Abstract
Chronic stress increases the probability of receiving an anxiety, depression, or chronic illness diagnosis. Pharmacological interventions that reduce the behavioral and physiological effects of chronic stress in animal models may represent novel approaches for the treatment of stress-related psychiatric disorders. Here, we examined the effects of cyclooxygenase-2 (COX-2) inhibition on anxiety-like behaviors and amygdala glutamatergic signaling after chronic non-invasive oral corticosterone (CORT) administration in mice. Treatment with the highly selective COX-2 inhibitor Lumiracoxib (LMX) reversed anxiety-like behavior induced by chronic CORT. Specifically, acute and repeated administration of LMX 5 mg kg−1 reduced chronic CORT-induced anxiety-like behavior measured using the elevated-plus maze, elevated-zero maze, and light-dark box tests. In contrast, LMX did not affect anxiety-like behaviors in naïve mice. Ex vivo electrophysiology studies revealed that repeated LMX treatment normalized chronic CORT-induced increases in spontaneous excitatory glutamatergic currents recorded from anterior, but not posterior, basolateral amygdala neurons. These data indicate COX-2 inhibition can reverse chronic CORT-induced increases in anxiety-like behaviors and amygdala glutamatergic signaling, and support further clinical investigation of selective COX-2 inhibitors for the treatment of affective and stress-related psychiatric disorders.
Keywords: Cyclooxygenase-2, Stress, Anxiety, Basolateral amygdala
1. Introduction
Chronic stress is detrimental to long-term health and increases the clinical diagnostic probability of anxiety disorders, depression, and debilitating physiological illness (McEwen, 2004; Alevizos et al., 2014). The International Classification of Diseases, 11th revision (ICD-11) now includes five official stress disorder diagnoses, which exhibit a 13–25% comorbidity with depression, anxiety, and substance use disorders (Gradus, 2017). Selective serotonin reuptake inhibitors (SSRIs) represent the first-line treatments for affective and stress-related disorders, yet many estimates indicate that nearly one-third of patients with mood disorders do not adequately respond to currently available monoamine-based treatments (Artigas et al., 2018; Rush et al., 2006). These data clearly identify an unmet need for additional therapeutic targets to treat affective and stress-related disorders. In addition to monoamine-based molecular targets, recent studies have begun to elucidate immunological and inflammatory molecular signaling systems as potential novel targets for therapeutics development (Muller, 2017; Liu et al., 2017).
In the CNS, COX-2 generates pro-inflammatory prostaglandins (PGs) from free arachidonic acid (AA) (Breder et al., 1995) and also metabolizes the endocannabinoids (eCBs) anandamide (AEA) and 2-Arachidonoylglycerol (2-AG) into prostaglandin ethanolamides (PG-EAs; prostamides) (Kozak et al., 2002), and PG-glycerol esters (PGGs) (Morgan et al., 2018). Basal expression of COX-2 in the brain is limited to stress-processing regions, including the basolateral amygdala (BLA) (Breder et al., 1995), where it is rapidly up-regulated by stress and the stress hormone corticosterone (CORT) (Serrats et al., 2017; Madrigal et al., 2003a). These data suggest elevated COX-2 activity may contribute to the pathophysiology of some affective and stress-related psychiatric disorders. Indeed, peripheral COX-2 expression is increased in some patients with major depression (Galecki et al., 2012, 2014). Importantly, pharmacological inhibition of COX-2 has shown promise in preclinical animal models of anxiety-like and depressive-like behaviors (Hermanson et al., 2013; Gamble-George et al., 2016; Wang et al., 2017; Myint et al., 2007; Guo et al., 2009), and in clinical trials, when used as an augmentation strategy with SSRI treatment in patients with major depression (Akhondzadeh et al., 2009; Faridhosseini et al., 2014). These findings suggest COX-2 inhibition may represent a novel approach for the treatment of affective disorders.
We and others have shown that pharmacological inhibition of COX-2 can reduce some acute stress-induced anxiety-like behaviors in mice (Madrigal et al., 2003a; Gamble-George et al., 2016; Kumari et al., 2007). For example, mice treated with a COX-2 inhibitor after foot-shock stress showed reduced anxiety-like behavior in the novelty-induced hypophagia test (NIH) and elevated-plus maze (Gamble-George et al., 2016). Other reports also indicate that COX-2 inhibition after immobilization stress can improve hypoactivity and memory retention (Kumari et al., 2007), and reduce anxiety in a mirror chamber test (Dhir et al., 2006). Treatment with COX inhibitors can reduce depressive-like behavior after chronic unpredictable stress (Guo et al., 2009) and improve mobility and memory in a model of chronic fatigue (Kumar et al., 2010). Despite these studies, the effects of COX-2 inhibition in long-term models of anxiety-like and depressive-like behaviors is limited, and additional investigation is critical for rigorous preclinical target validation. Moreover, the neurobiological mechanisms by which COX-2 inhibition reduces anxiety are poorly understood, suggesting further research in this area is needed.
Chronic stress and mood and anxiety disorders are closely linked. This association is partially mediated by elevated levels of glucocorticoids, reflecting an end-point of HPA-axis activation (Brown, 2009; Tata and Anderson, 2010). This effect is seen in humans (Van Uum et al., 2008; Meyer and Novak, 2012) as well as in rodent models (Goshen et al., 2008; Shanks et al., 1990), and forms the theoretical basis for using repeated exogenous CORT administration to model chronic stress-related behavioral and physiological phenotypes (David et al., 2009; Waters and McCormick, 2011). Chronic CORT exposure produces anxiety and depressive-like behaviors in rodents (Sterner and Kalynchuk, 2010), many of which are reversed by monoaminergic antidepressants, supporting the predictive validity of this model (Gulbins et al., 2013).
Here we examine the effects of COX-2 inhibition on CORT-induced anxiety-like behaviors in mice using the highly selective COX-2 inhibitor Lumiracoxib (LMX). LMX is highly selective for COX-2, having a 400-fold greater selectivity for COX-2 over COX-1 in vivo (Tannenbaum, 2004). Using well-validated tests of anxiety-like behavior in mice (Kulesskaya and Voikar, 2014; Lister, 1987; Kulkarni et al., 2007), we show that both acute and repeated LMX reduces some of the anxiogenic effects induced by chronic CORT administration. The anxiolytic behavioral effects of LMX were paralleled by a reduction in CORT-driven increases in amygdala glutamatergic transmission. These data further support the potential of selective COX-2 inhibitors for the treatment of affective and stress-related psychiatric disorders.
2. Material and methods
2.1. Animals
Male ICR (CD-1) mice aged 8–12 weeks were used for all experiments (Envigo, Indianapolis, IN). Mice were group housed in the animal care facilities at Vanderbilt University, where the colony rooms are climate-controlled and maintained at 21±2 °C, 30% ± 10% relative humidity on a 12L:12D cycle, with lights on at 0700 h. Food and water were provided ad libitum for the duration of the experiments. All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Animals and C. f. t. U. o. t. G. f. t. C. a. U. o. L, 2011), and were approved by the Vanderbilt University Institutional Animal Care and Use Committee (#M1600213).
2.2. Drugs and treatments
Lumiracoxib 5, 10, or 30 mg kg−1 (Selleck Chemicals, TX, USA), was administered via intraperitoneal (I.P.) route 2 h prior to behavioral testing for all experiments. For acute treatments, LMX was administered in dimethyl sulfoxide (DMSO; vehicle) at a volume of 1 mL kg−1. We have previously shown high levels of LMX molecules can be detected in mouse brain, following an I.P. injection of LMX 5 mg kg−1 in DMSO (Morgan et al., 2018). Published data also shows that up to three days of repeated I.P. injections with DMSO is well-tolerated in mice, but the toxicity of chronic DMSO could produce experimental confounds (SC et al., 2006). Considering this, for repeated (>9 repeated daily injections) treatments, LMX 5 mg kg−1 was administered in a formulation containing ethanol:kolliphor:saline (1:1:18; Sigma-Aldrich, St. Louis, MO) at a volume of 10 mL kg−1 injected once daily. For the chronic stress experiments, initially two doses of corticosterone were tested; this was done to replicate existing findings in the literature (Gulbins et al., 2013; Stone and Lin, 2008; van Donkelaar et al., 2014), and validate the ability of this route of administration to induce anxiety-like behaviors in ICR mice (Ersek et al., 2016). CORT (#27840; Sigma-Aldrich, St. Louis, MO) was administered continuously for 5 weeks at doses of 100 μg mL−1 or 250 μg mL−1 (0.1 mg/mL, 0.25 mg/mL) in homecage drinking water. CORT was first dissolved in 95% ethanol, sonicated, and added to the drinking water with a final ethanol concentration of 1%. The control group was given 1% ethanol-containing water. Vanderbilt University Department of Animal Care provided water bottles, which were wrapped in aluminum foil to avoid photodegradation of CORT. Bottles were weighed and fresh treatment water was supplied every 72 h throughout the duration of experiments.
