Abstract
Aims
Patients with metastatic gastrointestinal stromal tumours (GIST) are treated in first line with the oral tyrosine kinase inhibitor, imatinib, until progressive disease. With this fixed dosing regimen, only approximately 40% of patients reach adequate plasma levels within the therapeutic index. Therapeutic drug monitoring (TDM) is a solution to reach plasma levels within the therapeutic index. However, introducing TDM will also increase costs, due to prolonged imatinib use and laboratory costs. The aim of this study was to evaluate the cost‐effectiveness of TDM in patients with metastatic/unresectable GIST treated with imatinib as a first line treatment, compared with fixed dosing.
Methods
A survival model was created to simulate progression, mortality and treatment costs over a 5‐year time horizon, comparing fixed dosing vs TDM‐guided dosing. The outcomes measured were treatments costs, life‐years and quality‐adjusted life‐years.
Results
Total costs over the 5‐year time horizon were estimated to be €106 994.85 and €150 477.08 for fixed dosing vs TDM‐guided dosing, respectively. A quality‐adjusted life year gain of 0.74 (95% confidence interval 0.66–0.90) was estimated with TDM‐guided dosing compared to fixed dosing. An average incremental cost‐effectiveness ratio of €58 785.70 per quality‐adjusted life year gained was found, mainly caused by longer use and higher dosages of imatinib.
Conclusion
Based on the currently available data, this analysis suggests that TDM‐guided dosing may be a cost‐effective intervention for patients with metastatic/unresectable GIST treated with imatinib which will be improved when imatinib losses its patency.
Keywords: cost‐effectiveness, fixed dosing, gastrointestinal stromal tumours, imatinib, therapeutic drug monitoring
What is already known about this subject
Patients suffering from gastrointestinal stromal tumours (GIST) treated with fixed dose imatinib as a first‐line treatment, may receive suboptimal dosing and thereby suboptimal treatment outcomes. Therapeutic drug monitoring (TDM) has proven to be a simple and effective measure to increase the number of patients with drug levels within the therapeutic window.
What this study adds
This paper explores the effect of TDM on quality of life gains, occurrences of adverse events, and associated costs in GIST patients treated with imatinib.
TDM may be a cost‐effective intervention for GIST patients treated with imatinib.
1. INTRODUCTION
Gastrointestinal stromal tumours (GIST) are the most common type of soft tissue sarcoma. Worldwide, the annual incidence of GIST is about 10 cases per million people, corresponding to at least 8000 new cases per year in Europe.1, 2 Patients with metastatic or unresectable GIST receive fixed dosed imatinib as first‐line treatment until progressive disease.3, 4 When disease progression is noticed, the dose of imatinib is doubled, followed by second‐line treatment with sunitinib and third‐line treatment with regorafenib after each progression. Sunitinib, regorafenib and double‐dosed imatinib are regarded as more toxic with worse quality of life compared to standard dosed imatinib. In its palliative intent, the goal of treatment with imatinib, sunitinib and regorafenib in patients with GIST is to improve the progression free survival, with the lowest toxicity.5, 6, 7
Imatinib mesylate is an oral tyrosine kinase inhibitor that has been approved at fixed doses of once daily 400 mg for use in different types of cancer, e.g. BRC‐Abl positive chronic myeloid leukaemia and GIST.8, 9 However, since this drug shows large interpatient variability in pharmacokinetics sub‐ and supratherapeutic exposures may be encountered which could affect treatment outcome. In addition, in retrospective analyses improved efficacy was shown at plasma concentrations >1100 μg/L while more adverse events were observed at plasma concentrations >3200 μg/L.10, 11 Therefore, it is important to treat patients within the therapeutic index. Since many factors may influence the plasma exposure of imatinib, it is not possible to predict whether an individual patient will reach an adequate plasma exposure using a standard fixed dose of the drug.
