Abstract
Purpose
This study aimed to evaluate the specific roles of estrogen receptor β (ERβ) on the invasion and migration of osteosarcoma (OS) cells and explore the regulatory mechanisms relating with Wnt signaling pathway.
Methods
The expression of ERβ was detected in human OS tissues by quantitative real-time PCR and immunohistochemistry. U2-OS cells were transfected with siRNA-ERβ (si-ERβ) to downregulate ERβ and treated with FH535 to inhibit Wnt signaling. The migration and invasion ability was detected by scratch and transwell assay, respectively. The expression of β-catenin, MMP-7, and MMP-9 was detected by Western blot. Subcutaneous tumor-bearing model was established by injection of U2-OS cells into mice, and the tumor volumes were measured. Orthotopic transplantation model was established by transplantation of tumor tissues into the liver of mice, and the metastatic tumors were counted.
Results
ERβ was downregulated in human OS tissues and U2-OS cells. The transfection of si-ERβ significantly increased the scratch healing rate; the number of invasion cells; and the expression of β-catenin, MMP-7, and MMP-9 in U2-OS cells. The injection of si-ERβ-transfected U2-OS cells into mice significantly increased the subcutaneous tumor volume; the expression of β-catenin, MMP-7, and MMP-9; and the number of metastatic tumors in liver tissues. The promoting effects of si-ERβ on the invasion and migration of U2-OS cells were significantly reversed by FH535 in vitro and in vivo.
Conclusion
Silencing of ERβ promotes the invasion and migration of OS cells via activating Wnt signaling pathway.
Keywords: estrogen receptor β, osteosarcoma, Wnt signaling pathway, invasion, migration
Introduction
Osteosarcoma (OS) is a common malignant bone tumor that usually develops in teenagers.1 It is estimated that the incidence of OS is 4 million/year worldwide, with a peak at the age of 15–19 years.2 The clinical outcomes of patients with metastatic OS are extremely poor.3 The 5-year survival of localized OS is about 65–70%, while the 5-year survival of metastatic OS is only <20%.4 The discovery of novel therapeutic targets against metastatic OS is urgently needed.
Estrogen receptor β (ERβ), also known as nuclear receptor subfamily 3 group A (NR3A2), is an important transcription factor that is involved in the occurrence and development of cancers.5 ERβ has been considered as a potential therapeutic target in cancers, which can significantly inhibit the proliferation of diverse cancer cell lines, such as colon cancer SW480 cells,6 breast cancer MCF-7 cells,7 prostate cancer DU145 cells,8 and OS U2-OS cells.9 Noteworthily, ERβ also exerts an obvious inhibitory role on the invasion and migration of cancer cells. It has been reported that ERβ1 significantly inhibits the migration and invasion of breast cancer cells, as well as the tumor formation in mice.10,11 ERβ1 inhibits the invasion and migration of breast cancer cells in vitro, and the invasion, dissemination, and micrometastasis of breast cancer cells in vivo.12 In addition, a previous study has proved that ERβ significantly promoted the migration and invasion of OS cells.9 However, the regulatory mechanisms of ERβ on OS are not fully revealed.
Wnt signaling pathway is a β-catenin-dependent extracellular pathway that is involved in a multitude of cellular processes, such as proliferation, apoptosis, differentiation, and migration.13 The inhibition of the Wnt signaling pathway has been proved to suppress the migration and invasion of OS cells by various studies. For example, the upregulation of naked cuticle homolog 2 (NKD2), a negative regulator of Wnt signaling pathway, decreases the migration and invasion ability of OS cells in vitro and inhibits the tumor metastasis in vivo.14 The transfection of β-catenin siRNA decreases the invasion ability of OS cells through downregulating membrane type-1 matrix metalloproteinase (MT1-MMP).15 In addition, a previous study has proved that Erb-041, an ERβ agonist, inhibits skin photocarcinogenesis in mice through downregulating Wnt signaling pathway.16 However, the specific regulatory relationship between ERβ and Wnt signaling pathway on OS is still unclear.
In this study, ERβ was silenced by siRNA-ERβ (si-ERβ). The specific roles of si-ERβ on the migration and invasion of U2-OS cells were evaluated in both vitro and vivo. In addition, the regulatory mechanisms of ERβ relating with the Wnt signaling pathway were investigated. Our findings may provide a novel therapeutic target and a new insight into the underlying mechanisms for the treatment of metastatic OS.
Materials and methods
Clinical specimens
A total of 24 patients (11 male and 13 female; 14–51 years old) histologically diagnosed as OS (14 distal femur and 10 proximal tibia) were screened from our hospital between January 2016 and January 2018. Paraffin-embedded OS tissues and adjacent normal tissues were collected from these patients prior to administering any treatment.
This study was conducted after obtaining local ethical committee approval of Basic Medical College of Jiujiang University.
