Abstract
Background and purpose
Premature ovarian insufficiency (POI) refers to a hypergonadotropic hypoestrogenism and the condition of pre-onset ovarian function failure. Epimedium is a common traditional Chinese herbal medicine that is widely used to relieve POI in China. To systematically explore the pharmacological mechanism of epimedium on POI therapy, a network pharmacology approach was conducted at the molecular level.
Methods
In this study, we adopt the network pharmacology method, which mainly includes active ingredients prescreening, target prediction, gene enrichment analysis and network analysis.
Results
The network analysis revealed that 6 targets (ESR1, AR, ESR2, KDR, CYP19A1 and ESRRG) might be the therapeutic targets of epimedium on POI. In addition, gene-enrichment analysis suggested that epimedium appeared to play a role in POI by modulating 6 molecular functions, 5 cellular components, 15 biological processes and striking 52 potential targets involved in 13 signaling pathways.
Conclusion
This study predicted the pharmacological and molecular mechanism of epimedium against POI from a holistic perspective, as well as provided a powerful tool for exploring pharmacological mechanisms and rational clinical application of traditional Chinese medicine.
Keywords: network pharmacology, premature ovarian insufficiency, epimedium, infertility, GO, KEGG
Introduction
Premature ovarian insufficiency (POI) is the loss of ovarian follicular property and results in failure of normal cycle of ovarian function.1,2 POI is a common, spontaneous and heterogeneous disease characterized by amenorrhea and perimenopausal syndrome in woman before the age of forty.3–5 A series of complications are found in POI patients, including sexual dysfunction, infertility, osteoporosis, vasomotor symptoms and cardiovascular diseases.6 The main pathogen of POI is not very clear, but it is classified as genetic, iatrogenic and autoimmune.7 Presently, POI is ameliorated mostly by estrogen supplementation, but it has some side effects, like increasing risk of breast cancer and endometrial carcinoma.8 Chinese herbal medicine, as a relatively safe and effective medicine, supplies another choice for POI patients.
Epimedium, also called Yinyanghuo or Horny Goat Weed, is a perennial herbaceous plant.9 It is well renowned in Traditional Chinese Medicine (TCM) and has been widely used in Asian countries for hundreds of years since ancient times.10 In the theory of TMC, epimedium exerts effects in treating impotence, infertility, amnesia, osteoporosis, senile functional diseases and cardiovascular diseases.11,12 Moreover, in traditional use, epimedium has been used for treating POI, which is more effective in combination with other herbs.12 Modern pharmacological researches suggest that epimedium exhibits numerous pharmacological activities including anti-apoptotic, anti-oxidative, anti-neuroinflammatory, which may contribute to preventing and benefiting various diseases of nervous system.13–15 In addition, it is reported that the extract of epimedium promotes the production of sperm through inhibiting oxidative stress in luteinizing hormone-releasing hormone (LHRH) agonist-induced rat models of male infertility.16 The role of epimedium playing in ovarian-related diseases has been discovered. Epimedium and hyperin increase the secretion of estrogen and progesterone via upregulating CYP17 and CYP19, and further enhanced the ovarian endocrine function.17 However, its potential mechanisms in curing POI have not been clarified completely.
TCM holds the characteristics of multi-pathway, multi-target, multi-component and synergistic effects that result in indefinite substance bases, unclear mechanisms of action and other problems.18 Therefore, it is not easy to understand the potential molecular mechanism of TCM by conventional experimental methods. Hence, new strategies and new methods are required to explore its underlying mechanism of particular therapeutic efficacy systematically and comprehensively. The concept of network pharmacology is proposed to investigate synergistic effects and potential mechanisms of multiple compounds through analyzing complex and multi-layered networks, which is a novel research field based on pharmacology and pharmacodynamics.19–22 Network pharmacology expounds the role of TCM in human biological network from overall perspective via integrating pharmacology, omics, system biology and computational biology.
In this study, a comprehensive network pharmacology approach was established to probe the potential pharmacological mechanism of epimedium on POI by molecular docking and network analysis. Firstly, the active compounds of epimedium were downloaded from Traditional Chinese Medicine Systems Pharmacology (TCMSP) Database and Analysis Platform, and then were input into PharmMapper to get their targets. In addition, target genes of POI were obtained from MalaCards (the human disease database) and NCBI database. Afterward, the interactions among the common targets were gathered via String. All the targets were uploaded to DAVID 6.8 to do GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) analyses. Finally, the pharmacological data were integrated into compound-target and target-pathway networks. Thus, this study offered a forceful tool for investigating the active mechanisms of epimedium on treating POI. The workflow of this study on epimedium against POI based on network pharmacology was drawn (Figure 1).
Figure 1.
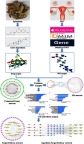
Workflow for epimedium against POI.
Abbreviation: POI, premature ovarian insufficiency.