Therapeutic drug monitoring (TDM) is a technique used to determine plasma exposure for certain drugs, and to adjust the dose in order to achieve plasma exposure within the therapeutic index. A study by Lankheet et al12 shows that the use of TDM in GIST patients treated with imatinib results in 95% of the patients to achieve adequate therapeutic plasma concentrations. Additionally, TDM has been shown to improve safety and efficacy of many targeted oral anticancer drugs.13, 14 A relationship between plasma exposure and treatment outcome has been retrospectively established for imatinib in patients with GIST,10 supporting the rationale for the use of TDM in GIST patients treated with imatinib. While the effect of TDM to redistribute patients to adequate plasma concentrations has been proven, it is currently unknown what the financial consequences of TDM is compared with fixed dosing. While an increase in clinical efficacy may result in a reduction in costs associated with slower disease progression and less adverse events, it is unclear whether these savings weigh up to the additional costs that come with the use of increased and prolonged dosages and laboratory handling costs associated with TDM.
The aim of this study is to evaluate the cost‐effectiveness of TDM in patients with metastatic/unresectable GIST treated with imatinib as first line treatment, compared with fixed dosing of imatinib.
2. METHODS
2.1. General considerations
To determine the possible gain of TDM over fixed dosing in GIST patients treated with imatinib, the costs and effects of both groups, from a societal perspective, were modelled over 5 years. The main comparison made in this model is the effect of TDM during the initial imatinib treatment on costs, life‐year and quality‐adjusted life‐year (QALY) gains, compared with fixed dosing. The difference TDM introduces in the treatment of metastatic GIST patients is fully located in the imatinib treatment health‐state, and allows for patients receiving a sub‐ or supratherapeutic dose of imatinib to be redistributed in the correct therapeutic index within the imatinib health‐state. This results in higher drug costs but prolonged time to progression when transferred from subtherapeutic to therapeutic and lower drug costs and a lower chance of side effects when transferred from supratherapeutic to therapeutic.
2.2. Model structure
A partitioned survival model was created using Microsoft Excel. This analysis included a fictitious cohort of 10 000 patients with metastatic and/or unresectable GIST, starting the first line of treatment with imatinib. The model consisted of 6 mutually exclusive health‐states: regular dose imatinib progression‐free (IPF); escalated dose imatinib progression‐free (EIPF); sunitinib progression‐free; regorafenib progression‐free; best supportive care (BSC); and death (Figure 1). The regular dose IPF health‐state was subdivided into subtherapeutic, therapeutic and supratherapeutic imatinib plasma concentrations, to allow optimization with TDM and decrease of imatinib plasma levels in the first treatment period15 to be noticeable in this health‐state, and its effect on subsequent health‐states. All patients entered the model in the IPF health‐state. Progression‐free survival (PFS) and overall survival (OS) curves for each treatment health‐state were used to determine the time‐dependent transition probabilities between health‐states. The model uses 14‐day cycles, based on the first moment TDM takes place after starting the IPF line of treatment. The different lines of treatments used in this model (IPF, EIPF, sunitinib progression‐free, regorafenib progression‐free and BSC) are in line with the clinical guidelines formulated by the European Society for Medical Oncology.16 Reduction of imatinib exposure of ~30% over the first 3 months is taken into account for the imatinib groups only, to further analyse the effect of TDM.15 Perfect adherence to the guidelines is assumed for each fictitious patient. The time horizon for this model is 5 years, based on available data for survival for the IPF treatment.10 Health outcomes were measured in life‐years and QALYs. Monetary values were measured and if needed converted into euros.
Figure 1.
