Abstract
Global food security is challenged by increasing levels of CO2, O3 and temperature trough their impacts on production and grain quality of wheat, one of the major C3 crops and staple food across the world. The present study was conducted to assess the effects of elevated levels of CO2 (EC; 550 ppm) and tropospheric O3 (EO; 70 ppb) as well as of combined interactive treatment [EC X EO; ECO] on plant growth, yield and grain quality of two wheat cultivars (HD-2967 and C-306) grown during 2016–17 and 2017–18 using free air ozone and carbon dioxide enrichment (FAOCE) facility under field conditions. Individually, EC, increased leaf area index (LAI; 15.9–28.2%), photosynthetic rate (Pn; 11.4–20.3%) and yield (8.2–20.9%) whereas EO declined LAI (5.1–12.5%), Pn (2.8–11.8%) and yield (2.2–14.2%) over ambient conditions (Amb: 405.2 ppm CO2 and 30.7 ppb O3). Under ECO condition, EC increased LAI (2.2–17.1%), Pn (2.8–17.6%) and grain yield parameters (4.4–24.3%) across the cultivars in both years, but reduced the positive effects of EO on quality as compared to Amb. Dilution effect of increased yield under EC condition have reduced total protein, micro- and macro-nutrient concentrations whereas EO increased them notably compared to Amb. Starch in grains increased under EC but reduced under EO as compared to Amb. AOT40, the sum of averaged difference of O3 h−1 concentration beyond 40 ppb for 7 hours (31233 ppb h−1) in FAOCEs rings during the crop growth period led to reduction in average grain yield of HD-2967 and C-306 by 11.6 and 8.5% or by 1.6 and 1.3% yield loss per ppb increase of O3, respectively. The growth, yield and quality parameters of both wheat cultivars responded similarly but to different extent to all treatments. EC was able to offset the negative effects of EO on yield and yield components only, but not those concerning the quality of grains. To stabilize global food security, precursor gases forming tropospheric ozone must be constrained.
Keywords: Agriculture, Atmospheric science, Environmental analysis, Environmental assessment, Environmental hazard, Environmental risk assessment, Leaf area index (LAI), Photosynthetic rate, Growth, Yield, Nutrients, Climate change
1. Introduction
The global average concentrations of greenhouse gases (GHGs) such as carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O) and tropospheric ozone (hereafter referred as O3) have increased significantly in the last two decades (NOAA, ESRL, 2019). Among these, CO2 and O3 are closely linked to plant growth, yield and quality of grains such as wheat, rice, maize and chickpea (Saha et al., 2015: Abebe et al., 2016). Increase in atmospheric CO2 works as carbon fertilizer, improves plant growth and productivity of crops (Van der Kooi et al., 2016; Deryang et al., 2016) and negatively impacts the nutrients such as iron, zinc and crude protein contents in the grains (Broberg et al., 2017). Since the pre-industrial era (1750), CO2 concentration has increased incessantly from 280 ppm to 409.7 ppm (NOAA, ESRL, 2019) and is expected to increase further if current emission rates continue. Burning of fossil fuel and biomass remain prime contributors of CO2, oxides of nitrogen (NOx) and volatile organic compounds (VOC). Tropospheric ozone, a secondary air pollutant formed via photochemical reactions among NOx and organic VOC is phytotoxic in nature and is deleterious to crops and ecosystems (CLRTAP, 2017; Lefohn et al., 2017). Global average ozone concentration fall in the range of 35–50 ppb compared to the average of 20 ppb in the beginning of the 20th century (Proietti et al., 2016; Sicard et al., 2017). With the current emission rates of precursor gases, ozone concentration across the Asian countries would continue to rise in the 21st century (Frei, 2015). Crop growth, productivity and grain quality have their own responses to the elevated CO2 and O3 in the ambient atmosphere.
Enhanced CO2 level can lead to positive effects more in C3 plants compared to C4 plants (Mishra et al., 2013; Pleijel and Hogy, 2015). Rising CO2 levels can cause accumulation of excess C and reduction in nutrients and thus could alter the C, N metabolism in C3 plants such as wheat (Hogy et al., 2013). Enhanced CO2 is known to cause reduction in rice milling percentage, head rice recovery, protein content and nutritional quality of grains in C3 plants such as wheat and rice (Myers et al., 2014). Ozone enters into plants during the normal gas exchange process through the stomatal pores (CLRTAP, 2017) and accumulates in leaves. It reacts with unsaturated biomolecules to form reactive species of oxygen (ROS) and causes programmed cell death that negatively impacts plant photosynthesis and metabolism in plants (Pleijel et al., 2018). Higher accumulation of surface ozone diminishes the storage of carbon in vegetation, resulting in reduced plant growth and yield (Tai et al., 2014). Besides, deterioration in plant growth and productivity, enrichment of O3 also impacts grain quality of crops (Broberg et al., 2015; Malin et al., 2015). Compared to individual effects of CO2 and O3, combined interactive environment of CO2 and O3 affects growth and development of crops as well as yield and nutrient levels in different ways (Phothi et al., 2016). There has been studies on individual effects of CO2 and O3 on wheat, rice, maize and chickpea crops and their yield in India and in other parts of world (Mishra et al., 2013; Saha et al., 2015; Abebe et al., 2016; Chaturvedi et al., 2017; Harmens et al., 2019). Most of these studies have been conducted using open top chambers in field conditions. However, it will be useful to conduct experiment on exposure of crops, particularly wheat, to interactive CO2–O3 which are otherwise scarcely reported. This becomes more meaningful in Indian sub-continent where levels of CO2 and O3 are increasing in parallel and wheat cultivars are most sensitive to O3 exposure (Mishra et al., 2013 and more references therein).
Wheat (Triticum aestivum L.) is an important staple food across the globe and its growth and yield are sensitive to tropospheric O3 (Broberg et al., 2015). It is grown on the largest area (220.1 Mha) on Earth's surface with a total production of 749.4 million tons (FAO, 2016). About 65 percent of wheat production is used for food, 12 percent for industrial and 17 percent for animal feed purposes (FAO, 2016). India cultivates wheat on 30.6 million hectares of land area and produces more than 98.4 million ton per year with an average productivity rate of 3.2 ton ha−1 (Economic Survey, 2016–17). Most of wheat in India is grown in fertile farm lands of Indo-Gangetic Plain (IGP) which is the most densely populated area of the country. This region experiences high levels of CO2 and O3 (formed from precursor gases, NOx, VOC) due to emissions from coal based thermal power plants, vehicles and large scale biomass burning at domestic level. Ozone levels have shown a steady increase in past years in IGP under tropical climatic conditions (Mahajan et al., 2012; Mishra et al., 2013). There exists a major apprehension that wheat crop productivity and quality of grains can go down significantly in this century due to increasing O3 levels in the region (Singh et al., 2018). The information about effects of elevated CO2 (EC) and elevated tropospheric O3 (EO) and their interactive (EC X EO together) exposure on plant growth, yield and yield components of wheat crop from the Indian sub-continent is too scarce. Such studies are essential from the food security point of view and developing more CO2 and O3 stress tolerant verities of wheat. To fill such knowledge gaps, this study was conducted on two wheat cultivars (HD-2967: hybrid variety and C-306: a traditional variety) using free air ozone and carbon dioxide enrichment (FAOCE) facility under field conditions to assess the effects of 1) projected elevated CO2 (EC; 550 ppm); 2) elevated tropospheric O3 (EO; 70 ppb) and 3) combined interactive effects of EC and EO (ECO) on plant growth, yield attributes, yield and quality of grains.
2. Materials and methods
2.1. Site description
This work was carried out at a FAOCE facility established at the Indian Agricultural Research Institute (IARI), New Delhi, India during Rabi season of the years 2016–17 and 2017–18. New Delhi, (28° 37′N and 77° 12′E about 228.6 m above mean sea level) represents a transition climatic zone from sub-arid climate in the west and sub-humid in the east and experiences sub-tropical climate with average annual precipitation of 797.4 mm. Weather parameters during the study period such as rainfall, maximum and minimum temperatures, sunshine hours and relative humidity were recorded from an agro-meteorological station situated within 100 meters distance from the FAOCE facility and are provided in Table 1. Soil at experimental site was sandy loam in texture.
Table 1.
Weather parameters during the wheat crop growing period for the years 2016–17 and 2017–18 around the free air ozone and carbon dioxide enrichment (FAOCE) facility at IARI, New Delhi.
