Abstract
Aims
The development of monoclonal antibodies (mAbs) requires an understanding of the interindividual variability (IIV) in pharmacokinetics (PK) at the population level facilitated by population PK (PopPK) modelling. However, there is no clear rationale for selecting which covariates to screen during PopPK model development. Here, we compare the effect of covariates on PK parameters for mAbs in oncology and identify the most commonly used covariates affecting PK parameters.
Methods
All 25 mAbs approved for therapeutic use in oncology until December 2017 by the Food and Drug Administration and the European Medicines Agency were selected for study. Literature searches revealed 23 available PopPK models for these mAbs. To understand the magnitude and types of covariate effect on PK parameters, all covariates included in the final PopPK model for each mAb were summarized.
Results
The most commonly identified covariates were baseline body weight (BW; 17 mAbs), baseline serum albumin (8 mAbs), and sex (7 mAbs) on clearance; and BW (16 mAbs) and sex (12 mAbs) on central volume of distribution. A reduced PopPK model was developed for nivolumab and ipilimumab using these covariates, and the percentage of explained IIV from the reduced model (20.3% and 16.8%, respectively) was compared with that from the full model (24.5% and 27.9%, respectively).
Conclusions
This analysis provides a uniform platform for selecting covariates and suggests that the effect of BW, albumin and sex should be included during the development of PopPK models for mAbs in oncology. The reduced model was able to explain IIV to a similar extent as the full model.
Keywords: cancer, monoclonal antibodies, pharmacokinetics, population analysis
What is already known about this subject
The pharmacokinetics of mAbs in oncology has been studied extensively, and almost 23 PopPK models of approved mAbs have been published. Although covariate selection is based on physiological relevance and clinical judgment (presumed clinical relevance), there is inconsistency in the approach to covariate selection.
What this study adds
Analyses of PopPK model of 23 mAbs in oncology provided information to support a uniform platform for selecting covariates and suggested that, in addition to other physiological factors (covariates) of clinical relevance, the effect of body weight, baseline albumin and sex should always be included during model development.
Including the effect of body weight, baseline albumin and sex on PK parameters in the reduced model provided reduction in IIV relative to the base model, which was comparable to the full model with all relevant covariates.
1. INTRODUCTION
Many therapeutic monoclonal antibodies (mAbs) have been approved since the first mAb approval in 1986, and the expected trend is for at least 6–9 first marketing approvals for mAbs each year in the short term in the USA and European Union (EU).1, 2, 3 These mAbs are highly target specific and are being developed for treatment in multiple therapeutic areas. Recent approvals and current clinical studies describe mAbs that are used across therapeutic areas, including cardiovascular disease, immunology, infectious diseases, oncology and ophthalmology, and approximately 40% of those currently in late stages of development are for oncology indications.3, 4 Specifically in oncology, rituximab was the first mAb to be approved (almost 20 years ago) for the treatment of patients with low‐grade or follicular B‐cell non‐Hodgkin lymphoma whose tumour did not respond to other therapies.5 Owing to the success of checkpoint inhibitors (ipilimumab, nivolumab, atezolizumab, durvalumab, avelumab and pembrolizumab) and other antibody‐based cancer therapies, many new mAbs are in clinical development.3, 4, 6
The pharmacokinetics (PK) of mAbs has been studied extensively and there is a good understanding of the physiological behaviour of mAbs.7, 8 The PK of mAbs differs from those of small‐molecule therapeutics in several ways. Due to their large size, mAbs have relatively slow tissue uptake and a low volume of distribution in the central compartment (VC, which is typically around 3–4 L), as they are largely confined to vascular and interstitial spaces. The primary route of clearance (CL) for mAbs is via proteolytic degradation, which occurs by nonspecific Fc‐receptor‐mediated mechanisms with linear kinetics and/or by a nonlinear target‐mediated CL pathway. The elimination half‐life of mAbs depends on the immunoglobulin G (IgG) subtype and is often long (up to 4 weeks), particularly in the absence of predominant target‐mediated drug disposition.7, 9, 10, 11
As for any therapeutic drug, development of mAbs requires an understanding of the optimal dose and/or exposure–effect relationship, which includes characterization of interindividual variability (IIV) in PK to optimize efficacy and safety in patients. Specifically, understanding of IIV in PK at the population level can be achieved using population PK (PopPK) modelling.12 In this approach, the PK of a mAb is characterized in the target patient population and IIV is investigated by assessing the effect of different covariates on PK parameters, including CL and volume of distribution (central and peripheral). Historically, the commonly tested covariates in PopPK models include body size (weight or surface area), sex, ethnicity and age.13 However, the influence of other demographic factors, disease status and comorbidities, immunogenicity status (defined by the presence or absence of antidrug antibodies), blood chemistry parameters, and concomitant medications is also often assessed.7 Of these many potential covariates, baseline body weight (BW) has most commonly been retained in the final model.9 As there is no clear rationale on which covariates are screened during PopPK model development, the choice of covariates for different analyses has been based on the covariates collected during the study, physiological relevance and clinical judgment. Hence, there is no consistent approach to the types of covariates being chosen for analyses, even for mAbs that are against similar targets and that exhibit similar pathways for distribution and elimination. Here, we reviewed specific covariates used in the final PopPK analysis of mAbs approved for 1 or more oncology indications, in the USA or EU, and analysed the magnitude of the effect of each of the covariates included in the final PopPK model to better understand how it impacts the PK of mAbs. The primary objective of this study is to provide a meaningful comparison of the effect of covariates on PK parameters for mAbs in oncology in order to identify the most relevant covariates affecting PK parameters.
The secondary objective of this study was to analyse the percentage of unexplained variability from the base model, and how it may be explained by including covariates affecting CL or VC. To understand the extent of variability that can be explained by including covariates on PK parameters, a comparison of IIV from a full model (including relevant prespecified covariates) with that from a reduced model (including selective covariates identified from our analyses) was done using published PopPK models for nivolumab and ipilimumab as case examples.14, 15 A reduced model was developed for both nivolumab and ipilimumab that included the effect of most relevant covariates, and the IIV in both full and reduced models was analysed with respect to the base model.
