Abstract
Aims
Rituximab is standard care in a number of lymphoma subtypes, including follicular lymphoma (FL), although many patients are resistant to rituximab, or develop resistance with repeated treatment, and a high proportion relapse. Obinutuzumab is a novel anti‐CD20 monoclonal antibody with improved efficacy over rituximab. It is approved for previously untreated chronic lymphocytic leukaemia (CLL), and for use with bendamustine in patients with rituximab‐relapsed/refractory FL.
Methods
Using a previously described population pharmacokinetic (PK) model of obinutuzumab in patients with non‐Hodgkin lymphoma and CLL, we conducted an exposure‐response analysis using data from 6 clinical trials in patients with CD20+ B‐cell malignancies (CLL11, GADOLIN, GATHER, GAUDI, GAUGUIN and GAUSS) to describe the PK properties of obinutuzumab, identify covariates influencing exposure, and explore how exposure affects safety, efficacy and pharmacodynamics.
Results
A 2‐compartment model with linear and time‐dependent clearance described obinutuzumab PK. Disease type and subtype, body weight, baseline tumour size, and sex had the largest effects on PK. Obinutuzumab exposure was not associated with occurrence or severity of adverse events, but higher exposure appeared to be associated with greater efficacy, particularly longer progression‐free survival. However, in multivariate Cox regression analysis, progression‐free survival benefit in the obinutuzumab plus bendamustine arm was independent of exposure.
Conclusion
The updated population PK model reported here accurately describes the PK of obinutuzumab patients with non‐Hodgkin lymphoma and CLL. The selected obinutuzumab dosing regimen offers clinical benefit in a majority of rituximab‐refractory FL patients treated with bendamustine, irrespective of variability in exposure, whilst minimising adverse events.
Keywords: drug exposure, follicular lymphoma, obinutuzumab, pharmacokinetics
What is already known about this subject
Rituximab, a type I anti‐CD20 monoclonal antibody, is the current standard of care in follicular lymphoma and a number of other lymphoma subtypes, although management of rituximab relapse and resistance presents a significant challenge.
Obinutuzumab is a novel, humanised, type II anti‐CD20 monoclonal antibody that has exhibited superior efficacy over rituximab, regardless of alternative dosing schedules.
What this study adds
The study provides a population pharmacokinetic model that accurately describes the pharmacokinetics of obinutuzumab in patients with non‐Hodgkin lymphoma and chronic lymphocytic leukaemia.
No association was found between obinutuzumab exposure and adverse events in rituximab‐relapsed/refractory follicular lymphoma patients, confirming the favourable safety profile of the fixed‐dose obinutuzumab regimen that is now approved for use with bendamustine in these patients.
1. INTRODUCTION
Follicular lymphoma (FL) is the most common type of indolent non‐Hodgkin lymphoma (iNHL).1 Lymphomas of this subtype are characterised by an ongoing pattern of relapse and are usually incurable in their advanced stages.1, 2, 3 Rituximab was the first monoclonal antibody (mAb) to target the CD20 B‐cell surface antigen,4 and its use in conjunction with chemotherapy significantly improved outcomes in patients with advanced (stage 3–4) FL in first‐line and salvage settings.5, 6, 7, 8, 9, 10 Although rituximab is standard care in FL and a number of other lymphoma subtypes,11 management of rituximab relapse and resistance presents a significant challenge.12
Obinutuzumab (GA101) is a novel, humanised anti‐CD20 mAb. In contrast to rituximab, which works primarily via complement‐dependent cytotoxicity and localises CD20 in lipid rafts by binding it as a tetramer,4 obinutuzumab is a type II mAb13, 14, 15 that binds to CD20 without forming cross‐links to CD20 tetramers, and so remains dispersed throughout the entire surface of the B cell. Obinutuzumab is glycoengineered to enhance binding affinity to FcγRIIIA/B expressed on effector cells such as natural killer cells, macrophages/dendritic cells and neutrophils, and works mainly by promoting direct cell death and antibody‐dependent cellular cytotoxicity.4 In clinical trials, obinutuzumab showed improved efficacy compared with rituximab16, 17, 18, 19; in particular, a retrospective pharmacokinetic (PK)/pharmacodynamic (PD) analysis of the CLL11 trial demonstrated that obinutuzumab exhibited superior efficacy over rituximab, regardless of alternative dosing schedules; even when the dose of rituximab was tripled, it could not match the levels of B‐cell depletion achieved by obinutuzumab.20 Obinutuzumab is approved for previously untreated chronic lymphocytic leukaemia (CLL), and for use with bendamustine in patients with rituximab‐relapsed/refractory FL.4
A predictive model describing the population PK of obinutuzumab, based on data from 678 patients with CLL or iNHL, has been described previously.21 This model used data from 4 clinical studies: CLL11 (NCT01010061; patients with CLL)22; GAUDI (NCT00825149; patients with iNHL)23; GAUGUIN (NCT00517530; patients with CLL, iNHL, diffuse large B‐cell lymphoma [DLBCL] or mantle cell lymphoma [MCL])18, 24; and GAUSS (NCT00576758; patients with CLL, iNHL, DLBCL or MCL).25 We report the development of an updated model based on a larger database including patients from 2 additional studies: GADOLIN (NCT01059630; patients with rituximab relapsed/refractory iNHL)26; and GATHER (NCT01414855; patients with DLBCL).27 Our objectives were to further characterise the PK of obinutuzumab in different CD20 B‐cell malignancies and to explore whether differences in drug exposure in patients from the GADOLIN study affected PD, safety or efficacy.
2. METHODS
2.1. Patients and study designs
Serum obinutuzumab concentrations from 961 patients with CD20+ B‐cell malignancies from 6 clinical studies (GAUSS [n = 105: 4 CLL, 96 iNHL, 4 DLBCL, 1 MCL]; GAUGUIN [n = 131: 30 CLL, 56 iNHL, 26 DLBCL, 19 MCL]; GAUDI [n = 134 iNHL]; CLL11 [n = 308 CLL]; GADOLIN [n = 183 iNHL]; and GATHER [n = 100 DLBCL]) were analysed using a validated sandwich enzyme‐linked immunosorbent assay with a lower limit of quantitation of 4.05 ng/mL,21 and were included in a population PK analysis using nonlinear mixed effect modelling techniques with NONMEM software (Version 7.3.0, ICON Development Solutions28; Table S1). Across the studies, obinutuzumab was given intravenously at doses of 50–2000 mg. All studies were carried out in accordance with the Declaration of Helsinki and the International Guidelines for Good Clinical Practice. Protocols were approved by the ethics committees at participating centres.