| Month, year | Rainfall (mm) | Temperature (°C) |
Sunshine (hour) | Relative humidity (%) | Monthly average O3 (ppb) | ||
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | |||||
| December, 16 | 0 | 23.3 | 5.3 | 14.3 | 4.5 | 72 | 29.5 |
| January, 17 | 2.1 | 20.1 | 7.7 | 13.9 | 4.2 | 76 | 29.2 |
| February, 17 | 0 | 23.9 | 9.9 | 17.1 | 7.1 | 70 | 31 |
| March, 17 | 0.6 | 29.7 | 14.2 | 21.7 | 8.1 | 62 | 30.4 |
| April, 17 | 0.3 | 38 | 20.7 | 29.6 | 8.4 | 56 | 32.8 |
| November, 17 | 0 | 26.8 | 10.6 | 18.7 | 2.5 | 69 | 27.4 |
| December, 17 | 0.2 | 23.1 | 7 | 15 | 4.5 | 69 | 31.2 |
| January, 18 | 0.4 | 20.7 | 4.2 | 12.4 | 4.4 | 68 | 29 |
| February, 18 | 0 | 28.3 | 7.9 | 18.1 | 6.3 | 71 | 33 |
| March, 18 | 0 | 31.6 | 12.7 | 22.2 | 8 | 57 | 30.6 |
| April, 18 | 0.8 | 38.6 | 19.2 | 28.9 | 7.7 | 43 | 32.6 |
2.2. Treatment and experiment design
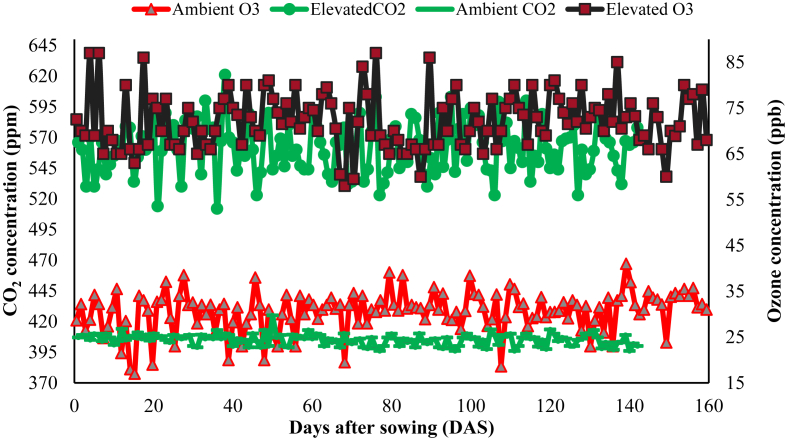
The treatment descriptions in this study included ambient atmospheric CO2 and tropospheric O3 (referred as Amb), EC and EO alone and interactive combined treatment of EC X EO (referred as ECO). With the operational constraints, two FAOCE rings, each of 19.8 m2 area were established for each of the four exposure conditions viz. Amb, EC, EO and ECO. Each ring was divided into four equal halves and each wheat cultivar variety was grown in two quadrants. Thus, there were total of four replicates of each wheat cultivar for each exposure condition. The targeted elevated CO2 (EC) and tropospheric O3 (EO) were 550 ppm and 70 ppb, respectively but the real exposure concentrations at field conditions were 561.2 ppm and 72.2 ppb, respectively. In the control ring, average ambient concentrations of CO2 and O3 were 405.2 ppm and 30.7 ppb, respectively during the experiment. Average ambient and elevated concentrations of EC and EO during crop growing period are shown graphically (Fig. 1). Crops were exposed to elevated CO2 and O3 during 10.00–17.00 h (7 h) throughout the crop growing period. Seven hour exposure of EC and EO is considered suitable to record the full response of crops and is practiced in such previous studies (Ashmore, 2005). Completely randomized design (CRD) was adopted for plot distribution. Anemometer and wind vane were installed in the center of FAOCE rings to record wind speed and direction, respectively. Compressed pure CO2 gas cylinders of 30 kg capacity were used for mixing CO2 with ambient air. Carbon dioxide and air mixture was supplied to FAOCE rings through polyethylene tubes having laser holes (0.3 mm in diameter; hole to hole distance was 30 cm). Solenoid valves were used to control the pressure of elevated CO2 in FAOCE in tune with prevailing wind speed and direction. Ozone generators fitted with ultra-violet lamps to convert atmospheric oxygen into O3 were used to generate ozone. Flappers were used to control the release of O3 and air mixture as per requirement in the rings. Mixture of tropospheric O3 and air was supplied through a common tin duct placed perpendicular to the FAOCE rings. Fuji Infrared Gas Analyzer (IRGA) (Serial Number: A7A0203T, Fuji Electronic System Japan) and ozone monitoring sensor (2B Technologies, USA) were installed in the center of each ring to monitor EC and EO concentrations, respectively. Air samples were taken from the center of each ring and analyzed for CO2 and O3 concentrations.
Fig. 1.
Daily average concentrations of ambient CO2, elevated CO2, ambient ozone and elevated ozone measured during the crop growing years 2016–2017 and 2017–2018 for complete crop growth period starting from days after sowing (DAS) until harvest. The two year average values were ambient CO2 (405.4 ± 4.7 ppm), elevated CO2 (561.2 ± 20.9 ppm), ambient ozone (30.7 ± 4.4 ppb) and elevated ozone (72.2 ± 5.9 ppb).
2.3. Crop fertilization
Crops were fertilized with N (20 kg ha−1), P2O5, K2O (60, kg ha−1), and Zn SO4 (25 kg ha−1). Full doses of P2O5, K2O and ZnSO4 and half (50%) dose of nitrogen were applied at the time of sowing. Remaining dose of N was applied in two equal parts at vegetative and flowering stages. Planting of wheat cultivars was done at 10 cm plant to plant distance and at 15 cm row to row distance during the last week of November month in of the years 2016–17 and 2017–18.
2.4. Measurement of plant growth parameters
The photosynthetic rate (Pn) were determined by using LI-6400 XT Portable Photosynthesis System (Model No. LI-6400XT; LI-COR, USA). Leaf area index (LAI) was measured by leaf area meter (LI-COR; Model No. LAI 2200C, USA) during the vegetative and flowering stages. The photosynthetically active radiation (PAR) was determined by using Line Quantum Sensor (Model: LI-COR, LI-1500 Light Sensor Logger, USA) along with Pn. It was determined between 11.00 to 12.00 h time of the clear sky days only. The factional interception of PAR (fIPAR) was calculated as
| fIPAR = (I0−It)//I0 | (1) |
where I0 is the incident PAR on the top of canopy, It is the transmitted PAR at the bottom of canopy (Monteith et al., 1981). The fIPAR was calculated on 46th day after sowing (indicative of vegetative growth stage) and on 82nd day after sowing (indicative of flowering stage) during each cropping year. The fIPAR varied in very small window from 0.94 to 0.95 (Avg: 0.945) during the vegetative growth stage and from 0.95 to 0.97 (Avg: 0.96) during the flowering stage of wheat crop across the cropping years. The average temperature on the Pn measuring dates at vegetative growth stages ranged from 12.4 to 15.0 °C and at flowering stage from 11.2 to 21.9 °C across the growing years.
2.5. Measurement of yield and yield components
For assessing yield components, plants from 1m2 area of each replicate i.e. total 240 plants of each of two wheat cultivars were harvested at maturity stage from rings designated for each of four exposures. Spikes (m−2), spike length (cm), grains (spike−1), grain weight (spike−1), biological yield (tonha−1), grain yield (tonha−1), 1000 grain weight (g) and harvest percent (ratio of grain yield to biological yield) were determined by following the standard procedures.
2.6. AOT40 calculation
AOT40 is the sum of averaged difference of O3 h−1 concentration beyond 40 ppb for 7 h in the FAOCEs rings during complete crop growth period (Mills et al., 2018). The sum of AOT40 was 31232.61 ppb in this experiment.
2.7. Analysis of soil and plant samples
Soil samples were collected from 0-10 cm depth from surface in three replicates from each part of the ring using a 5 cm diameter auger. The composite samples, prepared by mixing three replicates, were analyzed for electrical conductivity, pH, total nitrogen (N) (Kjeldahl method), total organic carbon (TOC; Blair et al., 1995), potassium (K) and phosphorus (P) (Jackson, 1973). The crude protein content of grains was calculated by multiplying N concentrations with conversion coefficient of 5.95 (Juliano, 1993). Starch content of grains was determined following the methodology of Sadasivam and Minickam (1992). The elemental analysis of acid digested grain samples was done using Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) (model: iCAP7000 series; Thermo Scientific, USA) (Yadav and Rajamani, 2003).
2.8. Statistical analysis
The plant growth, yield, yield components and grain quality parameters of both wheat cultivars grown during 2016–17 and 2017–18 were analyzed using 9.3 SAS statistical software. A two way analysis of variance (ANOVA) was used to analyze the effects of Amb, EC, EO and ECO treatments on the crop growth, yield and grain quality parameters of wheat crops. The data was compared with the ambient and EO alone conditions. Duncan's multiple range tests were performed. The statistically analysis of data was carried out at 95 % confidence level (p < 0.05) to test statistical significance.
3. Results
The observational data on responses of crop growth, yield attributes, yield and quality of grains of two wheat cultivars under EC, EO and ECO exposure are presented in Tables 2, 3, 4, 5, 6, 7, and 8, shown graphically in Figs. 2, 3, and 4 and are discussed hereafter in sub-sections.
Table 2.
Leaf area index (LAI) and photosynthetic rates (Pn) of HD-2967 and C-306 wheat cultivars grown under elevated CO2 (EC), tropospheric O3 (EO) and interactive condition of elevated CO2 X O3 (ECO)∗.
| Wheat variety | Treatment | Leaf Area Index (LAI) |
Photosynthetic Rate (Pn; μmolem−2s−1) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vegetative stage |
Flowering stage |
Vegetative stage |
Flowering stage |
||||||||||
| 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | ||
| HD- 2967 | Amb | 4.5 | 4.6 | 4.5 | 3.9 | 3.9 | 3.9 | 19.9 | 20.6 | 20.3 | 21 | 19.5 | 20.2 |
| EC | 5.7 | 5.6 | 5.6 | 5 | 4.8 | 4.9 | 23 | 24.8 | 23.9 | 23.8 | 22.3 | 23 | |
| EO | 4.1 | 4.2 | 4.2 | 3.5 | 3.7 | 3.6 | 18.9 | 19 | 18.9 | 19.9 | 18.7 | 19.3 | |
| ECO | 4.4 | 4.3 | 4.3 | 4.1 | 3.9 | 4 | 20.4 | 21.4 | 20.9 | 21.9 | 22 | 22 | |
| C-306 | Amb | 4.9 | 4.8 | 4.8 | 4.2 | 4.4 | 4.3 | 22.3 | 22 | 22.2 | 22.5 | 21 | 21.7 |
| EC | 5.9 | 5.7 | 5.8 | 5.2 | 5.1 | 5.2 | 25.1 | 25.8 | 25.5 | 25.2 | 23.4 | 24.3 | |
| EO | 4.5 | 4.2 | 4.3 | 3.8 | 4 | 3.9 | 20.1 | 19.4 | 19.8 | 21.6 | 20.4 | 21 | |
| ECO | 4.6 | 4.7 | 4.7 | 4.4 | 4.2 | 4.3 | 21.6 | 22.8 | 22.2 | 22.2 | 22.7 | 22.4 | |
Determination was on 40 plants (10 plants from each replicate for each treatment and growth stage during each year).
Table 3.