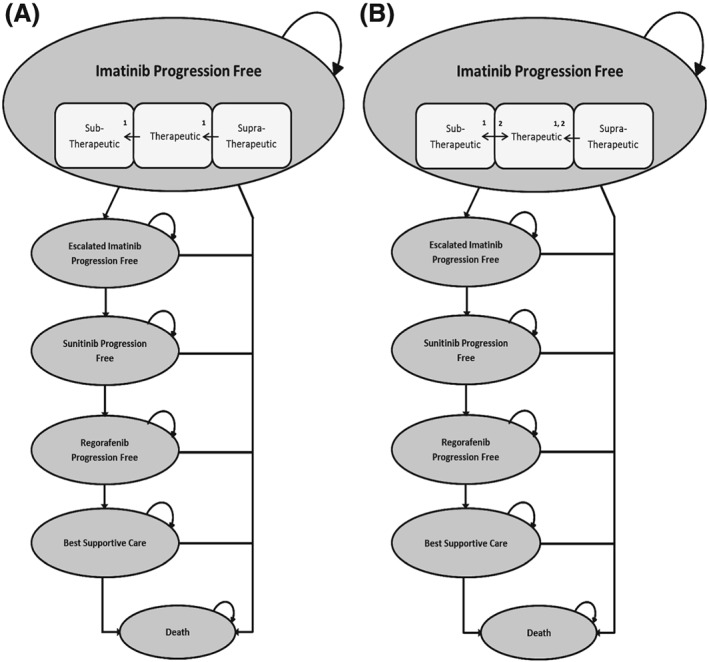
(A) Flowchart of metastatic gastrointestinal stromal tumour progression treated with fixed dosing imatinib. (B) Flowchart of metastatic gastrointestinal stromal tumour progression treated with therapeutic drug monitoring‐adjusted imatinib dosing. Arrows indicated by 1 represent the reduction of blood plasma concentration caused by lower bioavailability for imatinib over time. Arrows indicated by 2 represent the effect of therapeutic drug monitoring
2.3. Model data
2.3.1. Clinical data
Data for the distribution of patients in the IPF group over the therapeutic subgroups, and the effect of TDM, defined as the percentage of patients being redistributed of the therapeutic subgroups, was gathered from an study from Lankheet et al.12 This study was a retrospective cohort study in patients treated with imatinib in whom TDM was performed from August 2012 to April 2016. The intrapatient variability in imatinib PK was not taken into account since Abrantes et al demonstrated that for the best result one should exclude the portion of unexplained variability related to interoccasion variability in the individual parameters to calculate the future dose.17
2.3.2. Model data inputs
A complete list of all input parameters can be found in supplementary file 1. Progression probabilities from 1 line of treatment to the next were based on Kaplan–Meier PFS data gathered for each health‐state. This data was converted into time‐dependent probabilities for progression from 1 line of treatment to the next for each cycle in the model, to reflect reality as accurately as possible. Due to deaths being counted as progression in all found survival data, chances of death for each treatment group was determined by dividing the OS data by the PFS data, for each specific cycle, yielding the patients who died during treatment and excluding them from entering the next line of treatment.
Transition probabilities from IPF to EIPF were different for each therapeutic subgroup, based on the data from Demetri et al10 to account for differences in progression due to the effect of the respective therapeutic groups. PFS for the supratherapeutic health‐state was extrapolated beyond 42 months due to lack of data. OS data for the subtherapeutic imatinib health‐state was not available, and was extrapolated based on the PFS data for this health‐state. The further treatment groups of sunitinib and regorafenib were not split in therapeutic subgroups due to lack of data. Chances of adverse events occurring were determined for each treatment group. For each treatment group the most common adverse events have been used. Only severe (grade 3 or 4) adverse events have been taken into account in this model, since the costs of grade 1 and 2 adverse events are considered to be low.
2.3.3. Costs
Direct drug acquisition costs were gathered from the Dutch National Health Care Institute (www.medicijnkosten.nl, accessed October 2017), and based on the drug dose as described in the clinical guidelines for treatment of metastatic and unresectable GIST. Diagnostic and follow‐up procedures in line with the clinical guidelines were also implemented in the model. Prices of these procedures were gathered using the Dutch Healthcare Authority. The price for TDM was set at €65, being the average national tariff for chromatographic complex bioanalysis.
Costs of possible adverse events are based on an article by Mickisch et al18 where costs per event of a variety of grade 3 and 4 adverse events are reported, for the UK, Germany, Italy and France. By a lack of Dutch data, the adverse events costs of Germany are used in this model, as the Dutch healthcare is most comparable with that of Germany. and there are no Dutch data.
2.3.4. Utilities
Utility weights used in this model were European Quality of Life 5‐Item Questionnaire (EQ‐5D) scores. The utility score for imatinib was based on clinical trial data from Wilson et al19 and Chabot et al20 for regular dose and escalated dose, respectively. Sunitinib utility score was based on an article by Paz‐Ares et al21 which gathered EQ‐5D scores of a phase III clinical study comparing GIST‐patients treated with sunitinib and a placebo. Regorafenib utility scores were based on EQ‐5D scores determined by the GRID study,5, 22 a phase III trial reporting on the efficacy and safety of regorafenib as treatment for advanced GIST.