2. METHODS
2.1. Selection of monoclonal antibodies
This study was based on all mAbs approved for any cancer indication until December 2017 by the Food and Drug Administration in the USA and by the European Medicines Agency in the EU.1, 2, 3, 4, 16 Antibody–drug conjugates were excluded from this analysis. Monoclonal antibodies with both subcutaneous and intravenous routes of drug administration were included, although most of the approved mAbs in oncology are currently being administered through an intravenous route. Literature searches were conducted for available PopPK models of approved mAbs through PubMed for published manuscripts, or the Food and Drug Administration website for clinical pharmacology reports.
2.2. Structural model for monoclonal antibodies
The PK of mAbs was mostly described by a 2‐compartment model with linear elimination, as the target is saturated at a therapeutic dose with these mAbs. Few mAbs exhibit nonlinear (Michaelis–Menten kinetics) or parallel linear and nonlinear elimination pathways, as many therapeutic mAbs are eliminated via saturable target‐mediated mechanisms. Monoclonal antibodies with only a nonlinear elimination component in the model were excluded from our analyses. For mAbs with parallel linear and nonlinear elimination pathways, the effect of covariates was assessed on the linear component in most cases, and was therefore included in the analyses. Considering that the objective was to analyse the effect of covariates on PK parameters, this assumption was deemed suitable.
2.3. Covariate analyses
The available PopPK models for approved mAbs were reviewed to determine what covariates were evaluated and assessed for the magnitude of effect on PK parameters in these analyses. An ideal case would have been to analyse the effect of covariates using a full model that might include the effect of other relevant predictors on PK38, 39, 40; however, only the final model was available for most mAbs in published PopPK models or clinical pharmacology reports. Therefore, analysis in this manuscript used the estimates of covariate effects from published final PopPK models.
Specifically, parameter estimates for each covariate in the respective final models were tabulated, and the magnitude of the effect of covariates that were present in the PopPK model for more than 5 mAbs on CL and VC was analysed. The information related to the effect of covariates on intercompartmental CL or peripheral volume of distribution was very limited, and therefore was not included in the analyses. The magnitude of the effect of these covariates across different mAbs was analysed using forest plots. The forest plots were generated using the parameter estimate (95% confidence interval [CI]) values provided in the final PopPK model for the selected, evaluable (based on type of elimination, as described above) covariates for mAbs.
In some PopPK models, estimation errors for PK parameters were reported as relative standard error (RSE). In those cases, 95% CI was calculated based on the following equation:
Reference values for covariates were based on values provided in the respective published PopPK models or clinical pharmacology reports. The median value of a covariate was used as a reference in case the reference value used in the PopPK model was not specified. Median BW from different clinical trial data sets used in PopPK analyses of these mAbs ranged from 62 to 80 kg, and it was >70 kg for most studies. The 5th and 95th percentiles for BW (51–115 kg) were used from a previously published nivolumab analysis data set14 and were assumed to be similar for all mAbs based on previous analyses where BW distribution was similar across tumour types.9 Similarly, the 5th and 95th percentile values for baseline serum albumin (ALB) values were used from a nivolumab data set (3.1–4.7 g dL−1).14
2.4. Evaluating covariates that reduce unexplained IIV in the base model from nivolumab and ipilimumab PopPK analysis
The magnitude of unexplained IIV was evaluated by a PopPK approach using nivolumab and ipilimumab PopPK models as case examples, which included a base model (without any covariates on PK parameters), a full model (which included all prespecified relevant covariates on PK parameters),14, 15 and a reduced model (which included selected covariates on PK parameters). To evaluate IIV that can be explained by including covariates in a PopPK model for a mAb, further information about the base model was required, which was not included in published manuscripts or clinical pharmacology reports. Nivolumab and ipilimumab were therefore used as case examples because we had access to data for both drugs. The nivolumab full model included effects of tumour type, performance status, sex, race, hepatic function, ALB, baseline lactate dehydrogenase, estimated glomerular filtration rate (GFR), age and BW on CL, and BW and sex on VC.14 The ipilimumab full model included the effects of prior treatment, concomitant budesonide, sex, immunogenicity, human leucocyte antigen genotype, Eastern Cooperative Oncology Group (ECOG) performance status, alanine aminotransferase, direct bilirubin, age, ALB, GFR, lactate dehydrogenase and BW on CL, and of BW and sex on VC.15
A reduced model was developed for both nivolumab and ipilimumab that included the effect of only the most commonly identified covariates on PK parameters from covariate analyses (BW, ALB and sex on CL, and BW and sex on VC). This reduced model was run using a similar data set that was used in base and full models for both nivolumab and ipilimumab. IIV of CL and VC was determined for base, full and reduced models, and change or reduction of IIV in full and reduced models was analysed with respect to the base model.
2.5. Nomenclature of target and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,41 and are permanently achieved in the Concise Guide to PHARMACOLOGY 2017/2018.42
3. RESULTS
3.1. Selection of mAbs and summary of most common covariates affecting PK as reported in PopPK models
Our search identified a total of 25 mAbs that were approved for oncology indications in the USA and EU by December 2017. PopPK models were available for 23 of the 25 mAbs. No PopPK models were available for catumaxomab and tositumomab‐I131 in the public domain. Other mAb constructs, including antibody–drug conjugates, were not included in these analyses.
All covariates included in the final PopPK model for 23 mAbs, including the PK structural model, were summarized from the literature review (Table 1).14, 15, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 The effect of covariates was evaluated specifically for the linear portion of CL for mAbs exhibiting linear or parallel linear and nonlinear CL. Thus, mAbs that exhibited only nonlinear elimination (alemtuzumab, cetuximab and denosumab) were excluded from our analysis. The most common covariates, defined as those included in the final PopPK model for >5 mAbs, are summarized in Table 2. Blinatumomab was excluded because the effects of BW, ALB and sex were not included in the final model. For ofatumumab, the effect of body surface area was analysed on PK parameters in comparison with BW, and therefore was not included in the analyses. In addition, dinutuximab was not included in these analyses because the effect of BW was fixed at 0.75 and not estimated. Therefore, the effect of BW on CL was analysed for 17 mAbs.
Table 1.