Crop growth parameters of HD-2967 and C-306 wheat cultivars grown under elevated CO2 (EC), tropospheric ozone (EO) and their interactive condition of elevated CO2 X O3 (ECO).
| Variety | Treatments | Spikes (m−2)∗ |
Spike length (cm)∗∗ |
Number of spikelets (spike−)∗∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | ||
| HD-2967 | Amb | 400±8a | 394 ± 24a | 397 ± 12a | 8.2 ± 0.8a | 8.6 ± 1.0a | 8.4 ± 0.8a | 35 ± 1.5a | 37 ± 0.7a | 36 ± 1.3a |
| EC | 457 ± 14b | 431 ± 17b | 444±9b | 9.0 ± 0.1b | 9.0 ± 0.4a | 9.0 ± 0.1b | 37 ± 0.2a | 35 ± 1.0a | 36 ± 0.4a | |
| EO | 381 ± 30c | 371 ± 32c | 376 ± 27c | 8.1 ± 0.4a | 8.4 ± 0.3a | 8.3 ± 0.3a | 33 ± 1.3a | 31 ± 1.5b | 32 ± 0.9b | |
| ECO | 424 ± 35a | 398 ± 24a | 411 ± 19a | 8.5 ± 0.2a | 8.2 ± 0.7a | 8.3 ± 0.4a | 37 ± 0.5a | 31 ± 1.0a | 34 ± 0.8a | |
| C-306 | Amb | 450 ± 18a | 374 ± 18a | 412 ± 10a | 8.2 ± 0.3a | 8.7 ± 0.2a | 8.4 ± 0.3a | 33 ± 1.1a | 35 ± 0.6a | 34 ± 0.3a |
| EC | 470 ± 14b | 401 ± 53b | 435 ± 32b | 9.5 ± 0.4b | 9.8 ± 0.3b | 9.6 ± 0.4b | 37 ± 0.9a | 39 ± 1.3a | 38 ± 1.0b | |
| EO | 406±5c | 320 ± 19c | 363 ± 10c | 8.5 ± 0.3a | 8.8 ± 0.3a | 8.6 ± 0.3a | 31 ± 1.2a | 33 ± 1.0a | 32 ± 0.2a | |
| ECO | 460±9a | 392 ± 21a | 426 ± 11a | 8.6 ± 0.2a | 8.7 ± 0.2a | 8.6 ± 0.4a | 35 ± 1.1a | 31 ± 1.0a | 33 ± 0.6a | |
Different lower cases show significant difference (at p < 0.05 level) among different treatments within the same year according to Duncan's test.
Average of 240 plants (60 plants from each replicate for each year).
Average of 40 spikes (10 each from each replicate and each year)
Represents mean of two year response of yield parameter under respective treatment.
Table 4.
Yield parameters of HD-2967 and C-306 wheat cultivars grown under elevated CO2 (EC), tropospheric ozone (EO) and their interactive condition of elevated CO2X O3 (ECO).
| Variety | Treatments | Number of grain (spike−1)∗ |
Grain weight (g spike−1)∗ |
1000 grain weight (g)∗∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | ||
| HD-2967 | Amb | 41.3 ± 3.0a | 52.6 ± 2.5a | 46.9 ± 1.3a | 2.2 ± 0.2a | 2.4 ± 0.4a | 2.3 ± 0.2a | 35.9 ± 0.9a | 34.8 ± 1.3a | 35.4 ± 1.3a |
| EC | 44.2 ± 5.5a | 54.6 ± 5.0b | 49.4 ± 5.2a | 2.4 ± 0.1a | 2.4 ± 0.1a | 2.4 ± 0.03a | 36.4 ± 0.8a | 37.0 ± 0.8a | 36.7 ± 0.6a | |
| EO | 40.5 ± 3.0a | 45.6 ± 6.0c | 43.1 ± 3.7a | 1.8 ± 0.1b | 1.9 ± 0.1b | 1.9 ± 0.1b | 33.9 ± 1.0a | 33.1 ± 1.0a | 33.5 ± 0.4b | |
| ECO | 42.3 ± 2.0a | 49.7 ± 14a | 46.0 ± 8.1a | 2.2 ± 0.2a | 2.3 ± 0.2a | 2.2 ± 0.1a | 34.3 ± 1.1a | 35.6 ± 0.9a | 34.9 ± 0.9a | |
| C-306 | Amb | 42.2 ± 1.0a | 43.0 ± 3.0a | 42.6 ± 1.1a | 2.1 ± 0.2a | 2.3 ± 0.1a | 2.2 ± 0.2a | 36.1 ± 0.5a | 40.2 ± 1.0a | 38.2 ± 1.1a |
| EC | 52.0 ± 12.8b | 60.0 ± 7.3b | 56.0 ± 4.1b | 2.7 ± 0.2a | 2.8 ± 0.3b | 2.7 ± 0.3a | 36.3 ± 0.6a | 39.7 ± 1.0a | 38.0 ± 1.2a | |
| EO | 35.8 ± 1.6c | 42.1 ± 5.1a | 38.9 ± 2.7a | 1.8 ± 0.3b | 1.8 ± 0.2c | 1.8 ± 0.2b | 33.3 ± 0.8a | 36.6 ± 1.1b | 35.0 ± 0.8b | |
| ECO | 47.5 ± 4.1a | 47.3 ± 2.5c | 47.4 ± 2.2a | 2.2 ± 0.1a | 2.2 ± 0.1a | 2.2 ± 0.1a | 35.2 ± 1.0a | 38.4 ± 0.7a | 36.8 ± 0.9a | |
Different lower cases show significant difference (at the level of p < 0.05) among different treatments within the same year according to Duncan's test.
Average grains of 40 spikes (10 from each replicate for each year).
Average grain weight of 4000 grains (1000 grains from each replicate).
Represents mean of two year response of yield parameter under respective treatment.
Table 5.
Biological and Grain yields of HD-2967 and C-306 wheat cultivars grown under elevated CO2 (EC), tropospheric ozone (EO) and their interactive condition of elevated CO2 X O3 (ECO).
| Variety | Treatments |
∗Biological yield (ton ha−1) |
Grain yield (ton ha−1)∗∗ |
Harvest percent∗∗∗ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | 2016–17 | 2017–18 | Mean$ | ||
| HD-2967 | Amb | 13.5 ± 0.9a | 14.8 ± 1.3a | 14.1 ± 1.4a | 4.4 ± 0.4a | 4.3 ± 0.6a | 4.3 ± 0.4a | 32.5 ± 3.7a | 29.1 ± 7.1a | 30.8 ± 4.0a |
| EC | 14.7 ± 0.5b | 15.3 ± 0.8b | 15.0 ± 1.2b | 5.0 ± 0.2b | 5.2 ± 0.3b | 5.1 ± 0.5b | 34.0 ± 1.7a | 33.9 ± 4.4b | 33.9 ± 1.7a | |
| EO | 11.0 ± 1.0c | 12.0 ± 1.2c | 11.5 ± 1.5c | 3.9 ± 0.2c | 3.7 ± 0.3c | 3.8 ± 0.3c | 35.4 ± 1.0a | 30.8 ± 2.3a | 33.1 ± 1.6a | |
| ECO | 14.9 ± 1.3a | 14.1 ± 1.0a | 14.5 ± 1.3a | 4.2 ± 0.3a | 4.6 ± 0.1a | 4.4 ± 0.3a | 28.2 ± 3.5b | 32.6 ± 4.0a | 30.4 ± 1.2a | |
| C-306 | Amb | 13.3 ± 2.2a | 14.4 ± 1.0a | 13.8 ± 1.5a | 4.5 ± 0.3a | 4.9 ± 0.3a | 4.7 ± 0.4a | 33.8 ± 5.1a | 34.0 ± 2.0a | 33.9 ± 3.9a |
| EC | 15.2 ± 1.0b | 14.9 ± 1.3b | 15.0 ± 1.4b | 5.4 ± 0.3b | 5.3 ± 0.6b | 5.3 ± 0.3b | 35.5 ± 2.0a | 35.5 ± 5.2a | 35.5 ± 4.0a | |
| EO | 11.5 ± 0.7c | 13.3 ± 2.5c | 12.4 ± 1.6c | 4.4 ± 0.3a | 4.2 ± 0.2c | 4.3 ± 0.2c | 38.2 ± 1.6b | 31.5 ± 7.7b | 34.8 ± 5.3a | |
| ECO | 13.9 ± 1.1a | 15.1 ± 1.3a | 14.5 ± 1.5a | 4.6 ± 0.3a | 5.1 ± 0.3a | 4.8 ± 0.3a | 33.0 ± 1.0a | 33.7 ± 1.4a | 33.3 ± 1.0a | |
Different lower cases show significant difference (at p < 0.05 level) among different treatments within the same year according to Duncan's test.
Biological yield (total accumulated dry matter of the plant) of 240 plants (60 plants from each replicate in each year).
Grain yield of 240 plants.
Harvest percent (ratio of grain yield to biological yield times) of 240 plants.
indicate average of two years.
Table 6.
Effects of elevated CO2 (EC), tropospheric O3 (EO) and interactive condition of elevated CO2X O3 (ECO) on nitrogen, crude protein, starch and C: N ratio in the grains of two wheat cultivars∗.
| Wheat variety | Nitrogen content (%) |
Crude protein content (%) |
Starch content (%) |
C: N Ratio |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | |
| HD-2967 | Amb | 2.00 | 1.97 | 1.99 | 11.9 | 11.7 | 11.8 | 76 | 74 | 75 | 22.3 | 27.3 | 24.8 |
| EC | 1.77 | 1.81 | 1.79 | 10.5 | 10.7 | 10.7 | 86.7 | 84.3 | 85.5 | 30.8 | 26.4 | 28.6 | |
| EO | 2.20 | 2.3 | 2.25 | 13.0 | 13.8 | 13.4 | 67 | 65.3 | 66.2 | 16.7 | 20.1 | 18.4 | |
| ECO | 1.98 | 2.00 | 1.99 | 11.8 | 11.9 | 11.9 | 77 | 76 | 76.5 | 29.2 | 22.8 | 26 | |
| C-306 | Amb | 2.00 | 1.95 | 1.98 | 11.9 | 11.6 | 11.7 | 72 | 75 | 73.5 | 23.1 | 28.3 | 25.7 |
| EC | 1.80 | 1.83 | 1.82 | 10.7 | 10.8 | 10.8 | 82 | 84 | 83 | 27.6 | 31.8 | 29.7 | |
| EO | 2.33 | 2.22 | 2.26 | 13.9 | 13.2 | 13.5 | 68 | 66 | 67 | 21.6 | 18.4 | 20 | |
| ECO | 2.09 | 2.00 | 2.05 | 12.4 | 11.9 | 12.2 | 74 | 76.3 | 75.2 | 27.4 | 24.4 | 25.9 | |
Determination was done on 32 samples (8 samples from each replicate for each treatment during each year).
