Abstract
Aims
To evaluate the relative bioavailability of oral amoxicillin (AMX) tablets in comparison to AMX suspension in Roux‐en‐Y gastric bypass bariatric subjects.
Methods
A randomized, double‐blind, cross‐over study was performed on the bioavailability of oral AMX tablets and suspension in Roux‐en‐Y gastric bypass subjects operated at least 3 months previously . Doses of 875 mg of the AMX tablet or 800 mg of the AMX suspension were given to all the subjects, allowing a washout of 7 days between the periods. Blood samples were collected at 0, 0.25, 0.5, 1, 1.5, 2, 4, 6 and 8 hours after drug administration and the AMX levels were quantified by liquid chromatography coupled with triple quadrupole tandem mass spectrometry. The pharmacokinetic parameters were calculated by noncompartmental analysis, normalized to an 875 mg dose and the bioavailability of the AMX from the tablets was compared to that from the suspension formulation.
Results
Twenty subjects aged 42.65 ± 7.21 years and with a body mass index of 29.88 ± 4.36 kg/m2 were enrolled in the study. The maximum AMX plasma concentration of the tablets and the suspension (normalized to 875 mg) were 7.42 ± 2.99 mg/L and 8.73 ± 3.26 mg/L (90% confidence interval of 70.71–99.11), and the total area under the curve from time zero to infinity were 23.10 ± 7.41 mg.h/L and 27.59 ± 8.32 mg.h/L (90% confidence interval of 71.25–97.32), respectively.
Conclusion
The tablets presented a lower bioavailability than the suspension formulation and the total absorbed amount of AMX in these subjects was lower in comparison to the standard AMX absorption rates in nonbariatric subjects, regardless of the formulation.
Keywords: amoxicillin, gastric bypass, pharmacokinetic, relative bioavailability
What is already known on this subject
The anatomical changes of bariatric surgery, particularly gastric bypass, may present some issues related to drug absorption. For some drugs, absorption is reduced, while it increases for others.
The prescription of liquid formulations is preferred to tablets for bariatric subjects.
What this study adds
Evidence of the reduced oral bioavailability of amoxicillin tablets compared to suspension formulations in Roux‐en‐Y gastric bypass bariatric subjects.
The total absorbed amount of amoxicillin in Roux‐en‐Y gastric bypass bariatric subjects was found to be lower in comparison to nonbariatric subjects for both tablet and suspension formulations.
1. INTRODUCTION
Amoxicillin (AMX), an aminopenicillin, is one of the most commonly prescribed antibiotics for the treatment of upper and lower respiratory tract, skin, genitourinary tract and Helicobacter pylori infections. It is listed in the World Health Organization's 20th list of Essential Medicines (2017)1 and is available in many countries in tablet, capsule, powder (for oral suspension) and intravenous‐injection‐solution forms, the latter not being available in Brazil. Oral AMX forms are completely absorbed in the small intestine2 and present absolute bioavailability ranging from 89.4 to 97% for doses of 125 up to 1000 mg.3, 4
According to the Biopharmaceutical Classification System (BCS), AMX may be classified differently because it presents dose‐dependent behaviour. As such it can be considered a class I drug for doses up to 875 mg, class II between 875 and 1000 mg and class IV for doses over 1000 mg.5 Therefore, it is hypothesized that any physiological changes in the gastrointestinal (GI) tract may interfere in the AMX absorption rate, especially when given in tablet form at higher doses as the processes of disintegration and dissolution of the tablets are directly dependent on the fluid content, pH and motility of the GI tract, and any changes in this environment may therefore affect the rate and extension of the drug absorption.6
One population that presents these physiological changes are overweight patients who have undergone bariatric surgery, namely the Roux‐en‐Y gastric bypass (RYGB) procedure, a restrictive and malabsorptive surgery. Since their stomach has been reduced to a small proximal gastric pouch of around 50 mL, their duodenum and the initial part of the jejune (around 60 cm) were excluded from the intestinal tract. These patients may present with some drug‐absorption issues because of the reduced length of the small intestine.7 In particular, very little information is available about AMX absorption in bariatric subjects, which leads to uncertainty about how to prescribe oral antibiotics for this population in order to avoid the risk of therapeutic failure and the development of antimicrobial resistance.8, 9, 10, 11
Although liquid formulations are preferable to solid ones for bariatric subjects,6 the two main pharmaceutical forms of AMX have similar pharmacokinetic (PK) profiles in the general population. With regard to absorption, an absolute bioavailability of 89.4%–97% has been described for doses between 125 and 1000 mg, based on the percentage of total area under the plasma concentration vs time curve (AUC) calculated after oral vs intravenous administration. For oral doses of 250 , 500 and 875 mg, a proportional maximum observed plasma concentration (Cmax; 3.8–10.4, 5.9–10.8 and 17.83–18.59 mg/L) and AUC from time zero to infinity (AUC0–inf; 9.8–13.24, 18.8–27.4 and 51.29–55.42 mg.h/L) were described. With regard to distribution and elimination, AMX presents low plasma protein binding (17–20%), a volume of distribution of 0.26–0.41 L/kg and fast elimination, with a half‐life elimination of 1–1.5 hours, mainly through the renal route (57–80%).5
There is only one study published in previous literature12 suggesting a lower absorption of AMX after bariatric surgery. However, this study is a case report in which therapeutic failure was observed when oral AMX was administered to a pregnant patient, and no blood quantification or PK parameters were measured or calculated. It is not clear whether there was a loss in the AMX absorption or whether the therapeutic failure was inherent to the patient condition.