Covariates included on PK parameters to explain variability using PopPK analyses based on the final model in mAbs that are approved for treatment in cancer
| mAb (approval date) | PK | Patients (PK samples) | Parameters | Covariates in final model | Reference |
|---|---|---|---|---|---|
| Alemtuzumab (2001/2014) | 2‐CMT/nonlinear elimination | 67 (1565) | Vmax | WBC count | 17 |
| Atezolizumab (2016) | 2‐CMT/linear elimination | 906 (5811) | CL | ADA, ALB, BW, tumour sizea | 18 |
| VC | ALB, BW, sex | ||||
| Avelumab (2017) | 2‐CMT/linear elimination | 1629 (10,220) | CL | ALB, BW, dose level (3 mg/kg), sex, tumour size,a tumour type (MCC) | 19 |
| VC | BW, sex | ||||
| Bevacizumab (2004) | 2‐CMT/linear elimination | 1792 (8943) | CL | ALB, ALK, BW, IFNα, sex | 20 |
| VC | BW, sex | ||||
| Blinatumomab (2014) | 1‐CMT/linear elimination | 322 (3015) | CL | CrCL | 21 |
| VC | None | ||||
| Cetuximab (2004) | 2‐CMT/nonlinear elimination | 143 (912) | CL | Ideal BW, WBC | 22 |
| VC | Total BW | ||||
| Vmax | Ideal BW, WBC | ||||
| Daratumumab (2015) | 2‐CMT/linear and nonlinear elimination | 223 (2572) | CL | ALB, BW, formulation (phase 2 vs phase 3), type of myeloma (IgG vs non‐IgG) | 23 |
| VC | BW, sex | ||||
| Denosumab (2010) | 2‐CMT/nonlinear elimination | 1076 (14,228) | CL | BW, race, tumour type | 24 |
| VC | BW, race | ||||
| Dinutuximab (2015) | 2‐CMT/linear elimination | Not reported | CL | BW | 25 |
| VC | BW | ||||
| Durvalumab (2017) | 2‐CMT/linear and nonlinear elimination | 1409 (7407) | CL | ADA, ALB, BW, CrCL, ECOG PS, sex, tumour sizea | 26 |
| VC | BW, sex | ||||
| Vmax | sPD‐L1 | ||||
| Elotuzumab (2015) | 2‐CMT/linear and nonlinear elimination | 375 (6958) | CL | BW, concomitant lenalidomide/dexamethasone | 27 |
| VC | Asian race, B2MICG, BW, sex | ||||
| Vmax | Serum M‐protein | ||||
| Ipilimumab (2011) | 2‐CMT/linear | 499 (2095) | CL | BW, LDH | 15 |
| VC | BW | ||||
| Necitumumab (2015) | 2‐CMT/linear and nonlinear elimination | 807 (4920) | CL | BW | 28 |
| VC | BW | ||||
| Nivolumab (2014) | 2‐CMT/linear and time varying | 1895 (12,292) | CL | BW, ECOG PS, eGFR, race, sex | 14 |
| VC | BW, sex | ||||
| Obinutuzumab (2013) | 2‐CMT/linear and time‐dependent elimination | 678 (12,634) | CL | BW, tumour type, sex | 29 |
| VC | BW, sex | ||||
| Ofatumumab (2009) | 2‐CMT/linear and nonlinear elimination | 477 (6908) | CL | BSA, IgG | 30 |
| VC | BSA, sex | ||||
| Olaratumab (2016) | 2‐CMT/linear elimination | 171 (1501) | CL | BW, tumour sizea | 31 |
| VC | BW | ||||
| Panitumumab (2006) | 2‐CMT/linear and nonlinear elimination | 1200 (8482) | CL | BW, sex, tumour type | 32 |
| VC | BW, sex | ||||
| Vmax | Age, BW | ||||
| Pembrolizumab (2014) | 2‐CMT/linear elimination | 2195 (12,171) | CL | ALB, BW, ECOG PS, eGFR, ipilimumab status, sex, tumour size,a tumour type | 33 |
| VC | ALB, BW, ipilimumab status, sex | ||||
| Pertuzumab (2012) | 2‐CMT/linear elimination | 481 (4525) | CL | ALB, BWb | 34 |
| VC | BWb | ||||
| Ramucirumab | 2‐CMT/linear elimination | 1639 (6427) | CL | BW | 35 |
| VC | BW | ||||
| Rituximab (1997) | 2‐CMT/linear and nonlinear elimination | 29 (512) | CL | Age, BW | 36 |
| VC | Sex | ||||
| Trastuzumab (1998) | 2‐CMT/linear and nonlinear elimination | 266 (1419) | CL | ALB, BW, prior gastrectomy | 37 |
| VC | BW |
Antibody–drug conjugates and biosimilars were not included in this study.
Tumour size and tumour burden were defined as the sum of the longest diameter of target lesions. Tumour burden was renamed to tumour size for atezolizumab and pembrolizumab for consistency.
For pertuzumab, lean BW was used as the BW.
2‐CMT, 2‐compartment model; ADA, antidrug antibody; ALB, baseline serum albumin; ALK, alkaline phosphatase; B2MICG, serum β2‐microglobulin; BSA, body surface area; BW, baseline body weight; CL, clearance; CrCL, creatinine CL; ECOG, Eastern Cooperative Oncology Group; eGFR, estimated glomerular filtration rate; IFNα, interferon‐α treatment; IgG, baseline immunoglobulin G concentration; LDH, lactate dehydrogenase; mAb, monoclonal antibody; MCC, Merkel cell carcinoma; PK, pharmacokinetics; PopPK, population PK; PS, performance status; sPD‐L1, soluble programmed death ligand 1; VC, central volume of distribution; Vmax, maximum elimination rate; WBC, white blood cell.
Table 2.