Written informed consent was obtained from patients over the age of 18 years and parents of patients under the age of 18 years. This was conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry (IHC)
Paraffin-embedded tissues were sliced at 5 μm, dewaxed in xylene, dehydrated with graded ethanol, incubated in 0.3% H2O2 for 15 mins, and incubated in 10 mM EDTA for 15 mins under microwave irradiation. The sections were blocked with 10% BSA for 30 mins and incubated with primary antibody (anti-ERβ, 1:100, Cell Signaling, Danvers, MA, USA) for 3 hrs at 37°C. Then, the sections were washed with PBS for 5 times and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000, Cell Signaling) for 1 hr at 37°C. Followed by staining with diaminobenzidine (DAB), dehydration with graded ethanol, and vitrification with dimethyl benzene, positive stained cells (yellow-brown or brown) were observed under a microscope (Olympus, Japan) and counted in five randomly selected fields.
Cell culture and treatments
Human OS cell line U2-OS and human osteoblast cell line hFOB1.19 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and preserved in our laboratory. Cells were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin. Cells were maintained in a humidified incubator at 37°C with 5% CO2.
U2-OS cells in logarithmic growth phase were randomly divided into four groups: si-ERβ, U2-OS cells transfected with si-ERβ (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; si-ERβ + FH535, U2-OS cells transfected with siRNA-ERβ and treated with 20 μmol/L FH535 (an inhibitor of Wnt signaling) (Sigma, St. Louis, MO, USA) for 48 hrs; blank, U2-OS cells without transfection and treatment. Cell transfection was performed by using lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction.
Quantitative real-time PCR (qRT-PCR)
Total RNAs were extracted from specific tissues, and cells by using RNApreppure tissue kit (TIANGEN, Beijing, China), and RNApreppure cell kit (TIANGEN), respectively. cDNA was synthesized by using PrimeScript RT reagent kit (Takara, Dalian, China). The special primers were used as followed: ERβ-F, 5′-GCCGCCCCATGTGCTGAT-3′; ERβ-R, 5′-GGACCCCGTGATGGAGGACTT-3′; β-catenin-F, 5′-TGAGGACAAGCCACAAGATTAC-3′; β-catenin-R, 5′- TCCACCAGAGTGAAAAGAACG-3′. GAPDH was used as an internal control (GAPDH-F, 5ʹ-GAGTCAACGGATTTGGTCGT-3ʹ; GAPDH-R: 5ʹ- TTGATTTTGGAGGGATCTC-3ʹ). The PCR program included 95°C for 10 mins, 40 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 34 s. The relative expression levels of target genes were calculated using the 2−ΔΔCt method.17
Western blot
Cells of different groups were lysed in RIPA lysis buffer (Thermo Fisher Scientific). Total proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% skim milk in Tris Buffered Saline Tween (TBST) for 1 hr and incubated with primary antibody (anti-ERβ, -MMP-7, -MMP-9, and -β-catenin; 1:100, Cell Signaling) overnight at 4°C. After washing with TBST for three times, the membrane was incubated with HRP-conjugated secondary antibody (1:1000, Abcam, Cambridge, England) for 2 hrs at 25°C. The protein bands were visualized and quantified using a Gel Imaging System (Thermo Fisher Scientific).
Immunofluorescence
Cells of different groups were fixed in 4% paraformaldehyde for 20 mins at 4°C and permeated in 0.1% Triton X-100 (MP Biomedicals, Houston, TX, USA) for 5 mins. Then, cells were blocked with 5% BSA for 30 mins and incubated with primary antibody (anti-β-catenin, 1:100, Abcam) overnight at 4°C. After washed with PBS for 5 times, cells were incubated with Alexa Fluor 488-conjugated secondary antibody (1:500, Abcam) for 1 hr at 37°C. Followed by staining with DAPI (4,6-diamino-2-phenylindole), positive stained cells (green fluorescence) were observed under a fluorescence microscope (Olympus).
Scratch assay
Cells of different groups were seeded at a density of 0.5×106/well in 6-well plates and cultured overnight (more than 90% confluence). A wound track at approximately 5 mm was scored in each dish with a pipette head, and the debris was removed by 3 times of washing with PBS. After 48 hrs of culturing, the scratch healing state was observed under a microscope (Olympus).
Transwell assay
Transwell assay was performed by using transwell chambers (Corning, Corning, NY, USA). Cells of different groups were seeded at a density of 0.1×105/µL in the upper chamber (precoated with Matrigel). A total of 600 µL DMEM containing 100 ng/mL stromal cell-derived factor-1 (SDF-1) were placed in the lower chamber. After incubation at 37°C for 24 hrs, cells on the upper chamber were removed with cotton swabs. Cells on the lower chamber were fixed in formaldehyde for 30 mins and stained with 0.1% crystal violet for 20 mins. Positive stained cells were observed under a microscope (Olympus).