Most of the reviews presented in previous literature6 recommend the prescription of an oral solution for all drug classes, assuming that some problems related to tablet absorption may occur, but none of them demonstrated whether there are any differences in bioavailability between tablets and suspension in the RYGB population.
Therefore, the aim of this study was to carry out a randomized clinical study to evaluate the bioavailability of AMX tablets and AMX suspension in RYGB bariatric subjects.
2. METHODS
2.1. Study design and population
This was a single‐centre, randomized, double‐blind (clinical and analytical), cross‐over (2×2) study to compare the relative bioavailability of oral AMX tablets and suspension in subjects who have undergone RYGB surgery. The study protocol was registered in the Brazilian Clinical Trials Registry under the number RBR‐3DGCPV. The protocol was approved by the State University of Maringá Institutional Review Board (CAAE57476516.3.0000.0104) and all subjects provided written informed consent before inclusion in the prescreening study.
Adult subjects, aged 18–55 years, who had undergone RYGB surgery between 3 months and 10 years before the start of this study, and who resided in the city of Maringá, Paraná State, were included in the study. Subjects who were allergic to AMX and other antibiotics of the same class, had renal or hepatic impairment, were pregnant, had haemoglobin levels below 9.9 g/dL, were undergoing treatment with pro‐kinetics, or who had undergone any other type of bariatric surgery were excluded from the study.
2.2. Study protocol
To determine the sample size, the same rules that are applied for bioequivalence studies with healthy subjects for the determination of relative AMX bioavailability were followed for the RYGB subjects.
A total of 26 subjects were prescreened (haemogram, lipidogram, glycaemia, creatinine, urea, albumin, transaminases, bilirubin and β‐human chorionic gonadotropin [for women] examinations) and, of them, 20 subjects fulfilled the inclusion criteria.
Anthropometric and clinical data were collected; namely: sex, age, ethnicity, weight, height, body mass index (BMI, kg/m2), heart rate, blood pressure (systolic and diastolic) and time since surgery. Four visits were scheduled for the study: the first was for inclusion screening, the second and third visits were for the PK study, and the fourth was for the clinical evaluation and the closing of the study. On every visit, before any intervention, vital signs were measured for each subject.
2.3. AMX dosing and blood sampling
The AMX 875‐mg tablets (EMS Sigma Pharma, São Paulo, Brazil) dosage form was named as the Test and the AMX–400 mg/5 mL suspension (EMS Sigma Pharma, São Paulo, Brazil) dosage form was named as the Reference. The description of tablet and suspension excipients according to each drug Summary Product Characteristics (SmPC) are listed in Table 1. In Brazil, the 875‐mg dosage is only commercially available as a tablet.
Table 1.
Description of amoxicillin suspension vehicles and tablet excipients
| Ingredients | |
|---|---|
| Suspension | Gum xanthan, sodium saccharin, strawberry essence, sodium citrate dihydrate, sodium cyclamate, silicon dioxide and sucrose. |
| Tablets | Pregelatinized corn starch, croscarmellose sodium, methylene chloride, magnesium stearate, titanium dioxide, silicon dioxide, sodium starch glycolate, microcrystalline cellulose, and hypromellose + macrogol. |
On the second and third visits, the subjects were admitted to the Clinical Research Centre of the University Hospital of Maringá at 7 am for PK studies after overnight fasting. An Abocath catheter was placed in the forearm of the subjects for serial blood collection.
In the morning (07:30), all subjects were dosed with one AMX tablet (875 mg) or 10 mL of AMX suspension (400 mg/5 mL), which was freshly prepared, according to a randomized schedule, with up to 200 mL of water in small portions of 50 mL over a period of 2 minutes to help swallow the formulations.
The blood samples were collected at times 0 (before the drug intake), 0.25, 0.5, 1, 1.5, 2, 4, 6 and 8 hours after AMX intake. Standardized snacks were given 2 and 7 hours after drug administration and a standardized meal was given to all subjects 4 hours after drug administration.
