Abstract
There is growing recognition that variation in animal personality traits can influence survival and reproduction rates, and consequently may be important for wildlife population dynamics. Despite this, the integration of personality research into conservation has remained uncommon. Alongside the establishment of personality as an important source of individual variation has come an increasing interest in factors affecting the development of personality. Recent work indicates the early environment, including natal nutrition, may play a stronger role in the development of personality than previously thought. In this study, we investigated the importance of three personality metrics (activity, boldness and acclimation time) for estimating survival of a threatened species, the hihi (Notiomystis cincta), and evaluated the influence of early natal nutrition on those metrics. Our results showed that boldness (as measured from a one-off cage test) had a positive effect on the probability of juvenile hihi surviving to adulthood. There was also a tendency for juveniles that received carotenoid supplementation in the nest to be bolder than those that did not, suggesting that the early environment had some influence on the expression of boldness in juvenile hihi. Linking the development of personality traits with ultimate effects on vital rates may benefit conservation management, as it could enable developmentally targeted management interventions. To our knowledge, this study is the first to identify potential linkages between early natal nutrition, personality and fitness in a wild-living population.
This article is part of the theme issue ‘Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation’.
Keywords: personality, individual behaviour, nutrition, supplemental feeding, survival, hihi
1. Introduction
In recent years, it has become recognized that measured personality traits may reflect individual variation in vital rates, i.e. survival, reproduction and dispersal [1–4]. An understanding of individual variation in behaviour can therefore potentially improve our ability to understand and predict population dynamics.
Alongside this expanding literature linking individual behaviour with vital rates, there has been a growing interest in the development of personality [5]. The early natal environment is of critical importance to a wide range of life-history parameters [6–9], and there is evidence that early development can alter the development of personality [10]. Nutrition is an important component of the natal environment and phenotypic traits such as personality may be sensitive to nutritional conditions [11]. Several studies have now reported a link between personality traits and the early environment [12,13], in particular early nutrition. For example, nestling female zebra finches (Taeniopygia guttata) fed a low-quality diet were found to engage in exploratory and foraging behaviour more quickly than those fed a high-quality (protein enriched) diet [14]. Another study [11] similarly manipulated early micronutrient availability in zebra finches, finding this to affect neophobia and handling response in both sexes, with sex-specific effects on boldness and aggression. Neither study, however, investigated later life-history effects of this early diet manipulation.
Despite the growing evidence that vital rates can be affected by personality, which can, in turn, be influenced by early nutrition, research linking these three components has been lacking to date. Connecting these aspects may be useful to wildlife conservation because it provides a means to not only improve our understanding of the demography of threatened populations but also to improve our ability to effectively manage those populations through a better understanding of the mechanisms driving variation in vital rates. The value of connecting animal personality and applied conservation is well recognized [15,16] but remains underused [17].
We attempt to bridge this frequently cited disconnect between basic and applied research [16] by examining the importance of personality metrics for estimating survival of a threatened species. We investigate the role of early natal nutrition in shaping demographically important behavioural differences among individuals and consider the management implications of our findings.
We evaluated the influence of three personality metrics on survival up to 3 years post-fledging for a threatened forest passerine, the hihi (Notiomystis cincta). Hihi were once found throughout the northern parts of New Zealand, but by the late 1800s they had become restricted to a single offshore island (Hauturu). Hihi have since been reintroduced to seven sites and all extant reintroduced populations currently rely on the provision of supplementary sugar water. Previous research found no detectable effect of neonatal supplementary feeding in hihi juvenile survival from fledging to breeding age [18]. In this study, we investigated whether there might be an indirect effect of natal nutrition on survival via a behavioural pathway. To do this, we used experimental supplementation of protein and carotenoids (potential supplemental feeding options) to examine the effect of natal nutrition on personality metrics and tested whether these metrics were useful for predicting survival. Carotenoids are important biomolecules only obtained through dietary means and have previously been linked to a range of health benefits in hihi [19–22] and other species, including humans (e.g. [23]). In addition, some studies have demonstrated links between carotenoids and behavioural metrics [24–27], yet none have experimentally assessed the role of early carotenoid availability in the later expression of behaviour or long-term survival.
Our study followed free-living individuals throughout their lives, enabling the influences of experimental neonatal nutritional treatments on behaviour and survival to be tracked at later life-history stages.