Materials and methods
Chemical components in epimedium
We collected the chemical components from TCMSP (http://lsp.nwu.edu.cn/browse.php?qc=herbs),22 which is a system pharmacology platform designed for studying TCMs comprehensively. To maximize the discovery of fully active compounds, two conditions were set as the criteria for these screening models — oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18, which are the most important indicators for evaluating the characteristics of ADME (absorption, distribution, metabolism and excretion). Among these compounds, 23 active herbal ingredients of epimedium successfully satisfy all the criteria (Table S1).
Compound targets for epimedium
The PubChem Database (http://pubchem.ncbi.nlm.nih.gov/) is an online server for drug-target identification,23 which provides publicly available chemical information of chemical substances and their biological activities. We input all active compounds into the PubChem Database and have obtained the 3D molecular structure of epimedium. Eventually, only 19 compounds were recruited and 4 compounds were removed because of lacking precise structural information. These 3D molecular structure files were imported into PharmMapper (http://lilab.ecust.edu.cn/pharmmapper/index.php),24 which is an online tool for drug-target identification by a pharmacophore mapping method. Using this web tool, we acquired predicted drug targets of each compound. After merging the duplicate data, we chose the target genes with normalized fit score >0.9 as potential targets for epimedium (Table S2).
POI targets
POI-related genes were downloaded from public database sources, including the NCBI Gene database (http://www.ncbi.nlm.nih.gov/gene/),25 the Online Mendelian Inheritance in Man (OMIM) database (http://www.omim.org) and the DrugBank database (http://www.drugbank.ca/).26 As a result, a total of 127 target genes were obtained (Table S3). To acquire candidate targets of epimedium acting on POI, we integrated the compounds' predicted targets of epimedium with target genes of POI and chose those replicate genes. In the end, only 6 genes were identified as targets of 19 compounds of epimedium, including ESR1, AR, ESR2, KDR, CYP19A1 and ESRRG (Table S4).
Protein–protein interaction (PPI) data
The PPI data were gained from String (https://string–db.org, version 11.0) which is a database for predicting protein–protein interactions with confidence score ranges (low confidence score <0.4; medium: 0.4–0.7; high >0.7–0.9; highest confidence >0.9).27 The target proteins were selected with species limited to “Homo sapiens” and a confidence score >0.4. The associated proteins which directly or indirectly interacted with common targets of epimedium and POI were obtained through STRING.
Network construction method
The network construction was built as follows: (1) network between active compounds and targets of epimedium (compound–compound target); (2) network between epimedium active compounds and common targets between epimedium and POI (compound–common target); (3) network among compounds, common targets and associated proteins of epimedium and POI (compound–common target–PPI) and (4) network among active common targets, associated proteins and pathways (common target–PPI–pathway). The network analysis software Cytoscape (www.cytoscape.org; version 3.2.1) was used to visualize networks.28 The nodes represented targets, compounds, pathways and edges indicated interactions, respectively.
Gene ontology and pathway analysis
To investigate the functional annotation and involved pathways of genes, the GO- and KEGG-enrichment analyses were calculated and evaluated by DAVID version 6.8 (Database for Annotation, Visualization and Integrated Discovery, http://david.abcc.ncifcrf.gov/home.jsp).29 Difference was considered to be statistically significant at p<0.05.
Results and discussion
Compound–compound target network analysis
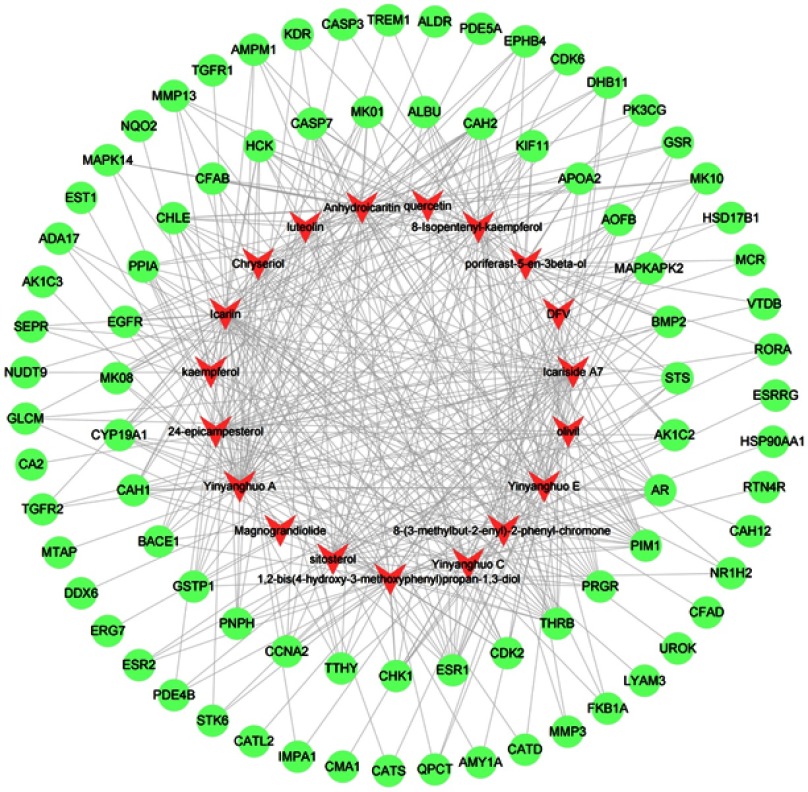
In this work, we obtained 19 active compounds of epimedium from TCMSP database, which conformed to the characteristics of various ingredients for TCM. As shown in Figure 2, the network of compound–compound target consisted of 101 nodes (19 active ingredient nodes and 82 ingredient target nodes) and 401 edges. In this network, compound targets in interior of the circle showed more interactions with compounds than those in the external part. Most targets were hit by multiple compounds, but 19 targets only could be modulated by one compound such as NQO2, EST1, CATL2, ESRRG and so on. AR, ESR1, CAH2, PIM1, THRB, CHLE, HCK and PPRG were modulated by more than ten ingredients, which may be the key targets in epimedium. For example, luteolin, chryseriol, yinyanghuo A/C/E, Anhydroicaritin synergistically act on AR, ESR1 and so on. Consequently, the compounds of epimedium may play a pharmacological role through regulating these targets in multiple diseases and we could have an approximate observation on the relevance between active compounds and targets via compound–compound target network (Figure 2).