Establishment of subcutaneous tumor-bearing model and orthotopic transplantation model in mice
Four-week-old specific pathogen-free (SPF) mice (male, 20–25 g) were purchased from the Medical College of Shanghai Jiaotong University (Shanghai, China). Mice were feeding at 25°C and 50% humidity with free access to water and food. A total of 100 μL U2-OS cells in different groups (si-ERβ, NC-ERβ, si-ERβ + FH535, and blank) were subcutaneously injected into the posterior limb of each mouse at a density of 0.1×108 cells/mL (subcutaneous tumor-bearing model). Mice were killed by cervical dislocation, and the tumor volumes were measured by vernier caliper every 5 days. After the injection for 20 days, small pieces of tumor tissues were transplanted into the liver of healthy mice (orthotopic transplantation model). Five weeks later, the metastatic tumors in liver tissues were counted and observed by HE staining. All animal experiments were approved by the local Institutional Review Board.
HE staining
The liver tissues of mice were fixed in 10% formaldehyde and embedded in paraffin. The tissue sections at 5 μm were dewaxed in xylene, rehydrated in graded ethanol, and stained with HE for 5 mins (Beyotime, Shanghai, China) for 2 mins. After dehydration with graded ethanol and vitrificated with dimethylbenzene, the tissues were observed under a microscope (Olympus).
Statistical analyses
All experiments were performed in triplicate, and all data were expressed as mean ± SD. Statistical analysis was performed by SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Comparison between different groups was determined by Student’s t-test (two groups) and one-way ANOVA (more than two groups). A P-value <0.05 was considered to be significantly different.
This study was conducted after obtaining Basic Medical College of Jiujiang University’s Ethics Committee. Basic Medical College of Jiujiang University’s Ethics Committee granted ethical and legal approval for the involvement of animals in this study.
Results
ERβ was downregulated in human OS tissues
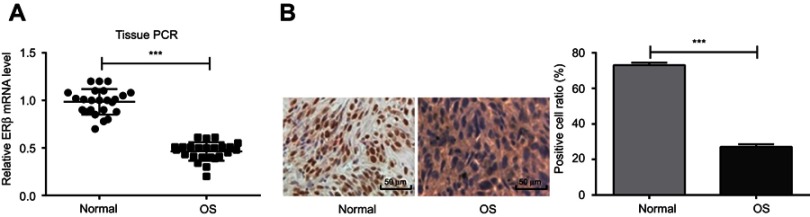
The expression of ERβ was detected in OS tissues of 24 OS patients. qRT-PCR showed that the expression of ERβ was significantly lower in OS tissues than in adjacent normal tissues (P<0.001) (Figure 1A). In addition, IHC showed that the positive cell rate was significantly lower in OS tissues than in adjacent normal tissues (27.1±1.49% vs 73.0±1.40%, P<0.001) (Figure 1B)
Figure 1.
The expression of ERβ in OS and adjacent normal tissues of 24 OS patients detected by qRT-PCR and IHC. (A) Relative expression of ERβ at mRNA level (qRT-PCR); (B) positive stained cells (Bar =50 μm, ×200). ***P<0.001.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; qRT-PCR, quantitative real-time PCR; IHC, immunohistochemistry.
ERβ was downregulated in U2-OS cells
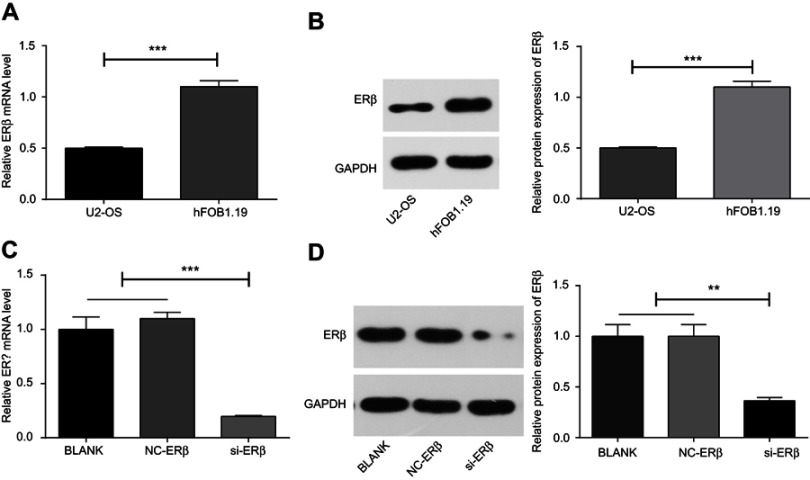
The expression of ERβ was detected in U2-OS and hFOB1.1 cells. qRT-PCR and Western blot showed that the expression of ERβ was significantly lower in U2-OS cells than in hFOB1.1 cells at both mRNA and protein level (P<0.001) (Figure 2A and B). Then, si-ERβ was used to silence ERβ in U2-OS cells. As shown in Figure 2C and D, the expression of ERβ in U2-OS cells was significantly inhibited by si-ERβ at both mRNA and protein level (P<0.01). No significant difference on the expression of ERβ was observed between blank and NC-ERβ group (Figure 2C and D).