Common demographic and disease specific covariatesa included in the final PopPK model for approved oncology mAbs included in the present analysis
| Drug name | CL | VC | |||
|---|---|---|---|---|---|
| BW | ALB | Sex | BW | Sex | |
| Atezolizumab | X | X | ‐ | X | X |
| Avelumab | X | X | X | X | X |
| Bevacizumab | X | X | X | X | X |
| Daratumumab | X | X | ‐ | X | X |
| Durvalumab | X | X | X | X | X |
| Elotuzumab | X | ‐ | ‐ | X | X |
| Ipilimumab | X | ‐ | ‐ | X | ‐ |
| Necitumumab | X | ‐ | ‐ | X | ‐ |
| Nivolumabb | X | ‐ | X | X | X |
| Obinutuzumab | X | ‐ | X | X | X |
| Ofatumumabc | ‐ | ‐ | ‐ | ‐ | X |
| Olaratumab | X | ‐ | ‐ | X | ‐ |
| Panitumumab | X | ‐ | X | X | X |
| Pembrolizumab | X | X | X | X | X |
| Pertuzumabd | X | X | ‐ | X | ‐ |
| Ramucirumab | X | ‐ | ‐ | X | ‐ |
| Rituximab | X | ‐ | ‐ | ‐ | X |
| Trastuzumab | X | X | ‐ | X | ‐ |
Antibodies with nonlinear elimination were excluded: alemtuzumab, cetuximab and denosumab. Blinatumomab was excluded because the common covariates were not included in the final model. For dinutuximab, BW was included in CL and VC, but the coefficient was fixed at 0.75 and therefore not included in the analysis.
The table shows covariates included in the final PopPK models for >5
mAbs identified and included in the present analysis.
For nivolumab, ALB was included in the sensitivity analyses but was not included in the final model.
For ofatumumab, body surface area was included for CL and VC instead of BW in the final model.
For pertuzumab, lean BW was used in the final model.
ALB, baseline serum albumin; BW, baseline body weight; CL, clearance; PopPK, population PK; VC, central volume of distribution.
During these analyses, BW, sex and ALB for CL, and BW and sex for VC were identified as the most commonly used covariates. The effect of ALB on CL was included for 8 mAbs. For nivolumab, ALB was analysed only in the sensitivity analysis, and therefore was not included in Table 2. The effect of sex on CL was included for 7 mAbs. The next most widely used covariate effect on CL was tumour size, the sum of the longest diameter of the target lesion, which was included in the final PopPK model for 5 mAbs (atezolizumab, avelumab, durvalumab, olaratumab and pembrolizumab; Table S1). Other covariates that were included for CL in the final model for a limited number of mAbs were tumour type (avelumab, obinutuzumab, panitumumab and pembrolizumab), ECOG performance status (durvalumab, nivolumab and pembrolizumab), creatinine CL (blinatumomab and durvalumab), estimated GFR (nivolumab and pembrolizumab) and immunogenicity status (atezolizumab and durvalumab). Other covariates were used only once in the available PopPK models.
BW as a covariate for VC was used in the final PopPK model for 16 mAbs, and sex as a covariate was used for 12 mAbs (Table 2). Less commonly used covariates were ALB and race (each used twice).
3.2. Covariate analyses
Covariate analyses were performed to assess the magnitude of effect on PK parameters. As described in the Methods, this analysis was done for BW, ALB and sex for CL, and for BW and sex for VC, as these were identified as the most commonly used covariates.
3.3. Assessment of magnitude of the effect of BW, ALB and sex on CL
The effect of BW on CL was assessed in our analyses for 17 mAbs (Figure 1A). For this analysis, the magnitude of the effect of BW was represented as the percentage change relative to the median BW reported in the PopPK model. CL of all mAbs increased with an increase in BW (blue bar), and the magnitude of this effect was >20% when compared with the reference in most cases, except for avelumab. For patients who had BW lower than the reference, the CL was lower than the reference (white bar), and the effect was >20% for all, except durvalumab and avelumab. For all 17 mAbs, the effect of BW had a statistically significant effect on mAb CL (the 95% CI did not include 1). For elotuzumab, rituximab and trastuzumab, the effect of BW on CL was markedly greater than for the other 14 antibodies analysed.
Figure 1.
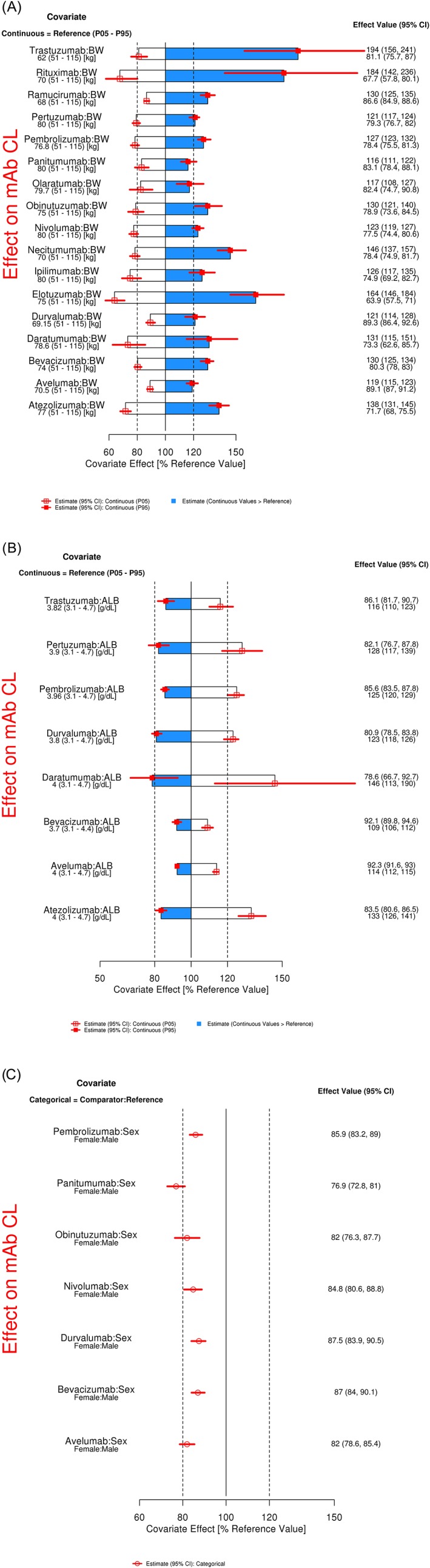
Magnitude of the effect of selected covariates on CL of mAbs in oncology. Graphical representation of percentage variability explained by A, continuous BW, B, continuous ALB, and C, categorical sex on CL based on final PopPK model. Categorical covariate effects (95% CI) are represented by open symbols (horizontal lines). Continuous covariate effects (95% CI) at the 5th/95th percentiles of the covariate are represented by the end of horizontal boxes (horizontal lines). Open/shaded area of boxes represents the range of covariate effects from the median to the 5th/95th percentile of the covariate. The reference values for the covariates used in analyses include BW of 80 kg, ALB of 4 g/dL and sex of male. The parameter estimate in the reference patient is considered 100% (vertical solid line), and dashed vertical lines are at 80% and 120% of this value. ALB, albumin; BW, body weight; CI, confidence interval; CL, clearance; mAb, monoclonal antibody; P05, 5th percentile; P95, 95th percentile; PopPK, population PK
The effect of ALB on CL was analysed for 8 mAbs that included this covariate in the final PopPK model (Figure 1B), and the magnitude of the effect was represented as the percentage change with respect to the reference patient (with median ALB used in the respective analyses). CL of mAbs increased with a decrease in ALB (white bar) and the effect was statistically significant in all cases, with a magnitude of effect >20% in 6 of 8 cases.