Figure 2.
Compound–target network. Red arrows represent active ingredients in epimedium. Green circles represent targets of epimedium. Edges represent interaction between ingredients and targets.
The active compounds of epimedium playing role in diseases through acting on targets have been recognized. Nam SY et al found that kaempferol reached its role in anti-inflammatory, antioxidant and anticancer properties through inducting inflammatory mediators like TSLP, IL-1β, TNF-α and IL-8.30 Anhydroicaritin, as a potent SREBP2 inhibitor, restrained the osteoclasts formation and ameliorated bone loss caused by diabetes.31 In 4T1 mammary tumor-bearing model, Yinyanghuo C improved the mRNA levels of endothelial markers, such as the endothelial-cell-specific molecule-1 (ESM-1), the platelet endothelial adhesive factor-1 (CD31) and the vascular von Willebrand factor (vWF), and promoted tumor-associated angiogenesis.32 Luteolin and apigenin synergistically dilapidated the AKT and AR signaling network, and further improved the therapeutic efficacy of androgen ablation in prostate cancer.33 The above studies indicated the important role of multiple compounds in different diseases.
Compound–common target between compound and POI network analysis
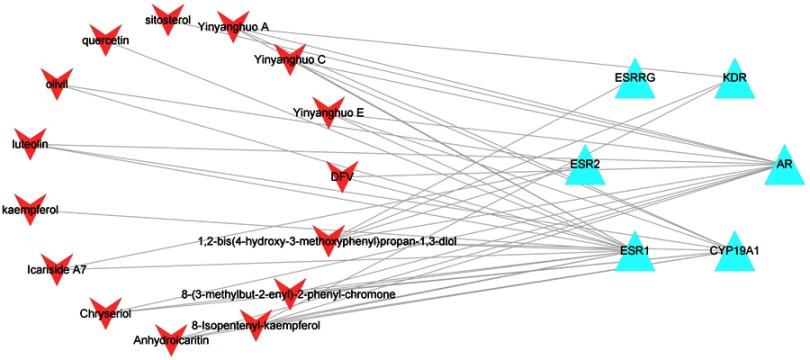
To further find the target of epimedium on POI, we built a compound–common target network (Figure 3). In this network, 6 hub targets and 15 central compounds were obtained, including 21 nodes and 41 edges. Thus, this suggested that only 15 ingredients of epimedium may act on POI via 6 hub targets consisting of androgen receptor (AR), estrogen receptor alpha (ESR1), estrogen receptor beta (ESR2), kinase insert domain receptor (KDR), cytochrome P450 family 19 subfamily A member 1 (CYP19A1) and estrogen-related receptor gamma (ESRRG).
Figure 3.
Compound–common target of epimedium and POI network. Red arrows represent active ingredients in epimedium. Blue triangles represent common targets of epimedium and POI. Edges represent interaction between ingredients and common targets.
Abbreviation: POI, premature ovarian insufficiency.