The subjects were discharged from the unit after the last blood sampling. A minimum of 7 days of drug washout was allowed between visit 2 and 3. During the whole protocol, the subjects were assisted by a physician from the Clinical Research Centre.
2.4. Analytical method and sample processing
Immediately after blood sampling, the tubes were coded for blinding purposes. The samples were centrifuged for 15 minutes (2000× g, 4°C) and the plasma was separated and kept at −80°C for a maximum of 30 days until analysis, according to the stability studies. The method was validated according to the International Conference Harmonization rules accomplished with the local and international regulations for bioanalytical methods.13, 14
Five replicate samples per concentration were used to determine the lower limit of quantification of 0.2 mg/L, intra‐ and interday coefficients of variation of <5% over a linear range of peak height ratios (r2 = 0.99) from 0.2 to 15 mg/L, and sample stability, obtained in analytical method validation.
AMX concentrations were quantified in the plasma samples by liquid chromatography coupled with triple quadrupole tandem mass spectrometry, according to the method described by Dong et al. (2013) with slight modifications for validation of the method.15 A USP reference standard of vancomycin was used as the internal standard.
The system used was the LC Waters Alliance 2695 coupled to the Quattro Premier XE Spectrometer, Masslinx software (Waters Technology, USA), with a reverse‐phase chromatography column ACE C18, 50 × 4.6 mm in size and a particle size of 3 μm. The mobile phase consisted of an aqueous solution of 0.1% formic acid and acetonitrile (77:23, v/v), pH 2.5, at a flow rate of 0.4 mL/min, at 30°C and an injection volume of 5 μL. A positive electrospray ionisation mode (ES +) and transition ion source m/z 348.5 → m / z 113.5 for AMX and m/z 725 → m/z 143.5 for vancomycin were used.
2.5. Data entry and statistical analyses
Standardized clinical report forms were used and the data files were inputted into Microsoft Excel datasheets (Version 2010; Microsoft Corp.).
A descriptive analysis was carried out for the anthropometric and clinical data. The correlation between the co‐variables: sex, age, adjusted weight, BMI and time since surgery and the variable AUC0–inf was determined by Pearson correlation coefficient for tablets and the suspension. A correlation was considered strong when the Pearson (r) coefficient was >0.7.
Noncompartmental analysis was carried out using the Phoenix WinNonlin® program, which was used to estimate the PK parameters: Cmax; time required to reach Cmax; AUC from time zero (predose) to the last sampling time (AUC0–8); AUC0–inf); residual area or percentage of extrapolated part of AUC0–inf (%AUC); apparent terminal elimination rate constant (Kel); apparent terminal elimination half‐life (t1/2); clearance (Cl/F) and volume of distribution (Vd/F). The AUC0–8 and AUC0–inf were determined from the concentration–time curve using the linear trapezoidal rule.16
Since the suspension dose (800 mg) was slightly lower than the tablet dose (875 mg), a correction factor of 1.093 was used when calculating the PK parameters of the suspension formulation. The difference in the amount administered between the tablet and suspension (75 mg) was small and the considered doses were within the described linearity limits of AMX.
An analysis of variance (ANOVA) was performed on all log‐transformed variables and the 90% confidence interval of the geometric mean for the individual test/reference ratios for AUC0–inf and Cmax was used to determine the relative bioavailability.
In this type of study, bioequivalence between the formulations can typically be concluded if these confidence intervals are within the acceptance range of 80–125%.17, 18
3. RESULTS
3.1. Demographic and clinical data
A total of 20 RYGB subjects (4 males and 16 females) were enrolled in the study and all completed both study periods. The main demographic and clinical data of the subjects are shown in Table 2. The mean age was 42.65 ± 7.21 years, mean weight 79.76 ± 12.55 kg and the mean BMI 29.88 ± 4.36 kg/m2. The mean time since surgery was 41.25 months with a range of 5–108 months.
Table 2.
Population demographic and clinical data
| Variable | Subjects (n = 20) |
|---|---|
| Sex, n (%) | |
| Male | 4 (20) |
| Female | 16 (80) |
| Age (y) | 42.65 (7.21) |
| Weight (kg) | 79.76 (12.55) |
| BMI (kg/m2) | 29.88 (4.36) |
| Months post‐bypass | 41.25 (36.76) |
| Systolic pressure (mmHg) | 109.35 (10.52) |
| Glycaemia (mg/dL) | 90.05 (19.42) |
| Haemoglobin (g/dL) | 12.53 (1.28) |
| Creatinine (mg/dL) | 0.67 (0.08) |
| ALT (U/L) | 34.45 (10.85) |
| Total cholesterol (mg/dL) | 151.00 (25.02) |
| Triglyceride (mg/dL) | 86.50 (28.42) |
| HDL (mg/dL) | 53.20 (16.22) |
| LDL (mg/dL) | 95.05 (21.77) |
| Hypertension, n (%) | 7 (35) |
| Type 2 diabetes, n (%) | 1 (5) |
| Hypothyroidism, n (%) | 3 (15) |
| Depression, n (%) | 6 (30) |
| Sleep apnoea, n (%) | 1 (5) |
Mean (standard deviation). ALT, alanine aminotransferase; BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
3.2. AMX PK
The curve of AMX concentration vs time after oral administration of the suspension and tablets in RYGB bariatric subjects shows that the mean amount of tablet formulation absorbed was lower in comparison to the suspension formulation (Figure 1).