2.3. Behavior measures
2.3.1. Elevated-plus and -zero mazes (EPM and EZM)
The EPM had two wall-free open-arms (30 × 10 cm; light illuminance ~100–115 lux), and 2 walled (“closed”) arms (30 × 10 × 15 cm; ~20–25 lux) anchored to a square 5 × 5 cm open center. To begin the test and trigger video tracking, mice were placed in the center, facing an open-arm. The annular EZM was divided into 4 quadrants, with two open (non-walled) arms, 60.9 cm each (~100–115 lux), and two walled (15 cm) arms (~20–25 lux), 50.8 cm long. Mice were placed in the middle of an open-arm, to begin the test and trigger tracking. Both mazes were made of black acrylonitrile butadiene styrene plastic and elevated 47 cm off the ground. Each mouse explored the EPM and EZM for 5 min per test, with ANY-maze (Stoelting, Wood Dale, Illinois) video tracking software used to monitor and analyze behavior during testing.
2.3.2. Open-field test (OFT)
A square sound-attenuating chamber with clear plexiglass walls (27.9 × 27.9 × 20.3 cm; MED-OFA-510; MED Associates, St. Albans, Vermont) was contained within a white sound-attenuating chamber box. Mice were placed in the center of the open field chamber to begin the test and recorded for 30 min, with beam breaks from 16 infrared light beams measuring movement and position with Activity Monitor v5.10 (MED Associates) software. The center of the OFT was designated as a square of the innermost 50% of the OFT arena floor, with the remainder considered the perimeter. The chamber was illuminated at ~200 lux and white noise was present at ~60 dB.
2.3.3. Light-dark box (LD box)
The light–dark (LD) box test was performed as previously described (Bedse et al., 2018). A black insert (Med Associates ENV-511; made of IRT to allow infrared beam transmission) was placed into a chamber with clear plexiglass walls (27.9 × 27.9 × 20.3 cm; MED-OFA-510) to split the chamber into equal halves light (~350–400 lux) and dark (<5 lux) divisions. Beam breaks from 16 infrared beams were recorded Activity Monitor v5.10 (MED Associates) to monitor position and behavior during the 10-min test. White noise was present at ~60 dB.
2.4. Ex vivo electrophysiological recordings
To examine effects of CORT and LMX on synaptic transmission in the amygdala, slice electrophysiological experiments were performed, as previously described (Bluett et al., 2017), following behavioral testing described above. Mice were deeply anesthetized with isoflurane and transcardially perfused with ice-cold oxygenated (95% v/v O2, 5% v/v CO2) N-methyl-D-glucamine (NMDG) based artificial cerebral spinal fluid (ACSF) containing (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Na-ascorbate, 3 Na-pyruvate, 5 N-acetylcyctine, 0.5 CaCl2·4H2O and 10 MgSO4·7H2O. The brain was quickly removed, a 3 mm coronal block containing the basolateral amygdala (BLA) was cut using an ice-chilled coronal brain matrix, and 250 μm hemisected coronal slices of the BLA were cut in ice fold NMDG solution using a Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL, USA). Slices were incubated for 8–15 min in 32 °C oxygenated NMDG-ACSF, and then moved into a HEPES-based ACSF containing (in mM): 92 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 ascorbate, 3 Na-pyruvate, 5 N-acetylcyctine, 2 CaCl2·4H2O and 2 MgSO4·7H2O. Slices were kept in this HEPES-ACSF until recording. Slices obtained from mice treated with LMX in vivo were incubated in LMX (20 mM) containing HEPES-ACSF for at least 1 h before recording. The order of recordings from each condition was alternated day by day to control for slice age and incubation time. Electrophysioogical recordings were performed in a submerged recording chamber during continuous perfusion of oxygenated ACSF containing (in mM): 113 NaCl, 2.5 KCl, 1.2 MgSO4·7H2O, 2.5 CaCl2·2H2O, 1 NaH2PO4, 26 NaHCO3, 1 ascorbate, 3 Na-pyruvate and 20 glucose; at a flow rate of 2–3 mL min-1. LMX (20 mM) was added to ACSF for LMX-receiving animals. For drug experiments, LMX was dissolved in DMSO. Vehicle slices without LMX had a parallel volume of DMSO added to HEPES-ACSF and ACSF for control. For all experiments, 0.5 g/L of fatty acid free bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) was added to HEPES-ACSF and ACSF to increase solubility of LMX. Slices were visualized using a Nikon microscope equipped with differential interference contrast video microscopy. Whole-cell voltage-clamp and current-clamp recordings from BLA pyramidal cells were obtained under visual control using a 40x objective. 2–5 MΩ borosilicate glass pipettes were filled with high [K+] based solution containing (in mM): 125 K+-gluconate, 4 NaCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, and 10 Na-phosphocreatine. The GABA-A receptor antagonist, picrotoxin (50 μM; Abcam, Cambridge, MA), was also added to the internal recording solution to isolate glutamatergic transmission. For spontaneous EPSC (sEPSC) measurements in voltage-clamp, neurons were held at −70 mV and only cells with access resistance of <20Ω were included. Recordings were performed using a MultiClamp 700B amplifier (Molecular Devices), and Clampex software (version 10.2; Molecular Devices).
2.5. Statistical analyses
Data were analyzed using the following statistical tests: two-tailed unpaired t-test, one-way factorial analysis of variance (ANOVA), two-way factorial ANOVA, or two-way repeated measures ANOVA. Following omnibus ANOVAs, post-hoc Holm–Sidak's tests were used for comparisons between groups, using Prism Graphpad 7 (San Diego, CA, USA) software. Effect size was calculated using formula for η (Alevizos et al., 2014) to reflect the proportion of total variability that is accounted for by water (CORT or vehicle) or drug (LMX or vehicle) treatment (Tabachnick and Linda, 2001). Sample sizes are noted as n-values on graphs and represent biological replicates, i.e. mice. Data are presented as mean ± SEM, with p < 0.05 considered statistically significant. Each dataset underwent a Rout Test for outlier identification. Sample sizes are not uniform across behavior tests, as each chronic CORT/COX-2 inhibitor experiment used a minimum of 3 separate cohorts of mice, not all of which were run through every behavioral task.
3. Results
3.1. LMX does not affect anxiety-like behaviors in naïve mice
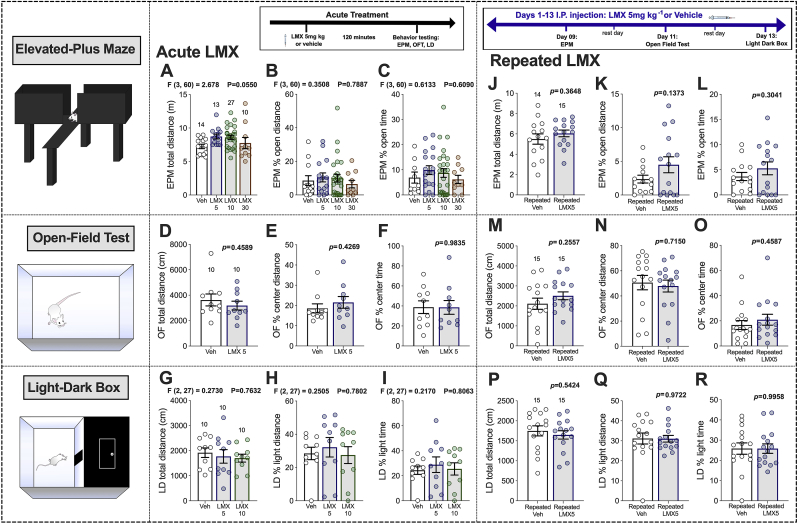
Acute LMX (5, 10, and 30 mg kg−1) did not affect anxiety-like behaviors in naïve mice at any dose tested. LMX did not significantly affect total distance traveled, % open-arm distance or open-arm time in the EPM (Fig. 1A–C). In a separate cohort of mice, the effect of LMX 5 mg kg−1 was evaluated in the OFT. No significant differences in total distance, % center distance, or % center time were observed in the OFT (Fig. 1D–F). Similarly, acute LMX treatment (5 mg kg−1 or 10 mg kg−1) did not affect total distance, % light distance, or % light time in the LD box test (Fig. 1G–I). These results indicate that under basal conditions LMX has no effect on anxiety-like behavior, which is consistent with previous findings from our group using a 1 mg kg−1 dose (Gamble-George et al., 2016).