Table 7.
Effects of elevated CO2 (EC), tropospheric O3 (EO) and their interactive condition of elevated CO2X O3 (ECO) on Fe, Cu Zn and Mn contents in ppm units in the grains of two wheat cultivars∗.
| Iron (Fe) |
Copper (Cu) |
Zinc (Zn) |
Manganese (Mn) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | |
| HD-2967 | Amb | 68 | 75.2 | 71.6 | 6.1 | 6.4 | 6.2 | 48 | 56 | 52 | 43.1 | 39.5 | 41.3 |
| EC | 63.1 | 65.5 | 64.3 | 5.2 | 6.0 | 5.6 | 50 | 40 | 45 | 34.2 | 41.4 | 37.8 | |
| EO | 86.2 | 88.4 | 87.3 | 6.3 | 6.4 | 6.3 | 54.6 | 60 | 57.3 | 52.1 | 46.3 | 49.2 | |
| ECO | 75 | 71 | 73 | 5.5 | 6.7 | 6.1 | 48.7 | 58.7 | 53.7 | 40.0 | 45 | 42.5 | |
| C-306 | Amb | 71.8 | 66.8 | 69.3 | 5.4 | 5.8 | 5.6 | 53.2 | 46.8 | 50 | 35.8 | 44.8 | 40.3 |
| EC | 64.4 | 67.6 | 66 | 5.3 | 4.9 | 5.2 | 38.9 | 46.1 | 42.5 | 39.4 | 33.8 | 36.6 | |
| EO | 78.3 | 80.1 | 79.2 | 6.3 | 6.6 | 6.4 | 55.6 | 61 | 58.3 | 48.1 | 56.3 | 52.2 | |
| ECO | 73 | 72.2 | 72.6 | 5.6 | 5.0 | 5.3 | 55.1 | 49.1 | 52.1 | 37.2 | 44.8 | 41 | |
Determination was done on 32 samples (8 from each replicate for each treatment during each year).
Table 8.
Effects of elevated CO2 (EC), tropospheric O3 (EO) and their interactive condition of elevated CO2X O3 (ECO) on calcium (Ca), magnesium (Mg), potassium (K) and phosphorus (P) contents in the grains of two wheat cultivars∗.
| Calcium (Ca) (ppm) |
Magnesium (Mg) (ppm) |
Potassium (K) (%) |
Phosphorus (P) (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | 2016–17 | 2017–18 | Mean | |
| HD-2967 | Amb | 26.1 | 33.5 | 29.8 | 88.1 | 98.7 | 93.4 | 0.3 | 0.34 | 0.32 | 0.28 | 0.33 | 0.3 |
| EC | 23.2 | 28.2 | 25.7 | 91 | 79.2 | 85.1 | 0.31 | 0.26 | 0.28 | 0.29 | 0.25 | 0.27 | |
| EO | 31.2 | 38 | 34.6 | 100 | 118 | 109 | 0.33 | 0.43 | 0.38 | 0.31 | 0.37 | 0.34 | |
| ECO | 31.3 | 26.7 | 29 | 93.4 | 104.2 | 98.9 | 0.35 | 0.31 | 0.33 | 0.29 | 0.31 | 0.30 | |
| C-306 | Amb | 34.4 | 28.2 | 31.3 | 97.6 | 89.2 | 93.4 | 0.29 | 0.33 | 0.31 | 0.3 | 0.34 | 0.32 |
| EC | 22 | 26.4 | 24.2 | 82 | 94 | 88 | 0.31 | 0.27 | 0.29 | 0.31 | 0.3 | 0.30 | |
| EO | 38.3 | 32.5 | 35.4 | 101.3 | 112.7 | 107 | 0.39 | 0.35 | 0.37 | 0.35 | 0.32 | 0.33 | |
| ECO | 26.1 | 30.3 | 28.2 | 106.5 | 86.7 | 96.6 | 0.28 | 0.34 | 0.31 | 0.34 | 0.3 | 0.32 | |
Determination was done on 32 samples (8 from each replicate for each treatment during each year).
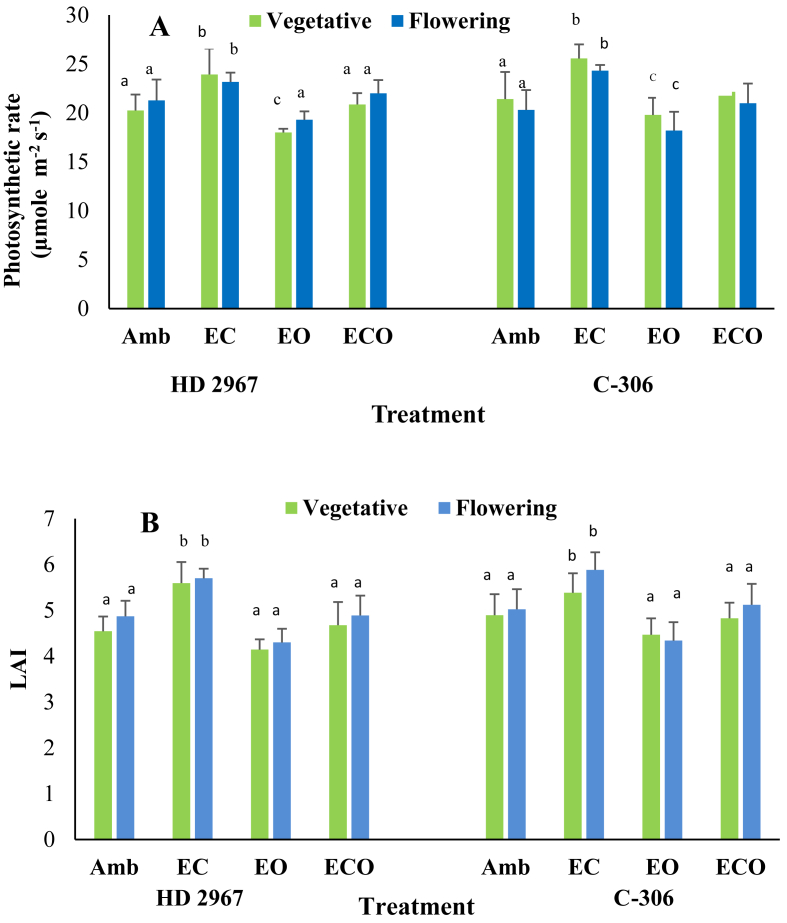
Fig. 2.
Average of two crop growing years (2016–17 and 2017–18) data on (A) leaf area index (LAI) and (B) photosynthetic rate (Pn) during vegetative and flowering stages of the growth of HD 2967 and C-306 wheat cultivars under elevated CO2 (EC), elevated O3 (EO) and the combined interactive treatment of EC X EO (ECO). Error bars show standard deviations. Different lower cases show significant difference (at p < 0.05 level) among different treatments within the same year according to Duncan's test.
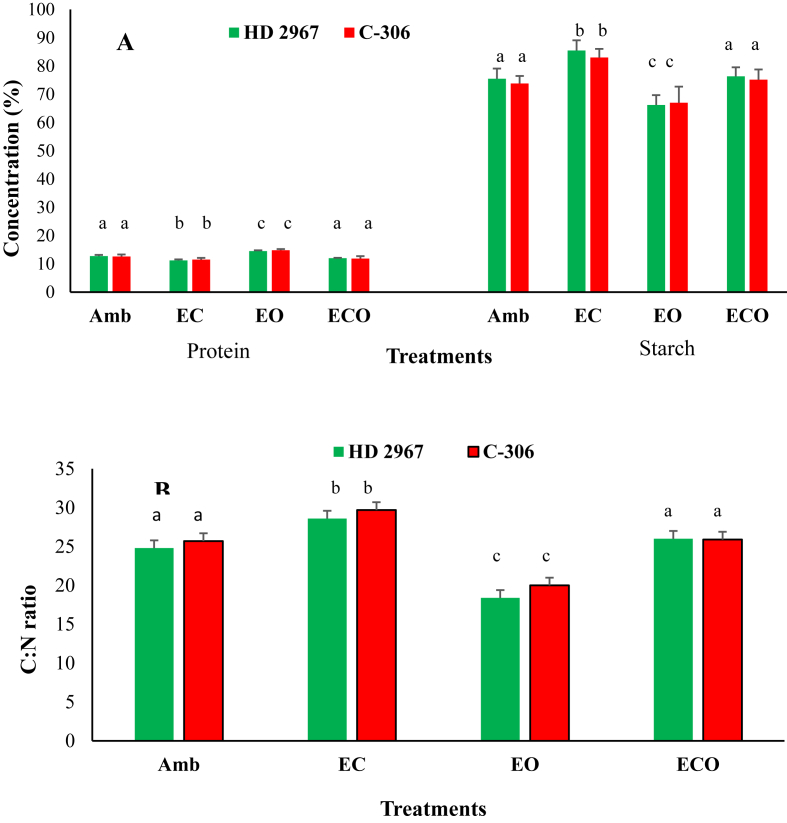
Fig. 3.
Average data of two cropping years (2016–17 and 2017–18) on (A) protein and starch contents and (B) C: N ratios in the grains of two wheat cultivars under different treatments.