2.3.5. Analyses
A probabilistic comparison of fixed dosing vs the TDM‐guided dosing in the first line of treatment of GIST patients was performed from a Dutch health‐care perspective. Additionally, sensitivity analyses were conducted in order to explore overall parameter uncertainty. Analyses were run using 5000 iterations, each stochastically sampling parameter values in the determined ranges. Beta‐ and γ‐distributions were used for transition probabilities and costs, respectively, and plausible uncertainty was taken into account for all parameter range distributions. Results of analyses were used to estimate incremental cost‐utility ratios (ICUR). Additionally, scatter‐plots and cost‐effectiveness acceptability curves were created to graphically describe cost‐effectiveness.
2.3.6. Sensitivity analyses
Currently, imatinib is patented by Novartis under the name Glivec/Gleevec. This patent is set to expire in the near future, resulting in drastic changes in the pricing of this drug. Decreased generic drug costs or discounts up to 99% are possible. To determine the effect of changes in drug pricing of imatinib on the cost‐effectiveness of TDM, decreased drug costs of 50%, 80%, 95% and 99% were used. Other sensitivity analyses performed are variations in imatinib and BSC utility values, as well as increased costs of adverse events and BSC to determine whether or not the analyses are robust. Discount rates of 4% and 1.5% were used for costs and QALYs, respectively, in agreement of our national guidelines for pharmaco‐economic research.23
3. RESULTS
3.1. Base case
Based on our model we estimated that over a 5‐year time horizon, 66% and 42% of the patients following the GIST treatment would die in the fixed dosing and TDM group, respectively (Table 1). In the fixed dosing group 14% of the patients would not progress beyond the initial imatinib health‐state, compared with 35% in the TDM health‐state. PFS in the TDM‐guided group was estimated to be delayed compared with the fixed dosing group, and a lower amount of deaths occurred in the TDM‐guided group. Subsequent treatment lines showed similar progression and mortality rates relative to the amount of patients entering these health‐states. Adverse events occurred more frequently as patients progressed to the next lines of treatment. BSC has the highest mortality rate for both fixed dosing and TDM‐guided dose, with 96% and 95% of patients entering this health‐state, respectively.
Table 1.
Health‐state transitions for therapeutic drug monitoring (TDM)‐guided dosing and fixed dosing
| Fixed dosing | TDM | ||||
|---|---|---|---|---|---|
| Health state | n | % | n | % | |
| Imatinib | Cohort | 10 000 | 100 | 10 000 | 100 |
| Not progressed | 1424 | 14 | 3453 | 35 | |
| Progressed total | 8576 | 86 | 6547 | 65 | |
| • progressed alive | 4562 | 46 | 4580 | 46 | |
| • progressed dead | 4014 | 40 | 1967 | 20 | |
| Adverse events | 731 | 7 | 949 | 9 | |
| Imatinib escalated | Cohort | 4562 | 100 | 4580 | 100 |
| Not progressed | 1535 | 34 | 1895 | 41 | |
| Progressed total | 3027 | 66 | 2685 | 59 | |
| • progressed alive | 1473 | 32 | 1318 | 29 | |
| • progressed dead | 1554 | 34 | 1367 | 30 | |
| Adverse events | 1322 | 29 | 1188 | 26 | |
| Sunitinib | Cohort | 1473 | 100 | 1318 | 100 |
| Not progressed | 136 | 9 | 170 | 13 | |
| Progressed total | 1337 | 91 | 1148 | 87 | |
| • progressed alive | 948 | 64 | 816 | 62 | |
| • progressed dead | 389 | 26 | 332 | 25 | |
| Adverse events | 163 | 17 | 141 | 11 | |
| Regorafenib | Cohort | 948 | 100 | 816 | 100 |
| Not progressed | 204 | 22 | 197 | 24 | |
| Progressed total | 744 | 78 | 618 | 76 | |
| • progressed alive | 504 | 53 | 425 | 52 | |
| • progressed dead | 240 | 25 | 194 | 24 | |
| Adverse events | 431 | 46 | 354 | 43 | |
| Best supportive care | Cohort | 504 | 100 | 425 | 100 |
| Alive | 18 | 4 | 20 | 5 | |
| Dead | 486 | 96 | 405 | 95 | |
| Total deaths | 6683 | 67 | 4264 | 43 | |
Average total costs over 5 years made were estimated to be €106 994.85 (95% confidence interval [CI]: €104 468.90–109 607.49) and €150 477.08 (95% CI: €145 862.62–155 475.62) for fixed dosing and TDM‐guided dose, respectively. An average life‐year gain of 0.78 (95% CI: 0.66–0.90) and a QALY gain of 0.74 (95% CI: 0.61–0.88) were estimated with the use of TDM‐guided dosing compared with fixed dosing (Table 2). Figure 2 shows the ICURs observed over 5000 iterations of the model, with an average ICUR of €58 785.70 (95% CI: €53 677.72–€66 750.60) per QALY. From the cost‐effectiveness acceptability curve, one can read that the decision maker will be 100% sure that TDM‐guided dosing is favourable over fixed dosing when society is willing to pay €72 000 or more per QALY gained (Figure 3).