AR function is essential for folliculogenesis of normal female. In female AR(-/-) mice model, mice appeared normal but developed POI phenotype with abnormal ovarian gene expression.34 Several studies reported that the occurrence of POI was related to genetic variation in ESR1 gene (PvuII polymorphism).35,36 The researches associated with ESR2, KDR and ESRRG in POI were gradually discovered.37–39
Compound–common target–other human proteins’ PPI network analysis
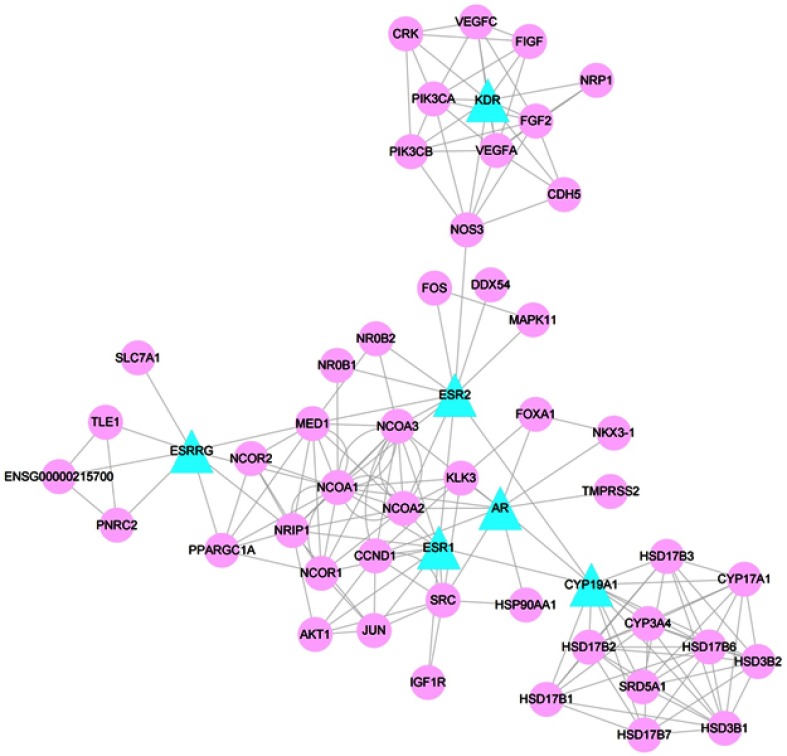
As shown in Figure 4, the PPI network contained candidate targets of epimedium remedying POI and their interacting proteins. In this network, there were 53 nodes (6 candidate target nodes and 47 associated protein nodes) and 183 edges. The network comprehensively summarized the internal net of epimedium in healing POI.
Figure 4.
PPI network. Blue triangles represent common targets of epimedium and POI. Pink circles represent interacting proteins that directly or indirectly interacted with common targets. Edges represent interaction between targets.
Abbreviation: POI, premature ovarian insufficiency.
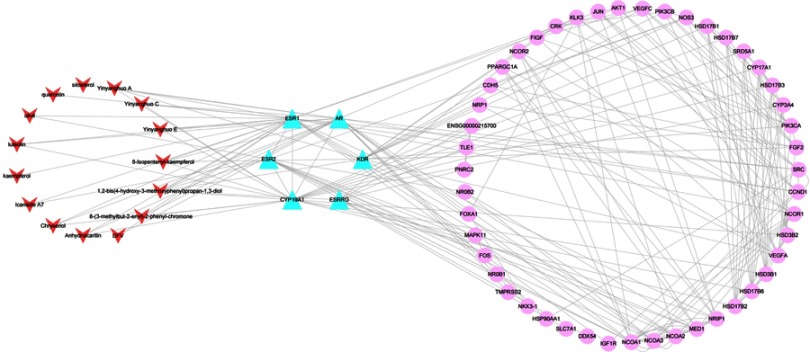
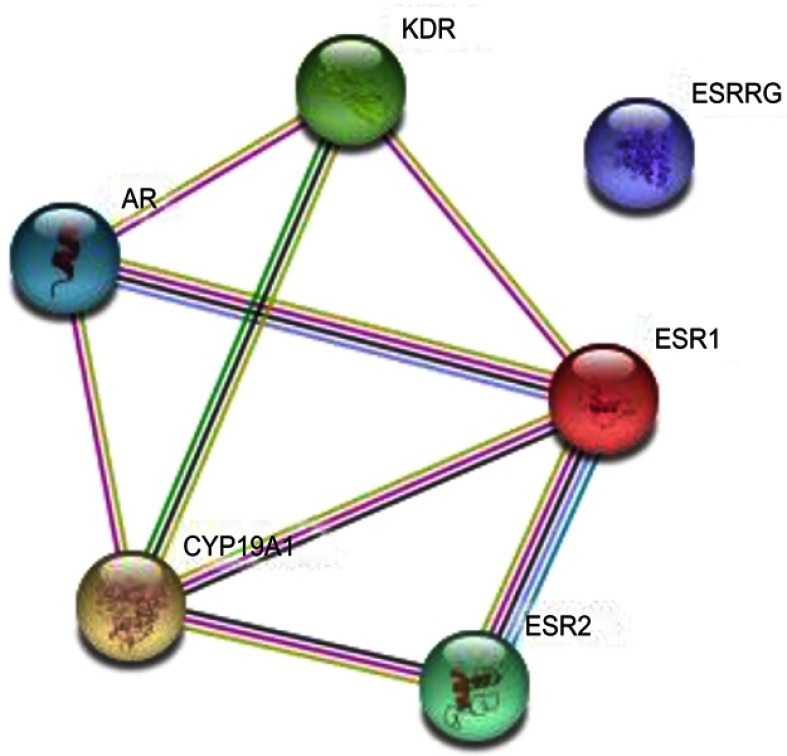
To elucidate the significance of compound targets, we further constructed a PPI network of compound–common target–other human proteins, which was composed of 68 nodes (15 central compounds, 6 candidate target nodes and 47 associated target nodes) and 224 edges (Figure 5). It was more clear to discover that each compound directly or indirectly acted on POI through specific protein. The String online server was used to build an interactive network for all hit genes. Remarkably, AR, KDR, ESR1, ESR2 and CYP19A1 constituted an interaction network, while ESRRG was independent from the network (Figure 6). And ESRRG only corresponded to one component. These dates suggested that epimedium probably acted on POI mainly through the network of AR-KDR-ESR1-ESR2-CYP19A1.