Figure 1.
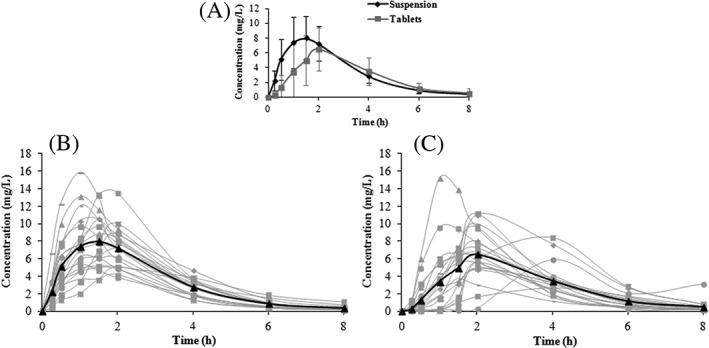
Spaghetti plotting of amoxicillin plasma concentrations vs time. (A) Mean suspension and tablet pharmacokinetic profiles. (B) Individual suspension pharmacokinetic profiles (mean in bold line). (C) Individual tablet pharmacokinetic profiles (mean in bold line)
This difference can be better observed by comparison of the relevant PK parameters presented in Table 3, where the mean Cmax of the suspension was higher than that of the tablets (8.73 ± 3.26 vs 7.42 ± 2.99 mg/L) and the mean time required to reach Cmax of the suspension was slightly lower than that of the tablets (1.7 ± 0.86 vs 2 ± 0.76 hours), both suggesting a slower absorption process for the tablets and a higher mean AUC0–8 and AUC0–inf for the suspension in comparison to the tablets (26.82 ± 8.18 vs 22.14 ± 7.51 mg.h/L and 27.59 ± 8.32 vs 23.10 ± 7.41 mg.h/L, respectively), which also indicates reduced absorption.
Table 3.
Pharmacokinetic parameters of amoxicillin after oral administration of tablets and suspension in Roux‐en‐Y gastric bypass bariatric subjects
| Pharmacokinetic parameters | Amoxicillin | ||
|---|---|---|---|
| Suspension (n = 20) | Tablets (n = 20) | Tablet/ suspension ratio interval (90% CI)* | |
| Cmax (mg/L) | 8.73 (3.26) | 7.42 (2.99) | 70.71–99.11 |
| AUC0–8 (mg.h/L) | 26.82 (8.18) | 22.14 (7.51) | 69.76–95.46 |
| AUC0–inf (mg.h/L) | 27.59 (8.32) | 23.10 (7.41) | 71.25–97.32 |
| %AUC | 97.14 (2.51) | 97.15 (1.73) | |
| tmax (h) | 1.7 (1–5) | 2 (1–4) | |
| t½ (h) | 1.32 (0.31) | 1.40 (0.68) | |
| Kel (h−1) | 0.55 (0.12) | 0.55 (0.13) | |
| Cl/F (L/h) | 34.81 (12.12) | 41.05 (14.30) | |
| Vd/F (L) | 68.82 (29.71) | 88.54 (69.30) | |
Mean (standard deviation).
CI, confidence interval; Cmax, maximum observed plasma drug concentration; AUC0–8, total area under the curve (from time 0 to 8 hours); AUC0–inf, total area under the curve (from time 0 to infinity); %AUC, percentage of extrapolated part of AUC0–inf; tmax, time to Cmax (range); t½, elimination half‐life; Kel, elimination rate constant; Cl, clearance; Vd, volume of distribution;
Bioequivalent when the interval between 80–125%.
The mean %AUC for both formulations (97.14 and 97.15% for the suspension and tablets, respectively) indicate that the sampling times were sufficient for the complete characterization of absorption, as AUC0–8 corresponded to >80% of AUC0–inf. There was no statistically significant difference between the tablets and the suspension for the disposition parameters, namely t½, Kel, Cl/F and Vd/F (Table 3).
The effects of period (Cmax, p = 0.92; AUC0–8, p = 0.75; AUC0–inf, p = 0.75) and sequence (Cmax, p = 0.96; AUC0–8, p = 0.98; AUC0–inf, p = 0.99) were not significant, indicating that there is no residual effect (carryover).