Figure 2.
The expression of ERβ in U2-OS and hFOB1.1 cells detected by qRT-PCR and Western blot. (A and C) Relative expression of ERβ at mRNA level (qRT-PCR); (B and D) relative expression of ERβ at protein level (Western blot). si-ERβ, U2-OS cells transfected with siRNA-ERβ for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; blank, U2-OS cells without transfection. **P<0.01; ***P<0.001.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; qRT-PCR, quantitative real-time PCR; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NC, negative control; si, small interfering RNA.
si-ERβ transfection activated Wnt signaling pathway
In order to evaluate the regulatory relationship between ERβ and the Wnt signaling pathway, the expression of β-catenin was detected. Western blot showed that the expression of β-catenin was significantly higher in U2-OS cells than in hFOB1.1 cells at the protein level (P<0.01) (Figure 3A). U2-OS cells in si-ERβ group exhibited significantly higher expression of β-catenin than those in NC- ERβ and blank group (P<0.001). However, the expression of β-catenin in si-ERβ-transfected U2-OS cells was significantly inhibited by the intervention of FH535 (P<0.01) (Figure 3B). In addition, immunofluorescence showed that si-ERβ transfection significantly increased the fluorescence intensity of β-catenin in U2-OS cells and promoted the nuclear aggregation of β-catenin. The intervention of FH535 significantly decreased the fluorescence intensity of β-catenin in si-ERβ-transfected U2-OS cells and inhibited the nuclear aggregation of β-catenin (Figure 3C).
Figure 3.
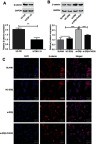
The expression of β-catenin in U2-OS and hFOB1.1 cells detected by Western blot and Immunofluorescence. (A and B) Relative expression of β-catenin at protein level (Western blot); (C) positive stained cells (bar =100 μm, ×100). si-ERβ, U2-OS cells transfected with siRNA-ERβ for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; si-ERβ + FH535, U2-OS cells transfected with siRNA-ERβ and treated with 20 μmol/L FH535 for 48 hrs; blank, U2-OS cells without transfection and treatment. **P<0.01; ***P<0.001.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DAPI, 4',6-diamidino-2-phenylindole; NC, negative control; si, small interfering RNA.
si-ERβ transfection promoted the migration of U2-OS cells
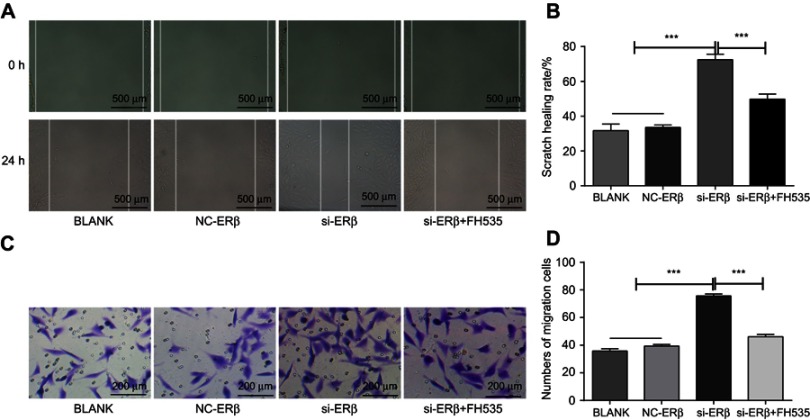
The effect of si-ERβ on the migration of U2-OS cells was evaluated. Scratch assay showed that U2-OS cells in si-ERβ group exhibited significantly higher scratch healing rate than those in NC-ERβ and blank group (72.47±3.19% vs 33.56±3.42% and 31.47±3.19, P<0.001) (Figure 4A and B). Transwell assay showed that the number of migration cells was significantly higher in the si-ERβ group than in NC-ERβ and blank group (75.48±2.89 vs 37.69±2.25% and 38.89±2.06, P<0.001) (Figure 4C and D). The intervention of FH535 significantly decreased the scratch healing rate (32.47±2.19 vs 72.47±3.19%, P<0.001) and the number of migration cells (42.45±2.46 vs 75.48±2.89%, P<0.001) in si-ERβ-transfected U2-OS cells (Figure 4A–D).
Figure 4.