2.2. Population PK analysis
The population PK analysis was performed as described previously.21 The base model, a 2‐compartment model in which clearance was a sum of time‐dependent clearance (CLT) and time‐independent clearance (CLinf), was updated with clinical data from the GATHER and GADOLIN studies. GATHER is an open‐label phase II trial to evaluate the safety and efficacy of obinutuzumab in combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy in patients with advanced DLBCL. In GATHER, patients received eight 21‐day cycles of obinutuzumab (1000 mg given intravenously on Day 1 of each cycle) alongside standard CHOP therapy for 6 cycles. GADOLIN, a phase III trial to assess the efficacy, safety and PK of obinutuzumab plus bendamustine (G‐Benda) vs bendamustine monotherapy in rituximab‐refractory iNHL, is described in detail elsewhere.29, 30 In GADOLIN, a fixed dose of 1000 mg obinutuzumab was given on days 1, 8 and 15 of cycle 1, and on day 1 of cycles 2–6 (28‐day cycles). Obinutuzumab maintenance therapy was subsequently given as 1000 mg every 2 months for 2 years, or until disease progression.
CLT decreased with time with a decay coefficient (Kdes). The Monte Carlo importance sampling expectation‐maximisation assisted by mode a posteriori (IMPMAP) estimation method was used (Supplementary Methods).31
Covariates investigated in the model are listed in Table S2. Graphical evaluation of the models and visual predictive check32 simulations were performed as described previously.21 Normalised prediction‐distribution‐error procedures33, 34 and stratification of model evaluation diagnostics were also undertaken (Supplementary Methods and Table S3). Individual predicted PK parameters were computed and summarised based on the standard iNHL dosing regimen, and individual concentration–time courses simulated (Supplementary Methods).
2.3. Exposure–safety and exposure–efficacy relationships for FL patients (GADOLIN study)
Exposure–safety and exposure–efficacy relationships in rituximab‐relapsed/refractory FL patients from the GADOLIN trial were explored using graphical analyses. Among patients with available PK assessments (n = 183), only patients who received at least 5 doses of obinutuzumab (i.e. ≥3 dosing cycles) were included in the analyses. Baseline patient/disease characteristics of the subpopulation treated with G‐Benda who contributed to the PK analysis were consistent with those of the intention‐to‐treat population.29, 30
Safety outcomes explored were occurrence of serious adverse events (SAEs), severity of infusion‐related reactions (IRRs), occurrence and severity of neutropenia and thrombocytopenia adverse events (AEs) and change in neutrophil/platelet counts. Relationships between exposure and PD outcomes were also explored (Supplementary Methods). Analyses included patients who received ≥1 dose of obinutuzumab.
For the exposure–efficacy analysis, mean obinutuzumab concentration over the induction period (Cmean) was used to represent obinutuzumab exposure as it accounted for the actual dosing history (including dosing delays and modifications) over the entire induction period. This is similar to using cumulative area under the curve (AUC) over induction period, but it better accounts for differences in the duration of induction between patients. This exposure measure has been used previously for obinutuzumab.35 Minimum serum concentration at a specific timepoint (Ctrough) would have been a better predictor of target saturation; however, it mainly accounts for the latest dose before Ctrough. Distributions of Cmean were compared for different best overall response (BOR) categories. Relationships of progression‐free survival (PFS) and overall survival (OS) with exposure were assessed by comparing Cmean in patients with or without events (progression, relapse, or death for PFS; death for OS). The risk of PFS and OS events associated with 3 categories of exposure (low, medium and high, defined using tertiles of exposure) was compared graphically using Kaplan–Meier plots for all patients (analysis cut‐off: 1 September 2014) and for patients with low and high baseline tumour size (defined based on the median value calculated from the sum of the products of diameters of the target lesion; Figure S1).
2.4. Cox proportional hazards analysis of PFS and OS
In a post‐hoc exploratory analysis, relationships between PK exposure, PFS and OS were characterised by semi‐parametric Cox proportional hazards (CPH) models to investigate the effect of potential confounding factors on exposure (Supplementary Methods). Models where exposure was treated as a continuous or categorical covariate were evaluated during model development. CPH models were based on a 120‐day safety update analysis of the GADOLIN trial (cut‐off: 1 May 2015).
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,36 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.37
3. RESULTS
3.1. Population PK analysis in CLL, DLBCL and NHL
A total of 16 301 serum obinutuzumab concentrations from 961 patients were captured for the population PK analysis; 469 of these patients had iNHL, of whom 406 had FL (8545 serum samples) and 183 were from the GADOLIN trial (2178 serum samples). Other disease types were CLL (n = 342), DLBCL (n = 130) and MCL (n = 20).
Summary statistics for the continuous covariates are presented in Table S4. The 2‐compartment linear model with both linear (CLinf) and time‐dependent clearances (CLT) described previously21 accurately described obinutuzumab PK in patients from the 6 clinical studies.
Parameter estimates for the final covariate model are summarised in Table S5. Values for CLinf, central volume of distribution (V1) and intercompartmental clearance (Q) were similar to previous estimates based on 678 patients,21 but estimates for the decay coefficient of time dependent clearance (kdes) and peripheral volume of distribution (V2) were higher (0.11 vs 0.036 day−1 and 1.23 vs 1.01 L, respectively) and CLT was lower (0.154 vs 0.231 L day−1). Unexplained interindividual variability was moderate (44.6%) for CLinf and was high for CLT (110%) and kdes (90.6%). These values are consistent with the previously reported values for obinutuzumab,21 except for kdes, where incorporation of additional covariates in the current analysis allowed lower unexplained interindividual variability from 201% (in21) to 90.6%. The interindividual variability values were also consistent with those reported for rituximab in CLL patients.38
As baseline B‐cell counts and disease type were confounded (e.g. by high B‐cell counts in CLL patients); of these 2 variables, disease type was tested as a covariate in the model.
According to the model, a patient with FL or DLBCL would have a CLinf of 74 mL day−1, V1 of 2.72 L, Q of 1.32 L day−1 and V2 of 1.23 L (analysed covariates set to reference values). The initial value of CLT was 154 mL day−1, with an estimated half‐life of 6.3 days. CLT half‐life varied according to disease type and treatment (marginal zone lymphoma, 21.1 days; CLL treated with chlorambucil, 20.4 days; iNHL treated with bendamustine, 10.7 days; FL treated with fludarabine and cyclophosphamide, 2.4 days). Initial CLT was higher in CLL or MCL than the reference subtype (FL or DLBCL), higher in men than women, and increased with increasing baseline tumour size (Table 1). Relative to a reference patient (65‐year‐old female with FL or DLBCL, baseline tumour size 3000 mm2), initial CLT was ~100% greater in a patient with high baseline tumour size (22 400 mm2) and 55% lower in a patient with low baseline tumour size (304 mm2). For CLinf, the variance from the reference value was ~20% for the same 2 baseline tumour size values (Table 1).