2. Material and methods
(a). Study site and species
The hihi was reintroduced to Tiritiri Matangi Island (36°36′ S, 174°53′ E, 220 ha, hereafter Tiritiri) in 1995. The island now supports approximately 100 breeding pairs and is intensively managed through the provision of supplementary food (sugar water) and nest-boxes [28]. Breeding activity is intensively monitored, and all nestling hihi are colour banded in the nest at 21 days of age. Juveniles fledge at approximately 30 days old and reach independence at one to two weeks post-fledging. Hihi become sexually mature and breed in their first year. Island-wide resighting surveys are carried out in September and February each year (pre- and post-breeding), when the identities of all hihi seen are recorded. Hence, a comprehensive sightings database exists.
(b). Personality testing
Ninety-seven juvenile hihi (56 males and 41 females) of known parentage were personality tested between 25 February and 23 March 2011 after capture in mist nets. Thirty-eight of these birds were recaptured and translocated to another site in April 2011 [29], and 35 of the other 61 birds were known to survive to their first breeding season (September 2011). Most (80) of the birds tested were from first clutches (94–134 days post-hatching when tested), with the remainder from second clutches (41–65 days post-hatching).
Traditional assays of personality generally involve a period of captivity (e.g. [30,31]) and repeated observations in different contexts to characterize consistency in behaviour. However, this is not always possible or preferable when working with wild populations and particularly with threatened species where handling should be minimized. We developed a method refined from these traditional assays that took 10–15 min per bird and enabled us to obtain three personality metrics. We note that these metrics were obtained at a single point in time under specific circumstances, but do not necessarily reflect an individual's personality—rather they provide a personality proxy obtainable with minimal handling and stress to individuals of a threatened species.
We tested birds in a specifically designed cage (150 × 50 × 50 cm) similar to that used in captive tests, with a removable divider across the middle modified from that used in another study [31]. Each end of the cage had three perches (two low and one high), and vegetation (a mixture of natural fern fronds and some artificial vegetation) was placed around the side and back edges of the cage. The cage was made from plywood except for the front which was covered with wire mesh; this enabled observation from one side, as well as enabling the observer (K.M.R.) to approach without being seen from the side or back. Each end had a small hole that could be opened to allow the bird to escape, and the front side had two large opening doors.
The cage was set up daily near the mist net site (within 50–100 m). Once captured, birds were promptly removed from the mist net and transferred into a black bird bag, weighed and then removed from the bag by hand. The bird was then released into the cage via the escape hole, and the observer would move out of sight. After 5 min, the observer would approach the cage from the back and remove the central divider. After another 5 min, the escape hole on the opposite side to the bird was opened. If the bird still remained after 5 min, the front doors would be left open until the bird left.
All tests were filmed using a Canon SX20 camera. This footage was used to calculate the following personality metrics: (i) activity score: the number of movements (hops or flights) made by the bird in the first 5 min in the cage, (ii) boldness: the latency (seconds) of the bird to move to the ‘novel’ side of the cage after removal of the divider, and (iii) acclimation time: the time between release into the cage and the first body shake. We devised this last measure after noting considerable variation in initial behaviour in the cage. Most birds underwent an initial, but highly variable, high movement phase when first placed inside (presumably an attempt to escape), but would then settle onto one of the perches, shake and ruffle their body feathers, and then sit still and survey the surroundings briefly before resuming movement.
(c). Natal nutrition experiment
In total, 287 nestlings from 84 first-clutch nests underwent supplementary feeding. Full details on the experimental treatment is provided in [18]. Eighty first-clutch individuals were subsequently caught for personality testing, creating four treatment groups of similar size (19 N+C+, 21 C+N−, 19 N+C−, 21 C−N−), with N+ nestlings fed a high protein dietary supplement (Wombaroo™ Lorikeet and Honeyeater Food, Wombaroo Food Products, Glen Osmond, SA, Australia), and N− a control supplement (sugar water, known to be fed to nestlings by parents [22]). Wombaroo was found to slightly increase fecundity (by 5%) in another hihi population [32]. C+ nestlings received the sugar water supplement enhanced with carotenoids (lutein and zeaxanthin in the form of OroGLO® liquid; Kemin Industries, Des Moines, IA, USA) and C− without. Lutein and zeaxanthin are associated with yellow feather pigmentation in hihi, and their ratio in OroGLO liquid is similar to these carotenoids natural ratio in the circulating plasma of the species [33]. Nestlings were fed every second day between 4 and 20 days of age. The volume fed (0.2–3.0 ml) was dependent on age and was estimated to comprise around 5–10% of a hihi nestling's daily intake [34]. Second clutch nestlings did not receive any supplementary feeding. All first-clutch nestlings within a brood shared the same nutritional treatment (N+ or N−), but carotenoid treatment was applied alternately down the pre-treatment weight ranking, with treatment group randomly assigned to the heaviest nestling in the brood (mean brood size = 3.63, s.d. = 0.93).