Fig. 1.
Effects of acute and repeated LMX on anxiety-like behavior in naïve mice. Effects of acute LMX (5, 10, and 30 mg kg−1) in EPM (A–C). Effects of acute LMX (5 mg kg−1) in the OFT (D–F). Effects of LMX (5 and 10 mg kg−1) in the LD Box (G–I). Effects of repeated LMX (5 mg kg−1) in the EPM (J–L) OFT (M–O) LD Box (P–R). Data presented as mean ± SEM with individual data points representing one mouse (n; noted above bar graphs). F and p-values for one-way ANOVA or p-values for unpaired t-test shown in individual panels.
3.2. Repeated LMX does not affect anxiety-like behaviors in naïve mice
We next hypothesized that repeated COX-2 inhibition might be required to reduce anxiety-like behavior under basal conditions. We selected 5 mg kg−1 as our dose for repeated administration based on a lack of effects of higher doses in our acute studies, and our previous studies examining brain PG levels and behavior after LMX treatment (Morgan et al., 2018; Gamble-George et al., 2016). Mice were treated with LMX once daily for 13 days. On treatment days 9, 11, and 13, injections were given 2 h before behavior testing. Repeated LMX had no effect on total distance, % open-arm distance, or % open-arm time in the EPM (Fig. 1J-L). Similarly, repeated LMX had no effect on total distance, % center distance, or % center time in the OFT (Fig. 1M–O). Lastly, LMX did not affect total distance, % light distance, or % light time in the LD Box (Fig. 1P–R). These data indicate that COX-2 inhibition with LMX does not alter anxiety-like behavior under basal conditions even after repeated administration.
3.3. Chronic CORT treatment increases anxiety-like behavior
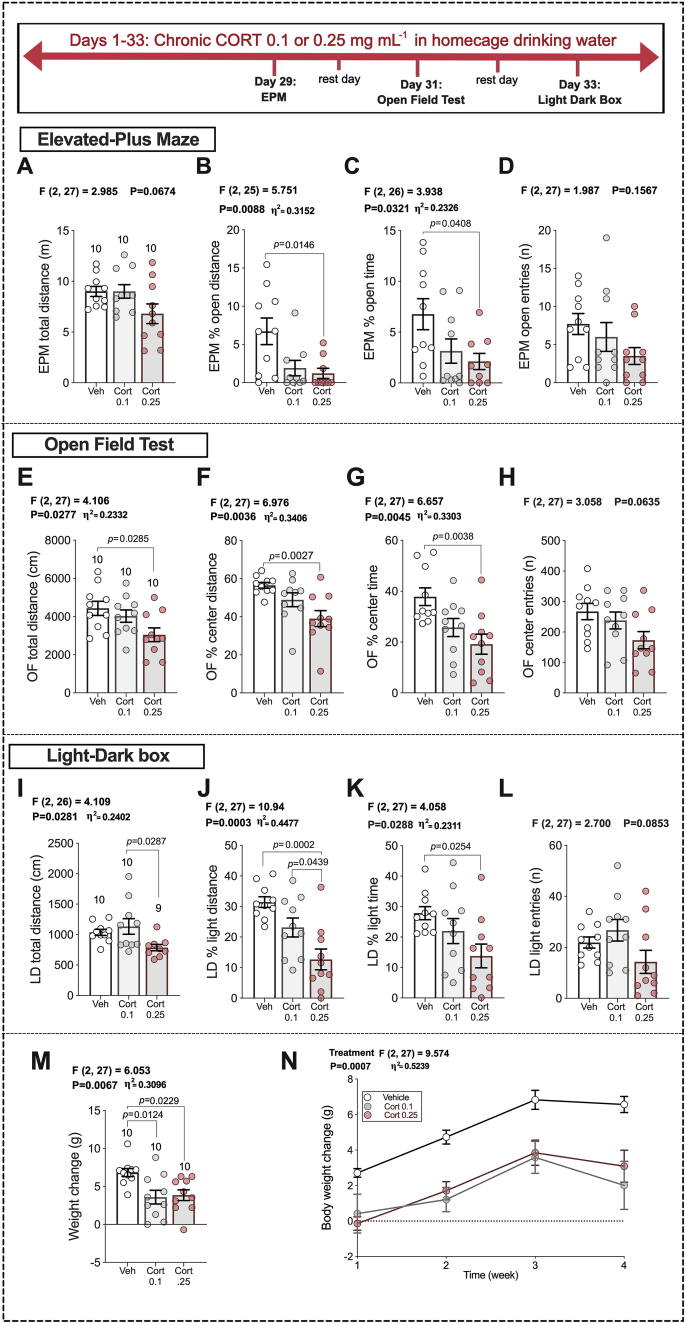
To examine the effects of LMX under conditions of increased anxiety we utilized a chronic non-invasive oral CORT administration paradigm. We first conducted a dose-response study with two doses of CORT administered in homecage drinking water for five weeks. Mice were exposed to either 100 μg mL−1 or 250 μg mL−1 CORT in homecage drinking water. Beginning on experimental day 29, mice were tested in the EPM, OFT and LD Box (see Fig. 2 timeline). CORT administration was continued throughout the behavioral testing period. CORT treatment dose-dependently increased anxiety-like behavior in all assays tested. High-dose CORT-exposed mice exhibited lower % open-arm distance and % open-arm time in the EPM (Fig. 2B and C) than vehicle-treated mice, without affecting total distance or open-arm entries (Fig. 2A and D). Similarly, in the OFT, mice exposed to high-dose CORT exhibited significantly reduced total distance traveled, % center distance, and % center time, without significantly affecting entries into the center zone (Fig. 2E–H). In the LD box test, high dose CORT significantly reduced % light distance, and % light time (Fig. 2J and K) without affecting total distance or light-zone entries relative to vehicle treatment (Fig. 2I and L). Mouse weights were comparable between groups on the first day of vehicle, low, or high-dose CORT treatment (M = 31.43, SEM = 0.6580; M = 29.53, SEM = 0.6180; and M = 29.78, SEM = 0.4138, respectively). Both low and high-dose CORT also reduced the rate of body weight gain, which is commonly seen in models of chronic stress that induce anxiety-like behavioral phenotypes (Jaggar et al., 2017; Gupta et al., 2014; Sadler and Bailey, 2016; Slattery et al., 2012; Monteiro et al., 2015) (Fig. 2M and N). Mice exposed to 250 μg mL−1 in drinking water showed increased latency to feed in the NIH test, spent less time in the center of OFT, and had increased immobility time in the OFT (Gulbins et al., 2013). Based on these data, the 250 μg mL−1 dose was chosen for subsequent chronic CORT experiments.
Fig. 2.
Chronic oral corticosterone increases anxiety-like behavior. Vehicle (1% EtoH), 0.1 or 0.25 mg/mL corticosterone, administered in homecage drinking water for 33 days. EPM results: (A–D). OFT results: (E–H). LD box: (I–L). The number of mice per group are included on the first graph for each measure (far left of each row). p-values (above bars) represent significance levels of Holm-Sidak pairwise comparisons between individual groups after Omnibus ANOVA (ANOVA F-scores above graphs). Data presented as individual values, with Mean ± SEM. Effect sizes depicted as η2 and shown for significant main effects.