Table 1.
Effects of covariates on key pharmacokinetic parameters in the final covariate model
| Parameter | Covariate | Reference value for covariate * | Illustrative value for covariate * | Effect on parameter, % (mean, 95% CLs) |
|---|---|---|---|---|
| CLinf | Body weight | 75 kg | 52 kg | −20.9 (−26, −15.4) |
| 115 kg | 31.4 (21.6, 42) | |||
| Serum albumin | 40 g/L | 28.7 g/L | 25.4 (35.1, 16.4) | |
| 48.7 g/L | −12.6 (−16.3, −8.6) | |||
| Sex | Female | Male | 17.6 (9.8, 26) | |
| Disease type | FL or DLBCL | CLL | 46.9 (36.7, 57.9) | |
| SLL | 38 (10.2, 72.9) | |||
| MCL | 106.9 (44.6, 195.9) | |||
| Age | 65 years | 38 years | 13.8 (5.7, 22.6) | |
| 83 years | −5.7 (−8.9, −2.5) | |||
| Baseline tumour size | 3000 mm2 | 304 mm2 | −18.6 (−24.4, −12.4) | |
| 22 400 mm2 | 19.8 (12.3, 27.8) | |||
| CLT | Sex | Female | Male | 45.1 (19.3, 76.6) |
| Disease type | FL or DLBCL | CLL | 125.1 (72.3, 194.1) | |
| MCL | 180.3 (28, 513.7) | |||
| Baseline tumour size | 3000 mm2 | 304 mm2 | −55.2 (−64.8, −42.9) | |
| 22 400 mm2 | 102.3 (63.6, 150.1) | |||
| V1 | Body weight | 75 kg | 52 kg | −12.9 (−15.3, −10.5) |
| 115 kg | 17.5 (13.9, 21.4) | |||
| Sex | Female | Male | 19.4 (15.8, 23) | |
| V2 | Body weight | 75 kg | 52 kg | −32.6 (−39.7, −24.6) |
| 115 kg | 58.4 (39, 80.5) | |||
| Kdes | Received CHOP | No | Yes | −69.1 (−75.1, −61.6) |
| Received fludarabine + cyclophosphamide | No | Yes | 164.4 (38.5, 404.8) | |
| Received bendamustine | No | Yes | −40.9 (−55.5, −21.6) | |
| Disease type | FL or DLBCL | MZL | −70.1 (−81.9, −50.7) |
CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone; CL: confidence limit; CLinf: nonspecific time‐independent clearance; CLT: initial value of time‐dependent clearance; CLL: chronic lymphocytic leukaemia; DLBCL: diffuse large B‐cell lymphoma; FL: follicular lymphoma; kdes: decay coefficient of time‐dependent clearance; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; PK: pharmacokinetic; SLL: small lymphocytic lymphoma; V1: central volume of distribution; V2: peripheral volume of distribution.
For continuous covariates, reference values are medians and illustrative values are the 2.5th and 97.5th percentiles of the values in the analysis data set.
Several other covariates had an effect on CLinf; the most marked differences relative to reference values were in patients with MCL, CLL and small lymphocytic lymphoma (Table 1).
Covariate model evaluation showed no model deficiencies (diagnostic plots) or unaccounted trends (dependencies of random effects). Visual predictive checks showed good agreement between simulated and observed data for all studies.
Sensitivity analyses were performed by refitting the final model developed on data from the 6 clinical studies from only those patients with iNHL and FL. Population predictions and goodness‐of‐fit for these 2 models were very similar to those for the final model, and individual predictions were nearly identical.
Summaries of mean PK parameters are shown in Table S6 and results of model‐based simulations with the iNHL dosing regimen are reported in Supplementary Results and Figures S2 and S3. Simulations showed that the loading doses enable concentrations to reach steady‐state values at the end of cycle 1, and that concentrations differ slightly according to lymphoma type, iNHL subtype, weight, sex and baseline tumour size, as well as the accompanying chemotherapy.
3.2. Relationships between exposure and study outcomes in GADOLIN relapsed/refractory FL patients
These analyses used efficacy and safety data from the 145 FL patients from GADOLIN.
3.3. Exposure–safety analysis
Analysis of the relationships between exposure and SAE occurrence were done for all reported SAEs and for seven System Organ Classes in which 4 or more SAEs (occurring at any time) were reported (Table S7). Graphical analysis showed no relationship between the severity of IRRs and the predicted obinutuzumab Cmax after the dose that preceded the IRR (Figure S4). The time course of reductions in neutrophil counts and platelet counts was similar for low (Cmean < 293 μg mL−1), middle (Cmean 293–388 μg mL−1) and high (Cmean > 388 μg mL−1) tertiles of obinutuzumab exposure. Distribution of Cmean values showed no relationship with the occurrence or severity of neutropenia or thrombocytopenia, although the numbers of patients with thrombocytopenia AEs of Common Terminology Criteria for Adverse Event grades 1–4 was small (data not shown).
Anti‐drug antibodies were detected in 1 patient from GADOLIN treated with G‐Benda; no influence on obinutuzumab PK was seen.
3.4. Exposure–pharmacodynamic relationships
In all obinutuzumab exposure tertiles, B‐cell counts decreased rapidly from baseline after the start of obinutuzumab treatment and remained depressed for the whole observation period (n = 143; data not shown).
The reduction in tumour size between baseline and end of induction was similar across exposure groups with a slightly higher reduction in the middle and high tertiles (median 80%, 86% and 84% in the low, middle and high tertiles, respectively; data not shown) suggestive of a possible relationship between exposure and tumour burden reduction. However, this might be subject to confounding by higher baseline tumour size in patients with low PK exposure (Figure S5), and is a visual comparison only that was not subject to formal statistical analysis.
3.5. Exploratory exposure–efficacy relationships
Graphical analyses of exposure–efficacy relationships in FL patients who received obinutuzumab in GADOLIN were performed on 128 patients for BOR and PFS. Patients with complete response had apparently higher obinutuzumab Cmean than patients in other response categories (partial response, stable disease or progressive disease; Figure S6), especially in the subset of patients with low baseline tumour size (below median value).
Patients with PFS events appeared to have a lower median obinutuzumab Cmean than patients without events, both for all patients and those with high baseline tumour size; in patients with low baseline tumour size, Cmean distributions were similar for patients with and without PFS events (Figure 1).
Figure 1.