(d). Data analysis
We modelled the data using OpenBUGS v.3.2.3, which uses Markov chain Monte Carlo techniques to fit Bayesian hierarchical models [35]. This approach allowed multiple random effects to be modelled and enabled imputation of missing values where these were present (between 2 and 5% of individuals for each personality metric, primarily owing to camera or mechanical failure during testing) by sampling from either a normal or Bernoulli distribution as appropriate [36]. We used uninformative priors for all fixed effects (normal distribution with mean 0 and precision 0.01) and hyper-parameters (uniform distribution from 0 to 2). Models were run with two chains for up to 200 000 iterations with an initial burn-in of 10 000 samples after checking convergence by examining the chains and autocorrelation plots.
We modelled hihi survival up to 3 years post-fledging using a state-space formulation of the Cormack–Jolly–Seber (CJS) model, with survival and detection probabilities modelled with logit link functions and Bernoulli error distributions [37]. Using this formulation, the state (alive or dead) of each individual is modelled as a missing value for each survey after it was last seen [37,38]. Fledglings entered the encounter history in March 2011 and were considered juveniles until the pre-breeding survey in September 2011. Individuals were considered adults for the six surveys (pre- and post-breeding) carried out over three breeding seasons (2011/2012, 2012/2013 and 2013/2014). An additional ‘survey’ was included in April 2011 when 38 juveniles were removed for translocation. Here, the harvested individuals were recorded as alive at the time of translocation, and the state of each remaining individual was modelled as a missing value because a resighting survey was not undertaken at that time. The model was coded so that the survival of translocated individuals was not estimated for the post-harvest intervals. Thus, the encounter history comprised eight encounter occasions for 97 individuals.
We initially created a survival model that included all parameters that we considered may affect survival. This model included fixed effects of activity, boldness and acclimation time applied separately to juvenile and adult survival, and fixed effects of sex and clutch applied across both age classes. We included mother of the individual as a random effect to account for the lack of independence among siblings. Detection was modelled with a fixed effect of sex on detection probability because males are more vocal and conspicuous than females and therefore expected to have higher detection probabilities. The detection model also included a random time effect on detection probability, meaning variation in detection probability among surveys was accounted for. Boldness displayed a distinctly bimodal distribution so we rescored this to 0 (did not move to novel side (=shy)) or 1 (did move to novel side (=bold)). We used standardized z-scores for acclimation time and activity score after doing square root transformations to improve normality. Spearman rank-order tests on the three personality metrics showed no correlation between acclimation time and activity (−0.03) or boldness (0.02), and a weak-moderate correlation between activity and boldness (−0.44).
The next phase of our analysis was to investigate the effects of natal nutritional treatment on any personality metric identified as being a ‘significant’ predictor of hihi survival (i.e. effect(s) where 95% credible interval (CRI) excluded zero). To do this, we reduced the CJS model by removing ‘insignificant’ effects (i.e. 95% CRI overlapping zero) and integrated this reduced CJS survival model with a logit-linear model estimating the effects of protein and carotenoid supplementation on personality metrics important for survival. This integrated model structure enabled us to derive estimates of indirect effects of nutritional supplementation on survival through effects on personality while accounting for uncertainties in all parameters. We included an effect of treatment in order to distinguish birds used as controls in the feeding experiment (i.e. given sugar water without carotenoids or protein) from those that had not been treated at all (but which received sugar water from their parents).
Finally, we examined the direct effect of nutritional supplementation on hihi survival by including nutritional treatment in the reduced CJS model, so that personality and nutrition were competing fixed effects on survival.
3. Results
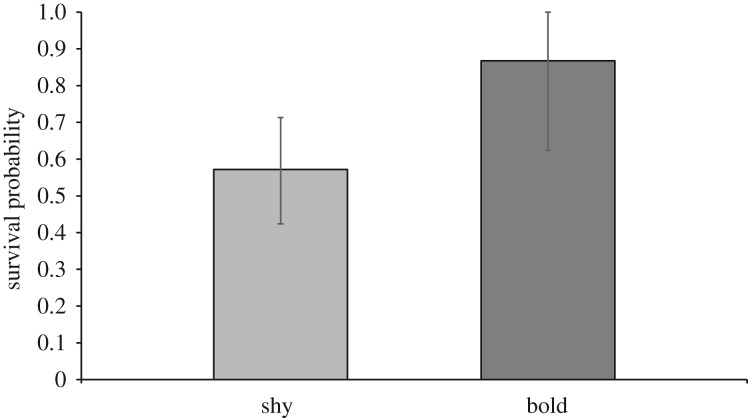
We found a clear effect of boldness on juvenile hihi survival probability (95% CRI = 1.73, 26.02), with bolder fledglings experiencing a higher probability of surviving to adulthood than shy fledglings (figure 1; parameter b.bold.phi.juv in table 1). There was also a tendency for juveniles with higher activity scores to have lower survival to adulthood (parameter b.act.phi.juv in table 1), but the effect was uncertain (95% CRI included zero; −13.18, 0.02). There was no evidence that adult hihi survival probabilities were influenced by any of the three personality metrics included in our analysis or that sex or clutch affected survival (table 1).