The effect of sex on CL was evaluated for 7 mAbs, and the magnitude of the effect of change in CL in females was compared with that in males, which was used as a reference (Figure 1C). In all cases, the CL of mAb was lower in females compared with males, and the change in CL was statistically significant (the 95% CI does not include 1). The decrease in CL in females compared with males was very similar across all 7 mAbs, with the median effect ranging from 13 to 23%.
3.4. Effect of BW and sex on VC
The BW effect on VC was analysed for 16 mAbs (Figure 2A). The VC of all mAbs increased with an increase in BW (blue bar), and the magnitude of this effect was >20% when compared with the reference in most cases, except avelumab. For patients who had BW less than the reference, the VC was low (white bar) and the effect was >20% for almost half of the mAbs.
Figure 2.

Magnitude of the effect of selected covariates on VC of mAbs in oncology. Graphical representation of percentage variability explained by A, continuous BW and B, categorical sex on VC in the final PopPK model. Categorical covariate effects (95% CI) are represented by open symbols (horizontal lines). Continuous covariate effects (95% CI) at the 5th/95th percentiles of the covariate are represented by the end of horizontal boxes (horizontal lines). Open/shaded areas of boxes represent the range of covariate effects from the median to the 5th/95th percentile of the covariate. The reference values for the covariates used in analyses include BW of 80 kg and male sex. The parameter estimate in the reference patient is considered 100% (vertical solid line), and dashed vertical lines are at 80% and 120% of this value. BW, body weight; CI, confidence interval; mAb, monoclonal antibody; P05, 5th percentile; P95, 95th percentile; VC, central volume of distribution
The effect of sex on VC was evaluated for 12 mAbs, where the females had lower VC compared with males and the median effect ranged from 11 to 22% (Figure 2B).
3.5. Unexplained IIV in the PopPK analyses after addition of covariates for nivolumab and ipilimumab
Figure 3 shows unexplained IIV in the base model that was published for nivolumab and ipilimumab.14, 15 Unexplained IIV for CL in the base model was 42.9% for nivolumab and 39.5% for ipilimumab. The data set and the full model were similar to previously published analyses and included similar covariates for CL and VC.14, 15 For the reduced PopPK model for both nivolumab and ipilimumab, the covariate effects of BW, ALB and sex on CL, and BW and sex on VC were included. Although the addition of covariates in the full and reduced models accounts for part of the unexplained IIV, most of it remains unexplained (see unexplained portion in full and reduced models in Figure 3). For nivolumab CL, the reduced model (which included selected covariates) was able to explain 20.3% of the total unexplained IIV in the base model, and the full model (which included the effect of additional covariates) was able to explain 24.5% of the total unexplained IIV in the base model. For nivolumab VC, both full and reduced models had the same covariates (BW and sex); therefore, there was no difference in the extent of variability explained from these models. Similarly, for ipilimumab CL, the reduced model was able to explain 16.8% of total unexplained IIV in the base model compared with 27.9% in the full model. The residual variabilities in base, full and reduced models for both nivolumab and ipilimumab were similar.
Figure 3.
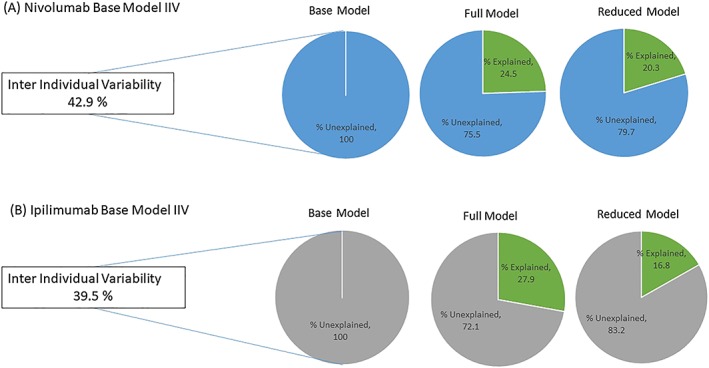
Plot showing percentage of explained and unexplained variability in base, full and reduced models for A, nivolumab and B, ipilimumab. Covariates were included on CL in full and reduced models for nivolumab and ipilimumab to minimize the unexplained variability. The plots show percentages of variability explained by adding the covariates on CL in full and reduced models for nivolumab and ipilimumab. The full model included all covariates that were present in the original published model,14, 15 and the reduced model included the effects of BW, ALB and sex on CL. ALB, albumin; BW, body weight; CL, clearance; IIV, interindividual variability
4. DISCUSSION
In this study, our aim was to identify the most commonly used covariates among the 23 mAbs approved for use in at least 1 oncology indication for which PopPK models were available in the public domain. The most commonly used covariates in the final PopPK models were BW, ALB and sex for CL, and BW and sex for VC. This analysis may provide a uniform platform for selection of covariates for PopPK analyses across studies. The assessment of magnitude of the effect of covariates on the PK parameter was based on an 80–120% boundary (or ± 20% of reference), which was used as a screening criterion for potential clinical relevance. Covariate effect within 80–120% is not expected to have clinical relevance. Additionally, this 80–120% boundary was not guided by any exposure–response relationship, and the significance of a given covariate impacting clinical efficacy and safety can be further evaluated in exposure–response analyses.