3.4. Acute LMX partially reduces chronic CORT-induced anxiety-like behavior
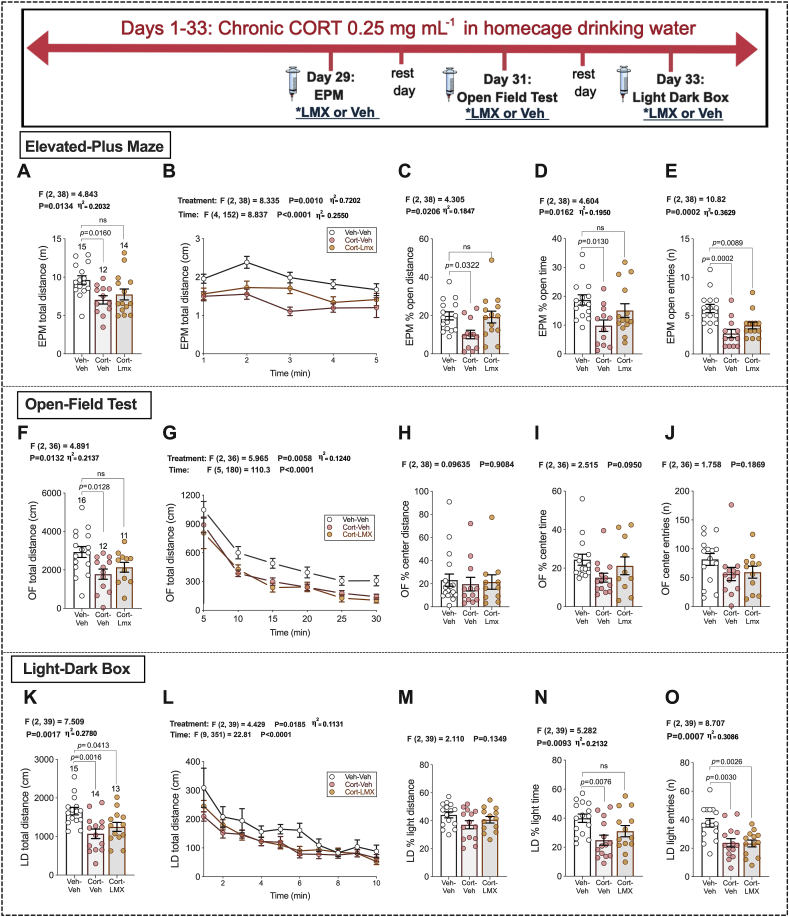
Initial testing indicated that plasma corticosterone levels were significantly increased in mice exposed to chronic CORT treatment (see Fig. 3 timeline), t = 3.884, df = 24, p < 0.0001; Vehicle H2O plasma CORT levels: M = 35.39 ng/mL, SEM = 10.41 ng/mL, n = 11; CORT H2O plasma CORT levels: M = 507.3 ng/mL, SEM = 154.6 ng/mL, n = 8, with plasma levels after chronic exogenous treatment comparable to those elicited by chronic stress in published data. In 2015, Gong et al. reported average CORT serum levels just above 600 ng/mL following 23 days of chronic restraint stress (Gong et al., 2015). Acute LMX 5 mg kg−1 treatment reduced anxiety-like behavior in the EPM and LD box following chronic CORT treatment. The CORT-LMX group was not significantly different from the Veh-Veh group in EPM total distance, % open-arm distance, or % open-arm time (Fig. 3A–D). However, both CORT-Veh and CORT-LMX treated animals made significantly fewer entries into the open-arms of the EPM (Fig. 3E). CORT-LMX was not different from Veh-Veh group in OFT total distance (Fig. 3F and G), and no group differences were seen for % center distance, % center time, or number of entries into the center (Fig. 3H–J). In the LD box, both the CORT-treatment groups had reduced total distance (Fig. 3K-L), but no group differences were seen for % light distance (Fig. 3M). CORT-LMX was not different from the Veh-Veh group in % light time (Fig. 3N). However, both CORTgroups made fewer entries into the light side relative to vehicle treatment (Fig. 3O). These data indicate that acute LMX treatment is able to attenuate some anxiogenic effects of chronic CORT treatment.
Fig. 3.
Acute COX-2 inhibition following high-dose chronic CORT in homecage drinking water. Vehicle water (1% EtoH) or 0.25 mg/mL CORT, administered in homecage for 33 days. EPM results (A–E), OFT (F–J), LD (K–O). The number of mice per group are included on the first graph for each measure (far left of each row). p-values (above bars) represent significance levels of Holm-Sidak pairwise comparisons between individual groups after Omnibus ANOVA (F-scores above graphs). Data graphs B, G, & L were analyzed with Two-Way ANOVA. Data points represent individual values, with Mean ± SEM. Data presented as individual values, with Mean ± SEM. Effect sizes depicted as η2 and shown for significant main effects.
3.5. Repeated LMX reduces chronic CORT-induced anxiety-like behavior
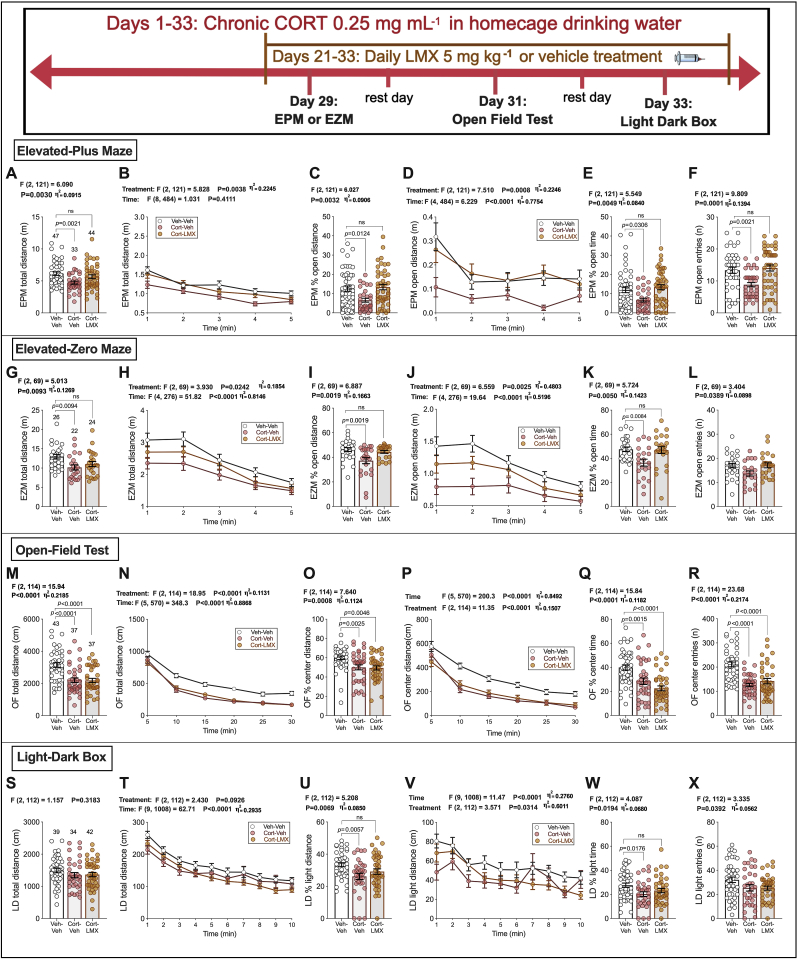
Although repeated LMX did not affect anxiety-like behaviors in naïve mice, based on the partial efficacy of acute LMX treatment in chronic CORT treated mice, we examined the effects of repeated LMX treatment in this model. Repeated LMX 5 mg kg−1 administration reversed anxiety-like behavior in the EPM, EZM, and LD box after chronic CORT (see Fig. 4 timeline). In the EPM, the CORT-Repeated LMX group did not statistically differ from Veh-Veh controls in total distance, % open-arm distance, % open-arm time, and open-arm entries (Fig. 4A–F). In a separate cohort of mice, we found that CORT-Repeated LMX groups were not different from Veh-Veh controls in EZM total distance, % open-arm distance, or % open-arm time (Fig. 4G–K). No group differences were seen for EZM entries into the open-arms (Fig. 4L). Both CORT treatment groups were significantly lower than the Veh-Veh controls in OFT total distance, % center distance, % center time, and center entries (Fig. 4M–R). In the LD box, CORT-Repeated LMX groups were not different from Veh-Veh controls in % light distance, % light time. No group differences were observed in total distance or light entries (Fig. 4S–X). As reported in earlier experiments (Fig. 2M and N), CORT treatment had a significant main effect on body weight gain, F(2, 124) = 58.86, p < 0.0001. Sidak's post-hoc comparison test revealed significant differences between Veh-Veh and CORT-Veh groups (p < 0.0001) as well as Veh-Veh and CORT-LMX groups (p < 0.0001). However, CORT-Veh and CORT-LMX groups were not significantly different on measured body weight gain (p = 0.5589). Mice consumed similar amounts of water across groups. No differences were seen in group-housed cage water bottle weights (weights recorded every 72 h) over time, with no main effect of Treatment F (2, 9) = 3.631 p = 0.0698, and no interaction of Treatment x Time, F (6, 27) = 1.820 p = 0.1327.
Fig. 4.