Figure 1.
Estimated survival probabilities from fledging to recruitment for shy and bold juvenile hihi on Tiritiri Matangi Island.
Table 1.
Posterior means, standard deviations and 95% credible intervals for effects (logit link) of personality traits (acclimation time, activity score and boldness) measured in juvenile hihi on their subsequent annual survival on Tiritiri Matangi Island. (Data were fitted to a modified version of the CJS model that combines generalized linear mixed models for survival and detection probability. The survival model included fixed effects of sex, age (juvenile versus adult), clutch (first versus second) and personality traits, with the mother of the bird included as a random effect. The detection model included a fixed sex effect and random time effects (i.e. random variation in detection probability among surveys). LCL, lower credible limit; UCL, upper credible limit.)
| parameter | meaning | mean | s.d. | LCL | UCL |
|---|---|---|---|---|---|
| a.phi | survival intercept (logit of annual adult female survival probability) | −0.428 | 1.049 | −2.398 | 1.613 |
| b.sex.phi | effect of sex (male) on survival probability | −0.142 | 0.760 | −1.608 | 1.158 |
| b.age.phi | effect of age (juvenile) on survival probability | 2.218 | 4.250 | −3.292 | 13.410 |
| b.clutch.phi | effect of clutch (second) on survival probability | 0.488 | 0.768 | −1.022 | 1.933 |
| b.acc.phi.juv | effect of acclimation time on juvenile survival probability | −3.695 | 4.603 | −13.360 | 4.958 |
| b.acc.phi.ad | effect of acclimation time on adult survival probability | 0.458 | 0.469 | −0.437 | 1.449 |
| b.act.phi.juv | effect of activity score on juvenile survival probability | −5.154 | 3.675 | −13.180 | 0.022 |
| b.act.phi.ad | effect of activity score on adult survival probability | 0.068 | 0.432 | −0.810 | 0.915 |
| b.bold.phi.juv | effect of boldness on juvenile survival probability | 11.880 | 6.433 | 1.731 | 26.020 |
| b.bold.phi.ad | effect of boldness on adult survival probability | 0.730 | 0.908 | −1.017 | 2.596 |
We found a potential link between carotenoid supplementation and the probability of boldness (i.e. the probability an individual was assigned a boldness score of 1) in hihi. Carotenoid supplementation was estimated to increase the probability of boldness from 0.63 for control-treated birds (N−C−) to 0.80 (based on parameters a.bold, b.feed.bold and b.C.bold in table 2), but this effect was uncertain (95% CRI = −0.12, 2.06 for parameter b.C.bold). Integrating this analysis with the effect of boldness on survival (table 2) showed that carotenoid supplementation did not have a significant effect on juvenile survival (95% CRI = −5.24, 11.29 for parameter b.C.phi.juv). Although carotenoid supplementation was estimated to increase juvenile survival probability from 0.73 (control treatment) to 0.79 owing to the higher probability of boldness, this indirect effect was highly uncertain owing to the combined uncertainties in all parameters. There was no indication of any effect of protein supplementation on any of the personality metrics or on juvenile or adult survival (95% CRIs all included zero), so this effect was not considered further.
Table 2.