Table 2.
Results base case analysis
| Life years (95% CI) | QALYs (95% CI) | Cost, € (95% CI) | Cost per life‐year, € (range) | Cost per QALY, € (range) | |
|---|---|---|---|---|---|
| Fixed dosing | 3.09 (2.97–3.21) | 2.80 (2.58–2.99) | 106 994.85 (104 468.90–109 607.49) | ||
| TDM‐guided dosing | 3.87 (3.81–3.73) | 3.54 (3.25–3.81) | 150 477.08 (145 862.62–155 475.62) | ||
| Incremental | 0.78 (0.66–0.90) | 0.74 (0.61–0.88) | 43 481.44 (36 107.33–50 636.58) | 55 744.87 (53 173.74–58 657.33) | 58 785.70 (53 677.72–66 750.60) |
CI, confidence interval; QALY, quality‐adjusted life‐year.
Figure 2.
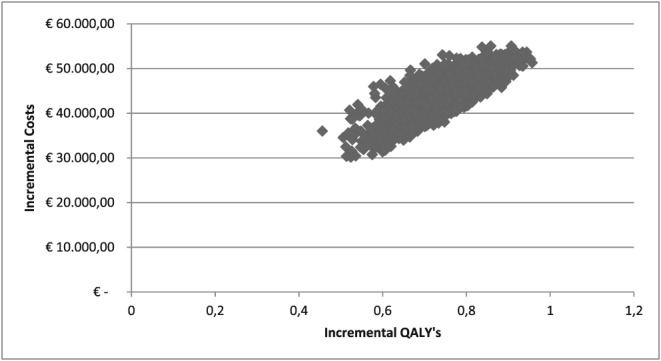
Incremental cost‐effectiveness ratio of fixed dosing vs therapeutic drug monitoring‐guided dosing. Base case analysis run with 5000 iterations
Figure 3.
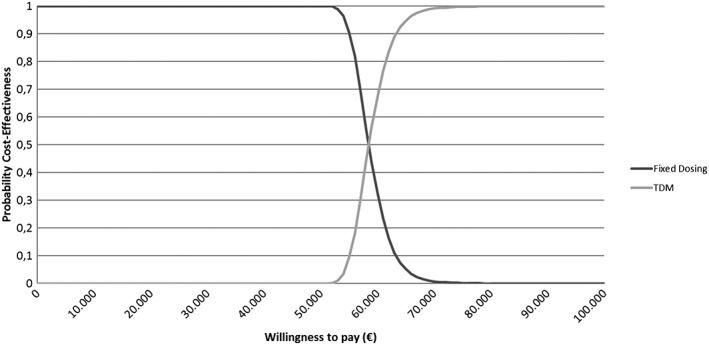
Cost‐effectiveness curve comparing fixed dosing vs therapeutic drug monitoring‐guided dosing
3.2. Sensitivity analyses
The 1‐way sensitivity analyses for possible discounts, ranging from 50 to 99% discount, in imatinib drug prices showed a reduction in the ICUR, yielding a cost of €21 993.54, €9094.02, €2627.29 and €907.25 per QALY gained for 50%, 80%, 95% and 99% decreased prizes, respectively (Figure 4).