Figure 5.
Compound–common target–other human proteins’ PPI network. Red arrows represent active ingredients in epimedium. Blue triangles represent common targets of epimedium and POI. Pink circles represent interacting proteins that directly or indirectly interacted with common targets. Edges represent interaction between targets.
Abbreviations: POI, premature ovarian insufficiency; PPI, protein–protein interaction.
Figure 6.
Interaction network of all hit genes.
GO, KEGG pathway enrichment analysis and reactome analysis
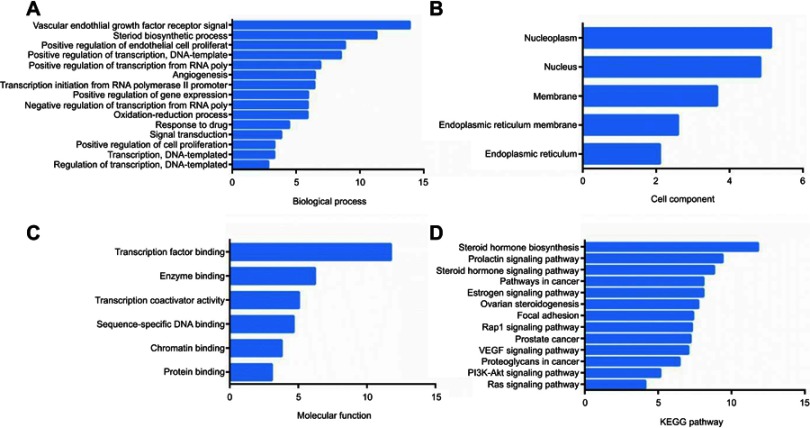
To further investigate the multiple mechanisms of epimedium on POI from a systematic level, GO-enrichment analysis for the biological process, molecular function and cellular component of the 53 selected targets were performed using DAVID 6.8. The analysis results indicated that epimedium acted on POI by regulating multiple biological processes (BP) (p<0.01), and the top five of them were vascular endothelial growth factor receptor signal (GO:0048010), steroid biosynthetic process (GO:0006694), positive regulation of endothelial cell proliferation (GO:0001938), positive regulation of transcription, DNA-template (GO:0045893) and positive regulation of transcription from RNA polymerase II promoter (GO:0045944), respectively (Figure 7A and Table S5). The main cellular components (CC) terms (p<0.01) were nucleoplasm (GO:0005654), nucleus (GO:0005634), membrane (GO:0016020), endoplasmic reticulum membrane (GO:0005789) and endoplasmic reticulum (GO:0005783) (Figure 7B, Table S6), while the top five molecular functions (MF) terms (p<0.01) included transcription factor binding (GO:0008134), enzyme binding (GO:0019899), transcription coactivator activity (GO:0003713), sequence-specific DNA binding (GO:0043565) and chromatin binding (GO:0003682) (Figure 7C, Table S7). As shown in Figure 7D and Table S8, the 53 proteins were further mapped to 13 KEGG pathways with p<0.01. The data indicated that epimedium confronted with POI primarily depending on hormone-regulation-related signaling pathway, including steroid hormone biosynthesis (hsa00140), prolactin signaling pathway (hsa04917), thyroid hormone signaling pathway (hsa04919), pathways in cancer (hsa05200), estrogen signaling pathway (hsa04915), etc.
Figure 7.
GO and KEGG analysis of targets. (A) Biological process. (B) Cell Component. (C) Molecular function. (D) KEGG pathway. The y-axis shows significantly enriched categories of the targets and the x-axis shows the enrichment scores of these terms (p<0.01).