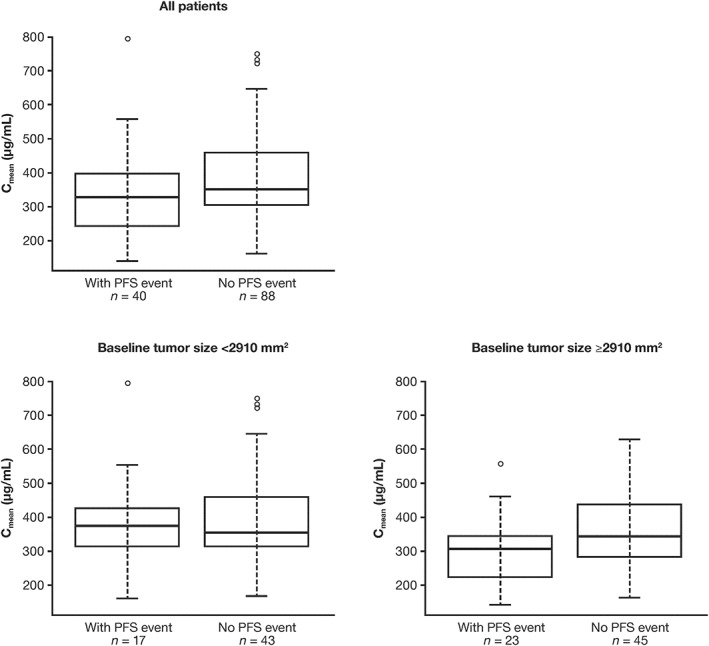
Relationships between PFS and obinutuzumab exposure (Cmean) in patients with FL participating in GADOLIN who received at least 3 dosing cycles of obinutuzumab (n = 128). Lines inside boxes denote medians. Boxes denote IQR. Error bars: limits of 1.5 × IQR. Circles show outliers. Cmean: mean obinutuzumab exposure over induction period; FL: follicular lymphoma, IQR: interquartile range; PFS: progression‐free survival
3.6. CPH analysis of exposure–efficacy relationships
The CPH models included 294 patients for PFS (obinutuzumab [G‐Benda], n = 128; control [bendamustine‐only], n = 166) and 295 for OS (129 and 166, respectively). Parameters of the final models are presented in Table 2. OS data were not analysed by exposure tertiles because of immaturity of the data. Model predictions were in good agreement with observed data for both PFS and OS.
Table 2.
Parameters of the final cox proportional hazards models for PFS and OS based on data in patients with follicular lymphoma in GADOLIN
| Covariate/tertile | HR (95% CI) * | β | SE | RSE |
|---|---|---|---|---|
| PFS, model with exposure as continuous covariate (C mean ) | ||||
| Cmean (as a continuous covariate) | 0.9974 (0.9965 to 0.9983) | −0.0027 | 0.0005 | 17.42 |
| Bone marrow involvement at baseline, yes vs no | 1.689 (1.217 to 2.346) | 0.5244 | 0.1675 | 31.95 |
| PFS, with exposure as categorical covariate (C mean tertiles) | ||||
| Low tertile of Cmean | 0.3695 (0.2219 to 0.6154) | −0.9956 | .2603 | 26.14 |
| Middle tertile of Cmean | 0.4466 (0.2701 to 0.7383) | −0.8062 | 0.2565 | 31.82 |
| High tertile of Cmean | 0.3031 (0.1782 to 0.5156) | −1.194 | 0.2711 | 22.71 |
| Bone marrow involvement at baseline, yes vs no | 1.758 (1.259 to −2.454) | 0.564 | 0.1703 | 30.19 |
| OS | ||||
| Log Cmean (as a continuous covariate) | 0.8484 (0.7731 to 0.9309) | −0.1645 | 0.04736 | 28.8 |
| Age (as a continuous covariate), increase of 1 y | 1.06 (1.034 to 1.086) | 0.05785 | 0.01257 | 21.73 |
| Bone marrow involvement at baseline, yes vs no | 2.401 (1.473 to 3.914) | 0.8759 | 0.2494 | 28.47 |
CI: confidence interval; Cmean: mean obinutuzumab exposure over induction period; FL: follicular lymphoma; HR: hazard ratio; OS: overall survival; PFS: progression‐free survival; SE: standard error; RSE: relative standard error.
Patients in obinutuzumab + bendamustine arm who received <5 obinutuzumab doses (3 cycles) were excluded from this analysis. Cmean tertiles: low = 141–313 μg/mL; middle = 313–400 μg/mL; high = 400–794 μg/mL.
Computed as exp(β).
The PFS analysis with continuous exposure confirmed that the risk of a PFS event was lower in the G‐Benda arm than the bendamustine‐only arm, as previously reported.18
CPH models indicated that the risk of progression or death decreased with increasing obinutuzumab PK exposure; by approximately 40% and 80% at the 5th (Cmean 195 μg/mL) and 95th (Cmean 604 μg/mL) percentiles of exposure, respectively (hazard ratio [HR] [95% confidence interval (CI)] 0.60 [0.50–0.71] and 0.20 [0.12–0.35], respectively). In patients with bone marrow involvement at baseline, the risk of progression or death increased by 69% (HR [95% CI] 1.69 [1.22–2.35]).
For OS, patient age was a significant predictor of shorter survival, although the effect was small. No effect of baseline tumour size on PFS or OS was seen. Figure 2 illustrates the effects of exposure and prognostic factors on the PFS hazard. Longer PFS was observed in all obinutuzumab groups compared to the bendamustine‐only group, with longer PFS for patients in the higher tertile of exposure vs those in the lower and middle tertiles (Figure 3), particularly in patients with high baseline tumour size (data not shown).
Figure 2.