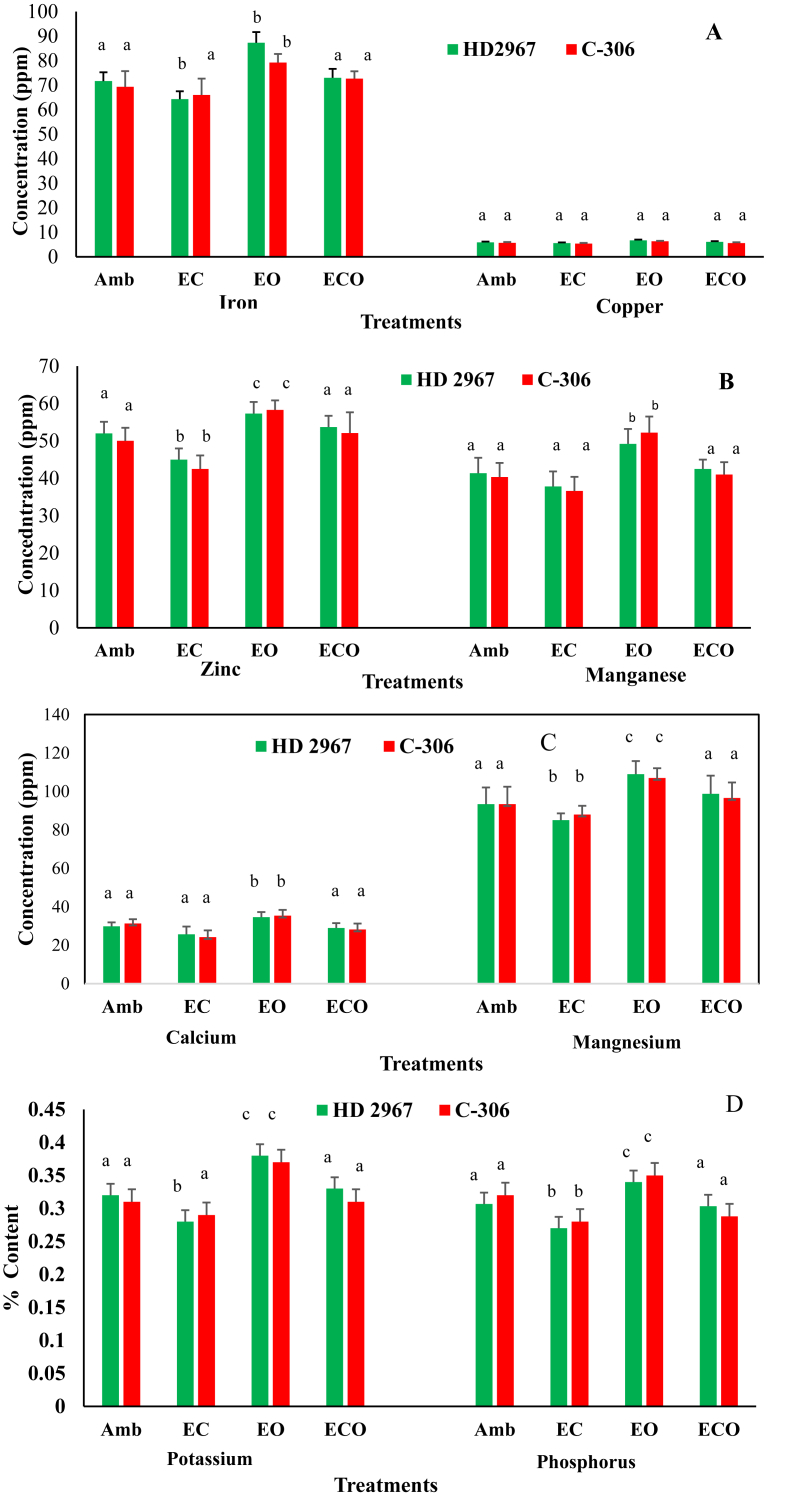
Fig. 4.
Average values of two year (2016–17 and 2017–18) data on (A) iron and copper (B) zinc and manganese (C) calcium and magnesium (D) potassium and phosphorus contents in the grains of two wheat cultivars under elevated CO2 (EC), elevated O3 (EO) and the combined interactive treatment of EC X EO (ECO). Error bars show standard deviations. Different lower cases show significant difference (at p < 0.05 level) among different treatments within the same year according to Duncan's test.
3.1. Effects of EC, EO and ECO on plant growth parameters
3.1.1. Photosynthetic rate (Pn) and leaf area index (LAI)
Average Pn across the wheat cultivars and the growing years varied from 22.3 to 25.8 (μmole m−2 s−1) under EC and from 18.7 to 21.6 (μmole m−2 s−1) under EO treatments (Table 2; Fig. 2, panel A). The EC increased Pn in the range of 13.8–20.3 % in HD-2967 and in the range of 11.4–20.0% (p < 0.05) in C-306 cultivar across the crop growing stages and years (Table 2). Opposite to this, Pn was reduced in range of 4.0–7.7% in HD-2967 and in range of 2.8–11.8% in C-306 cultivar under EO condition as compared to the Amb. The EC under combined interactive ECO conditions increased Pn by 7.9–17.6% in HD-2967 and by 2.8–17.5% in C-306 as compared to EO alone (Table 2). Pn under ECO condition did not show noticeable difference compared to Amb condition suggesting that EC and EO offset the effects of each other under ECO conditions. The LAI varied from 4.8 to 5.9 under EC and from 3.5 to 4.5 under EO alone across the cultivars and growing years (Table 2; Fig. 2, panel B). The LAI increased (at p < 0.05 level) under EC and decreased (at p < 0.05 level) under EO alone in both cultivars as compared to Amb during crop growing years. Under ECO treatment, EC caused an increase in LAI in the range of 2.4–17.1% in HD-2967 and in the range of 2.2–15.7% in C-306 cultivar over EO alone across the crop growing stages and years (Table 2). LAI between ECO and Amb conditions was comparable in both cultivars (Fig. 2, panel B).
3.1.2. Effects of EC, EO and ECO on yield and yield parameters
3.1.2.1. Number of spikes (m−2), spike length (cm) and grain weight (g spike−1)
The average number of spikes (m−2) ranged between 401 m−2 to 470 m−2 under EC and between 320 m−2 to 406 m−2 under EO across the crop cultivars as well as crop growing years (Table 3). The two year average of number of spikes in both cultivars were very close to each other (Table 3). Individually, EC increased the number of spikes (m−2) by 9.4–14.2% in HD-2967 and by 4.5–7.2% in C-306 cultivar. While, EO reduced the number of spikes by 4.8–5.8% in HD-2967 and by 9.8–14.7% in C-306 cultivar. These comparisons were made with Amb treatment. The EC under ECO conditions increased average number of spikes (m−2) by 9.3% and 17.9% in HD-2967 and C-306, respectively as compared to EO alone. Moreover, numbers of spikes (m−2) under ECO conditions were non-significantly different from the Amb (Table 3). The percentage increase/decrease in number of spikes (m−2) under EC, EO and ECO treatments compared to Amb was specific to the cultivar (s).
The spike length of wheat cultivars varied from 9.0 cm to 9.8 cm under EC and from 8.1 cm to 8.8 cm under EO for HD-2967 and C-306 cultivars, respectively. It does not vary significantly in HD-2967 (p < 0.05) under various treatments but significantly increased in C-306 (p < 0.05) under EC as compared to Amb condition (Table 3). Number of spikelets (spike−1) under EC ranged from 35 to 39 and from 31 to 33 under EO across the cultivars and growing years. Under EC, average spikelets were significantly more in C-306 (p < 0.05) as compared to Amb. EO reduced spikelets significantly (p < 005) in HD-2967 cultivar over Amb. The spikelets under ECO treatment were at par with ambient condition in both cop cultivars (Table 3).
3.1.2.2. Number of grains (spike−1), grain weight (spike−1)
The number of grains per spike varied from 44.2 to 60 under EC treatment and from 35.8 to 45.6 under EO (Table 4). EC alone increased average number of grains (spike−1) by 5.3% in HD-2967 and by 31.4% in C 306 as compared to the ambient (Amb). The number of grains per spike under EO decreased by 8.1% in HD-2967 and by 8.7% in C-306 cultivar compared to Amb. Number of grains (spike−1) under ECO were non-significantly different from Amb in HD-2967 but significantly high in C-306 (p < 0.05) as compared to Amb (Table 4). Grain weight per spike is another parameter to assess the yield of wheat crop. Average grain weights per spike under EC increased by 4.4% in HD-2967 and by 23.2% (p < 0.05) in C-306 (Table 3). Nonetheless, it was significantly decreased (p < 0.05) in both crop cultivars under EO treatment compared to Amb. Under ECO treatment, EC increased averaged grain weight by 15.8% (p < 0.05) in HD-2967 and by 22.2 % (p < 0.05) in C-306 as compared to EO alone. The grain weights (spike−1) of cultivars under ECO treatment were at par with Amb (Table 4). Test weight (1000 grain weight) of wheat cultivars responded similarly to Amb, EC and ECO treatments but reduced by 5.4% in HD-2967 and by 8.4% in C-306 under EO alone as compared to Amb (Table 4).
3.1.2.3. Biological yield, grain yields and harvest percent
Biological yield (total accumulated dry matter by a plant) per hectare for wheat cultivars ranged from 14.7 ton to 15.3 ton under EC and from 11.0 ton to 13.3 ton under EO (Table 5). EC treatment increased biological yield of HD-2967 in the range of 3.4–8.9% and of C-306 in the range of 3.5–14.3% during both crop growing years as compared to Amb. However, EO abated it significantly (p < 0.05) in both cultivars as compared to Amb (Table 5). The EC under ECO treatment increased biological yield of HD-2967 (17.5–35.4%) and C-306 (13.5–20.8%) as compared to EO alone. Biological yield did not change significantly between ECO and Amb treatment (Table 5).