Figure 4.
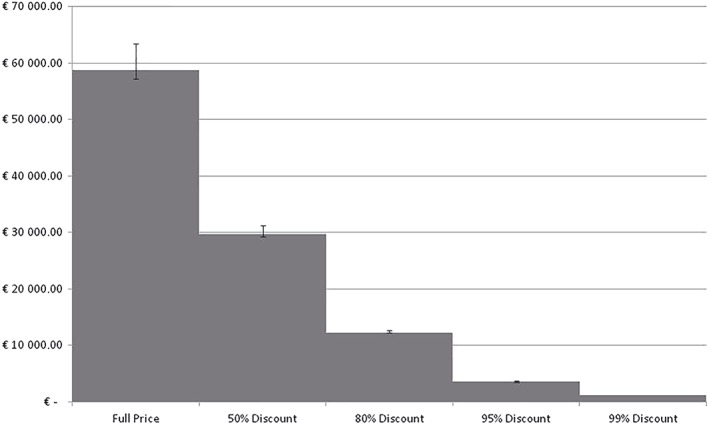
Effect of imatinib drug discounts on incremental cost ratio
Sensitivity analyses performed with variations in utility of the health‐states, and costs of adverse events and BSC resulted in minor changes in the ICUR. Only variations in the utility of the imatinib heath state yielded relatively large changes in the ICUR. ICURs of €76 321.64 and €54 464.70 were found when the health utility of imatinib was decreased by 20%, and increased to perfect health, respectively. A full overview of results of the sensitivity analyses is presented in supplementary file 2.
4. DISCUSSION
We hypothesized that TDM would result in longer time to progression and delayed costs related to second‐ and third‐line drugs. However, TDM‐guided dosing was also expected to be more expensive due to the laboratory costs made for TDM and higher doses of imatinib that need to be given in subtherapeutic patients. The question was whether the gain in health and related cost‐savings would outweigh these extra costs.
Given the available data, these analyses suggest that the use of TDM provides additional clinical benefit and may be cost‐effective for patients with metastatic/unresectable GIST starting with imatinib as a first line of treatment. An average ICUR of €58 758.70 per QALY gained was found. The use of TDM is shown to be cost‐effective in 100% of the cases when a willingness to pay of €72 000.00 per QALY gained is used. As a reference, in the Netherlands, a maximum cost of €80 000 per QALY gained is widely used as the threshold value for cost‐effectiveness in patients with highest burden of disease.24 Using this metric, TDM can be regarded as a cost‐effective intervention for use in the Netherlands, however this may not be the case for other countries with different cost‐effectiveness thresholds. Ultimately, it is up to the decision‐maker to decide whether ICURs are acceptable.
Our sensitivity analyses on potential decreased costs of imatinib in the nearby future might support the uptake of our conclusion, showing that ICUR will dramatically decrease with expected discounts up to 99%. This can be explained by the fact that the imatinib drug cost is the main cost‐driver in this analysis, due to the additional imatinib doses given to patients to be treated within the therapeutic index.
The additional sensitivity analyses showed no large effect on the ICUR when parameters concerning the intermediary health‐states were increased or decreased. Changes in parameters for health‐states beyond the initial imatinib health‐state will affect both TDM‐guided dosing and fixed dosing equally, showing minimal effect on the ICUR. This shows that the results from our analyses are robust.
To our knowledge, this is the first cost‐effectiveness analyses comparing the use of TDM‐guided dosing vs fixed dosing imatinib in GIST patients. Moreover, no cost‐effectiveness analyses have been performed on the effect of dose optimization based on TDM for oral tyrosine kinase inhibitors so far. Therefore, our results are a valuable addition to the existing knowledge.
TDM is set to be used in other sarcoma treatment drugs, such as sunitinib and pazopanib, as well. While sunitinib is part of the line of treatment used in this model, it was decided to not implement the TDM of sunitinib in this model, due to an exponential increase in complexity of the model. However, based on the results of this analysis, it can be expected that the use of TDM in the sunitinib group as well as the imatinib group may further increase the cost‐effectiveness, due to additional prolonging of the time it takes to progress to regorafenib and best supportive care.