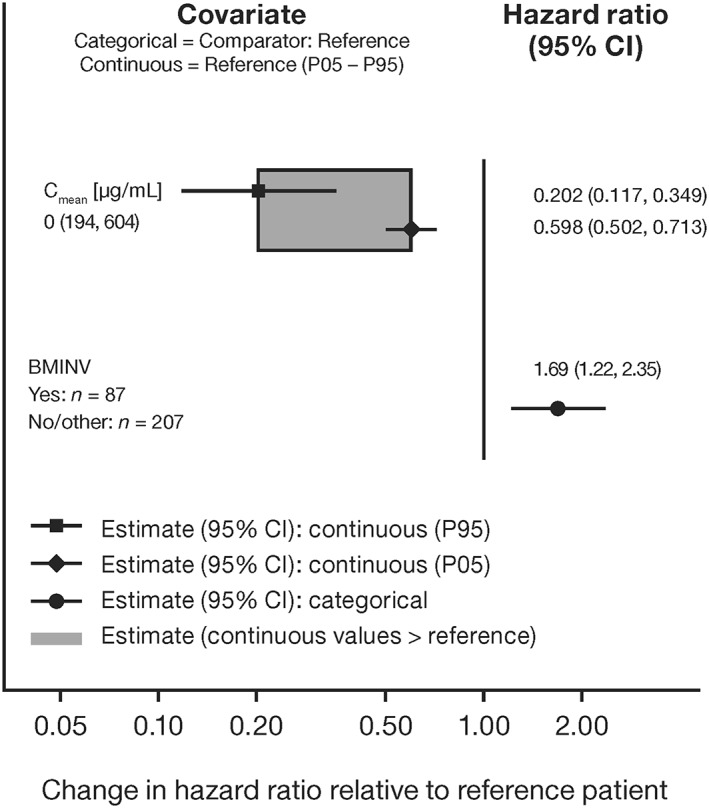
Covariate effects on HR in the final cox proportional hazards model for PFS (n = 128) in patients with FL participating in GADOLIN who received at least 3 dosing cycles of obinutuzumab (combined with bendamustine). BMINV: bone marrow involvement at baseline; CI: confidence interval; Cmean: mean obinutuzumab exposure over induction period; FL: follicular lymphoma; HR: hazard ratio; PFS: progression‐free survival
Figure 3.
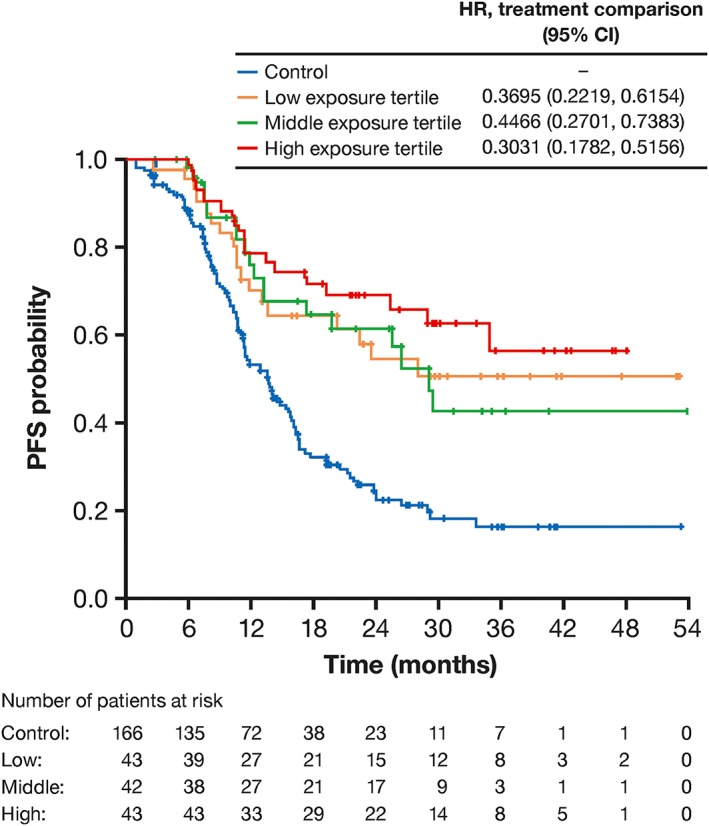
Kaplan–Meier plot, showing relationship between PFS (n = 128) and obinutuzumab exposure (by tertiles of Cmean) in patients with FL participating in GADOLIN who received at least 3 dosing cycles of obinutuzumab (combined with bendamustine). The upper table shows HR for PFS in obinutuzumab plus bendamustine arm relative to control arm (n = 166; bendamustine monotherapy) from the final CPH model. Cmean tertile ranges: lower, 141–313 μg/mL; middle, 313–400 μg/mL; higher, 400–794 μg/mL. CI: confidence interval; Cmean: mean obinutuzumab exposure over induction period; FL: follicular lymphoma; HR: hazard ratio; PFS: progression‐free survival
A CPH model with a categorical exposure (defined by tertiles of Cmean) was also implemented for PFS (Table 2). For all 3 exposure groups, risk of progression was significantly lower than for the bendamustine‐only arm. However, HRs were not ordered between exposure categories (0.37, 0.45, and 0.30 for low, middle and high exposure categories relative to the bendamustine‐only arm) and 95% CIs overlapped for all 3 categories.
4. DISCUSSION
The obinutuzumab population PK model described here is consistent with an earlier model based on a smaller number of CLL and NHL patients,21 and with previous experience with rituximab that showed time‐dependent PK in the targeting of CD20 on B‐cells by antibody.38, 39, 40 The expanded model, based on data from 6 rather than 4 clinical studies (283 additional patients) and examining a greater number of covariates, describes a 2‐compartment linear PK model with both time‐independent and time‐dependent clearance components.
The time‐dependent pathway is thought to be due to target‐mediated drug disposition. CD20+ B‐cells, the therapeutic target of obinutuzumab, also mediate its clearance; as treatment continues, fewer target cells are able to bind to and clear obinutuzumab. Once the majority of target cells are destroyed after being bound by obinutuzumab, target‐mediated drug disposition contributes much less to drug clearance. The model showed that clearance increased with increases in baseline tumour size, with a much more profound effect on CLT than CLinf, which is consistent with target‐mediated elimination. The model also showed substantial differences in clearance between types of lymphoma. This was most evident for MCL patients, in whom both CLT and CLinf, were higher when compared with a typical FL or DLBCL patient. Rate of clearance decline was also affected by lymphoma type; this may reflect differences between disease types in how CD20 is expressed or distributed41 and/or the number of circulating lymphocytes in blood.
The clearance parameters estimated by our model for a reference patient are typical for mAbs.42, 43 Based on our model estimate for CLT, it would take approximately 1 month (5 half‐lives) for CLT to reach a near‐zero value, suggesting that most CD20 target cells are saturated within the first month of dosing. Conditional simulations confirmed that steady state exposure to obinutuzumab is reached by the end of the first dosing cycle. By administering 2 extra 1000‐mg doses during cycle 1, the saturation of CD20+ cells is reached rapidly in a majority of patients and maintained throughout the treatment period.44
The increase in obinutuzumab CLinf, V1 and V2 with body weight is typical for mAbs, but model‐based conditional simulations of concentration–time profiles following the iNHL dosing regimen and the absence of a clear relationship between exposure and efficacy suggest that this association is probably not clinically relevant for dosing in patients with iNHL. Higher CLinf in patients with low albumin levels may be linked to the correlation of albumin levels with efficiency of the neonatal Fc receptor.45 There were also associations of CLinf and V1 with sex and of CLinf with age, but the dependencies were mild and are not considered clinically relevant. The association between CLT and baseline tumour size was deemed to be not clinically relevant, because 2 loading doses were able to provide sufficient exposure during cycle 1 even in subjects with high baseline tumour size. The large interindividual variability in the parameters characterising the time dependency, even if unexplained, was expected as it reflects the large heterogeneity in the individual target expression at baseline, and in the reduction of target over time following treatment initiation.