Posterior means, standard deviations and 95% credible intervals for indirect effects (logit link) of protein (N) and carotenoid (C) supplementation to hihi nestlings on their subsequent annual survival owing to changes in boldness. (Here, the CJS model described above (table 1) was simplified by removing insignificant factors (b.sex.phi, b.clutch.phi, b.acc.phi.juv, b.acc.phi.ad, b.act.phi.juv and b.act.phi.ad) and integrated with an additional logit-linear model estimating the effects of nestling nutritional supplementation on the probability of the bird being scored as bold. The parameter ‘b.feed.bold’ is included to distinguish birds used as controls in the feeding experiment (i.e. given sugar water without carotenoids or protein) from those that had not been treated at all (but which received sugar water from their parents). The indirect effects shown in the lower part of the table are derived from the main effects shown in the upper part. LCL, lower credible limit; UCL, upper credible limit.)
| parameter | meaning | mean | s.d. | LCL | UCL |
|---|---|---|---|---|---|
| a.phi | survival intercept (logit of annual adult survival probability) | −0.002 | 0.547 | −1.055 | 1.097 |
| b.age.phi | effect of age (juvenile) on survival probability | −1.035 | 0.910 | −2.813 | 0.728 |
| b.bold.phi.juv | effect of boldness on juvenile survival probability | 3.728 | 4.251 | 0.243 | 16.820 |
| b.bold.phi.ad | effect of boldness on adult survival probability | 0.373 | 0.653 | −0.916 | 1.668 |
| a.bold | boldness intercept (logit of probability of bird being bold) | −0.215 | 0.512 | −1.236 | 0.782 |
| b.feed.bold | effect of nestling feeding on probability of boldness | 0.782 | 0.663 | −0.508 | 2.090 |
| b.C.bold | effect of C supplementation on probability of boldness | 0.929 | 0.551 | −0.121 | 2.056 |
| b.N.bold | effect of N supplementation on probability of boldness | −0.071 | 0.530 | −1.107 | 0.971 |
| b.C.phi.juv | indirect effect of C supplementation on juvenile survival probability | 0.688 | 3.843 | −5.244 | 11.290 |
| b.C.phi.ad | indirect effect of C supplementation on adult survival probability | 0.065 | 0.487 | −1.018 | 1.293 |
| b.N.phi.juv | indirect effect of N supplementation on juvenile survival probability | −0.040 | 4.145 | −9.945 | 9.723 |
| b.N.ad | indirect effect of N supplementation on adult survival probability | −0.003 | 0.520 | −1.224 | 1.212 |
When carotenoid supplementation was included in the CJS model as a competing fixed effect with boldness, there was no indication that nestling supplementation of carotenoids had a direct effect on their survival as adults (95% CRI = −0.98, 1.24). We found that carotenoid supplementation was associated with higher juvenile survival, but this effect was uncertain (95% CRI = −1.71, 15.58). The effect of boldness on juvenile survival remained significant (95% CRI excluded zero, table 3).
Table 3.
Posterior means, standard deviations and 95% credible intervals for direct effects (logit link) of carotenoid supplementation versus boldness on annual survival of hihi. (Here, the effects of carotenoid supplementation were added as fixed factors to the simplified CJS model (table 2). LCL, lower credible limit; UCL, upper credible limit.)
| parameter | meaning | mean | s.d. | LCL | UCL |
|---|---|---|---|---|---|
| a.phi | survival intercept (logit of annual adult survival probability) | −0.133 | 0.610 | −1.369 | 1.067 |
| b.age.phi | effect of age (juvenile) on survival probability | −1.006 | 1.312 | −3.173 | 1.523 |
| b.bold.phi.ad | effect of boldness on adult survival probability | 0.473 | 0.654 | −0.813 | 1.751 |
| b.bold.phi.juv | effect of boldness on juvenile survival probability | 4.311 | 4.739 | 0.083 | 18.100 |
| b.C.phi.ad | effect of carotenoid supplementation on adult survival probability | 0.126 | 0.578 | −0.980 | 1.243 |
| b.C.phi.juv | effect of carotenoid supplementation on juvenile survival probability | 2.626 | 4.286 | −1.705 | 15.580 |
4. Discussion
There is growing recognition that personality may contribute to population-level processes and can therefore be relevant to models of population dynamics [16]. This increasing awareness of the direct link between behaviour and vital rates has coincided with an expanding literature on the development of animal personality that examines the role of the early environment (e.g. [11,39,40]). Here, we aimed to form a connection between these two fields of research by investigating the influence of personality metrics on survival of a threatened species and evaluating whether natal nutrition played a role in shaping demographically important personality traits.
As in other studies [1,41], we found a relationship between personality traits of juveniles and their subsequent survival. Our results showed that while the survival of adult hihi was not significantly affected by any of the personality metrics we examined, hihi scored as bold experienced a higher probability of surviving to adulthood than those scored as shy. Previous research on rodents has similarly found that neophobic (shy) individuals had significantly lower survival than their neophilic (bold) counterparts [42,43]. Cavigelli and McClintock [42] found that neophobic rats had a greater hypothalamic–pituitary–adrenal axis response to novelty. This greater response was identified as a potential mechanism for reduced longevity through associated effects on health and physiology. Other physiological links, such as variation in the oxidative costs associated with personality traits (e.g. [44–46]), have also been explored, although little is known about whether oxidative stress affects survival in natural populations [47].