One of the limitations in this study is that due to limited available data, not all differences in the imatinib health‐state subgroups could be taken into account. No clear consensus could be found whether or not the occurrences of adverse events were significantly different between these subgroups,9 resulting in the same occurrence of adverse events being used among these subgroups. This may have resulted in an underestimation of the effect of TDM in the imatinib group, due to a lower occurrence of adverse events in the supratherapeutic imatinib health‐state. Additionally, OS for the subtherapeutic imatinib group were not available, and have been extrapolated based on the PFS of the imatinib subgroups, which may result in either an over‐ or underestimation of the effect of TDM‐guided dosing. Finally, the increase in PFS and OS induced by targeting the predefined threshold is based on retrospective analyses of PK data collected in several clinical studies while a prospective validation of this target is currently still lacking. In patients with chronic myeloid leukaemia, 1 small prospective study was conducted in only 55 patients in which no added value of routine TDM was demonstrated mainly contributed to the nonadherence to dose‐adjustment advice given.25
This model makes a comparison based on an ideal situation, where adherence from patients to all parts of the treatment is 100%. In reality, this may not the case.26, 27 This may have resulted in an overestimation of the cost‐effectiveness of TDM‐guided dosing. The effect of GIST and its treatment on absence from work and associated costs were explored but ultimately not taken into account in this model due to lack of available data. Due to prolonged time to progression, a larger number of patients may be able to participate in the workforce, decreasing costs associated by absence from work, and further increasing the cost‐effectiveness of TDM‐guided dosing vs fixed dosing.
5. CONCLUSION
This analysis suggests that TDM‐guided dosing provides additional clinical benefit and may be cost‐effective compared to fixed dosing in patients suffering from metastatic/unresectable GIST, using imatinib as a first line of treatment, especially when imatinib loses its patent whereby drugs costs will significantly decrease.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
I.M.E.D., W.K. and N.P.v.E. designed the research. S.Z. and W.K. performed the research. S.Z., W.K., N.P.v.E. and I.M.E.D. analyzed the data. W.K. contributed analytical tools. All authors wrote the manuscript.
Supporting information
Table S1.
Input Parameters.
Supplementary file 2.
Overview results sensitivity analyses
Zuidema S, Desar IME, van Erp NP, Kievit W. Optimizing the dose in patients treated with imatinib as first line treatment for gastrointestinal stromal tumours: A cost‐effectiveness study. Br J Clin Pharmacol. 2019;85:1994–2001. 10.1111/bcp.13990
Précis: The use of TDM is a cost‐effective intervention in patients with GIST.
REFERENCES
- 1. Nilsson B, Bumming P, Meis‐Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era‐‐a population‐based study in western Sweden. Cancer. 2005;103(4):821–829. [DOI] [PubMed] [Google Scholar]
- 2. Verschoor AJ, Bovee J, Overbeek LIH, PALGA group , Hogendoorn PCW, Gelderblom H. The incidence, mutational status, risk classification and referral pattern of gastro‐intestinal stromal tumours in the Netherlands: a nationwide pathology registry (PALGA) study. Virchows Arch. 2018;472:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl 2):S‐1–S‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT‐positive malignancies. J Clin Oncol. 2002;20(6):1692–1703. [DOI] [PubMed] [Google Scholar]
- 5. Demetri GD, Reichardt P, Kang Y‐K, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu CC, Wu CE, Chen JS, et al. Imatinib escalation or sunitinib treatment after first‐line imatinib in metastatic gastrointestinal stromal tumor patients. Anticancer Res. 2014;34(9):5029–5036. [PubMed] [Google Scholar]
- 7. Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic‐phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111(8):4022–4028. [DOI] [PubMed] [Google Scholar]