Simulations of the iNHL dosing regimen in FL patients, i.e. 28‐day dosing cycles, showed that obinutuzumab concentrations were lower during cycles 2–5 of induction therapy for CHOP than for other partner chemotherapy. The recommended higher dosing frequency of obinutuzumab when given with CHOP (eight 21‐day cycles) compared to bendamustine (6 28‐day cycles) accounts for this difference.
In patients with FL participating in GADOLIN, graphical analysis revealed no relationships between exposure and safety parameters, and changes in neutrophil and platelet counts during study treatment were unaffected by the level of obinutuzumab exposure. This is not entirely surprising, as cytopenia during treatment with anti‐CD20 mAbs appears to have several possible causes.46 In early treatment cycles, tumour destruction by the immune system leads to release of cytokines such as interleukins, which have the potential to induce cytopenia. In later cycles, other causes of cytopenia, such as reduction in bone marrow reserve, are more likely.
As stated previously, B‐cell counts decrease rapidly after initiation of obinutuzumab treatment, and remain low during the entire treatment period in all exposure groups. This finding is consistent with our hypothesis that with the optimised dosing regimen of obinutuzumab (i.e. 1000‐mg fixed dose on days 1, 8 and 15 of cycle 1 and then on day 1 of subsequent cycles), the CD20 pool in the body is saturated quickly, with saturation maintained throughout the entire dosing period in all patients despite the between‐patient variability in PK exposure. Although there was a tendency for a greater reduction in tumour size during induction in patients with higher exposure, a confounding effect of tumour burden on exposure cannot be excluded. Recently, Tout et al.47 reported that lower rituximab exposure in DLBCL patients was a consequence of high tumour burden leading to poorer prognosis rather than a cause of inferior response. Similarly, exposure–response analyses of trastuzumab in patients with HER2+ cancers suggested that patients with low exposure had shorter overall survival times; however, they also had poor clinical factors at baseline and increasing the dose in this type of patient did not improve efficacy.48
Exploratory analysis of exposure–efficacy relationships suggested that patients with higher exposure to obinutuzumab could have better efficacy results (BOR, PFS), especially in patients with high tumour burden at baseline; however, this analysis was limited as it did not account for other prognostic factors. These results are consistent with graphical analyses for patients with CLL who received obinutuzumab that similarly were not adjusted for prognostic factors.21 In cancer patients, exposure–response analyses often indicate poorer response in the low exposure group; however, it is often not a causal relationship as poor prognostic factors lead to low exposure.48
CPH modelling confirmed that G‐Benda treatment significantly increased PFS compared with bendamustine alone in patients with FL, while differences in PFS between obinutuzumab exposure groups (tertiles of Cmean) were small, with overlapping CIs. In addition, bone marrow involvement at baseline significantly shortened both PFS and OS, consistent with the published literature.49, 50, 51 Increasing age also had a statistically relevant effect on survival (for OS only), in agreement with existing data.21 Differences in OS between patients with low and high exposure were not meaningful; however, at the time of the analysis cut off (1 May 2015), OS data were too immature to allow any definite conclusions. These results suggest that the current dosing regimen of obinutuzumab is offering clinical benefit to a majority of FL patients in GADOLIN, irrespective of the variability in drug exposure.
5. CONCLUSION
In conclusion, the updated population PK model reported here accurately describes the concentration–time course of obinutuzumab in patients with NHL and CLL, in accordance with earlier findings in this patient population.21 In rituximab‐relapsed/refractory FL patients in the GADOLIN study, no association was found between obinutuzumab exposure and AEs, confirming the favourable safety profile of the fixed‐dose regimen that is now approved for use with bendamustine in these patients. Graphical analysis suggested that an increase in obinutuzumab exposure was associated with better response rate and PFS in FL patients, but a multivariate Cox analysis of PFS that accounted for prognostic factors produced less conclusive results. These findings confirm the suitability of the recommended fixed dose regimen of 1000 mg of obinutuzumab for rituximab‐relapsed/refractory FL that provides clinical benefit in a majority of patients whilst minimising AEs.
COMPETING INTERESTS
The authors declare the following conflicts of interest: E.G. and L.G. were consultants for F. Hoffmann‐La Roche Ltd, and V.B., N.F., M.B. and C.J. are employees of F. Hoffmann‐La Roche Ltd. G.F‐.R. is a former employee of F. Hoffman‐La Roche. G.F.‐R. owns stock in F. Hoffmann‐La Roche Ltd.
CONTRIBUTORS
E.G. and L.G.: data analysis and data interpretation. V.B.: data analysis. N.F.: study, analyses and interpretation of the results. M.B.: study conduct and data collection, analysis and interpretation. G.F.‐R.: study conduct, data analysis and data interpretation. C.J.: study, analyses, interpretation of the results and co‐wrote the paper. All authors reviewed and contributed to the manuscript and approved the final version.
Supporting information
Table S1.
Studies of obinutuzumab included in the population pharmacokinetic analysis
Table S2. Covariates investigated in the population pharmacokinetic model
Table S3. Plots used for graphical evaluation of the model
Table S4. Summary statistics of continuous covariates (means and standard deviations)
Table S5. Parameter estimates for final covariate model
Table S6. Summary of conditional predictions for individual estimates of pharmacokinetic parameters by indolent non‐Hodgkin lymphoma subtype
Table S7. Patients and events from GADOLIN (study GAO4753g) in the graphical exposure–response analysis
Figure S1. Kaplan–Meier plots for progression‐free survival by exposure group and tumour size in patients with follicular lymphoma from GADOLIN who received at least 3 dosing cycles of obinutuzumab (n = 128).
Figure S2. Conditional simulations for the indolent non‐Hodgkin lymphoma dosing regimen. Medians (red), and 5th and 95th percentiles (blue) of the simulated concentrations are plotted. (A) Disease type; (B) indolent non‐Hodgkin lymphoma subtype; (C) baseline tumour size; (D) sex and body weight.
Figure S3. Predictive simulations in follicular lymphoma patients receiving the indolent non‐Hodgkin lymphoma dosing regimen. (A) Males and female patients with follicular lymphoma; (B) monotherapy with obinutuzumab; (C) obinutuzumab as monotherapy or co‐administered with CHOP, fludarabine/cyclophosphamide or bendamustine in follicular lymphoma.