Abbreviations: GO, gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
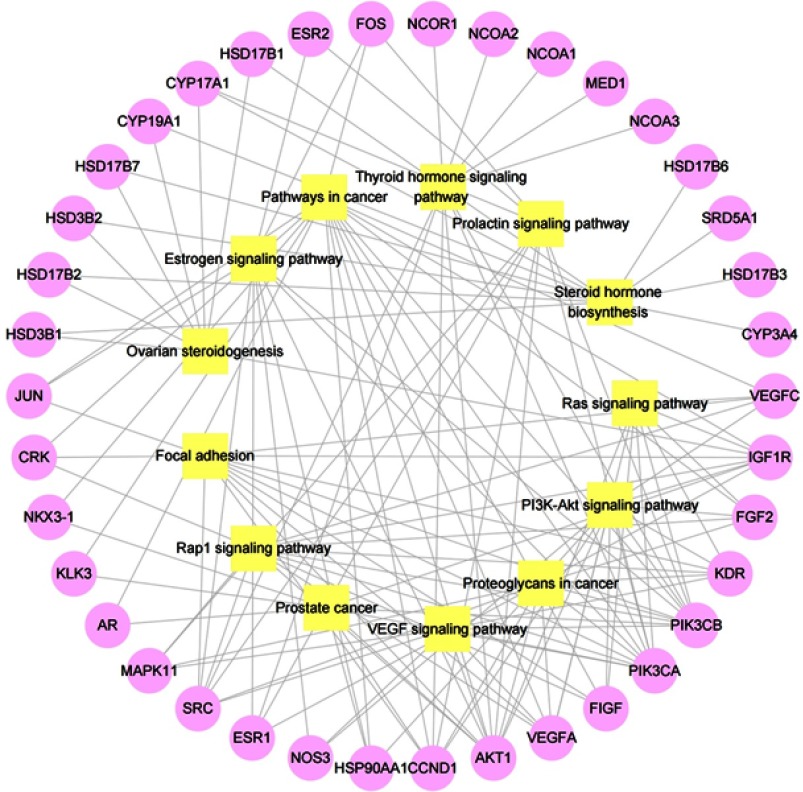
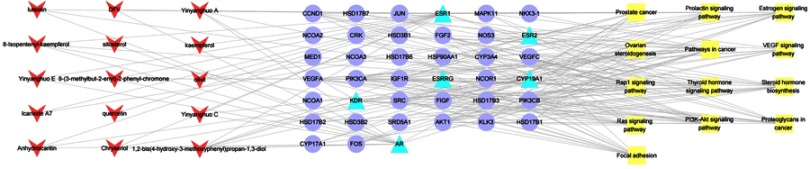
To further explore the relationship of compound, common target and pathway, we performed a “common target-pathway” network (Figure 8) and a “compound-common target-pathway” network (Figure 9). These networks revealed that epimedium retained multiple targets, multiple components and multiple pathways against POI.
Figure 8.
Target–pathway network. Yellow squares represent enriched pathways. Pink circles represent common targets and proteins that directly or indirectly interacted with common targets.
Figure 9.
Compound–target–pathway network. Red arrows represent active ingredients in epimedium. Blue triangles represent common targets of epimedium and POI. Purple circles represent proteins that directly or indirectly interacted with common targets. Yellow squares represent enriched pathways.
Abbreviation: POI, premature ovarian insufficiency.
Hormone-regulation-related signaling pathways were the main approach for epimedium against POI. The imbalance of steroid hormone biosynthesis, including GnRH, prolactin, FSH, GH- (growth hormone) IGF-1, LH, thyroid hormone and adrenocorticotropic hormone, led to follicular failure and no dominant follicle.40 Thyroid hormone signaling pathway acted an important role in the occurrence of POI. A study reported that thyroid autoimmunity was the most common autoimmune disease associated with POI.41 POI patients had lower serum androgen levels than normal person, which were from ovarian theca-derived cells.42 Vascular endothelial growth factor (VEGF) level was low in patients with POI because of genes mutation.43 However, in chicken models, epimedium induced the expression of VEGF.44 Epimedium inhibited EGFR and ER-α36 expression, and decreased cyclin D1 induction by estrogen and ER-α36-mediated MAPK/ERK signaling pathway.45 The PI3K-Akt signaling pathway46 and Rap1 signaling pathway47 strongly correlated with the occurrence and development of POI as well. Therefore, they were important mechanisms for POI and may play a key role in treatment. Overall, a pathway contained multiple targets, and each target could work on multiple pathways, thereby creating a complex network. However, the mechanisms could not be effective in vivo because of internal complex mechanisms. This study mainly provided an effective way to predict and discover new medicines treating specific diseases.
Conclusion
Currently, perfect therapy has not been discovered in treating POI. Western medicine’s therapeutic strategy is the main treatment plan but produces side-effect with long-term application. In this study, system's pharmacology and genomics were combined to evaluate epimedium-treating POI. Nineteen active herbal ingredients of epimedium and their respective targets were obtained from TCMSP database and PharmMapper Database, respectively. The target genes of POI were predicted via NCBI, DrugBank, OMIM databases and were mapped to targets of epimedium active compounds. As a result, only 6 targets were selected as candidate targets for epimedium against POI, namely AR, ESR1, ESR2, KDR, CYP19A1 and ESRRG. Then these 6 candidate targets and their 47 interaction proteins were analyzed by GO and KEGG analysis. The results indicated that epimedium may affect POI via regulating 15 biological processes, 5 cell components, 6 molecular functions and 13 signaling pathways. It suggested epimedium consisted of multiple compounds and acted on numerous distinct targets of POI via multiple pathways. However, we can only speculate but not confirm if such a mechanism really has an impact, because in evidence-based medicine the conclusion can not be determined until randomized trials are properly conducted. In addition, epimedium compound exerted dose-dependent action and accompanied certain adverse events. Therefore, network pharmacology was mainly applied to the action mechanism research of Chinese herbal formulas, promoted its modernization and developed into a new strategy for new drug research and development.