According to the accepted confidence interval of the guidelines for bioequivalence studies,17, 18 the geometric mean for the individual test/reference ratios for AUC0–inf and Cmax of AMX (Table 3) showed that the tablets were not bioequivalent to the suspension formulation, and presented a lower bioavailability. The mean relative bioavailability of the tablets as assessed by AUC comparison was only 83.43% of that of the suspension.
None of the covariables—sex, age, adjusted weight, BMI and time since surgery—correlated with AUC0–inf, for either the tablets or the suspension.
4. DISCUSSION
The present study has shown that AMX in tablet formulation presented both a slower rate of absorption and lower bioavailability in comparison to suspension formulation in RYGB bariatric subjects.
In nonbariatric subjects, differences between tablets and suspensions of BCS class I drugs are not expected to occur, presumably due to the higher amount of gastric fluid available in the healthy stomach, which leads to complete dissolution before the drug is driven into the small intestine, as demonstrated by Zhang et al. (2014) with another BCS class I drug studied in healthy volunteers.19 However, with regard to the absorption rate of AMX in bariatric patients, although it is a BCS class I drug5 at the dose used in this study, a lower Cmax and a delay in the Tmax were observed for the solid‐dosage form.
The tablets require prior disintegration and dissolution for the drug to be absorbed, as partial gastrectomy results in a significantly reduced gastric emptying time20, 21, 22 and, therefore, the loss of the normal retention time in the stomach. In the bariatric patient, AMX tablets move at the same speed as, or slightly slower than, water directly into the jejunum,20 where the water is rapidly absorbed. This results in the tablets losing contact with the water at a faster rate than is necessary for the optimal disintegration and dissolution process to occur.
Differences in bioavailability may, therefore, be consequences of the reduced length of the intestine and accelerated intestinal transit,22 as the AMX from tablets may not dissolve completely and therefore not be absorbed. The suspension formulation, by contrast, has fewer limitations, since there is no need for disintegration and the drug is therefore immediately available for absorption. It should be observed, however, that the changes in the intestinal transit rate and motility in RYGB subjects are still unclear, with contrasting results published in previous literature.23, 24, 25
Another possible explanation for the differences observed in the present study could be due to the differences in the excipients between the two formulations. It is known that some excipients and vehicles, such as polyethylene glycol 400 (PEG 400), mannitol and xylitol, among others, may decrease the GI transit time, resulting in decreased drug bioavailability.26 However, none of the tablet and suspension excipients used in this study (Table 1) are known to produce this effect. For example, according to Adkin et al. (1995),27 the intestinal transit time of saccharine and sucrose solutions, the vehicles used in the suspension formulation in this study, are similar to that of water, thereby not affecting the bioavailability of the suspensions.
Finally, differences could also be due to the slightly different dosage between the tablet and suspension formulations used. In fact, some studies have shown results of nonlinear kinetics for AMX,28 which could help to justify the difference between the tablets and suspension observed in this study. However, most of the data available in previous literature support a saturation absorption process of AMX at doses only above 1000 mg.5 Moreover, increasing the AMX dose from 250 to 1000 mg resulted in an increased AUC, countering the existence of the nonlinear disposition of AMX in the doses used in the present study.4
In addition to the differences between the two formulations (tablet and suspension) evidenced by our results, this study also shows that the total amount of AMX absorbed by RYGB subjects, for both pharmaceutical forms, was lower than the total amount of AMX absorbed by healthy volunteers.29, 30, 31
Several studies are available in previous literature in which healthy subjects receiving a dosage of 875 mg of AMX in tablet form (alone or in combination with Clavulanic acid) presented AUC0–inf and Cmax values from 43.80 to 51.29 mg.h/L and 12.13 to 15.30 mg/L, respectively.29, 30, 31 Compared to these studies, the RYGB subjects in the present study appear to present a mean reduction of AMX absorption of 40% for the suspension and 50% for tablets.
One of the possible explanations for the reduced amount of AMX absorbed in RYGB subjects may be that AMX has an absorption process mediated by PEPT‐1 carriers, which are present mainly in the proximal region of the intestine.
The deviation of the duodenum and part of the jejunum may decrease the active absorption sites of AMX, leading to a lower degree of absorption. For this reason, the reduced bioavailability that occurs only at doses higher than 1000 mg due to the transport saturation process32, 33, 34, 35 in healthy volunteers can be observed at lower doses in RYGB subjects due to the reduction of this process. Furthermore, with the expected increase in GI pH in the RYGB population, which corresponds to the pH of the healthy distal intestine, AMX is partially deprotonated in this group.36 As it is not entirely in its neutral form, the amount absorbed by the passive diffusion process may also be decreased.