Repeated, Subchronic COX-2 inhibition following high-dose chronic CORT in homecage drinking water. Vehicle water (1% EtoH) or 0.25 mg/mL CORT, administered in homecage for 33 days. EPM results: (A–F). EZM: (G–L) OFT results: (M–R) LD box: (S–X). p-values (above bars) as indicated from Holm-Sidak pairwise comparisons after two-way ANOVA (below graphs B, D, H, J, N, P, T, V) or Omnibus ANOVA (remaining graphs). Data presented as individual values, with Mean ± SEM. Effect sizes depicted as η2 and shown for significant main effects.
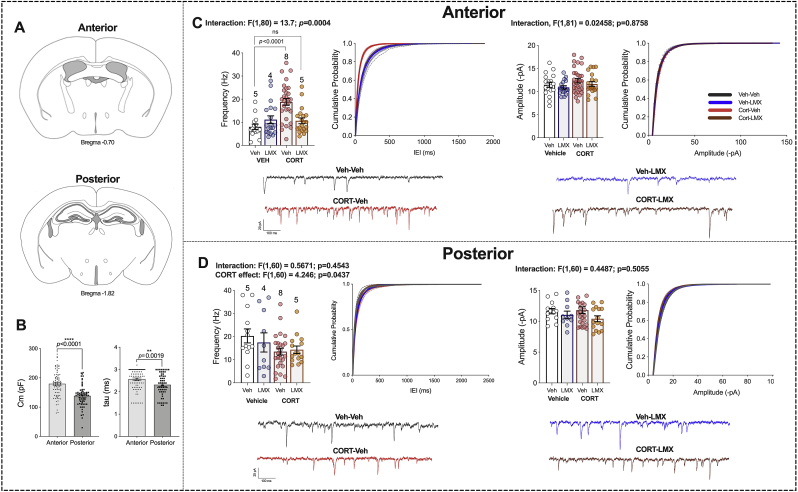
3.6. Repeated LMX normalizes chronic CORT-induced increases in glutamatergic transmission in anterior BLA neurons
We next determined if repeated LMX affected neurophysiological adaptations induced by chronic CORT treatment. We focused on examination of amygdala glutamatergic signaling given the well-established role of enhanced amygdala glutamatergic transmission in anxiety and stress responsivity (Song et al., 2017; Masneuf et al., 2014). Both positive and negative valence stimuli activate BLA cells, and distinct BLA output neurons drive opposing behavioral responses to positive and negative valence stimuli (Gore et al., 2015; Stuber et al., 2011; Kim et al., 2016). The BLA is spatially anatomically divided into anterior (aBLA) and posterior (pBLA) regions (McDonald, 1984) (Fig. 5A) with some studies suggesting aBLA neurons preferentially encode and initiate aversive/avoidance responses to negative valence stimuli while pBLA neurons encode and initiate appetitive/approach responses to positive valence stimuli. Consistent with previous data (Kim et al., 2016), we found aBLA cells had a higher mean membrane capacitance (Cm, Fig. 5B) and time constant (tau Fig. 5B) values than pBLA neurons. Chronic oral CORT treatment increased spontaneous excitatory postsynaptic current (sEPSC) frequency in the aBLA, without affecting sEPSC amplitude (Fig. 5C). Importantly, in brain slices from CORT mice treated with repeated LMX, sEPSC frequency was not significantly different from Veh-Veh controls (Fig. 5C). No effect of LMX was observed in pBLA neurons recorded from the same mice, however there was a main effect of CORT to reduce sEPSC frequency, but not amplitude, in pBLA neurons (Fig. 5D).
Fig. 5.
Chronic LMX treatment prevents CORT-induced changes in excitatory transmission in anterior BLA. (a) Representative depiction comparing anterior BLA to posterior BLA. (b) Comparison of mean membrane capacitance (Cm; ****, p < 0.0001) and time constant (tau; **, p < 0.01) of pyramidal neurons from anterior and posterior BLA slices (c) Spontaneous EPSC (sEPSC) frequency (left) and amplitude (right) recorded from pyramidal neurons in anterior BLA and representative traces from each group (bottom). (d) sEPSC frequency (left) and amplitude (right) from posterior BLA principal neurons and representative traces (bottom). F and p-values for two-way ANOVA shown above (c–d). P values shown for pairwise comparisons derived from Holm-Sidak multiple comparisons test after ANOVA or unpaired student's two-tailed t-test (b). For cumulative probability curves, p < 0.0001 by K–S test. Each point represents one cell. Data are presented as mean ± SEM.
4. Discussion
Here, we show that both acute and repeated treatment with the highly selective COX-2 inhibitor LMX reduces anxiety-like behavior following chronic CORT treatment, without affecting basal anxiety in naïve mice. We also found that repeated LMX normalized CORT-induced increases in aBLA glutamatergic transmission. Taken together with previous data, our findings further support the notion that COX-2 may represent a potential therapeutic target approach for the treatment of anxiety domain symptoms in the context of mood, anxiety, and trauma-related psychiatric disorders.
Here, we focused on traditional behavioral tests of conflict anxiety over assays of despair or anhedonia, for several reasons. First, we have previously shown no significant effect of pharmacological inhibition of COX-2 on sucrose preference and immobility in the tail-suspension test. This was seen in naïve mice, as well as in mice exposed to acute foot-shock stress (Gamble-George et al., 2016). Secondly, we did not observe clear changes in despair-like behavior or measures of anhedonia after chronic CORT treatment in preliminary studies (data not shown). With regard to anxiety-like behaviors, significant differences were seen in the EZM and EPM after repeated LMX treatment. Interestingly, we did not see significant improvements in overall exploratory behavior in the OFT with either acute or repeated LMX treatment. This finding suggests that COX-2 inhibition may preferentially improve high-contrast conflict-driven behavioral responses, such as those elicited in the EPM and EZM. These data are generally consistent with our previous findings that COX-2 inhibition reduces anxiety-like behavior after foot-shock stress in the NIH test and EPM (Gamble-George et al., 2016). Published studies have also shown that pharmacological inhibition of COX-2 reduces anxiety-like behavior in the mirror-chamber test after 6 h of immobilization stress, as revealed by increased number of mirror entries and time spent in the mirror side of the a chamber (Kumari et al., 2007; Dhir et al., 2006), without affecting exploratory behaviors in the OFT (Kumari et al., 2007). Following 15 days of forced swimming, treatment with a COX-2 inhibitor significantly decreased the latency to enter the mirror side of the mirror chamber test, and increased number of entries and time spent in the mirror side (Kumar et al., 2010). Cumulatively, these data suggest COX-2 inhibition may preferentially affect anxiety-domain behaviors over hedonic, despair-like, or general exploratory behaviors. These data also suggest future clinical studies should examine the effects of pharmacological inhibition of COX-2 on anxiety-domain symptoms in patients with mood, anxiety, and trauma-related disorders.
Another interesting observation from these studies and our previously published work, is the conditional efficacy of COX-2 inhibition. Generally, few behavioral effects of COX-2 inhibition are seen in naïve rodents; i.e., animal models which do not exhibit increases in anxiety-like behaviors at baseline. We have previously reported that pharmacological inhibition of COX-2 in naïve rodents had no effect on anxiety-like behavior in the EPM or NIH tests. Using 1 mg kg−1of LMX, no treatment differences were seen for latency to enter EPM open arms, or time spent in the open arms in naïve mice (Gamble-George et al., 2016). Similarly, the NIH test revealed no effects of COX-2 inhibition on latency to feed, or amount consumed in naïve mice (Gamble-George et al., 2016). This is in agreement with the findings reported here, wherein pharmacological inhibition of COX-2 did not alter anxiety-like behavior in naïve mice in the EPM, OFT, or LD box. Furthermore, we found that daily LMX injections did not affect anxiety-like behavior in naïve mice tested in the EPM, OFT, or LD box. In contrast, anxiolytic effects of LMX were clearly observed in CORT-treated mice in the EPM and EZM. These data highlight the conditional efficacy of pharmacological inhibition of COX-2 as it relates to the reduction of anxiety-like behavior, with effects seen only when anxiety is heightened by stress or CORT exposure. This conditional efficacy may be related to several factors, including the expression pattern of COX-2 in the brain, and the inducible nature of the enzyme (Breder et al., 1995; Yamagata et al., 1993; Feng et al., 1993). COX-2 exists constitutively in the CNS at low levels, but its induction has been demonstrated by various forms of stress in rodents (Yamagata et al., 1993; Munhoz et al., 2008). In rodent models, COX-2 in the CNS is upregulated by forced swim stress (Kumar et al., 2010; Yamagata et al., 1993), acute but prolonged immobility and restraint (Madrigal et al., 2003a, 2003b; Kumari et al., 2007; Dhir et al., 2006), chronic unpredictable stress (Guo et al., 2009), and chronic social defeat (Rivat et al., 2010). In addition, one published paper by Onaka and colleagues reports that 21 days of chronic CORT exposure significantly increased COX-2 mRNA in the amygdala (Onaka et al., 2015). These findings, combined with new research reported here suggesting conditional efficacy of pharmacological inhibition of COX-2, indicate a need for additional research examining the potential conditional efficacy of COX-2 inhibition, and if this is contingent upon expression levels of COX-2 in the brain.