Acknowledgments
This work is supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2017PH047) and the Research Foundation of Yantai Yuhuangding Hospital (Grant No. 201604).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x [DOI] [PubMed] [Google Scholar]
- 2.Nippita TA, Baber RJ. Premature ovarian failure: a review. Climacteric. 2007;10(1):11–22. doi: 10.1080/13697130601135672 [DOI] [PubMed] [Google Scholar]
- 3.Goswami D, Conway GS. Premature ovarian failure. Horm Res. 2007;68(4):196–202. doi: 10.1159/000102537 [DOI] [PubMed] [Google Scholar]
- 4.Del Mastro L, Ceppi M, Poggio F, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40(5):675–683. doi: 10.1016/j.ctrv.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Xiao L, Li J, et al. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2019;3:CD008018. doi: 10.1002/14651858.CD008018.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol off J Am Soc Clin Oncol. 2008;26(5):753–758. doi: 10.1200/JCO.2007.14.1655 [DOI] [PubMed] [Google Scholar]
- 7.Kovanci E, Schutt AK. Premature ovarian failure: clinical presentation and treatment. Obstet Gynecol Clin North Am. 2015;42(1):153–161. doi: 10.1016/j.ogc.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 8.Pinelli S, Artini PG, Basile S, et al. Estrogen treatment in infertile women with premature ovarian insufficiency in transitional phase: a retrospective analysis. J Assist Reprod Genet. 2018;35(3):475–482. doi: 10.1007/s10815-017-1096-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinese Flora Compilation Committee of Chinese Academy of Sciences.Flora of China (volume 27). Beijing, China: Science Press; 1979:296–299. [Google Scholar]
- 10.Chen XJ, Tang ZH, Li XW, et al. Chemical constituents, quality control, and bioactivity of epimedii folium (Yinyanghuo). Am J Chin Med. 2015;43(5):783–834. doi: 10.1142/S0192415X15500494 [DOI] [PubMed] [Google Scholar]
- 11.Indran IR, Liang RL, Min TE, et al. Preclinical studies and clinical evaluation of compounds from the genus epimedium for osteoporosis and bone health. Pharmacol Ther. 2016;162:188–205. doi: 10.1016/j.pharmthera.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 12.Chao SL, Huang LW, Yen HR. Pregnancy in premature ovarian failure after therapy using Chinese herbal medicine. Chang Gung Med J. 2003;26(6):449–452. [PubMed] [Google Scholar]
- 13.Sheng C, Xu P, Zhou K, et al. Icariin attenuates synaptic and cognitive deficits in an A-induced rat model of alzheimer’s disease. Biomed Res Int. 2017;2017:7464872. doi: 10.1155/2017/7464872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GQ, Li DD, Huang C, et al. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front Mol Neurosci. 2018;9(10):441. doi: 10.3389/fnmol.2017.00441 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Wei Z, Deng X, Hong M, et al. Icariin has synergistic effects with methylprednisolone to ameliorate EAE via modulating HPA function, promoting anti-inflammatory and anti-apoptotic effects. Int J Clin Exp Med. 2015;8(11):20188–20197. [PMC free article] [PubMed] [Google Scholar]
- 16.Park HJ, Koo YK, Park MJ, et al. Restoration of spermatogenesis using a new combined herbal formula of epimedium koreanum nakai and angelica gigas nakai in an luteinizing hormone-releasing hormone agonist-induced rat model of male infertility. World J Mens Health. 2017;35(3):170–177. doi: 10.5534/wjmh.17031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie X, Sheng W, Hou D, et al. Effect of Hyperin and Icariin on steroid hormone secretion in rat ovarian granulosa cells. Clin Chim Acta. 2019;495:646–651. doi: 10.1016/j.cca.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Liu AL, Du GH. [Network pharmacology: new guidelines for drug discovery]. Yao Xue Xue Bao. 2010;45(12):1472–1477. [PubMed] [Google Scholar]
- 19.Zhang Y, Guo X, Wang D, et al. A systems biology-based investigation into the therapeutic effects of Gansui Banxia Tang on reversing the imbalanced network of hepatocellular carcinoma. Sci Rep. 2014;24(4):4154. doi: 10.1038/srep04154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118 [DOI] [PubMed] [Google Scholar]