The migration of U2-OS cells detected by Scratch assay. (A) scratch healing under microscope (Bar =500 μm, ×4); (B) scratch healing rate; (C) migration cells under microscope (bar =200 μm, ×20); (D) number of migration cells. si-ERβ, U2-OS cells transfected with siRNA-ERβ for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; si-ERβ + FH535, U2-OS cells transfected with siRNA-ERβ and treated with 20 μmol/L FH535 for 48 hrs; Blank, U2-OS cells without transfection and treatment. ***P<0.001.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; NC, negative control; si, small interfering RNA; h, hours.
si-ERβ transfection promoted the invasion of U2-OS cells
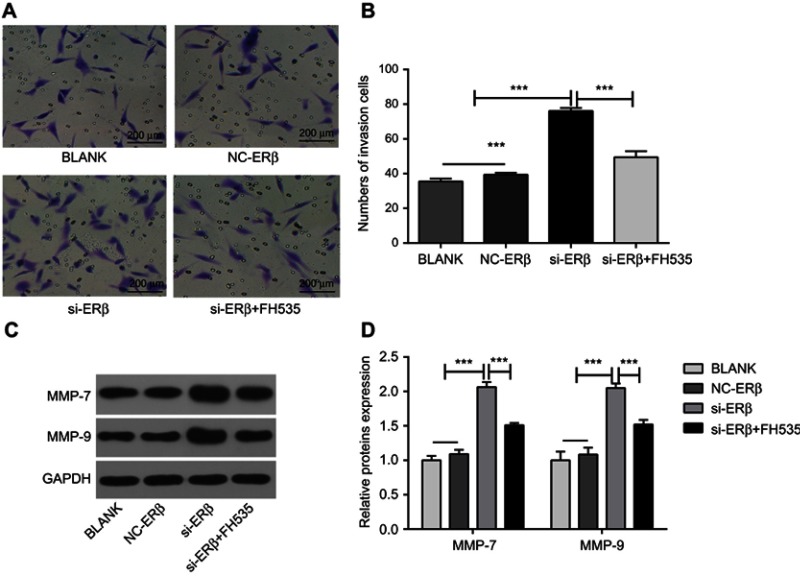
The effect of si-ERβ on the invasion of U2-OS cells was evaluated. Transwell assay showed that the number of invasion cells was significantly higher in the si-ERβ group than in NC-ERβ and blank group (76.11±2.97% vs 39.41±1.89% and 35.51±3.84, P<0.001). The intervention of FH535 significantly decreased the number of invasion cells in si-ERβ-transfected U2-OS cells (36.11±2.97 vs 76.11±2.97%, P<0.001) (Figure 5A and B). In addition, Western blot showed that the expression of MMP-7 and MMP-9 in U2-OS cells was significantly higher in the si-ERβ group than in NC-ERβ and blank group (P<0.001). The intervention of FH535 significantly decreased the expression of MMP-7 and MMP-9 in si-ERβ-transfected U2-OS cells (P<0.001) (Figure 5C and D).
Figure 5.
The invasion of U2-OS cells detected by Transwell assay and Western blot. (A) Invasion cells under a microscope (bar =200 μm, ×20); (B) the number of invasion cells; (C) protein brands of Western blot; (D) relative expression of MMP-7 and MMP-9 at protein level (Western blot). si-ERβ, U2-OS cells transfected with siRNA-ERβ for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; si-ERβ + FH535, U2-OS cells transfected with siRNA-ERβ and treated with 20 μmol/L FH535 for 48 hrs; blank, U2-OS cells without transfection and treatment. ***P<0.001.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP, matrix metalloproteinase; NC, negative control; si, small interfering RNA.
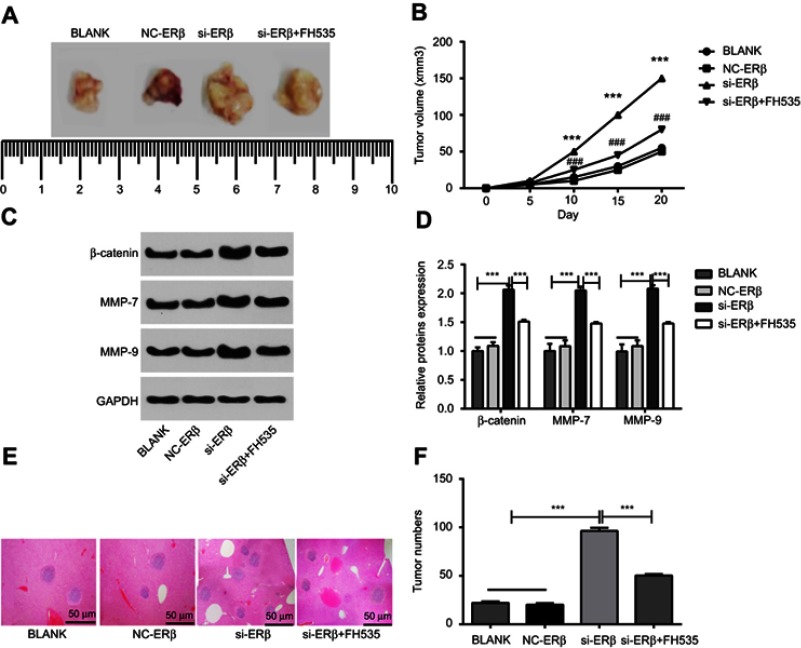
si-ERβ transfection promoted the growth and metastasis of OS tumors in mice
In order to further evaluate the promigratory and proinvasive effects of si-ERβ in vivo, subcutaneous tumor-bearing model was established in mice. As shown in Figure 6A and B, the tumor volumes in mice of different groups were all significantly increased in a time-dependent manner. The tumor volumes were significantly higher in the si-ERβ group than in NC-ERβ and blank group, beginning from the 10th day (P<0.001). The intervention of FH535 significantly decreased the tumor volumes in mice injected with si-ERβ-transfected U2-OS cells (P<0.001) (Figure 6A and B). In addition, Western blot showed that the expression of β-catenin, MMP-7, and MMP-9 in tumor tissues was significantly higher in the si-ERβ group than in the NC-ERβ and blank groups (P<0.001). The intervention of FH535 significantly decreased the expression of β-catenin, MMP-7, and MMP-9 in tumor tissues of mice injected with si-ERβ-transfected U2-OS cells (P<0.001) (Figure 6C and D). Furthermore, the metastatic ability of si-ERβ-transfected U2-OS cells was evaluated in a mouse model of orthotopic transplantation. As shown in Figure 6E and F, more metastatic tumors were observed in the liver tissues of mice in the si-ERβ group than in the NC-ERβ and blank group (P<0.001). The intervention of FH535 significantly decreased the number of metastatic tumors in the liver tissue of mice injected with si-ERβ-transfected U2-OS cells (P<0.001) (Figure 6E and F).