From a clinical point of view, as described by Jacobs (2003), the time (T) above the minimum inhibitory concentration (MIC) of 40% of the dosing interval has been taken as predictive of the bacteriological efficacy for β‐lactams.37
According to White et al., AMX associated with clavulanic acid required a 30–40% T > MIC for the maximal bacteriological efficacy against the key respiratory pathogen Streptococcus pneumonia.38 Based on the mean concentrations of the tablets and suspension in our RYGB subjects, infections caused by pathogens with a MIC below 4 mg/L would be covered in RYGB subjects for both formulations at the doses used in this study. However, and contrary to what can be assumed based on normal weight subjects, for pathogens with an MIC of >4 mg/L, such as Enterobacteriaceae or resistant Enterococcus spp., the time period above the MIC37 is not reached for either tablets or suspension in RYGB subjects, which could represent a therapeutic failure in severe infections. In addition, if we consider the individual profiles, there were cases where the 30–40% T > MIC above 4 mg/L was not reached.
To the best of our knowledge, this is the first randomized clinical study to investigate the absorption of AMX in RYGB subjects comparing two pharmaceutical forms (tablets and suspension), and it was carried out to clarify if there is any impairment in the process of absorption of AMX tablets in the modified GI tract of RYGB subjects.
This study has, however, some limitations: (i) it was not possible to unequivocally calculate the absolute oral bioavailability of AMX in bariatric patients since the intravenous dosage form of AMX is not approved in Brazil; and (ii) it was decided not to administer the exact same dose of the tested formulations to the subjects because of a potential lack of accuracy in the syringe volume of the suspension. By using a dosing syringe, a precise volume (10 mL) was considered instead of a broken volume (10.9375 mL), which could lead to more errors than benefits. Therefore, and to correct this difference, we numerically normalised the AMX plasma concentrations of the suspension to be comparable to the tablets.
5. CONCLUSIONS
According to the results of this study, AMX tablets presented a lower bioavailability than the suspension formulation in RYGB subjects. This is unlikely to cause a therapeutic failure, since the recommended dose of AMX for the treatment of general infections is very high and, despite the difference between the formulations, both reached the time above MIC for most of the pathogens for which AMX is recommended. A better characterisation of the oral absorption process of drugs prescribed for RYGB subjects is still needed, especially for drugs with a narrow therapeutic index, as this can be significantly altered.
5.1. Outlook
Although some drugs have already been studied in bariatric patients,8, 9, 10, 11 further studies to investigate the impact of gastric bypass surgery on the PK of drugs not yet studied are necessary. In addition, studies that investigate neuro‐enteric stimulation, transport carrier sites, hormonal changes in the GI tract and the intestinal‐transit time and its impact on drug absorption in this population are also deemed necessary. Finally, the development of physiologically based absorption models could enable dosage optimisation for this patient population, with therapeutic advantages.
COMPETING INTERESTS
There are no competing interests to declare.
All procedures performed in this study involving human participants were in accordance with, and previously approved by, the local Ethics Committee Review Board under the number CAAE57476516.3.0000.0104.
Informed consent was obtained from all individuals who participated in the study.
CONTRIBUTORS
We would like to thank the following persons for their invaluable help: T.F.d.S.M. in extraction and quantification of plasma samples, as chemical analyst. C.d.S.A., S.S.Y., L.E.S.d.O. and D.N., in recruitment and clinical evaluation of patients, as study physicians. C.F.S. and N.N.J. in the anthropometric evaluation of patients, as physical education professionals. S.R.B.S. and C.M.K. in the blood samples collection, as study nurses. J.M. in the statistical analysis, as statistician. A.D., P.J.P.A.P. and E.K. who were involved in data extraction, pharmacokinetic analysis and helpful comments.
Supporting information
Data S1. Supplement 1. Anthropometric and clinical data.
Supplement 2. Randomization list.
Supplement 3. Pearson correlation analysis of clinical data and total area under the curve from time zero to infinity.
Supplement 4. Individual pharmacokinetic profiles in normal scale.
Supplement 5. Individual pharmacokinetic profiles in log scale.
ACKNOWLEDGEMENTS
We would like to thank Dr Silvia Maria Tintori for technical assistance and the Clinical Analysis laboratory, medical outpatient, and Centre for Clinical Research and Bioequivalence staff for their assistance, all from the University Hospital of Maringá where the entire clinical protocol was performed. Also, we would like to thank Gustavo Mendes and the Therapeutic Equivalence Coordination (CETER) from ANVISA who kindly assisted us in the study of bioequivalence. We would like to thank the patients who have agreed to participate to the study.
This work was supported by the National Council of Technological and Scientific Development Foundation (CNPq), the Coordination of Improvement of Higher Education Personnel (CAPES) and the Parana State Science and Technology Secretariat—UGF‐SETI‐PR, Brazil.