Grain yield per hectare varied from 5.0 ton to 5.4 ton under EC and from 3.7 ton to 4.4 ton under EO treatment in both cultivars during both crop growing years (Table 5). Separately under EC treatment, grain yields in HD-2967 and C-306 were increased by 13.6–20.9% (p < 0.05) and by 8.1–20% (p < 0.05), respectively during both crop growing years (Table 5). Compared to EC, EO treatment decreased the average grain yield of HD-2967 (12.7%) (p < 0.05) and C-306 (8.3%) (p < 0.05) as compared to Amb. Under ECO treatment, EC increased the grain yield by 7.7–24.3% in HD-2967 and by 4.4–21.4% in C-306 as compared to EO alone. Moreover, grain yields of both wheat cultivars under ECO treatment were non-significantly (p < 0.05) different from the grain yield under ambient conditions. The average harvest percent was non-significantly different across the treatments and crop cultivars (Table 5). We assessed the effects of AOT40 on crop yield difference between Amb and EO treatments and found that the yield of crop per molecule of O3 of AOT40 decreased by 1.6% in HD-2967 and by 1.3% in C-306 cultivar.
3.2. Effects of EC, EO and ECO on wheat grain nutrients
3.2.1. Effects on starch and protein content of wheat grains
Data on starch and protein contents in the grains of two cultivars are provided in Table 6 and are shown graphically in Fig. 3, panel A. Starch, the main component of carbohydrates in plants and their grains, ranged from 82 to 86.7% under EC and from 65.3 to 68% under EO in both wheat cultivars. Under EC treatment, starch content was higher by 14% in HD-2967 and by 12–13.8% in C-306 as compared to Amb (Fig. 3, panel A). However, EO declined starch content by 11.7–11.8% in HD-2967 and by 5.6–12% in C-306 as compared to Amb. Under ECO treatment, EC significantly increased starch content by 14.9–16.4% in HD-2967 and by 8.8–15.6% in C-306 crop cultivars as compared to EO alone (Table 6; Fig. 3, panel A). Moreover, starch content under ECO treatment was non-significantly different from that observed under Amb conditions.
Protein content in grains of wheat cultivars ranged from 10.5 to 10.8% under EC and 13.0–13.9% under EO during both crop growing years (Table 6). EC alone significantly reduced the protein content in both cultivars. Protein content in grains of both cultivars increased notably (p < 0.05) under EO compared to Amb. Under ECO treatment, EC declined protein by 9.2–13.7% in HD-2967 and by 9.8–10.8% in C-306 as compared to EO alone. Furthermore, protein contents of grains under ECO were non-significantly different from the protein content observed under Amb condition (Fig. 3, panel A). Total carbon and Kjeldahl nitrogen (C: N) ratios in the grains of both wheat cultivars were calculated. The C: N ratio ranged from 26.4 to 31.8 under EC and from 16.7 to 21.6 under EO, irrespective of crop cultivar and crop growing years (Table 6 and Fig. 3, panel B). It significantly increased (p < 0.05) under EC and notably reduced under EO (p < 0.05) for both wheat crops in comparison to Amb (Table 6 and Fig. 3, panel B). It was non-significantly different between ECO and Amb.
3.2.2. Effects on micro- and macro-nutrients in wheat grains
Data on micronutrients Fe, Cu, Zn and Mn and macro nutrients Ca, Mg, K and P in the grains of two wheat cultivars under different treatments during both crop growing years are provided in Tables 7 and 8 and are shown graphically in Fig. 4 (panels A–D). The EC reduced average iron (Fe) content in the grains of HD-2967 and C-306 by 10.2% and 4.7%, respectively across the cropping years (Table 7 and Fig. 4, panel A). Contrary to EC treatment, EO significantly increased Fe content in HD-2967 (21.8%) (p < 0.05) and in C-306 (14.3 %) (p < 0.05) in both years as compared to Amb. Under ECO, EC treatment reduced the positive effect of EO on iron content by 16.3 % (p < 0.05) (p < 0.05) in HD-2967 and by 8.3% (p < 0.05) in C-306 as compared to EO alone (Fig. 4 panel A). Iron content of wheat grains under ECO and under ambient were similar. Cu content in the grains was much lower than Fe but similar to Fe, Cu contents in grains of HD-2967 and C-306 decreased by 9.6% and 7.2%, respectively under EC treatment. However, EO increased Cu in grains of both cultivars compared to Amb. Under ECO treatment, EC reduced the mean positive effect of EO for HD-2967 and C-306 by 3.2% and 17.2%, respectively as compared to EO alone. Copper content of wheat grains under ECO was at par with Amb (Fig. 4, panel A). Zinc content in grains varied from 38.9 ppm to 50 ppm under EC and from 54.6 ppm to 61 ppm under EO and from in both crop cultivars (Table 7). Zn content of wheat grains of HD (13.4%) and C-306 (15%) responded negatively to the EC. However, EO significantly increased zinc in HD-2967 and in C-306 (p < 0.05) grains as compared to Amb. Furthermore, Zn concentrations in the grains were non-significantly different between ECO and Amb treatments (Fig. 4, panel B). Similar to zinc, EC treatment declined manganese (Mn) content by 8.5% in HD-2967 and by 9.2% in C-306. Nonetheless, EO significantly increased Mn in grains of HD (p < 0.05) and in C-306 (p < 0.05) as compared to Amb. The EC in ECO treatment nullified the positive influence of EO (13.6–21.4%) across the crop cultivars and crop growing years as compared to the EO alone (Fig. 4, panel B). Manganese concentrations of grains under ECO and Amb were not significantly different from each other in both crop cultivars.
Similar to micronutrients, calcium (Ca) in grains of HD-2967 and C-306 was decreased by 13.7% and 22.6% (P < 0.05) under EC treatment. EO significantly increased Ca by 16.1% (p < 0.05) in HD-2967 and by 13% (p < 0.05) in C-306 as compared to ambient (Table 8). Under ECO treatment, EC countered the positive effects of EO on Ca concentrations in grains of HD-2967 (16.2%) and C-306 (20.3%) with respect to EO alone. Calcium content of grains was non-significantly different under ECO from ambient (Fig. 4, panel C). Magnesium (Mg) content of HD-2967 (8.8%) and C-306 (5.7%) wheat grains negatively responded to EC over the Amb (Fig. 4, panel C). However, EO alone increased average Mg concentration in grains in the range of 14.6–16.7% across the cultivars. Mg content in grains under ECO treatment was at par with ambient. EC significantly declined (p < 0.05) average potassium (K) content in the grains of both cultivars over Amb. EO significantly increased (p < 0.05) potassium as compared to Amb (Table 8 and Fig. 4, panel D). Under ECO treatment, EC negated the favorable effects of EO on mean K concentration of wheat grains for both crop cultivars (13.2–16.2%) compared to EO alone. Phosphorus concentration in grains responded negatively to EC and decreased in the range of 6.2–10%. EO increased (3.1–13.3%) it across the crop cultivars as compared to the ambient (Fig. 4, panel D).
4. Discussion
Rising levels of CO2 and tropospheric O3 have own effects on responses to crop growth, yield and quality of grains. Elevated atmospheric CO2 is expected to enhance wheat production (Mishra et al., 2013; Wang et al., 2013; Fitzgerald et al., 2016) whereas elevated tropospheric O3 is likely to reduce it across the world (Mills et al., 2018). It is important to note that concentrations of CO2 and O3 would increase concurrently under the projected future climate changing scenario (Proietti et al., 2016; Sicard et al., 2017). Previous studies indicated that EC negates the adverse effects of EO on plant growth and yield but reduces the positive effects on grain quality under the combined interactive treatment of EC and EO (ECO) (Broberg et al., 2015; Phothi et al., 2016). In the present study, EC significantly increased Pn and LAI in both wheat cultivars and EO reduced them significantly (p < 0.05) (Fig. 2, panels A and B). It is important to mention that Pn appears to be least influenced by fIPAR and temperature in this experiment as fIPAR varied in very small window from 0.94 to 0.95 during the vegetative growth stage (46 DAS) and from 0.95 to 0.97 during the flowering stage (82 DAS). The average temperature on the Pn measuring dates too showed limited changes at vegetative growth stages and at flowering stage (Table 1) across the growing years. Rather, this increase in Pn under EC might be due to increased carboxylation activities and the reduction in Pn under EO condition might be due to entering of O3 molecules in plants during normal gas exchange that create reactive oxygen species (ROS), resulting in programmed cell death and changes in physiology of plants (Ainsworth and Rogers, 2007; Keiser et al., 2017; Pleijel et al., 2018). Similar observations on Pn rate under EC have been reported by Ashraf and Harris (2013). The Pn rates were similar in ECO and Amb treatments. Similar to our results, Phothi et al. (2016) described that EC mitigates the negative influence of O3 on growth attributes in rice crop under ECO treatments and take them to at par with the ambient. Wang et al. (2013) did a meta-analysis of 59 experimental data pertaining to photosynthesis of wheat crops grown across the world under EC (450–800 ppm) and recorded an increase of 33% in Pn compared to ambient conditions. The observations made here are in conformity with their findings. The LAI too responded similar to Pn in both cultivars under EC and EO treatments. Earlier research has also documented that LAI of crops were significantly increased under EC and reduced under EO treatments (Tomer et al., 2015; Abebe et al., 2016).
Leisner and Ainworth (2012) and Feng et al. (2015) expressed that EO damages crops during the reproductive stage of growth due to increased abiotic stress sensitivity and high demand of resources for seed development. The production of ROS in apoplast triggers metabolic defense mechanism and programmed cell death and promotes leaf senescence, which diverts resources during growth and seed production and ultimately influences crop yield (Ainsworth, 2017). In our experiment, EO declined crop growth and yield significantly (p < 0.05) (Tables 2, 3, 4, and 5). A comparative assessment between ECO and EO divulged that EC under ECO treatment significantly increased some of the yield and yield attributes (p < 0.05) as compared to EO alone. It is evident from data that EC counteracted by the negative effects of EC under ECO treatment and took plant growth and yield components at par with Amb conditions (Tables 2, 3, 4, and 5). This may be attributed to reduced stomatal conductance and declined stomatal density due to EC that mitigated the adverse effects of EO (Ainsworth et al., 2008). Declined stomatal conductance under EC treatment limits O3 flux into the apoplast and consequently reduces the damaging effects of O3 in crops (Feng and Kobayashi, 2009). Lobell et al. (2012) pointed out that enforced senescence declines shoots (plant−1), number of grains (spike−1) and 1000 grain weight of wheat crops without showing any adverse effect. In similar pattern, Singh et al. (2017) and Pandey et al. (2018) have observed that the plant growth and yield of two wheat cultivars (HD-2967 and Somalia) have reduced under EO treatment. Ainsworth (2017) reported a reduction in grain yield from 2 to 16% in rice, maize, wheat and soybean crops based on modeling of empirical data gathered under EO condition and potential EO effects on crop productivity. But, Tai et al. (2014) stated that models were unable to quantify the interactive effects of EO with other abiotic stresses on the crops. Reduction in grain yield beyond AOT40 (40 ppb threshold limit of O3) in our study was similar to previous studies (Pleijel et al., 2018).