BW was a key covariate identified in this analysis for both CL and VC for all screened mAbs used in oncology. Although the effect of BW on the PK of mAbs is well established,9, 13 we have shown here that both CL and VC increased with an increase in BW, and the magnitude of the effect of BW for respective PK parameters was similar across the majority of mAbs analysed. It is also notable that the magnitude of effect of BW on CL was substantially higher for elotuzumab,27 rituximab36 and trastuzumab37 when compared with other mAbs. Along with several other mAbs included in the analysis (daratumumab,23 durvalumab26 and panitumumab32), these 3 mAbs exhibit parallel linear and nonlinear kinetics; nevertheless, it should be noted that for elotuzumab, rituximab and trastuzumab, the effect of BW was included only on the linear component and not on the nonlinear part of CL. In our opinion, BW might also influence the nonlinear component of CL and, if not included as a covariate, this effect of BW on the nonlinear CL may be distributed to linear CL. In the case of panitumumab, where the effect of the covariate was also analysed on the nonlinear component, the BW effect was within 20%.
For most mAbs, total BW was included as covariate, and lean BW and body surface area were used as other measures of body size. Previous analyses have shown that all of these measures of body size were highly correlated and performed similarly,43 and use of any of these measures was justified in PopPK models. We propose use of a uniform approach and thus recommend using BW in PopPK models, as BW is recorded during all clinical visits and does not require any further calculations.
BW‐based dosing has traditionally been used in the development of therapeutic mAbs, as body size was believed to account for a major source of variability across individuals.8, 9 This is supported by the argument that larger individuals have a higher volume of distribution due to a greater volume of plasma and interstitial fluid when compared with smaller individuals, and that there is lower linear elimination in smaller compared with larger individuals.13 Considering that BW has a significant effect on CL and VC, it may seem logical to use BW‐based dosing with mAbs compared with flat dosing. However, for most of these antibodies, the estimated effect of BW on CL in their respective PopPK analyses had a coefficient ranging from 0.4 to 0.6, indicating the effect was less than proportional and both flat or BW‐based dosing will be suitable for these mAbs. Additionally, modelling and simulation studies using a range of mAbs have now suggested that BW‐based and flat dosing resulted in similar PK or pharmacodynamic variability, so BW‐based dosing does not always offer an advantage over flat dosing.9, 44, 45
This study showed that CL of mAbs increased with a decrease in ALB. Our results also highlight the importance of ALB on CL for some mAbs in oncology, with an effect >20% in 75% of mAbs included in our analysis. Although the effect of ALB on nivolumab CL is significant, nivolumab was not considered in the present analysis because the final PopPK model of nivolumab did not include the effect of ALB on nivolumab CL. It is important to note that ALB was not included in the final PopPK model, as ALB values were not available for all patients in the analyses data set.14 However, the effect of ALB on nivolumab CL was determined using a sensitivity analysis in the subgroup of patients who had ALB values, and the effect was found to be significant.14 ALB has also been shown to affect the PK of 2 mAbs used in ulcerative colitis—infliximab46 and vedolizumab47—with higher CL observed in patients with lower ALB.
The exact mechanism linking ALB with CL of mAb is unclear and can be related to 2 hypotheses. One possible reason for this strong association between CL and ALB of mAbs could be cancer‐related cachexia, characterized by loss of muscle mass due to the hypermetabolic state.48, 49, 50 Lower ALB is one of the indicators of cachexia and hypermetabolic state, as albumin synthesis rates are decreased in hypoalbuminaemic cachectic cancer patients.51 This strong association of CL and ALB suggests that the elimination or turnover of proteins including mAbs and albumin is higher in cachectic patients, as they are in a hypermetabolic state. Another hypothesis is related to the neonatal Fc receptor (FcRn) rescue pathway shared by IgG and ALB.52 FcRn is widely expressed throughout the body and has a critical role in homeostatic regulation of these 2 proteins by salvaging them from cellular catabolism and allowing them to be recycled back into the extracellular fluid.7, 52 Lower serum ALB may indicate that an individual has lower FcRn levels, resulting in higher ALB CL by catabolism; likewise, an individual with lower FcRn levels might be expected to exhibit higher CL of any administered IgG mAb. Recent data from preclinical studies showed that loss of expression of the recycling receptor, FcRn, promotes tumour cell growth by increasing albumin consumption,53 which shows how FcRn and ALB can be correlated. However, if there is an association between FcRn levels and disease severity, it still remains to be determined.
We found a consistent and significant effect of sex on both CL and VC of mAbs evaluated in our study, where both CL and VC were lower in females than in males. Considering the effect of sex was significant and consistent across the mAbs evaluated, it was surprising that sex was not included in the final model for other mAbs. Potential reasons for the difference in PK of mAbs between males and females may, in theory, include differences in the lymph flow rate, which affects the rate of mAb absorption after intramuscular or subcutaneous injection and distribution; differences in mAb target levels, which affects target‐mediated disposition; and differences in expression of the Fcγ receptors, which are implicated in CL of mAb.13
Our analyses were limited, first, by the nature of our data sources, which relied only on published information. For example, it is possible that not every patient included in the PopPK model for each mAb had a serum ALB measurement. We knew this to be the case for nivolumab, and therefore excluded this mAb in our analysis of ALB as a covariate. Another example would be ipilimumab, where ALB and sex were present in the model after backward elimination, but were not included in the final model based on the criterion of limiting the number of covariates as the effect was within 20%. Similar to ipilimumab, criteria for selection of covariates in the final model for other mAbs can be different, which may have led to exclusion of mAbs from our analyses. Second, our decision to exclude mAbs displaying nonlinear PK may have led to bias. For those mAbs with parallel linear and nonlinear PK, the nonlinear component of elimination was considered null to assess the effect of the covariate in this analysis. However, this assumption is justifiable in that, at higher or therapeutic doses, the majority of mAbs are eliminated through the linear elimination pathway compared with the nonlinear component.