Montanha MC, dos Santos Magon TF, de Souza Alcantara C, et al. Reduced bioavailability of oral amoxicillin tablets compared to suspensions in Roux‐en‐Y gastric bypass bariatric subjects. Br J Clin Pharmacol. 2019;85:2118–2125. 10.1111/bcp.14023
The authors confirm that the PI for this paper is Sérgio Seiji Yamada and that he had direct clinical responsibility for the patients.
REFERENCES
- 1. Organization WH . Model List of Essential Medicines:20th list. Geneva, Switzerland, 2017.
- 2. Lennernäs H, Knutson L, Knutson T, et al. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: experience from a regional perfusion technique. Eur J Pharm Sci. 2002;15(3):271‐277. [DOI] [PubMed] [Google Scholar]
- 3. Ali B, Amin S, Ahmad J, Ali A, Mir SR, Ali M. Bioavailability enhancement studies of amoxicillin with nigella. Indian J Med Res. 2012;135(4):555‐559. [PMC free article] [PubMed] [Google Scholar]
- 4. Spyker DA, Rugloski RJ, Vann RL, O'Brien WM. Pharmacokinetics of amoxicillin: dose dependence after intravenous, oral, and intramuscular administration. Antimicrob Agents Chemother. 1977;11(1):132‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thambavita D, Galappatthy P, Mannapperuma U, et al. Biowaiver monograph for immediate‐release solid oral dosage forms: amoxicillin trihydrate. J Pharm Sci. 2017;106(10):2930‐2945. [DOI] [PubMed] [Google Scholar]
- 6. Azran C, Wolk O, Zur M, et al. Oral drug therapy following bariatric surgery: an overview of fundamentals, literature and clinical recommendations. Obes Rev. 2016;17(11):1050‐1066. [DOI] [PubMed] [Google Scholar]
- 7. Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007;132(6):2253‐2271. [DOI] [PubMed] [Google Scholar]
- 8. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 9. Srinivas NR. Impact of Roux‐en‐Y gastric bypass surgery on pharmacokinetics of administered drugs: implications and perspectives. Am J Ther. 2016;23(6):e1826‐e1838. [DOI] [PubMed] [Google Scholar]
- 10. Yska JP, van der Meer DH, Dreijer AR, et al. Influence of bariatric surgery on the use of medication. Eur J Clin Pharmacol. 2016;72(2):203‐209. [DOI] [PubMed] [Google Scholar]
- 11. Hachon L, Declèves X, Faucher P, Carette C, Lloret‐Linares C. RYGB and drug disposition: how to do better? Analysis of pharmacokinetic studies and recommendations for clinical practice. Obes Surg. 2017;27(4):1076‐1090. [DOI] [PubMed] [Google Scholar]
- 12. Magee SR, Shih G, Hume A. Malabsorption of oral antibiotics in pregnancy after gastric bypass surgery. J Am Board Fam Med. 2007;20(3):310‐313. [DOI] [PubMed] [Google Scholar]
- 13. ANVISA . Resolução‐RE n° 27, de 17 de maio de 2012. Available at: http://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0027_17_05_2012.html2012. Accessed September 04, 2018.
- 14. Food and Drug Administration . FDA guidance for industry: bioanalytical method validation. In: US, Department of Health and Human Services FaDA, Center, for Drug Evaluation and Research: Rockville M, editors. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm070107.Pdf2001. Accessed September 04, 2018.
- 15. Dong X, Ding L, Cao X, Jiang L, Zhong S. A sensitive LC‐MS/MS method for the simultaneous determination of amoxicillin and ambroxol in human plasma with segmental monitoring. Biomed Chromatogr. 2013;27(4):520‐526. [DOI] [PubMed] [Google Scholar]
- 16. Shargel L, Yu A, Wu‐Pong S. Applied Biopharmaceutics & Pharmacokinetics. 6th ed. The United States of America; 2015. [Google Scholar]
- 17. ANVISA . Manual de Boas Práticas em Biodisponibilidade e Bioequivalência . Available at: http://portal.anvisa.gov.br/registros‐e‐autorizacoes/medicamentos/produtos/bioequivalencia‐e‐biodisponibilidade/manuais2002. Accessed September 04, 2018.
- 18. Drug and Food Administration . Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs–general Considerations. In: Rockville MF, Administration aD, editors. 2014.