In our experiment, crop yield under EC treatment increased significantly (p < 0.05) (Table 5) as compared to that under Amb. It may be attributed to the increase in crop growth parameters such as Pn (8.8–19.8%) and LAI, and yield attributes such as number of grains (spike−1), increased grain weight (spike−1) and number of spikes (m2). Increased Pn in crop cultivars accumulate more carbohydrates in plants and result in improved yield (Abebe et al., 2016). Previous studies have indicated that plant growth and yield of wheat and rice crops increase under elevated CO2 and decline under EO treatment (Pingale et al., 2017; Guarin et al., 2019). Dubey et al. (2015) have assessed results of 19 studies across 9 countries and concluded that plant growth and yield components of wheat responded positively to various levels of EC. They have also opinioned that response of elevated atmospheric CO2 would become adverse under the projected climate change scenario even though it has potential to compensate the effects of other changes in the climate.
Under EC and EO scenarios, grain nutrient status is becoming a global concern for future requirement of human nutrition (Pleijel et al., 2018; Jin et al., 2019). Studies have documented that EC can alter the availability and uptake of nutrients in crops (Broberg et al., 2015; Malin et al., 2015; Pleijel and Hogy, 2015). Pleijel et al. (2018) reported that EO increases mineral content of wheat grains but declines starch content. In present study, EC treatment significantly (p < 0.05) declined protein contents in grains of wheat crops but increased starch content, similar to previous study (Asseng et al., 2019). Dilution of nitrogen content due to increased photosynthesis and more accumulation of carbohydrate under EC have been associated with decline in grain protein (Panozo et al., 2014). However, EO is known for grain filling process and influences carbohydrate and total protein accumulation in wheat (Zhou et al., 2015; Pleijel et al., 2018).
Similar to total protein content, micro- and macro-nutrients under EC treatment were low (P < 0.05) in both crop cultivars as compared to the ambient. EO impacted them in reverse manner. Dilution effect under EC reduces the mineral content of wheat grains (Pandey et al., 2018; Zhu et al., 2018) but EO compensated the effect of EC (Pleijel and Uddling, 2012; Pleijel et al., 2018). There has been reports on inconsistent changes in micro- and macro-nutrients levels in wheat grains under elevated atmospheric CO2 condition (Hogy, 2008). Elevated O3 conditions significantly reduced 31crude protein levels under non-ethylene diurea (NEDU) treated seeds of mung bean (Vigna radiata Cv. MN-98) as compared to ethylene diurea (EDU) (Jan, 2018). Chaturvedi et al. (2017) observed reduction in mineral contents in grains of rice under EC. Reduction in mineral contents of wheat grains under EC could be attributed to EC led declination in stomatal conductance and reducing transpiration-driven mass flow of minerals from root to apex organs (Fernando et al., 2014; Houshmandfar et al., 2015). The EC led dilution effect could also be reason for reduced nutrient concentrations in milled rice with unbalanced translocation of minerals from vegetative parts viz., leaf, stem and husk to grains (Yang et al., 2006). The increase in carbon content was accompanied with reduction in total N in both the wheat cultivars. The increase in C: N ratio in wheat grains might be due to more carbon accumulation under EC (51.5–52.2%) treatment and reduced nitrogen concentration (1.80–1.84 %) due to the dilution effect. However, reduced C:N ratio under EO treatment can be explained by lower concentration of carbon (43–44.9%) and higher concentration of nitrogen in grains. Similar observations on C: N ratios in chickpea grains have been made under EC treatment by Saha et al. (2015).
5. Conclusions
The EC impacted positively Pn, LAI, C:N ratio in grains, growth and yield parameters and therefore, the yield was higher under EC whereas EO declined them in both wheat cultivars. The average yield loss per O3 molecule exposure varied from 1.3 to 1.6% in two cultivars but EC mitigated the adverse effects of elevated tropospheric O3 of (AOT40) on wheat growth, yield and yield attributes. Both wheat cultivars responded in similar manner to EC, EO and ECO treatments but the extents of responses or sensitivity of each cultivar were different. Therefore, results of this study, delimited to two wheat cultivars from sub-tropical climate shall be interpreted with caution as effects of EC, EO and ECO may change with the cultivar(s) and the climatic zone. There is a need to develop more data on different genotypes of wheat grown under different geographical regions. Such study may help in selection or development of high yield and O3 stress tolerance varieties from the available diverse pool of wheat germplasm for combating future climate change scenario and food security.
Declarations
Author contribution statement
Achchhelal Yadav: Performed the experiments; Wrote the paper.
Arti Bhatia: Conceived and designed the experiments; Wrote the paper.
Sudesh Yadav: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vinod Kumar, Bhupinder Singh: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the National Innovations on Climate Resilient Agriculture (NICRA) project, Indian Council of Agricultural Research (ICAR), New Delhi.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors extend thank to the editor and two anonymous reviewers for their help in improving the quality of MS. Authors are thankful to Director, ICAR- Indian Agricultural Research Institute, New Delhi for providing the facilities to execute the experimental work. Authors extend their sincere thanks to Dr. S.D. Singh, Head, CESCRA, IARI, New Delhi for his help and guidance to carry out the experiment. The ICP-OES facility created under JNU-UPOE II project grant (ID98) and used in this work is duly acknowledged.
References
- Abebe A., Pathak H., Singh S.,D., Bhatia A., Harit R., Kumar Vinod. Growth, yield and quality of maize with elevated atmospheric carbon dioxide and temperature in North West India. Agric. Ecosyst. Environ. 2016;218:66–72. [Google Scholar]
- Ainsworth E.A. Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 2008;14:1642–1650. [Google Scholar]
- Ainsworth E.A. Understanding and improving global crop responses to ozone. Plant J. 2017;90:886–897. doi: 10.1111/tpj.13298. [DOI] [PubMed] [Google Scholar]
- Ainsworth E.A., Rogers A. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ. 2007;30(3):258–270. doi: 10.1111/j.1365-3040.2007.01641.x. [DOI] [PubMed] [Google Scholar]
- Ashmore M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005;28:949–964. [Google Scholar]
- Ashraf M., Harris P.J.C. Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51(2):163–190. [Google Scholar]
- Asseng S., Martre P., Maiorano A. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019;25(1):155–173. doi: 10.1111/gcb.14481. [DOI] [PubMed] [Google Scholar]
- Blair G.J., Leffro Rod D.B., Leanne F. Soil Carbon Fractions Based on their degree of oxidation and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995;46:1559–1566. [Google Scholar]
- Broberg M.C., Feng Z.Z., Xin Y., Pleijel H. Ozone effects on wheat grain quality-a summary. Environ. Pollut. 2015;193:203–213. doi: 10.1016/j.envpol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Broberg M.C., Uddling J.M.G., Peijel H. Fertilizer efficiency in wheat is reduced by ozone pollution. Sci. Total Environ. 2017;607–608:876–880. doi: 10.1016/j.scitotenv.2017.07.069. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A.K., Bahuguna R.N., Pal M., Shah D., Maurya S., Krishana S.V.J. Elevated CO2 and heat stress interaction affect grain yield, quality and mineral nutrients composition in rice under field condition. Field Crop. Res. 2017;206:149–157. [Google Scholar]
- CLRTAP . 2017. Mapping Critical Levels for Vegetation, Chapter III of Manual on Methodologies and Criteria for Modelling and Mapping Critical Loads and Levels and Air Pollution Effects, Risks and Trends.http://icpvegetation.ceh.ac.uk/ UNECE Convention on Long-range Trans boundary Air Pollution. [Google Scholar]
- Deryang D., Joshua Elliot., Christian F., Christoph M., Thomas A.M.P., Kenneth J.B., Declan C., Alex C.R., Dieter G., James W.,J., Nikolay Khabarov., Stefan O., Sibyll S., Erwin S., Hong Y., Cynthia R. Regional disparities in the beneficial effects of rising CO2 concentrations on crop water productivity. Nat. Clim. Chang./Lett. 2016;6:786–790. [Google Scholar]
- Dubey S.K., Tripathi S.K., Pranuthi G. Effect of elevated CO2 on wheat crop: mechanism and impact. Crit. Rev. Environ. Sci. Technol. 2015;45:2283–2304. [Google Scholar]
- Economic Survey . vol. 2. Ministry of Agriculture, department of agriculture and co-operation, Government of India; 2016-17. (Agriculture and Food Management, India). 164-154. [Google Scholar]
- FAO . Food and Agriculture Organization of The United Nations; Rome: 2016. Agricultural Data.http://faostat.fao.org/ Online at. [Google Scholar]
- Feng Z., Hu E., Wang X., Jiang L., Liu X. Ground-level O3 pollution and its impacts on food crops in China: a review. Environ. Pollut. 2015;199:42–48. doi: 10.1016/j.envpol.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Feng Z., Kobayashi K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with a meta-analysis. Atmos. Environ. 2009;43:1510–1519. [Google Scholar]