Figure 6.
The growth and metastasis of OS tumors in mice. (A) Subcutaneous tumors under naked eye; (B) subcutaneous tumor volumes at different time points; (C) protein brands of Western blot; (D) relative expression of β-catenin, MMP-7 and MMP-9 at protein level (Western blot); (E) metastatic tumors in the liver tissues of mice under microscope (HE staining) (bar =50 μm, ×40); (F) the number of metastatic tumors. si-ERβ, U2-OS cells transfected with siRNA-ERβ for 48 hrs; NC-ERβ, U2-OS cells transfected with siRNA-negative control-ERβ for 48 hrs; si-ERβ + FH535, U2-OS cells transfected with siRNA-ERβ and treated with 20 μmol/L FH535 for 48 hrs; blank, U2-OS cells without transfection and treatment. ***P<0.001 vs NC-ERβ and blank; ###P<0.001 vs si-ERβ.
Abbreviations: ERβ, estrogen receptor β; OS, ostemsarcoma; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP, matrix metalloproteinase; HE, hematoxylin-eosin; NC, negative control; si, small interfering RNA.
Discussion
ERβ is an estrogen-regulated transcription factor that plays a critical role in the progression of cancers.18 A large number of previous studies have proved that ERβ is downregulated in diverse cancers, such as breast cancer,19 ovarian cancer,20 prostatic cancer,21 and colon cancer.22 In this study, the expression of ERβ was detected in both human OS tissues and U2-OS cells. We found that the expression of ERβ was significantly lower in OS tissues than in adjacent normal tissues and significantly lower in U2-OS cells than in hFOB1.1 cells. Our findings are just consistent with previous studies and illustrate that ERβ is downregulated in OS.
The inhibition of cell invasion and migration is an important antitumor manifestation of ERβ on cancers.23,24 In this study, the invasion and migration abilities of si-ERβ-transfected U2-OS cells were evaluated. We found that the transfection of si-ERβ into U2-OS cells significantly increased the scratch healing rate and the number of invasion cells. These findings are just consistent with a previous study that the knockdown of ERβ significantly increases the migration and invasion abilities of U2-OS cells.9 In order to further identify the promigratory and proinvasive effects of si-ERβ in vivo, subcutaneous tumor-bearing model and orthotopic transplantation model were established in mice. We found that the injection of si-ERβ-transfected U2-OS cells significantly increased the subcutaneous tumor volume and the number of metastatic tumors in liver tissues of mice. These phenomena indicate that si-ERβ promotes the growth and metastasis of OS tumors in vivo, which are consistent with previous studies on animal models of breast cancer. It has been reported that exogenous ERβ expression significantly inhibits the growth of MCF-7 tumor xenografts in mice, and tumors are only observed in 2/6 mice injected with MCF-7-ERβ.25 MDA-MB-231 cells are disseminated away from the injection site of zebrafish at 5th day postinjection, while ERβ1-expressing MD-MB-231 cells remain at the primary site.12 In addition, we also found that si-ERβ significantly increased the expression of MMP-7 and MMP-9 in U2-OS cells and OS tumors of mice. Since MMP-7 and MMP-9 are positively associated with tumor metastasis,26,27 the upregulated MMP-7 and MMP-9 contribute to the promoting effects of si-ERβ on the invasion and migration of U2-OS cells in vitro and on the growth and metastasis of OS tumors in vivo.