- 8. de Wit D, Guchelaar HJ, den Hartigh J, Gelderblom H, van Erp NP. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today. 2015;20(1):18–36. [DOI] [PubMed] [Google Scholar]
- 9. Lankheet NA, Knapen LM, Schellens JH, et al. Plasma concentrations of tyrosine kinase inhibitors imatinib, erlotinib, and sunitinib in routine clinical outpatient cancer care. Ther Drug Monit. 2014;36(3):326–334. [DOI] [PubMed] [Google Scholar]
- 10. Demetri GD, Wang Y, Wehrle E, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009;27(19):3141–3147. [DOI] [PubMed] [Google Scholar]
- 11. Guilhot F, Hughes TP, Cortes J, et al. Plasma exposure of imatinib and its correlation with clinical response in the tyrosine kinase inhibitor optimization and selectivity trial. Haematologica. 2012;97(5):731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lankheet NAG, Desar IME, Mulder SF, et al. Optimizing the dose in cancer patients treated with imatinib, sunitinib and pazopanib. Br J Clin Pharmacol. 2017;83(10):2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verheijen RB, Yu H, Schellens JHM, Beijnen JH, Steeghs N, Huitema ADR. Practical recommendations for therapeutic drug monitoring of kinase inhibitors in oncology. Clin Pharmacol Ther. 2017;102(5):765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu H, Steeghs N, Nijenhuis CM, Schellens JHM, Beijnen JH, Huitema ADR. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53(4):305–325. [DOI] [PubMed] [Google Scholar]
- 15. Eechoute K, Fransson MN, Reyners AK, et al. A long‐term prospective population pharmacokinetic study on imatinib plasma concentrations in GIST patients. Clin Cancer Res. 2012;18(20):5780–5787. [DOI] [PubMed] [Google Scholar]
- 16. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2014;25(Suppl 3):iii21–iii26. [DOI] [PubMed] [Google Scholar]
- 17. Abrantes JA, Jonsson S, Karlsson MO, et al. Handling interoccasion variability in model‐based dose individualization using therapeutic drug monitoring data. Br J Clin Pharmacol. 2019; 85(6):1326-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mickisch G, Gore M, Escudier B, Procopio G, Walzer S, Nuijten M. Costs of managing adverse events in the treatment of first‐line metastatic renal cell carcinoma: bevacizumab in combination with interferon‐alpha2a compared with sunitinib. Br J Cancer. 2010;102(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson J, Connock M, Song F, et al. Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess. 2005;9(25):1–142. [DOI] [PubMed] [Google Scholar]
- 20. Chabot I, LeLorier J, Blackstein ME. The challenge of conducting pharmacoeconomic evaluations in oncology using crossover trials: the example of sunitinib for gastrointestinal stromal tumour. Eur J Cancer. 2008;44(7):972–977. [DOI] [PubMed] [Google Scholar]
- 21. Paz‐Ares L, García del Muro X, Grande E, González P, Brosa M, Díaz S. Cost‐effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) after progression or intolerance with imatinib. Clin Transl Oncol. 2008;10(12):831–839. [DOI] [PubMed] [Google Scholar]
- 22. Poole CD, Connolly MP, Chang J, Currie CJ. Health utility of patients with advanced gastrointestinal stromal tumors (GIST) after failure of imatinib and sunitinib: findings from GRID, a randomized, double‐blind, placebo‐controlled phase III study of regorafenib versus placebo. Gastric Cancer. 2015;18(3):627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakkaart‐van Roijen L, Tan SS, Bouwmans CAM. Handleiding voor kostenonderzoek. Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. 2010.
- 24. J.M.G. MMHvB‐WAMBHBDDMGWNJL . Rechtvaardige en Duurzame Zorg. In: Gezondheidszorg Rvd , ed. Advies Rechtvaardige en duurzame zorg door Raad voor de Volksgezondheid & Zorg 2007, https://www.raadrvs.nl/documenten/publicaties/2007/10/17/rechtvaardige‐en‐duurzame‐zorg
- 25. Gotta V, Widmer N, Decosterd LA, et al. Clinical usefulness of therapeutic concentration monitoring for imatinib dosage individualization: results from a randomized controlled trial. Cancer Chemother Pharmacol. 2014;74(6):1307–1319. [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long‐term therapy. Blood. 2011;117(14):3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Input Parameters.
Supplementary file 2.
Overview results sensitivity analyses