To investigate the ability of LMX to modulate CORT-induced physiological adaptations, we used ex vivo whole-cell patch-clamp electrophysiology approaches to record synaptic activity in the BLA. The BLA is a critical site in the regulation of anxiety and the stress-response (Zhang and Rosenkranz, 2012; Janak and Tye, 2015; Felix-Ortiz et al., 2016) and increases in activity after chronic stress (Zhang et al., 2019; Rosenkranz et al., 2010). Our group has shown that acute foot-shock stress can elevate intrinsic excitability of BLA neurons, and this stress effect is decreased by COX-2 inhibition (Gamble-George et al., 2016). Here we found chronic CORT treatment increases sEPSC frequency, but not amplitude, onto aBLA neurons. These data suggest CORT results in an increase in presynaptic release of glutamate onto aBLA pyramidal neurons consistent with previous studies (Sterner and Kalynchuk, 2010; Popoli et al., 2011). Importantly, LMX completely normalized this increase without affecting glutamatergic transmission in naïve mice, paralleling our behavioral findings. COX-2 is expressed at low levels in the BLA where it localizes to dendritic spines (Breder et al., 1995; Sang et al., 2005; Kaufmann et al., 1996), but, as noted above, is rapidly up-regulated by CORT, stress, and high-frequency stimulation associated with the induction of long-term potentiation (Madrigal et al., 2003a; Cao et al., 1995; Chen et al., 2017; Quan et al., 1998; Bliss and Collingridge, 1993). Furthermore, increased COX-2 expression and activity has previously been shown to enhance glutamatergic transmission via prostaglandin receptor activation, and potentially via generation of PG-Gs (Sang et al., 2005; Yang et al., 2008). Taken together, these data suggest the possibility that the reduction in sEPSC frequency observed after LMX treatment is due to reduced PG or PG-G formation induced by chronic CORT-induced up-regulation of COX-2 expression.
BLA connections with other anatomically distinct neuronal populations are thought to drive both aversive and appetitive forms of associative learning (Burgos-Robles et al., 2017a; Beyeler et al., 2016; Beyeler A et al., 2018). Briefly, BLA projections to nucleus accumbens (BLA-NAc) can be activated when a mouse is presented cues predicting appetitive stimuli (Beyeler et al., 2016). In comparison, cells in the BLA projecting to the central amygdala (BLA-CeA), as well as the BLA to prelimbic (BLA-PL) projector population are excited in response to aversive cues (Beyeler et al., 2016; Burgos-Robles et al., 2017b). These studies have suggested an intermingling or dorsal-ventral coding of valence within the BLA. However, a recent study by Shen and colleagues reports the anatomical segregation of cells within the anterior-posterior axis of the BLA associated with aversive stimuli (Shen et al., 2019). Their findings indicate a negative valence-coding BLA-NAc circuit, wherein social stress preferentially activates aBLA cholecystokinin (CCK)-positive neurons projecting to NAc D2 medium spiny neurons (Shen et al., 2019). Additionally, they report CCK-positive cells were almost exclusively co-localized with genetically distinct neuronal populations expressing the gene Rspo282. Previously published research has found the fear-associated behavior of ‘freezing’ can be elicited with optogenetic stimulation of Rspo2 cells in the aBLA (Kim et al., 2016). In contrast, photostimulation of Ppp1r1b-expressing neurons, located in the pBLA, can drive the appetitive behavior of intra-cranial self-stimulation (Kim et al., 2016). That CORT selectively increases sEPSC frequency onto aBLA is consistent with the anxiogenic phenotype observed after CORT administration and the known dissociation between aBLA and pBLA neurons with regard to valence-coding observed in some studies (Kim et al., 2016). These data suggest the possibility that LMX reduces anxiety-like behavior after CORT treatment via reductions in excess glutamatergic drive to negative valence-coding aBLA neurons, however this hypothesis remains to be tested experimentally.
Despite these important insights, the current work also has several limitations. First, the drinking water route of administration is chosen here because it allows for relatively easy non-invasive chronic administration that preserves the diurnal rhythm of hormone secretion (Herrmann et al., 2009; Karatsoreos et al., 2010), and produces plasma corticosterone levels comparable to chronic stress (Gong et al., 2015). However, the drinking water route of administration may result in variability of circulating CORT concentrations between mice depending on the amount of water they consume (Herrmann et al., 2009). Our early CORT experiments were focused on establishing a chronic oral administration model and optimizing oral dosing of CORT. This resulted in mice remaining essentially undisturbed (besides weights and water bottle changes) until behavior testing. Subsequent to this, every CORT experiment reported here included between 3 and 13 days of I.P. injections (vehicle or LMX 5 mg kg−1). Likely due to the stress of undergoing a scruff hold and multiple needle injections, some of the behavioral data in the dose-response study had lower variability compared to later cohorts in which drug effects were assessed (For example, see OFT data, Fig. 2F–H vs. Fig. 3H–J). Future studies will optimize the inclusion of a COX-2 inhibitor in the drinking water to circumvent this issue. Similarly, although one published report shows chronic exogenous CORT exposure can upregulate COX-2 mRNA in the amygdala (Onaka et al., 2015), additional research is needed to validate the ability of the current model to upregulate COX-2 in the BLA and BLA negative valence circuits. While these experiments focused on behavioral metrics, measured as a function of chronic CORT exposure and pharmacological inhibition of COX-2, this opens the door for continued inquiries into any possible dysregulation in HPA-axis function caused by oral CORT exposure, and the ability of LMX to revert these potential adaptations. Lastly, although our data indicate an association between increases in anxiety-like behavior and increases in aBLA glutamatergic transmission, whether these two phenomena are casually related is not known. Similarly, whether the anxiolytic effects of LMX are mediated via reductions in aBLA glutamatergic transmission will need to be examined in subsequent studies examining direct injections of LMX into the aBLA, for example.
5. Conclusions
In summary, we report that treatment with a selective COX-2 inhibitor reduces anxiogenic behavior observed after chronic oral CORT administration in mice. Our electrophysiological studies revealed that chronic CORT increases sEPSC frequency onto aBLA, but not the pBLA, neurons and that repeated LMX treatment reversed this effect. Both effects of LMX were limited to CORT-treated mice highlighting the conditional nature of COX-2 inhibition efficacy. These data support the preclinical efficacy of COX-2 inhibitors in validated animal models and suggest continued clinical investigation of novel and currently available COX-2 inhibitors for the treatment of anxiety and stress-related psychiatric disorders.
Conflicts of interest
The authors declare no competing financial interests relevant to the present study. S.P. has received consultation fees from Psy Therapeutics and Atlas Ventures in areas unrelated to the presented work.
Acknowledgements
This research was supported by NIH Grants 4T32MH065215-14 (AM), R01MH100096 (SP), and Brain & Behavior Research Foundation Young Investigator Awards (GB and RB). Behavioral studies were carried out at the Vanderbilt Neurobehavioral Core (supported by the EKS NICHD Award U54HD083211).