The antitumor mechanisms of ERβ are complex, which related to diverse regulatory factors, such as E-cadherin,10 EGFR,28 transforming growth factor β (TGFβ),29 p53-upregulated modulator of apoptosis,30 nuclear factor-kB/B-cell lymphoma-2 (NF-kB/BCL-2), and phosphatidylinositol-3 kinase/Akt (PI3K/Akt).9 In this study, the regulatory relationship between ERβ and Wnt signaling pathway was evaluated on U2-OS cells. We found that the si-ERβ significantly upregulated β-catenin in U2-OS cells, which indicates that the downregulation of ERβ activates the Wnt signaling pathway in U2-OS cells. The activation of Wnt signaling pathway contributes to promoting the invasion and migration of OS cells. Previous studies have proved that the upregulation of NKD2 (a negative regulator of Wnt signaling) and the transfection of β-catenin siRNA can both decrease the migration and invasion abilities of OS cells through inhibiting Wnt signaling pathway.14,15 Therefore, we suspect that si-ERβ may promote the invasion and migration of U2-OS cells through activating Wnt signaling pathway. This hypothesis is further confirmed by the intervention of FH535. We found that the intervention of FH535 significantly decreased the scratch healing rate, the number of invasion cells, and the expression of MMP-7 and MMP-9 in U2-OS cells transfected with si-ERβ. In addition, β-catenin was also upregulated in OS tumors of mice injected with si-ERβ-transfected U2-OS cells. The intervention of FH535 in mice injected with si-ERβ-transfected U2-OS cells significantly decreased the subcutaneous tumor volume; the expression of β-catenin, MMP-7, and MMP-9; as well as the number of metastatic tumors in liver tissues. These results further illustrate that the promoting effects of si-ERβ on the growth and metastasis of OS tumors in mice may attribute to the activation of the Wnt signaling pathway.
Conclusion
In conclusion, si-ERβ significantly promoted the invasion and migration of U2-OS cells in vitro and the growth and metastasis of OS tumors in vivo. The promoting effects of si-ERβ on OS metastasis were closely related with the activation of the Wnt signaling pathway. ERβ might be a potential therapeutic target for metastatic OS. However, this study is limited in si-ERβ. Further researches on the specific roles and regulatory mechanisms of ERβ overexpression on OS metastasis are still needed.
Acknowledgment
This study was funded by National Nature Science Foundation of China (No. 81360364): Antitumor role and mechanisms of liquiritigenin-mediated ERβ isoform in the hypoxia microenvironment of hepatocellular carcinoma.
Ethics approval and consent to participate
This study was conducted after obtaining local ethical committee approval of Basic Medical College of Jiujiang University. Written informed consent was obtained from patients over the age of 18 years and parents of patients under the age of 18 years. This was conducted in accordance with the Declaration of Helsinki. All animal experiments were conducted after obtaining Basic Medical College of Jiujiang University’s Ethics Committee. Basic Medical College of Jiujiang University’s Ethics Committee granted ethical and legal approval for the involvement of animals in this study.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jackson TM, Bittman M, Granowetter L. Pediatric malignant bone tumors: a review and update on current challenges, and emerging drug targets. Curr Prob Pediatr Adolesc Health Care. 2016;46:213–228. doi: 10.1016/j.cppeds.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L, Troisi R. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2010;115:1531–1543. doi: 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome–the French pediatric experience. Cancer. 2010;104:1100–1109. doi: 10.1002/cncr.21263 [DOI] [PubMed] [Google Scholar]
- 4.Siegel HJ, Pressey JG. Current concepts on the surgical and medical management of osteosarcoma. Expert Rev Anticancer Ther. 2014;8:1257. doi: 10.1586/14737140.8.8.1257 [DOI] [PubMed] [Google Scholar]
- 5.Guillette TC, Jackson TW, Belcher SM. Duality of estrogen receptor β action in cancer progression. Curr Opin Pharmacol. 2018;41:66–73. doi: 10.1016/j.coph.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johan H, Karin E, Karolina L, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009;69:6100–6106. doi: 10.1158/0008-5472.CAN-09-0506 [DOI] [PubMed] [Google Scholar]
- 7.Sreenivasan P, Hema P, Vaishali K, et al. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423. doi: 10.1158/0008-5472.CAN-03-2446 [DOI] [PubMed] [Google Scholar]
- 8.Shan Y, Wang X, Chan FL. Overexpression of estrogen receptor-related receptor β (ERRβ) suppresses the cell proliferation and tumor growth of androgen-dependent and -independent prostate cancer cells via induction of p21Cip1/Waf1. Cancer Res. 2006;66:779. [Google Scholar]