The association we found between higher survival and boldness contrasts with the findings of other studies where boldness was associated with lower survival [1]. For example, bolder juvenile largemouth bass (Micropterus salmoides) were found to have much lower survival than shy bass, most probably owing to differential predation-related mortality [48]. This negative association between boldness and survival is often attributed to bolder individuals experiencing greater vulnerability to predation as a result of being less risk-averse. Hihi have native avian predators on Tiritiri (e.g. ruru, Ninox novaeseelandiae), but exotic mammalian predators such as ship rats (Rattus rattus) and stoats (Mustela ermina) that pose the greatest threat to hihi are absent. As such, predation may not be a strong limitation on juvenile survival. Instead, we suggest that resource limitation (e.g. territory availability) is likely to be a key factor affecting recruitment because the hihi population is at a relatively high density on Tiritiri. Bolder individuals tend to be at the more proactive end of the behavioural spectrum, meaning that they may also be more aggressive and exploratory [41,49]. These traits are likely to provide bolder juveniles with an advantage when establishing a territory among a high density of older conspecifics. In addition, the Tiritiri hihi population relies on sugar water supplementation, which is provided at feeding stations where birds are required to enter a cage-like structure to access the feeder. As such, it is also plausible that bolder juveniles will be more inclined to enter the feeding stations, which could, in turn, provide them with a survival advantage through higher energy intake. Alternatively, there could be a physiological factor (e.g. oxidative status) underlying the relationship. Further work is required to understand the mechanism by which boldness increases juvenile hihi survival to recruitment.
In line with recent suggestions that the early environment, particularly nutrition, plays a role in the development (or expression) of personality traits [11,50,51], there was some evidence that carotenoid supplementation in the nest had a tendency to influence boldness in juvenile hihi. Carotenoid supplementation has been previously linked to health benefits in a range of bird species (e.g. [52,53]). Experimental manipulations of carotenoid availability in adults of two congeneric waterfowl (Anas spp.) showed an effect on exploratory behaviour [24]. Here, carotenoid availability in the nest may have affected boldness, with hihi that received a carotenoid supplement as nestlings tending to be bolder as juveniles. As discussed above, we found that bold individuals had higher juvenile survival probabilities. The effect of carotenoids on survival both directly and indirectly (through their effect on probability of boldness) was very uncertain, although there was a tentative indication that carotenoid supplementation could be associated with higher juvenile survival.
One limitation of our study was our inability to obtain repeated observations of behaviour for the individuals that underwent personality testing, as hihi is a wild-living threatened species and handling/stress needs to be minimized. This meant that the data we collected could only be considered a personality metric (i.e. a proxy for personality) rather than a personality trait linked to consistent individual behaviour. Nevertheless, the ability to collect snapshot data that are useful for making predictions for threatened populations, as was achieved here, is inherently valuable from both a population and resource perspective. Indeed, we suggest that personality metrics for threatened species would ideally require no handling or intervention for the animals involved.
Supplementary feeding is one of the primary management actions implemented to support reintroduced hihi populations. Given our results suggest that carotenoid supplementation may have positive implications for hihi survival, it is now possible for managers to make an informed decision about the merit of adding carotenoids to sugar water throughout the breeding season and also understand the uncertainty around the effectiveness of this management action (table 3). Considering this decision in a structured-decision making framework [54] will enable managers to identify whether this management action will help achieve the identified management objectives for the population (including weighing up expected benefits to the population with financial costs and parameter uncertainty).
The potential for behavioural research to improve our understanding of wildlife populations has led to increasing calls for behavioural ecology to be better integrated into conservation management [15,17]. However, awareness of the importance of behaviour has seldom been translated into relevant management tools [17] resulting in an ongoing disconnect between behavioural ecology and conservation management. Our study helps to bridge this divide by examining the survival consequences of personality in a wild-living population and experimentally manipulating natal nutrition to assess the influence of supplementary feeding treatments on personality and, ultimately, survival. Understanding such links between early environment, behaviour and vital rates enables informed consideration of management actions based on behavioural research.