References
- Akhondzadeh S., Jafari S., Raisi F., Nasehi A.A., Ghoreishi A., Salehi B., Mohebbi-Rasa S., Raznahan M., Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress. Anxiety. 2009;26(7):607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Alevizos M., Karagkouni A., Panagiotidou S., Vasiadi M., Theoharides T.C. Stress triggers coronary mast cells leading to cardiac events. Ann. Allergy Asthma Immunol. 2014;112(4):309–316. doi: 10.1016/j.anai.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animals, C. f. t. U. o. t. G. f. t. C. a. U. o. L . Institute for Laboratory Animal Research; Washington, D.C.: 2011. Guide for the Care and Use of Laboratory Animals; pp. 1–246. 2011. [Google Scholar]
- Artigas F., Bortolozzi A., Celada P. Can we increase speed and efficacy of antidepressant treatments? Part I: general aspects and monoamine-based strategies. Eur. Neuropsychopharmacol. 2018;28(4):445–456. doi: 10.1016/j.euroneuro.2017.10.032. [DOI] [PubMed] [Google Scholar]
- Bedse G., Bluett R.J., Patrick T.A., Romness N.K., Gaulden A.D., Kingsley P.J., Plath N., Marnett L.J., Patel S. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: comparative profiling of FAAH, MAGL and dual inhibitors. Transl. Psychiatry. 2018;8(1):92. doi: 10.1038/s41398-018-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A C.C., Silvestre M., Lévêque C., Namburi P., Wildes C.P., Tye K.M. Organization of valence-encoding and projection-defined neurons in the basolateral amygdala. Cell Rep. 2018;22(4):905–918. doi: 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A., Namburi P., Glober G.F., Simonnet C., Calhoon G.G., Conyers G.F., Luck R., Wildes C.P., Tye K.M. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron. 2016;90(2):348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bluett R.J., Baldi R., Haymer A., Gaulden A.D., Hartley N.D., Parrish W.P., Baechle J., Marcus D.J., Mardam-Bey R., Shonesy B.C., Uddin M.J., Marnett L.J., Mackie K., Colbran R.J., Winder D.G., Patel S. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat. Commun. 2017;8:14782. doi: 10.1038/ncomms14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder C.D., Dewitt D., Kraig R.P. Characterization of inducible cyclooxygenase in rat brain. J. Comp. Neurol. 1995;355(2):296–315. doi: 10.1002/cne.903550208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. N. Y. Acad. Sci. 2009;1179:41–55. doi: 10.1111/j.1749-6632.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A., Kimchi E.Y., Izadmehr E.M., Porzenheim M.J., Ramos-Guasp W.A., Nieh E.H., Felix-Ortiz A.C., Namburi P., Leppla C.A., Presbrey K.N., Anandalingam K.K., Pagan-Rivera P.A., Anahtar M., Beyeler A., Tye K.M. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 2017;20(6):824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A., Kimchi E.Y., Izadmehr E.M., Porzenheim M.J., Ramos-Guasp W.A., Nieh E.H., Felix-Ortiz A.C., Namburi P., Leppla C.A., Presbrey K.N., Anandalingam K.K., Pagan-Rivera P.A., Anahtar M., Beyeler A., Tye K.M. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 2017;20(6):824–835. doi: 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Matsumura K., Yamagata K., Watanabe Y. Induction by lipopolysaccharide of cyclooxygenase-2 mRNA in rat brain; its possible role in the febrile response. Brain Res. 1995;697(1–2):187–196. doi: 10.1016/0006-8993(95)00839-i. [DOI] [PubMed] [Google Scholar]
- Chen Q., Luo Y., Kuang S., Yang Y., Tian X., Ma J., Mai S., Xue L., Yang J. Cyclooxygenase-2 signalling pathway in the cortex is involved in the pathophysiological mechanisms in the rat model of depression. Sci. Rep. 2017;7(1):488. doi: 10.1038/s41598-017-00609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I., Drew M., Craig D.A., Guiard B.P., Guilloux J.P., Artymyshyn R.P., Gardier A.M., Gerald C., Antonijevic I.A., Leonardo E.D., Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir A., Padi S.S., Naidu P.S., Kulkarni S.K. Protective effect of naproxen (non-selective COX-inhibitor) or rofecoxib (selective COX-2 inhibitor) on immobilization stress-induced behavioral and biochemical alterations in mice. Eur. J. Pharmacol. 2006;535(1–3):192–198. doi: 10.1016/j.ejphar.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Ersek A., Santo A.I., Vattakuzhi Y., George S., Clark A.R., Horwood N.J. Strain dependent differences in glucocorticoid-induced bone loss between C57BL/6J and CD-1 mice. Sci. Rep. 2016;6:36513. doi: 10.1038/srep36513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faridhosseini F., Sadeghi R., Farid L., Pourgholami M. Celecoxib: a new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum. Psychopharmacol. 2014;29(3):216–223. doi: 10.1002/hup.2401. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Burgos-Robles A., Bhagat N.D., Leppla C.A., Tye K.M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Sun W., Xia Y., Tang W.W., Chanmugam P., Soyoola E., Wilson C.B., Hwang D. Cloning two isoforms of rat cyclooxygenase: differential regulation of their expression. Arch. Biochem. Biophys. 1993;307(2):361–368. doi: 10.1006/abbi.1993.1601. [DOI] [PubMed] [Google Scholar]
- Galecki P., Galecka E., Maes M., Chamielec M., Orzechowska A., Bobinska K., Lewinski A., Szemraj J. The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. J. Affect. Disord. 2012;138(3):360–366. doi: 10.1016/j.jad.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Galecki P., Talarowska M., Bobinska K., Szemraj J. COX-2 gene expression is correlated with cognitive function in recurrent depressive disorder. Psychiatry Res. 2014;215(2):488–490. doi: 10.1016/j.psychres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Gamble-George J.C., Baldi R., Halladay L., Kocharian A., Hartley N., Silva C.G., Roberts H., Haymer A., Marnett L.J., Holmes A., Patel S. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife. 2016;5 doi: 10.7554/eLife.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S., Miao Y.L., Jiao G.Z., Sun M.J., Li H., Lin J., Luo M.J., Tan J.H. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore F., Schwartz E.C., Brangers B.C., Aladi S., Stujenske J.M., Likhtik E., Russo M.J., Gordon J.A., Salzman C.D., Axel R. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell. 2015;162(1):134–145. doi: 10.1016/j.cell.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I., Kreisel T., Ben-Menachem-Zidon O., Licht T., Weidenfeld J., Ben-Hur T., Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Gradus J.L. Prevalence and prognosis of stress disorders: a review of the epidemiologic literature. Clin. Epidemiol. 2017;9:251–260. doi: 10.2147/CLEP.S106250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E., Palmada M., Reichel M., Luth A., Bohmer C., Amato D., Muller C.P., Tischbirek C.H., Groemer T.W., Tabatabai G., Becker K.A., Tripal P., Staedtler S., Ackermann T.F., van Brederode J., Alzheimer C., Weller M., Lang U.E., Kleuser B., Grassme H., Kornhuber J. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat. Med. 2013;19(7):934–938. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- Guo J.Y., Li C.Y., Ruan Y.P., Sun M., Qi X.L., Zhao B.S., Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur. J. Pharmacol. 2009;612(1–3):54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Gupta D., Radhakrishnan M., Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic–pituitary–adrenocortical and brain serotonergic system. Pharmacol. Biochem. Behav. 2014;124:129–136. doi: 10.1016/j.pbb.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Hermanson D.J., Hartley N.D., Gamble-George J., Brown N., Shonesy B.C., Kingsley P.J., Colbran R.J., Reese J., Marnett L.J., Patel S. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat. Neurosci. 2013;16(9):1291–1298. doi: 10.1038/nn.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Henneicke H., Street J., Modzelewski J., Kalak R., Buttgereit F., Dunstan C.R., Zhou H., Seibel M.J. The challenge of continuous exogenous glucocorticoid administration in mice. Steroids. 2009;74(2):245–249. doi: 10.1016/j.steroids.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Jaggar M., Weisstaub N., Gingrich J.A., Vaidya V.A. 5-HT2A receptor deficiency alters the metabolic and transcriptional, but not the behavioral, consequences of chronic unpredictable stress. Neurobiol Stress. 2017;7:89–102. doi: 10.1016/j.ynstr.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos I.N., Bhagat S.M., Bowles N.P., Weil Z.M., Pfaff D.W., McEwen B.S. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151(5):2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann W.E., Worley P.F., Pegg J., Bremer M., Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 1996;93(6):2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Pignatelli M., Xu S., Itohara S., Tonegawa S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 2016;19(12):1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K.R., Crews B.C., Morrow J.D., Wang L.H., Ma Y.H., Weinander R., Jakobsson P.J., Marnett L.J. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J. Biol. Chem. 2002;277(47):44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N., Voikar V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiol. Behav. 2014;133:30–38. doi: 10.1016/j.physbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Kulkarni S.K., Singh K., Bishnoi M. Elevated zero maze: a paradigm to evaluate antianxiety effects of drugs. Methods Find. Exp. Clin. Pharmacol. 2007;29(5):343–348. doi: 10.1358/mf.2007.29.5.1117557. [DOI] [PubMed] [Google Scholar]