- Fernando N., Panozzo J., Tausz M., Norton R.M., Neumann N., Fitzgerald G.J., Seneweera S. Elevated CO2 alters grain quality of two bread wheat cultivars grown under different environmental conditions. Agric. Ecosyst. Environ. 2014;185:24–33. [Google Scholar]
- Fitzgerald G.N., Tauz M., O’ Leary G., Mollahi M.R., Tauszposch S., Seneveera S., Markus V., Partington D.L., Neil A., Norton R.M. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Glob. Chang. Biol. 2016;22:2269–2284. doi: 10.1111/gcb.13263. [DOI] [PubMed] [Google Scholar]
- Frei M. Breeding of ozone resistant rice: relevance, approaches and challenges. Environ. Pollut. 2015;197:144–155. doi: 10.1016/j.envpol.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Guarin J.F., Kassie B., Mashaheet A.M., Burkey K., Asseng S. Modeling the effects of tropospheric ozone on wheat growth and yield. Eur. J. Agron. 2019;105:13–23. [Google Scholar]
- Harmens H., Hayes F., Sharps K., Sharps Katrina, Radbourne Alan, Mills Gina. Can Reduced irrigation mitigate ozone Impacts on an ozone-sensitive African wheat variety. Plants. 2019;8(7):220. doi: 10.3390/plants8070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogy P., Brunnbaur M., Koehler P., Schwadorf K., Breuer J., Franzaring J. Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ. Exp. Bot. 2013;88:11–18. [Google Scholar]
- Hogy P. Effects of elevated CO2 on grain quality of wheat. J. Cereal Sci. 2008;48(3):580–591. [Google Scholar]
- Houshmandfar A., Fitzgerald G.J., Tausz M. Elevated CO2 decreases both transpiration flow and concentrations of Ca and Mg in the xylem sap of wheat. J. Plant Physiol. 2015;174:157–160. doi: 10.1016/j.jplph.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Jackson M.L. Prentice Hall of India; New Delhi: 1973. Soil Chemical Analysis; pp. 41–330. [Google Scholar]
- Jan Effects of tropospheric ozone (O3) on yield and nutritional quality of mung bean (Vigna Radiata Cv. MN 98): evidence from Pakistan. J. Environ. Anal. Toxicol. 2018;8(2) [Google Scholar]
- Jin J., Armstrong R., Tang C. Impact of elevated CO2 on grain nutrient concentration varies with crops and soils - a long-term FACE study. Sci. Total Environ. 2019;651(2):2641–2647. doi: 10.1016/j.scitotenv.2018.10.170. [DOI] [PubMed] [Google Scholar]
- Juliano . Inter. Rice Res. Inst; 1993. Rice in Human Nutrition, No.26. [Google Scholar]
- Kaiser E., Zhou D., Heuvelink E., Harbinson J., Morales A., Marcelies L.F.M. Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. J. Exp. Bot. 2017;68(20):5629–5640. doi: 10.1093/jxb/erx357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefohn A.S., Malley C.S., Simon H., Wells B., Xu X. Responses of human health and vegetation exposure metrics to changes in ozone concentration distributions in the European Union, United States, and China. Atmos. Environ. 2017;152:123–145. [Google Scholar]
- Leisner C.P., Ainworth E.A. Quantifying the effects of ozone on plant reproductive growth and development. Glob. Chang. Biol. 2012;18:606–616. [Google Scholar]
- Lobell D.B., Sibley A., Ortiz-Monasterio J.I. Extreme heat effects on wheat senescence in India. Nat.Clim.Change. 2012 [Google Scholar]
- Mahajan G., Singh, Chauhan B.S. Impact of climate change on weeds in rice-wheat cropping system. Curr. Sci. 2012;102(9):1254–1255. [Google Scholar]
- Malin C.B., Feng Z., Xin Yue., Pleijel H. Ozone effects on wheat grain quality-A summary. Environ. Pollut. 2015;197:203–213. doi: 10.1016/j.envpol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Mills G., Sharps K., Simpson D., Pleijel H., Broberg M., Uddling J., Jaramillo F., Davies W.J., Agrawal M., Agrawal S.B., Ainsworth E.A., Buker P., Dentener F., Emberson L., Feng Z., Harmens H., Hayes F., Kobayashi K., Paoletti E., Van den Berg M., Van D.R. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018;24(8):3560–3574. doi: 10.1111/gcb.14157. [DOI] [PubMed] [Google Scholar]
- Mishra A.K., Rai R., Agrawal S.B. Differential response of dwarf and tall tropical wheat cultivars to elevated ozone with and without carbon dioxide enrichment: growth, yield and grain quality. Field Crop. Res. 2013;145:21–32. [Google Scholar]
- Monteith J.L., Gregory P.J., Marshall B., Ong C.K., Saffell R.A., Squire G.R. Physical measurements in crop physiology I. Growth and gas exchange. Exp. Agric. 1981;17(2):113–126. [Google Scholar]
- Myers S.S., Zanobetti A., Kloog I., Huybers P., Leaky A.D., Bloom A.J., Carlisle E., Dietterich L.H., Fitzgerald G., Hasegawa T., Holbrook N.M. Increasing CO2 threatens human nutrition. Nature. 2014;510:139–142. doi: 10.1038/nature13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA, ESRI . 2019. Mauna Loa CO2 Annual Mean Data. [Google Scholar]
- Pandey A.K., Ghosh A., Agrawal M., Agrawal S.B. Effect of nitrogen and varying levels of soil nitrogen in two wheat (Triticum aestivum L.) cultivars: growth, gas exchange, antioxidant status, grain yield and quality. Ecotoxicol. Environ. Saf. 2018;158:59–68. doi: 10.1016/j.ecoenv.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Panozo J.F., Walker C.K., Partington D.L., Neumann N.C., Tausz M., Seneveera S., Fitzgerald G.J. Elevated carbon dioxide changes grain protein concentration and composition and compromises baking quality, A FACE study. J. Cereal Sci. 2014;60:461–470. [Google Scholar]
- Phothi R., Umponstira C., Sarin C.S.W., Nabheerong N. Combining effects of ozone and carbon dioxide application on photosynthesis of Thai jasmine rice (Oryza sativa L.) cultivar Khao Dawk Mali 105. Aus. J. Crop Sci. 2016;10(4):591–597. [Google Scholar]
- Pingale B.N., Singh S.D., Yadav A. Increasing atmospheric carbon dioxide Potential impacts on yield and plant growth of rice (Oryza sativa L.) and maize (Zea mays L) crops. Indian J. Agric. Sci. 2017;87(8):1041–1044. [Google Scholar]
- Pleijel H., Broberg M.C., Uddling J., Mils Gina. Current surface ozone concentrations significantly decrease wheat growth yield and quality. Sci. Total Environ. 2018;613–614:687–692. doi: 10.1016/j.scitotenv.2017.09.111. [DOI] [PubMed] [Google Scholar]
- Pleijel H., Hogy P. CO2 dose response functions for wheat grains, protein and mineral yield based on FACE and open top chamber experiments. Environ. Pollut. 2015;198:70–77. doi: 10.1016/j.envpol.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Pleijel H., Uddling J. Yield vs. Quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2012;18:596–605. doi: 10.1111/j.1365-2486.2011.02489.x. [DOI] [PubMed] [Google Scholar]
- Proietti C., Anav A., De Marco A., Sicard P., Vitale M. A multi-sites analysis on the ozone effects on gross primary production of European forests. Sci. Total Environ. 2016;556:1–8. doi: 10.1016/j.scitotenv.2016.02.187. [DOI] [PubMed] [Google Scholar]
- Sadasivam S., Manickam A. Biochemical Methods for Agricultural Sciences. Wiley Eastern Limited; New Delhi: 1992. pp. 12–13. [Google Scholar]
- Saha S., Chakraborty D., Sehgal V.K., Pal M. Potential impact of rising atmospheric CO2 on quality of grains in chickpea (Cicer arietinum L) Food Chem. 2015;187:431–436. doi: 10.1016/j.foodchem.2015.04.116. [DOI] [PubMed] [Google Scholar]
- Sicard Pierre, Anav A., De Marco Alessandra, Paoletti E. Projected global tropospheric ozone impacts on vegetation under different emission and climate scenarios. Atmos. Chem. Phys. 2017;17:12177–12196. [Google Scholar]
- Singh A.A., Fatima A., Mishra A.K., Chaudhary N., Mukherjee A., Agrawal M., Agrawal S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: growth and productivity. Environ. Monit. Assess. 2018;190(4):190–203. doi: 10.1007/s10661-018-6563-0. [DOI] [PubMed] [Google Scholar]
- Singh S.R., Pandey B., Agrawal M. Development of resistance in two wheat cultivars against constant fumigation of ozone. Proc. Natl. Acad. Sci. India. 2017 [Google Scholar]
- Tai A.P.K., Val Martin M., Heald C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014;4:817–821. [Google Scholar]
- Tomer R., Bhatia A., Kumar V., Kumar A., Singh R., Singh B., Singh S.D. Impact of elevated ozone on growth, yield and nutritional quality of two wheat species in Northern India. Airo. Air Qual. Res. 2015;15:329–340. [Google Scholar]
- Van der Kooi C.J., Reich M., Low M., De Kok L.J., Tausz M. Growth and yield stimulation under elevated CO2 and drought: a meta-analysis on crops. Environ.Exp. Biol. 2016;122:150–157. [Google Scholar]
- Wang L., Feng Z., Schjoerring J.K. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): a meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013;178:57–63. [Google Scholar]
- Yadav S., Rajamani V. Aerosols of NW India-A potential Cu source! Curr. Sci. 2003;84(3):278–280. [Google Scholar]
- Yang L., Wang Y., Dong G., Gu H., Huang J., Zhu J., Yang H., Liu G., Han Y. The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crop. Res. 2007;102(2):128–140. [Google Scholar]
- Zhou X., Zhou J., Wang Y., Peng B., Zhu j., Yang L., Wang Y. Elevated tropospheric ozone increased grain protein and amino acids content of hybrid rice without manipulation by planting density. J. Sci. Food Agric. 2015;95:72–78. doi: 10.1002/jsfa.6684. [DOI] [PubMed] [Google Scholar]
- Zhu C., Kobayshi K., Loladze I., Zhu J., Jiang Q., Xu X., Liu G., Seneweera S., Ebi K.L., Drewnowski A., Fukagawa N.K., Zisla L.H. Carbon dioxide (CO2) levels this century will alter the protein, Micronutrients and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018;4:1–8. doi: 10.1126/sciadv.aaq1012. [DOI] [PMC free article] [PubMed] [Google Scholar]