- 9.Yang M, Bing L, Jin L, et al. Estrogen receptor β exhibited anti-tumor effects on osteosarcoma cells by regulating integrin, IAP, NF-kB/BCL-2 and PI3K/Akt signal pathway. J Bone Oncol. 2017;9:S2212137417300933. doi: 10.1016/j.jbo.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Ming J, Xu Y, et al. ERβ1 inhibits the migration and invasion of breast cancer cells through upregulation of E-cadherin in a Id1-dependent manner. Biochem Biophys Res Commun. 2015;457:141–147. doi: 10.1016/j.bbrc.2014.12.038 [DOI] [PubMed] [Google Scholar]
- 11.Li H, Tu Z, An L, et al. Inhibitory effects of ERβ on proliferation, invasion, and tumor formation of MCF-7 breast cancer cells–prognostication for the use of ERβ-selective therapy. Pharm Biol. 2012;50:839–849. doi: 10.3109/13880209.2011.637506 [DOI] [PubMed] [Google Scholar]
- 12.Thomas C, Rajapaksa G, Nikolos F, et al. ERβ1 represses basal-like breast cancer epithelial to mesenchymal transition by destabilizing EGFR. Breast Cancer Res. 2012;14:R148. doi: 10.1186/bcr3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985. doi: 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Kurenbekova L, Gao Y, et al. NKD2, a negative regulator of Wnt signaling, suppresses tumor growth and metastasis in osteosarcoma. Oncogene. 2015;34:5069. doi: 10.1038/onc.2014.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Z, Anmin C, Jianfeng C, et al. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac J Cancer Prev. 2011;12:239–245. [PubMed] [Google Scholar]
- 16.Chaudhary SC, Tripti S, Talwelkar SS, et al. Erb-041, an estrogen receptor-β agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by downregulating the WNT signaling pathway. Cancer Prev Res. 2014;7:186. doi: 10.1158/1940-6207.CAPR-13-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))Method. METHODS. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 18.Warner M, Huang B, Gustafsson JA. Estrogen receptor β as a pharmaceutical target. Trends Pharmacol Sci. 2017;38:92. doi: 10.1016/j.tips.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 19.Alnakhle H, Burns PA, Cummings M, et al. Estrogen receptor β1 expression is regulated by miR-92 in breast cancer. Cancer Res. 2010;70:4778–4784. doi: 10.1158/0008-5472.CAN-09-4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazennec G. Estrogen receptor beta, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett. 2006;231:151–157. doi: 10.1016/j.canlet.2005.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daniels G, Gellert LL, Melamed J, et al. Decreased expression of stromal estrogen receptor α and β in prostate cancer. Am J Transl Res. 2014;6:140–146. [PMC free article] [PubMed] [Google Scholar]
- 22.Campbellthompson M, Lynch IJ, Bhardwaj B. Expression of Estrogen Receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 23.Thomas C, Rajapaksa G, Nikolos F, et al. Abstract 1384: Estrogen receptor beta inhibits migration and invasion of breast cancer cells by regulating E-cadherin levels. Cancer Res. 2011;71:1384. [Google Scholar]
- 24.Tu Z, Ma Y, Tian J, et al. Estrogen receptor β potentiates the antiproliferative effect of raloxifene and affects the cell migration and invasion in HCT-116 colon cancer cells. J Cancer Res Clin Oncol. 2012;138:1091–1103. doi: 10.1007/s00432-011-1145-3 [DOI] [PubMed] [Google Scholar]
- 25.Hui L, Zhenzhen T, Lianxiao A, et al. Inhibitory effects of ERβ on proliferation, invasion, and tumor formation of MCF-7 breast cancer cells–prognostication for the use of ERβ-selective therapy. Pharm Biol. 2012;50:839–849. doi: 10.3109/13880209.2011.637506 [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Pyun JA, Lee KJ, et al. Study on association between single nucleotide polymorphisms of MMP7, MMP8, MMP9 genes and development of gastric cancer and lymph node metastasis. Korean J Gastroenterol. 2011;58:245. doi: 10.4166/kjg.2011.58.5.245 [DOI] [PubMed] [Google Scholar]
- 27.Hsu TI, Lin SC, Lu PS, et al. MMP7-mediated cleavage of nucleolin at Asp255 induces MMP9 expression to promote tumor malignancy. Oncogene. 2015;34:826. doi: 10.1038/onc.2014.462 [DOI] [PubMed] [Google Scholar]
- 28.Pinton G, Thomas W, Bellini P, et al. Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. Plos One 2010;5:e14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao C, Dahlmanwright K, Gustafsson JA. Estrogen receptor β: an overview and update. Nucl Recept Signal. 2008;4:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey P, Ström A, Gustafsson JÅ. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene. 2013;33:4213–4225. doi: 10.1038/onc.2013.384 [DOI] [PubMed] [Google Scholar]