- Kumar A., Kumari B., Kumar P. Protective effects of selective and non-selective cyclooxygenase inhibitors in an animal model of chronic stress. Neurosci Bull. 2010;26(1):17–27. doi: 10.1007/s12264-010-0713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari B., Kumar A., Dhir A. Protective effect of non-selective and selective COX-2-inhibitors in acute immobilization stress-induced behavioral and biochemical alterations. Pharmacol. Rep. 2007;59(6):699–707. [PubMed] [Google Scholar]
- Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berlin) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: the common pathway of stress-related Diseases. Front. Hum. Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal J.L., Moro M.A., Lizasoain I., Lorenzo P., Fernandez A.P., Rodrigo J., Bosca L., Leza J.C. Induction of cyclooxygenase-2 accounts for restraint stress-induced oxidative status in rat brain. Neuropsychopharmacology. 2003;28(9):1579–1588. doi: 10.1038/sj.npp.1300187. [DOI] [PubMed] [Google Scholar]
- Madrigal J.L., Garcia-Bueno B., Moro M.A., Lizasoain I., Lorenzo P., Leza J.C. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur. J. Neurosci. 2003;18(6):1701–1705. doi: 10.1046/j.1460-9568.2003.02888.x. [DOI] [PubMed] [Google Scholar]
- Masneuf S., Lowery-Gionta E., Colacicco G., Pleil K.E., Li C., Crowley N., Flynn S., Holmes A., Kash T. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–197. doi: 10.1016/j.neuropharm.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A.J. Neuronal organization of the lateral and basolateral amygdaloid nuclei in the rat. J. Comp. Neurol. 1984;222(4):589–606. doi: 10.1002/cne.902220410. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Meyer J.S., Novak M.A. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro S., Roque S., de Sa-Calcada D., Sousa N., Correia-Neves M., Cerqueira J.J. An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front. Psychiatry. 2015;6:6. doi: 10.3389/fpsyt.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A.J., Kingsley P.J., Mitchener M.M., Altemus M., Patrick T.A., Gaulden A.D., Marnett L.J., Patel S. Detection of cyclooxygenase-2-derived oxygenation products of the endogenous cannabinoid 2-arachidonoylglycerol in mouse brain. ACS Chem. Neurosci. 2018;9(7):1552–1559. doi: 10.1021/acschemneuro.7b00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N. Immunological aspects of the treatment of depression and schizophrenia. Dialogues Clin. Neurosci. 2017;19(1):55–63. doi: 10.31887/DCNS.2017.19.1/nmueller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz C.D., Garcia-Bueno B., Madrigal J.L., Lepsch L.B., Scavone C., Leza J.C. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz. J. Med. Biol. Res. 2008;41(12):1037–1046. doi: 10.1590/s0100-879x2008001200001. [DOI] [PubMed] [Google Scholar]
- Myint A.M., Steinbusch H.W., Goeghegan L., Luchtman D., Kim Y.K., Leonard B.E. Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression. Neuroimmunomodulation. 2007;14(2):65–71. doi: 10.1159/000107420. [DOI] [PubMed] [Google Scholar]
- Onaka Y., Shintani N., Nakazawa T., Haba R., Ago Y., Wang H., Kanoh T., Hayata-Takano A., Hirai H., Nagata K.Y., Nakamura M., Hashimoto R., Matsuda T., Waschek J.A., Kasai A., Nagayasu K., Baba A., Hashimoto H. CRTH2, a prostaglandin D2 receptor, mediates depression-related behavior in mice. Behav. Brain Res. 2015;284:131–137. doi: 10.1016/j.bbr.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N., Whiteside M., Herkenham M. Cyclooxygenase 2 mRNA expression in rat brain after peripheral injection of lipopolysaccharide. Brain Res. 1998;802(1–2):189–197. doi: 10.1016/s0006-8993(98)00402-8. [DOI] [PubMed] [Google Scholar]
- Rivat C., Becker C., Blugeot A., Zeau B., Mauborgne A., Pohl M., Benoliel J.-J. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150(2):358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J.A., Venheim E.R., Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.J., Trivedi M.H., Wisniewski S.R., Nierenberg A.A., Stewart J.W., Warden D., Niederehe G., Thase M.E., Lavori P.W., Lebowitz B.D., McGrath P.J., Rosenbaum J.F., Sackeim H.A., Kupfer D.J., Luther J., Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sadler A.M., Bailey S.J. Repeated daily restraint stress induces adaptive behavioural changes in both adult and juvenile mice. Physiol. Behav. 2016;167:313–323. doi: 10.1016/j.physbeh.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Sang N., Zhang J., Marcheselli V., Bazan N.G., Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J. Neurosci. 2005;25(43):9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SC G., CD C., N A., B S., H R. Nonclinical vehicle use in studies by multiple routes in multiple species. Int. J. Toxicol. 2006;25(6):499–521. doi: 10.1080/10915810600961531. [DOI] [PubMed] [Google Scholar]
- Serrats J., Grigoleit J.S., Alvarez-Salas E., Sawchenko P.E. Pro-inflammatory immune-to-brain signaling is involved in neuroendocrine responses to acute emotional stress. Brain Behav. Immun. 2017;62:53–63. doi: 10.1016/j.bbi.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Shanks N., Griffiths J., Zalcman S., Zacharko R.M., Anisman H. Mouse strain differences in plasma corticosterone following uncontrollable footshock. Pharmacol. Biochem. Behav. 1990;36(3):515–519. doi: 10.1016/0091-3057(90)90249-h. [DOI] [PubMed] [Google Scholar]
- Shen C.J., Zheng D., Li K.X., Yang J.M., Pan H.Q., Yu X.D., Fu J.Y., Zhu Y., Sun Q.X., Tang M.Y., Zhang Y., Sun P., Xie Y., Duan S., Hu H., Li X.M. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 2019;25(2):337–349. doi: 10.1038/s41591-018-0299-9. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Uschold N., Magoni M., Bär J., Popoli M., Neumann I.D., Reber S.O. Behavioural consequences of two chronic psychosocial stress paradigms: anxiety without depression. Psychoneuroendocrinology. 2012;37(5):702–714. doi: 10.1016/j.psyneuen.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Song C., Zhang W.H., Wang X.H., Zhang J.Y., Tian X.L., Yin X.P., Pan B.X. Acute stress enhances the glutamatergic transmission onto basoamygdala neurons embedded in distinct microcircuits. Mol. Brain. 2017;10(1):3. doi: 10.1186/s13041-016-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner E.Y., Kalynchuk L.E. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):777–790. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Stone E.A., Lin Y. An anti-immobility effect of exogenous corticosterone in mice. Eur. J. Pharmacol. 2008;580(1–2):135–142. doi: 10.1016/j.ejphar.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Stuber G.D., Sparta D.R., Stamatakis A.M., van Leeuwen W.A., Hardjoprajitno J.E., Cho S., Tye K.M., Kempadoo K.A., Zhang F., Deisseroth K., Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B., Linda S.F. vol. 1. 2001. (Fidell Using Multivariate Statistics). [Google Scholar]
- Tannenbaum H. Lumiracoxib. Drugs. 2004;64(19):2. [Google Scholar]
- Tata D.A., Anderson B.J. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol. Behav. 2010;99(2):186–193. doi: 10.1016/j.physbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar E.L., Vaessen K.R., Pawluski J.L., Sierksma A.S., Blokland A., Canete R., Steinbusch H.W. Long-term corticosterone exposure decreases insulin sensitivity and induces depressive-like behaviour in the C57BL/6NCrl mouse. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0106960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uum S.H., Sauve B., Fraser L.A., Morley-Forster P., Paul T.L., Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stressforskningsrapporter. 2008;11(6):483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- Wang B., Jin X., Kuang X., Tian S. Chronic administration of parecoxib exerts anxiolytic-like and memory enhancing effects and modulates synaptophysin expression in mice. BMC Anesthesiol. 2017;17(1):152. doi: 10.1186/s12871-017-0443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters P., McCormick C.M. Caveats of chronic exogenous corticosterone treatments in adolescent rats and effects on anxiety-like and depressive behavior and hypothalamic-pituitary-adrenal (HPA) axis function. Biol. Mood Anxiety Disord. 2011;1(1):4. doi: 10.1186/2045-5380-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Andreasson K.I., Kaufmann W.E., Barnes C.A., Worley P.F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11(2):371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yang H., Zhang J., Andreasson K., Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol. Cell. Neurosci. 2008;37(4):682–695. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y., Liu T.H., He Y., Pan H.Q., Zhang W.H., Yin X.P., Tian X.L., Li B.M., Wang X.D., Holmes A., Yuan T.F., Pan B.X. Chronic stress remodels synapses in an amygdala circuit-specific manner. Biol. Psychiatry. 2019;85(3):189–201. doi: 10.1016/j.biopsych.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Rosenkranz J.A. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]