- 19. Zhang J, Zhang Y, Wang R, et al. Pharmacokinetics and bioequivalence comparison of 600 mg single‐dose linezolid Oral suspension and tablet formulation in healthy Chinese subjects. J Bioequivalence Bioavailability. 2014;6:153‐157. [Google Scholar]
- 20. Stano S, Alam F, Wu L, et al. Effect of meal size and texture on gastric pouch emptying and glucagon‐like peptide 1 after gastric bypass surgery. Surg Obes Relat Dis. 2017;13(12):1975‐1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wölnerhanssen BK, Meyer‐Gerspach AC, Peters T, Beglinger C, Peterli R. Incretin effects, gastric emptying and insulin responses to low oral glucose loads in patients after gastric bypass and lean and obese controls. Surg Obes Relat Dis. 2016;12(7):1320‐1327. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen NQ, Debreceni TL, Burgstad CM, et al. Effects of posture and meal volume on gastric emptying, intestinal transit, Oral glucose tolerance, blood pressure and gastrointestinal symptoms after Roux‐en‐Y gastric bypass. Obes Surg. 2015;25(8):1392‐1400. [DOI] [PubMed] [Google Scholar]
- 23. Dirksen C, Damgaard M, Bojsen‐Møller KN, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux‐en‐Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346‐e255. [DOI] [PubMed] [Google Scholar]
- 24. Pellegrini CA, Deveney CW, Patti MG, Lewin M, Way LW. Intestinal transit of food after total gastrectomy and Roux‐Y esophagojejunostomy. Am J Surg. 1986;151(1):117‐125. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux‐en‐Y gastric bypass in rat model. Surgery. 2005;138(2):283‐290. [DOI] [PubMed] [Google Scholar]
- 26. Schulze JD, Ashiru DA, Khela MK, et al. Impact of formulation excipients on human intestinal transit. J Pharm Pharmacol. 2006;58(6):821‐825. [DOI] [PubMed] [Google Scholar]
- 27. Adkin DA, Davis SS, Sparrow RA, Huckle PD, Phillips AJ, Wilding IR. The effects of pharmaceutical excipients on small intestinal transit. Br J Clin Pharmacol. 1995;39(4):381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Velde F, de Winter BC, Koch BC, van Gelder T, Mouton JW. Non‐linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother. 2016;71(10):2909‐2917. [DOI] [PubMed] [Google Scholar]
- 29. Medicines Evaluation Board (MEB) . Public Assessment Report; AMXe/Clavulaanzuur Actavis 500/125 mg and 875/125 mg, film‐coated tablets. NL/H/2782/001–002/MR (2015). Available at: https://db.cbg‐meb.nl/Pars/h103893.pdf. Accessed October 18, 2018.
- 30. Medicines Evaluation Board (MEB) . Public Assessment Report; Amoxiclav Aristo 500 mg/125 mg and 875 mg/125 mg film‐coated tablets. NL/H/3468/001–002/DC (2016). Available at: https://db.cbg‐meb.nl/Pars/h116980.pdf. Accessed October 18, 2018.
- 31. Baglie S, Rosalen PL, Franco LM, et al. Comparative bioavailability of 875 mg amoxicillin tablets in healthy human volunteers. Int J Clin Pharmacol Ther. 2005;43(7):350‐354. [DOI] [PubMed] [Google Scholar]
- 32. Chulavatnatol S, Charles BG. Determination of dose‐dependent absorption of amoxycillin from urinary excretion data in healthy subjects. Br J Clin Pharmacol. 1994;38(3):274‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sala‐Rabanal M, Loo DD, Hirayama BA, Turk E, Wright EM. Molecular interactions between dipeptides, drugs and the human intestinal H+ ‐oligopeptide cotransporter hPEPT1. J Physiol. 2006;574(Pt 1):149‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bretschneider B, Brandsch M, Neubert R. Intestinal transport of beta‐lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco‐2 cell monolayers and the transepithelial flux. Pharm Res. 1999;16(1):55‐61. [DOI] [PubMed] [Google Scholar]
- 35. Nakashima E, Tsuji A, Kagatani S, Yamana T. Intestinal absorption mechanism of amino‐beta‐lactam antibiotics. III. Kinetics of carrier‐mediated transport across the rat small intestine in situ. J Pharmacobiodyn. 1984;7(7):452‐464. [DOI] [PubMed] [Google Scholar]
- 36. Tsuji A, Nakashima E, Hamano S, Yamana T. Physicochemical properties of amphoteric beta‐lactam antibiotics I: stability, solubility, and dissolution behavior of amino penicillins as a function of pH. J Pharm Sci. 1978;67(8):1059‐1066. [DOI] [PubMed] [Google Scholar]
- 37. Jacobs MR. How can we predict bacterial eradication? Int J Infect Dis. 2003;7:S13‐S20. [DOI] [PubMed] [Google Scholar]
- 38. White AR, Kaye C, Poupard J, Pypstra R, Woodnutt G, Wynne B. Augmentin® (amoxicillin/clavulanate) in the treatment of community‐acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent. J Antimicrob Chemother. 2004;53(suppl 1):i3‐i20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplement 1. Anthropometric and clinical data.
Supplement 2. Randomization list.
Supplement 3. Pearson correlation analysis of clinical data and total area under the curve from time zero to infinity.
Supplement 4. Individual pharmacokinetic profiles in normal scale.
Supplement 5. Individual pharmacokinetic profiles in log scale.