Supplementary Material
Acknowledgements
We thank the New Zealand Department of Conservation, the Supporters of Tiritiri Matangi, Ngtiwai, Ngti Manuhiri, Ngti Rehua and Ngti Paoa for allowing us to conduct this study on Tiritiri Matangi Island, and all those involved in the collection of survival data 2011–2014. We gratefully acknowledge Bryan Jenkins for building the cage used for personality testing. Kemin Industries donated carotenoids for the nestling nutrition experiment, and Annette Fayet assisted with fieldwork. We thank Isabel Castro, Todd Dennis, Jim Dale and David Saltz for providing helpful comments on early versions of this manuscript.
Ethics
Personality testing was carried out under a High Impact Research Permit from the New Zealand Department of Conservation (AK-30463-FAU) and with approval from the Massey University Animal Ethics Committee (11/02). In addition, approved methods were assessed and refined in the field to minimize or eliminate any potential adverse effects to individual animals, as noted above. The nestling nutritional experiments were carried out under a High Impact Research Permit from the Department of Conservation (AK-24128-FAU), with animal ethics permission from the Zoological Society of London.
Data accessibility
Dataset is provided in the electronic supplementary material, table S1.
Authors' contributions
K.M.R. conceived of, designed and coordinated the personality testing, carried out fieldwork, helped conduct the initial data analysis and helped draft the manuscript. E.H.P. participated in the final data analysis and drafted the manuscript. L.K.W. conceived of, designed and coordinated the nestling nutritional experiments, carried out fieldwork and helped draft the manuscript. K.A.P. participated in design of the study and helped draft the manuscript. J.G.E. and D.P.A. assisted in the design and coordination of the study and helped draft the manuscript, and D.P.A. conducted the final data analysis. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The study was supported by Marsden grant no. 17-MAU-091 and Leverhulme Trust Research grant no. (F/00 390/E), a Massey University Doctoral Scholarship to K.M.R., a NERC Research Studentship to L.K.W. and a RCUK Fellowship to J.G.E.
References
- 1.Smith BR, Blumstein DT. 2007. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. ( 10.1093/beheco/arm144) [DOI] [Google Scholar]
- 2.Nicolaus M, Tinbergen JM, Ubels R, Both C, Dingemanse NJ. 2016. Density fluctuations represent a key process maintaining personality variation in a wild passerine bird. Ecol. Lett. 19, 478–486. ( 10.1111/ele.12584) [DOI] [PubMed] [Google Scholar]
- 3.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 4.Spiegel O, Leu ST, Bull CM, Sih A. 2017. What's your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol. Lett. 20, 3–18. ( 10.1111/ele.12708) [DOI] [PubMed] [Google Scholar]
- 5.Trillmich F, Hudson R. 2011. The emergence of personality in animals: the need for a developmental approach. Dev. Psychobiol. 53, 505–509. ( 10.1002/dev.20573) [DOI] [PubMed] [Google Scholar]
- 6.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 8.Blount JD, Metcalfe NB, Arnold KE, Surai PF, Devevey GL, Monaghan P. 2003. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc. R. Soc. Lond. B 270, 1691–1696. ( 10.1098/rspb.2003.2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasumovic MM. 2013. The multidimensional consequences of the juvenile environment: towards an integrative view of the adult phenotype. Anim. Behav. 85, 1049–1059. ( 10.1016/j.anbehav.2013.02.009) [DOI] [Google Scholar]
- 10.Rödel HG, Meyer S. 2011. Early development influences ontogeny of personality types in young laboratory rats. Dev. Psychobiol. 53, 601–613. ( 10.1002/dev.20522) [DOI] [PubMed] [Google Scholar]
- 11.Noguera JC, Metcalfe NB, Surai PF, Monaghan P. 2015. Are you what you eat? Micronutritional deficiencies during development influence adult personality-related traits. Anim. Behav. 101, 129–140. ( 10.1016/j.anbehav.2014.12.029) [DOI] [Google Scholar]
- 12.Dingemanse NJ. 2004. The relation between dominance and exploratory behavior is context-dependent in wild great tits. Behav. Ecol. 15, 1023–1030. ( 10.1093/beheco/arh115) [DOI] [Google Scholar]