Figure S4. Relationship between severity (maximum CTC‐AE grade) of infusion‐related reactions (IRRs) and predicted Cmax preceding the IRR in patients with follicular lymphoma in the GADOLIN study (n = 145). Lines inside boxes denote medians and boxes denote interquartile range; error bars are limits of 1.5 × interquartile range and circles show outliers.
Figure S5. Relationships between baseline tumour size and obinutuzumab exposure (Cmean) in patients with follicular lymphoma participating in GADOLIN. In the top panel, lines inside boxes denote medians and boxes denote interquartile interquartile range; error bars are limits of 1.5 × interquartile range and circles show outliers.
Figure S6. Relationships between best overall response and obinutuzumab exposure (Cmean) in patients with follicular lymphoma participating in GADOLIN who received at least 3 dosing cycles of obinutuzumab (n = 128). Lines inside the boxes denote medians, boxes denote interquartile range, error bars are limits of 1.5 × IQR, and circles show outliers.
ACKNOWLEDGEMENTS
Support for third‐party writing assistance under the directions of the lead authors was provided by Helen Cathro at Gardiner‐Caldwell Communications and was funded by F. Hoffmann‐La Roche Ltd.
This work was supported by F. Hoffmann‐La Roche Ltd.
Gibiansky E, Gibiansky L, Buchheit V, et al. Pharmacokinetics, exposure, efficacy and safety of obinutuzumab in rituximab‐refractory follicular lymphoma patients in the GADOLIN phase III study. Br J Clin Pharmacol. 2019;85:1935–1945. 10.1111/bcp.13974
Clinicaltrials.gov identifiers: NCT01010061, NCT01059630, NCT01414855, NCT000825149, NCT00517530, NCT00576758.
Data Availability Statement: Qualified researchers may request access to individual patient level data through the clinical study data request platform.52 Further details on Roche's criteria for eligible studies are available from this site.53 Further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available from the Roche website.54
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient level data through the clinical study data request platform.52 Further details on Roche's criteria for eligible studies are available from this site.53 Further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available from the Roche website.54
REFERENCES
- 1. Bai B, Huang HQ. Individualized management of follicular lymphoma. Chin Clin Oncol. 2015;4:7. [DOI] [PubMed] [Google Scholar]
- 2. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122(6):981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nowakowski GS, Ansell SM. Therapeutic targeting of microenvironment in follicular lymphoma. Hematology Am Soc Hematol Educ Program. 2014;2014:169–173. [DOI] [PubMed] [Google Scholar]
- 4. Suresh T, Lee LX, Joshi J, Barta SK. New antibody approaches to lymphoma therapy. J Hematol Oncol. 2014;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–1423. [DOI] [PubMed] [Google Scholar]
- 6. Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German low‐grade lymphoma study group. Blood. 2005;106(12):3725–3732. [DOI] [PubMed] [Google Scholar]
- 7. Herold M, Haas A, Srock S, et al. Rituximab added to first‐line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an east German study group hematology and oncology study. J Clin Oncol. 2007;25(15):1986–1992. [DOI] [PubMed] [Google Scholar]
- 8. Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. [DOI] [PubMed] [Google Scholar]
- 9. Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German low‐grade lymphoma study group. Blood. 2004;104(10):3064–3071. [DOI] [PubMed] [Google Scholar]
- 10. van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non‐Hodgkin's lymphoma: long‐term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28(17):2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solimando AG, Ribatti D, Vacca A, et al. Targeting B‐cell non Hodgkin lymphoma: new and old tricks. Leuk Res. 2016;42:93–104. [DOI] [PubMed] [Google Scholar]
- 12. Zappasodi R, de Braud F, Di Nicola M. Lymphoma immunotherapy: current status. Front Immunol. 2015;6:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118(2):358–367. [DOI] [PubMed] [Google Scholar]
- 14. Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti‐CD20 antibody with enhanced direct and immune effector cell‐mediated B‐cell cytotoxicity. Blood. 2010;115(22):4393–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein C, Lammens A, Schafer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2012;5(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salles GA, Morschhauser F, Solal‐Celigny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non‐Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–2926. [DOI] [PubMed] [Google Scholar]
- 17. Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti‐CD20 monoclonal antibody obinutuzumab (GA101) in B‐cell lymphoma patients. Blood. 2012;119(22):5126–5132. [DOI] [PubMed] [Google Scholar]
- 18. Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b‐cell lymphoma or mantle‐cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2912–2919. [DOI] [PubMed] [Google Scholar]
- 19. Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20‐positive B‐cell malignancies. Blood. 2012;119(22):5118–5125. [DOI] [PubMed] [Google Scholar]
- 20. Kamisoglu K, Phipps A, Jamois C, et al. Greater efficacy and potency of obinutuzumab compared with rituximab in chronic lymphocytic leukemia patients confirmed by a semi‐mechanistic pharmacokinetic/pharmacodynamic model. Blood. 2017;130(1):1267.28679739 [Google Scholar]
- 21. Gibiansky E, Gibiansky L, Carlile DJ, et al. Population pharmacokinetics of Obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non‐Hodgkin's lymphoma and exposure‐response in CLL. CPT Pharmacometrics Syst Pharmacol. 2014;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. [DOI] [PubMed] [Google Scholar]
- 23. Radford J, Davies A, Cartron G, et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122(7):1137–1143. [DOI] [PubMed] [Google Scholar]
- 24. Salles GA, Morschhauser F, Solal‐Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non‐Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–2926. [DOI] [PubMed] [Google Scholar]
- 25. Sehn LH, Goy A, Offner FC, et al. Randomized phase II trial comparing obinutuzumab (GA101) with rituximab in patients with relapsed CD20+ indolent B‐cell non‐Hodgkin lymphoma: final analysis of the GAUSS study. J Clin Oncol. 2015;33(30):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheson BD, Trnĕný M, Bouabdallah K, et al. Obinutuzumab plus bendamustine followed by obinutuzumab maintenance prolongs overall survival compared with bendamustine alone in patients with rituximab‐refractory indolent non‐Hodgkin lymphoma: updated results of the GADOLIN study. Blood. 2016;128(22):615.27288518 [Google Scholar]
- 27. Sharman JP, Forero‐Torres A, Costa LJ, et al. Obinutuzumab plus CHOP is effective and has a tolerable safety profile in previously untreated, advanced diffuse large B‐cell lymphoma: the phase II GATHER study. Leuk Lymphoma. 2019;60(4):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504. [DOI] [PubMed] [Google Scholar]