It was difficult to quantify the proportion of IIV that was explained on PK parameters in the final PopPK models, as most of the available publications lacked information for the base and full models. To estimate this, we used published data from nivolumab14 and ipilimumab15 for which information was available from base and full models, and we had access to the data used in the PopPK model. We evaluated both the full model and the reduced model to understand how much unexplained IIV from the base model was addressed or minimized by including covariates from these 2 models. In our analyses, we found that the full model was able to explain only 24.5% and 27.9% of the total unexplained IIV determined from the base model for nivolumab and ipilimumab, respectively. Most of the total IIV in the base model (up to 75–80%) was still left unexplained even after including additional covariates in the full model. Our analyses also showed that the reduced model (with selected covariates: BW, ALB, and sex for CL, and BW and sex for VC) was able to explain only 20.3% and 16.8% of the total unexplained IIV on CL in the base model for nivolumab and ipilimumab, respectively. These data provided an explanation for BW, ALB and sex being the most relevant covariates for mAbs in oncology, as including the effect of these covariates was able to explain most of the total unexplained IIV determined in the base model. Additionally, the current analyses evaluated only the most common covariates that were included in PopPK models for more than 5 mAbs (of the 23 identified). Tumour size was included in the final PopPK model for 5 mAbs, whereby a larger tumour size was associated with higher CL.26, 31, 33 In addition to BW, ALB, and sex, other covariates might also influence PK of mAbs in oncology (e.g. tumour type, ECOG performance status, antidrug antibodies, estimated GFR, creatinine clearance), and it might be worth considering additional covariates if the data are available. However, further analysis is needed to confirm how much effect or variability is explained by adding these covariates into the model.
5. CONCLUSIONS
We suggest the following strategy for selection of covariates during development of mAbs in oncology based on our review of the PopPK analyses for available mAbs. At the time of screening, the covariates BW, ALB and sex should always be included in the development of a PopPK model. This would also enable uniform evaluation and consistency in the interpretation of the effect of important covariates on PK of mAbs. The suggested parsimonious reduced model (with selected covariates: BW, ALB and sex for CL, and BW and sex for VC) can provide reduction in IIV relative to the base model, which may be comparable to the full model with all relevant prespecified covariates. Additionally, it might be worth considering other relevant covariates that might influence PK of mAbs in oncology supported by analyses. We believe this approach can also be applied to mAbs in other therapeutic areas; however, a thorough analysis needs to be done to understand the effect of covariates on PK parameters for mAbs approved for the treatment of other diseases.
COMPETING INTERESTS
S.S. and A.R. are employees of Bristol‐Myers Squibb, which funded the study. G.B. and M.G. are currently employees of Genmab and were employees of Bristol‐Myers Squibb at the time these analyses were conducted.
CONTRIBUTORS
All authors planned the study and provided suggestions for development of the manuscript. G.B. conducted the literature search. G.B., S.S. and M.G. selected the studies. G.B. and S.S. performed the data analyses. All authors reviewed and approved the final manuscript.
6.
Supporting information
Table S1. Additional baseline and disease‐specific covariates for clearance included in the final population pharmacokinetic model for approved oncology monoclonal antibodies included in the present analysis
ACKNOWLEDGEMENTS
We thank Dr Chaitali Passey (Genmab), Dr Kinjal Sanghavi (BMS) and Dr Sumit Rawal (Eisai) for scientific discussions. This study was funded by Bristol‐Myers Squibb. Professional medical writing and editorial assistance were provided by Kakoli Parai, PhD, and Cara Hunsberger at StemScientific, an Ashfield company, funded by Bristol‐Myers Squibb.
Bajaj G, Suryawanshi S, Roy A, Gupta M. Evaluation of covariate effects on pharmacokinetics of monoclonal antibodies in oncology. Br J Clin Pharmacol. 2019;85:2045–2058. 10.1111/bcp.13996
Data Availability Statement:BMS policy on data sharing may be found at https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharing‐request‐process.html.
DATA AVAILABILITY STATEMENT
BMS policy on data sharing may be found at https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharing‐request‐process.html.
REFERENCES
- 1. Reichert JM. Antibodies to watch in 2015. MAbs. 2015;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reichert JM. Antibodies to watch in 2016. MAbs. 2016;8(2):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reichert JM. Antibodies to watch in 2017. MAbs. 2017;9(2):167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10(2):183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ault A. First monoclonal antibody for cancer treatment on way to market. Lancet. 1997;350:416. [Google Scholar]
- 6. Mahoney KM, Freeman GJ, McDermott DF. The next immune‐checkpoint inhibitors: PD‐1/PD‐L1 blockade in melanoma. Clin Ther. 2015;37(4):764–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633–659. [DOI] [PubMed] [Google Scholar]
- 8. Mould DR, Meibohm B. Drug development of therapeutic monoclonal antibodies. BioDrugs. 2016;30(4):275–293. [DOI] [PubMed] [Google Scholar]
- 9. Bai S, Jorga K, Xin Y, et al. A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet. 2012;51(2):119–135. [DOI] [PubMed] [Google Scholar]
- 10. Zhao L, Ren TH, Wang DD. Clinical pharmacology considerations in biologics development. Acta Pharmacol Sin. 2012;33(11):1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryman JT, Meibohm B. Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2017;6(9):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER) . Guidance for industry: population pharmacokinetics. 1999. Available from: https://www.fda.gov/downloads/drugs/guidances/UCM072137.pdf (accessed 11 September 2017).
- 13. Gill KL, Machavaram KK, Rose RH, Chetty M. Potential sources of inter‐subject variability in monoclonal antibody pharmacokinetics. Clin Pharmacokinet. 2016;55(7):789–805. [DOI] [PubMed] [Google Scholar]
- 14. Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model‐based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feng Y, Masson E, Dai D, Parker SM, Berman D, Roy A. Model‐based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol. 2014;78(1):106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. AntibodySociety.org . Approved antibodies. Available from: https://www.antibodysociety.org/news/approved‐antibodies/ (accessed 1 May 2018).