- 21.Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1111. doi: 10.1038/nbt1007-1110 [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Wang XD, Niu YY, et al. Molecular targets of Chinese herbs: a clinical study of hepatoma based on network pharmacology. Sci Rep. 2016;4(6):24944. doi: 10.1038/srep24944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Thiessen PA, Bolton EE, et al. PubChem substance and compound databases. Nucleic Acids Res. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(W1):W356–W360. doi: 10.1093/nar/gkx374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown GR, Hem V, Katz KS, et al. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 2015;43(Database issue):D36–D42. doi: 10.1093/nar/gku1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amberger J, Bocchini CA, Scott AF, et al. McKusick’s Online Mendelian Inheritance in Man (OMIM). Nucleic Acids Res. 2009;37(Database issue):D793–D796. doi: 10.1093/nar/gkn665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi: 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 30.Nam SY, Jeong HJ, Kim HM. Kaempferol impedes IL-32-induced monocyte-macrophage differentiation. Chem Biol Interact. 2017;274:107–115. doi: 10.1016/j.cbi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 31.Xu F, Ding Y, Guo Y, et al. Anti-osteoporosis effect of Epimedium via an estrogen-like mechanism based on a system-level approach. J Ethnopharmacol. 2016;177:148–160. doi: 10.1016/j.jep.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 32.Shui YM, Lv GY, Shan LT, et al. Epimedin C promotes vascularization during BMP2-induced osteogenesis and tumor-associated angiogenesis. Am J Chin Med. 2017;45(5):1093–1111. doi: 10.1142/S0192415X17500598 [DOI] [PubMed] [Google Scholar]
- 33.Tsai CH, Tzeng SF, Hsieh SC, et al. A standardized extract overcomes the feedback activation of HER2/3 Signaling upon androgen-ablation in prostate cancer. Front Pharmacol. 2017;8:721. doi: 10.3389/fphar.2017.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiina H, Matsumoto T, Sato T, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103(1):224–229. doi: 10.1073/pnas.0506736102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Dalgleish R, Vujovic S, et al. Microsatellite variation of ESR1, ESR2, and AR in Serbian women with primary ovarian insufficiency. Climacteric. 2018;21(5):472–477. doi: 10.1080/13697137.2018.1476967 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Tan R, Cui Y, et al. Estrogen receptor α gene (ESR1) polymorphisms associated with idiopathic premature ovarian failure in Chinese women. Gynecol Endocrinol. 2013;29(2):182–185. doi: 10.3109/09513590.2012.731113 [DOI] [PubMed] [Google Scholar]
- 37.Ding C, Li H, Wang Y, et al. Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Res Ther. 2017;8(1):173. doi: 10.1186/s13287-017-0601-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frampton JE. Triptorelin: a review of its use as an adjuvant anticancer therapy in early breast cancer. Drugs. 2017;77(18):2037–2048. doi: 10.1007/s40265-017-0849-3 [DOI] [PubMed] [Google Scholar]
- 39.Yorgun H, Tokgözoğlu L, Canpolat U, et al. The cardiovascular effects of premature ovarian failure. Int J Cardiol. 2013;168(1):506–510. doi: 10.1016/j.ijcard.2012.09.197 [DOI] [PubMed] [Google Scholar]
- 40.Medenica S, Nedeljkovic O, Radojevic N, et al. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. Eur Rev Med Pharmacol Sci. 2015;19(6):977–987. [PubMed] [Google Scholar]
- 41.Ayesha, Jha V, Goswami D. Premature ovarian failure: an association with autoimmune diseases. J Clin Diagn Res. 2016;10(10):QC10–QC12. doi: 10.7860/JCDR/2016/22027.8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bachelot A, Meduri G, Massin N, et al. Ovarian steroidogenesis and serum androgen levels in patients with premature ovarian failure. J Clin Endocrinol Metab. 2005;90(4):2391–2396. doi: 10.1210/jc.2004-1734 [DOI] [PubMed] [Google Scholar]
- 43.Jeon YJ, Choi Y, Shim SH, et al. Vascular endothelial growth factor gene polymorphisms in Korean patients with premature ovarian failure. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):138–142. doi: 10.1016/j.ejogrb.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Mehmood K, Li K, et al. Icariin ameliorate thiram-induced tibial dyschondroplasia via regulation of WNT4 and VEGF expression in broiler chickens. Front Pharmacol. 2018;9:123. doi: 10.3389/fphar.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Zheng N, Dong J, et al. Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J Steroid Biochem Mol Biol. 2017;171:318–327. doi: 10.1016/j.jsbmb.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 46.Yan Z, Dai Y, Fu H, et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J Mol Endocrinol. 2018;60(3):261–271. doi: 10.1530/JME-17-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Borgne M, Chartier N, Buchet-Poyau K, et al. The RNA-binding protein Mex3b regulates the spatial organization of the Rap1 pathway. Development. 2014;141(10):2096–2107. doi: 10.1242/dev.108514 [DOI] [PubMed] [Google Scholar]