4:7152. 10.1038/srep07152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriainen S., Karp M., Chang W., Virta M. (1997). Recombinant luminescent bacteria for measuring bioavailable arsenite and antimonite. Appl. Environ. Microbiol. 63 4456–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoney J. F., Port J., Giles J., Spanier J. (1978). Heavy-metal and antibiotic resistance in the bacterial flora of sediments of New York Bight. Appl. Environ. Microbiol. 36 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdt R., Monchy S., Leys N., Mergeay M. (2009). New mobile genetic elements in Cupriavidus metallidurans CH34, their possible roles and occurrence in other bacteria. Antonie Van Leeuwenhoek 96 205–226. 10.1007/s10482-009-9345-4 [DOI] [PubMed] [Google Scholar]
- World Health Organization [WHO] (2014). Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization; 237. [Google Scholar]
- Wright M. S., Peltier G. L., Stepanauskas R., Mcarthur J. V. (2010). Bacterial tolerances to metals and antibiotics in metal-contaminated and reference streams. FEMS Microbiol. Ecol. 58 293–302. 10.1111/j.1574-6941.2006.00154.x [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Udagawa T., Sawai T. (1990). Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J. Biol. Chem. 265 4809–4813. [PubMed] [Google Scholar]
- Yang S. X., Liao B., Li J. T., Guo T., Shu W. S. (2010). Acidification, heavy metal mobility and nutrient accumulation in the soil-plant system of a revegetated acid mine wasteland. Chemosphere 80 852–859. 10.1016/j.chemosphere.2010.05.055 [DOI] [PubMed] [Google Scholar]
- Zafar S., Aqil F., Ahmad I. (2007). Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 98 2557–2561. 10.1016/j.biortech.2006.09.051 [DOI] [PubMed] [Google Scholar]
- Zhai Y., Chen H., Xu B., Xiang B., Chen Z., Li C., et al. (2014). Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresour. Technol. 159 72–79. 10.1016/j.biortech.2014.02.049 [DOI] [PubMed] [Google Scholar]
- Zhao H., Xia B., Fan C., Zhao P., Shen S. (2012). Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Sci. Total Environ. 41 45–54. 10.1016/j.scitotenv.2011.12.047 [DOI] [PubMed] [Google Scholar]
- Zhu Y. G., Johnson T. A., Su J. Q., Qiao M., Guo G. X., Stedtfeld R. D., et al. (2013). Diverse and abundant antibiotic resistance genes in Chinese swine farms. PNAS 110 3435–3440. 10.1073/pnas.1222743110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.