- 17. Mould DR, Baumann A, Kuhlmann J, et al. Population pharmacokinetics‐pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64(3):278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroh M, Winter H, Marchand M, et al. Clinical pharmacokinetics and pharmacodynamics of atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther. 2017;102(2):305–312. [DOI] [PubMed] [Google Scholar]
- 19. US Food and Drug Administration, Center for Drug Evaluation and Research . Multi‐discipline review: avelumab. 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761049Orig1s000MultidisciplineR.pdf (accessed 18 July 2017).
- 20. Han K, Peyret T, Marchand M, et al. Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother Pharmacol. 2016;78(2):341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu M, Wu B, Brandl C, et al. Blinatumomab, a bispecific t‐cell engager (BiTE(®)) for CD‐19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55(10):1271–1288. [DOI] [PubMed] [Google Scholar]
- 22. Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48(3):267–278. [DOI] [PubMed] [Google Scholar]
- 23. US Food and Drug Administration, Center for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review(s): daratumumab. 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/761036Orig1s000ClinPharmR.pdf (accessed 18 July 2017).
- 24. Gibiansky L, Sutjandra L, Doshi S, et al. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin Pharmacokinet. 2012;51(4):247–260. [DOI] [PubMed] [Google Scholar]
- 25. US Food and Drug Administration, Center for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review(s): dinutuximab. 2014. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125516Orig1s000ClinPharmR.pdf (accessed 13 September 2017).
- 26. Baverel PG, Dubois VFS, Jin CY, et al. Population pharmacokinetics of durvalumab in cancer patients and association with longitudinal biomarkers of disease status. Clin Pharmacol Ther. 2018;103(4):631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gibiansky L, Passey C, Roy A, Bello A, Gupta M. Model‐based pharmacokinetic analysis of elotuzumab in patients with relapsed/refractory multiple myeloma. J Pharmacokinet Pharmacodyn. 2016;43(3):243–257. [DOI] [PubMed] [Google Scholar]
- 28. Long A, Chigutsa E, Wallin J. Population pharmacokinetics of necitumumab in cancer patients. Clin Pharmacokinet. 2017;56(5):505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibiansky E, Gibiansky L, Carlile DJ, Jamois C, Buchheit V, Frey N. Population pharmacokinetics of obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non‐Hodgkin's lymphoma and exposure‐response in CLL. CPT Pharmacometrics Syst Pharmacol. 2014;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Struemper H, Sale M, Patel BR, et al. Population pharmacokinetics of ofatumumab in patients with chronic lymphocytic leukemia, follicular lymphoma, and rheumatoid arthritis. J Clin Pharmacol. 2014;54(7):818–827. [DOI] [PubMed] [Google Scholar]
- 31. Mo G, Baldwin JR, Luffer‐Atlas D, et al. Population pharmacokinetic modeling of olaratumab, an anti‐PDGFRα human monoclonal antibody, in patients with advanced and/or metastatic cancer. Clin Pharmacokinet. 2018;57(3):355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma P, Yang BB, Wang YM, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49(10):1142–1156. [DOI] [PubMed] [Google Scholar]
- 33. Ahamadi M, Freshwater T, Prohn M, et al. Model‐based characterization of the pharmacokinetics of pembrolizumab: a humanized anti‐PD‐1 monoclonal antibody in advanced solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garg A, Quartino A, Li J, et al. Population pharmacokinetic and covariate analysis of pertuzumab, a HER2‐targeted monoclonal antibody, and evaluation of a fixed, non‐weight‐based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol. 2014;74(4):819–829. [DOI] [PubMed] [Google Scholar]
- 35. O'Brien L, Westwood P, Gao L, Heathman M. Population pharmacokinetic meta‐analysis of ramucirumab in cancer patients. Br J Clin Pharmacol. 2017;83(12):2741–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rozman S, Grabnar I, Novaković S, Mrhar A, Jezeršek Novaković B. Population pharmacokinetics of rituximab in patients with diffuse large B‐cell lymphoma and association with clinical outcome. Br J Clin Pharmacol. 2017;83(8):1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cosson VF, Ng VW, Lehle M, Lum BL. Population pharmacokinetics and exposure‐response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol. 2014;73(4):737–747. [DOI] [PubMed] [Google Scholar]
- 38. Gastonguay MR. A full model estimation approach for covariate effects: inference based on clinical importance and estimation precision. AAPS Annual Meeting, 7–11 November 2004. Baltimore, Maryland, USA: Abstract W4354.
- 39. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 40. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer Science+Business Media; 2001. [Google Scholar]
- 41. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alexander SPH, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol. 2017;174(Suppl 1):S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McLeay SC, Morrish GA, Kirkpatrick CM, Green B. The relationship between drug clearance and body size: systematic review and meta‐analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51(5):319–330. [DOI] [PubMed] [Google Scholar]
- 44. Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size‐based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–1024. [DOI] [PubMed] [Google Scholar]
- 45. Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit‐risk profile of a 240‐mg flat dose relative to a 3‐mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28(8):2002–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48(05):297–308. [DOI] [PubMed] [Google Scholar]
- 47. Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics‐pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn's disease. Aliment Pharmacol Ther. 2015;42(2):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. [DOI] [PubMed] [Google Scholar]
- 49. Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44(8):1124–1132. [DOI] [PubMed] [Google Scholar]
- 50. McMillan DC. The systemic inflammation‐based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. [DOI] [PubMed] [Google Scholar]
- 51. Fearon KC, Falconer JS, Slater C, McMillan DC, Ross JA, Preston T. Albumin synthesis rates are not decreased in hypoalbuminemic cachectic cancer patients with an ongoing acute‐phase protein response. Ann Surg. 1998;227(2):249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sand KM, Bern M, Nilsen J, Noordzij HT, Sandlie I, Andersen JT. Unraveling the interaction between FcRn and albumin: opportunities for design of albumin‐based therapeutics. Front Immunol. 2015;5:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Swiercz R, Mo M, Khare P, Schneider Z, Ober RJ, Ward ES. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget. 2017;10:3528–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Additional baseline and disease‐specific covariates for clearance included in the final population pharmacokinetic model for approved oncology monoclonal antibodies included in the present analysis
Data Availability Statement
BMS policy on data sharing may be found at https://www.bms.com/researchers‐and‐partners/independent‐research/data‐sharing‐request‐process.html.