- 29. Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab‐refractory indolent non‐Hodgkin lymphoma (GADOLIN): a randomised, controlled, open‐label, multicentre phase 3 trial. Lancet Oncol. 2016;17(8):1081–1093. [DOI] [PubMed] [Google Scholar]
- 30. Cheson BD, Trask PC, Gribben JG, et al. Health‐related quality of life and symptoms in patients with rituximab‐refractory indolent non‐Hodgkin lymphoma treated in the phase III GADOLIN study with obinutuzumab plus bendamustine versus bendamustine alone. Ann Hematol. 2017;96(2):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gibiansky L, Gibiansky E, Bauer R. Comparison of Nonmem 7.2 estimation methods and parallel processing efficiency on a target‐mediated drug disposition model. J Pharmacokinet Pharmacodyn. 2012;39(1):17–35. [DOI] [PubMed] [Google Scholar]
- 32. Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28(2):171–192. [DOI] [PubMed] [Google Scholar]
- 33. Brendel K, Comets E, Laffont C, Laveille C, Mentré F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mentre F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed‐effects models. J Pharmacokinet Pharmacodyn. 2006;33(3):345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gibiansky E, Gibiansky L, Carlile DJ, Jamois C, Buchheit V, Frey N. Population pharmacokinetics of obinutuzumab (GA101) in chronic lymphocytic leukemia (CLL) and non‐Hodgkin's lymphoma and exposure–response in CLL. CPT Pharmacometrics Syst Pharmacol. 2014. Oct;3:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexander SPH, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol. 2017;174(Suppl 1):S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li J, Zhi J, Wenger M, et al. Population pharmacokinetics of rituximab in patients with chronic lymphocytic leukemia. J Clin Pharmacol. 2012;52(12):1918–1926. [DOI] [PubMed] [Google Scholar]
- 39. Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti‐CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45(7):792–801. [DOI] [PubMed] [Google Scholar]
- 40. Yin A, Li J, Hurst D, Visich J. Population pharmacokinetics (PK) and association of PK and clinical outcomes of rituximab in patients with non‐Hodgkin's lymphoma. J Clin Oncol. 2010;28(Suppl 15):e13108. [Google Scholar]
- 41. Lim SH, Vaughan AT, Ashton‐Key M, et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood. 2011;118(9):2530–2540. [DOI] [PubMed] [Google Scholar]
- 42. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507. [DOI] [PubMed] [Google Scholar]
- 43. Gibson CR, Sandu P, Hanley WD. Monoclonal antibody pharmacokinetics and pharmacodynamics In: An Z, ed. Therapeutic monoclonal antibodies: from bench to clinic. New Jersey (NJ): John Wiley & Son, Inc; 2009: pp 439–60. John Wiley & Son, Inc.: Hoboken, NJ. [Google Scholar]
- 44. Cartron G, Hourcade‐Potelleret F, Morschhauser F, et al. Rationale for optimal obinutuzumab/GA101 dosing regimen in B‐cell non‐Hodgkin lymphoma. Haematologica. 2016;101(2):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex‐related fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197(3):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tout M, Casasnovas O, Meignan M, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a Lymphoma Study Association report. Blood. 2017;129(19):2616–2623. [DOI] [PubMed] [Google Scholar]
- 48. Kaagedal M, Claret L, Marchard M, et al. Herceptin in HER2‐positive gastric cancer: evaluation of exposure‐response with two dose levels. Population Approach Group in Europe (PAGE) 2017; 26: Abstract 7329.
- 49. Solal‐Celigny P, Cahu X, Cartron G. Follicular lymphoma prognostic factors in the modern era: what is clinically meaningful? Int J Hematol. 2010;92(2):246–254. [DOI] [PubMed] [Google Scholar]
- 50. Solal‐Celigny P, Lepage E, Brousse N, et al. Doxorubicin‐containing regimen with or without interferon alfa‐2b for advanced follicular lymphomas: final analysis of survival and toxicity in the Groupe d'Etude des Lymphomes Folliculaires 86 trial. J Clin Oncol. 1998;16(7):2332–2338. [DOI] [PubMed] [Google Scholar]
- 51. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562. [DOI] [PubMed] [Google Scholar]
- 52. Clinical study data request. Available from: www.clinicalstudydatarequest.com. Accessed April 2019.
- 53. Clinical study data request: Roche sponsor specific details. Available from: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Roche.aspx. Accessed April 2019.
- 54. Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents. Available from: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm. Accessed April 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Studies of obinutuzumab included in the population pharmacokinetic analysis
Table S2. Covariates investigated in the population pharmacokinetic model
Table S3. Plots used for graphical evaluation of the model
Table S4. Summary statistics of continuous covariates (means and standard deviations)
Table S5. Parameter estimates for final covariate model
Table S6. Summary of conditional predictions for individual estimates of pharmacokinetic parameters by indolent non‐Hodgkin lymphoma subtype
Table S7. Patients and events from GADOLIN (study GAO4753g) in the graphical exposure–response analysis
Figure S1. Kaplan–Meier plots for progression‐free survival by exposure group and tumour size in patients with follicular lymphoma from GADOLIN who received at least 3 dosing cycles of obinutuzumab (n = 128).
Figure S2. Conditional simulations for the indolent non‐Hodgkin lymphoma dosing regimen. Medians (red), and 5th and 95th percentiles (blue) of the simulated concentrations are plotted. (A) Disease type; (B) indolent non‐Hodgkin lymphoma subtype; (C) baseline tumour size; (D) sex and body weight.
Figure S3. Predictive simulations in follicular lymphoma patients receiving the indolent non‐Hodgkin lymphoma dosing regimen. (A) Males and female patients with follicular lymphoma; (B) monotherapy with obinutuzumab; (C) obinutuzumab as monotherapy or co‐administered with CHOP, fludarabine/cyclophosphamide or bendamustine in follicular lymphoma.
Figure S4. Relationship between severity (maximum CTC‐AE grade) of infusion‐related reactions (IRRs) and predicted Cmax preceding the IRR in patients with follicular lymphoma in the GADOLIN study (n = 145). Lines inside boxes denote medians and boxes denote interquartile range; error bars are limits of 1.5 × interquartile range and circles show outliers.
Figure S5. Relationships between baseline tumour size and obinutuzumab exposure (Cmean) in patients with follicular lymphoma participating in GADOLIN. In the top panel, lines inside boxes denote medians and boxes denote interquartile interquartile range; error bars are limits of 1.5 × interquartile range and circles show outliers.
Figure S6. Relationships between best overall response and obinutuzumab exposure (Cmean) in patients with follicular lymphoma participating in GADOLIN who received at least 3 dosing cycles of obinutuzumab (n = 128). Lines inside the boxes denote medians, boxes denote interquartile range, error bars are limits of 1.5 × IQR, and circles show outliers.
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform.52 Further details on Roche's criteria for eligible studies are available from this site.53 Further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents are available from the Roche website.54