- 13.Carere C, Drent PJ, Koolhaas JM, Groothuis TGG. 2005. Epigenetic effects on personality traits: early food provisioning and sibling competition. Behaviour 142, 1329–1355. ( 10.1163/156853905774539328) [DOI] [Google Scholar]
- 14.Krause ET, Honarmand M, Wetzel J, Naguib M. 2009. Early fasting is long lasting: differences in early nutritional conditions reappear under stressful conditions in adult female zebra finches. PLoS ONE 4, e5015 ( 10.1371/journal.pone.0005015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greggor AL, et al. 2016. Research priorities from animal behaviour for maximising conservation progress. Trends Ecol. Evol. 31, 953–964. ( 10.1016/j.tree.2016.09.001) [DOI] [PubMed] [Google Scholar]
- 16.Merrick MJ, Koprowski JL. 2017. Should we consider individual behavior differences in applied wildlife conservation studies? Biol. Conserv. 209, 34–44. ( 10.1016/j.biocon.2017.01.021) [DOI] [Google Scholar]
- 17.Berger-Tal O, Blumstein DT, Carroll S, Fisher RN, Mesnick SL, Owen MA, Saltz D, St Claire CC, Swaisgood RR. 2016. A systematic survey of the integration of animal behavior into conservation. Conserv. Biol. 30, 744–753. ( 10.1111/cobi.12654) [DOI] [PubMed] [Google Scholar]
- 18.Walker LK, Armstrong DP, Brekke P, Chauvenet ALM, Kilner RM, Ewen JG. 2013. Giving hihi a helping hand: assessment of alternative rearing diets in food supplemented populations of an endangered bird. Anim. Conserv. 16, 538–545. ( 10.1111/acv.12026) [DOI] [Google Scholar]
- 19.Ewen JG, Thorogood R, Karadas F, Pappas AC, Surai PF. 2006. Influences of carotenoid supplementation on the integrated antioxidant system of a free living endangered passerine, the hihi (Notiomystis cincta). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 143, 149–154. ( 10.1016/j.cbpa.2005.11.006) [DOI] [PubMed] [Google Scholar]
- 20.Ewen JG, Thorogood R, Karadas F, Cassey P. 2008. Condition dependence of nestling mouth colour and the effect of supplementing carotenoids on parental behaviour in the hihi (Notiomystis cincta). Oecologia 157, 361–368. ( 10.1007/s00442-008-1073-3) [DOI] [PubMed] [Google Scholar]
- 21.Ewen JG, Thorogood R, Brekke P, Cassey P, Karadas F, Armstrong DP. 2009. Maternally invested carotenoids compensate costly ectoparasitism in the hihi. Proc. Natl Acad. Sci. USA 106, 12 798–12 802. ( 10.1073/pnas.0902575106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorogood R, Kilner RM, Karadaş F, Ewen JG. 2008. Spectral mouth colour of nestlings changes with carotenoid availability. Funct. Ecol. 22, 1044–1051. ( 10.1111/j.1365-2435.2008.01455.x) [DOI] [Google Scholar]
- 23.Johnson EJ. 2002. The role of carotenoids in human health. Nutr. Clin. Care 5, 56–65. ( 10.1046/j.1523-5408.2002.00004.x) [DOI] [PubMed] [Google Scholar]
- 24.Rowe M, Pierson KL, McGraw KJ. 2015. Exploratory behavior is associated with plasma carotenoid accumulation in two congeneric species of waterfowl. Behav. Processes 115, 181–190. ( 10.1016/j.beproc.2015.04.008) [DOI] [PubMed] [Google Scholar]
- 25.Pryke SR, Andersson S, Lawes MJ, Piper SE. 2002. Carotenoid status signaling in captive and wild red-collared widowbirds: independent effects of badge size and color. Behav. Ecol. 13, 622–631. ( 10.1093/beheco/13.5.622) [DOI] [Google Scholar]
- 26.Grunst ML, Grunst AS, Parker CE, Romero LM, Rotenberry JT. 2014. Pigment-specific relationships between feather corticosterone concentrations and sexual coloration. Behav. Ecol. 26, 706–715. ( 10.1093/beheco/aru210) [DOI] [Google Scholar]
- 27.Backström T, Heynen M, Brännäs E, Nilsson J, Magnhagen C. 2015. Dominance and stress signalling of carotenoid pigmentation in Arctic charr (Salvelinus alpinus): lateralization effects? Physiol. Behav. 138, 52–57. ( 10.1016/j.physbeh.2014.10.003) [DOI] [PubMed] [Google Scholar]
- 28.Taylor S, Castro I, Griffiths R. 2005. Hihi/stitchbird (Notiomystis cincta) recovery plan 2004–2009. In Threatened species recovery plan, p. 31 Wellington, New Zealand: Department of Conservation. [Google Scholar]
- 29.Richardson KM. 2015. Dispersal: the effects of phenotype and habitat selection in reintroduced populations. Palmerston North, New Zealand: Massey University. [Google Scholar]
- 30.Verbeek MEM, Drent PJ, Wiepkema PR. 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim. Behav. 48, 1113–1121. ( 10.1006/anbe.1994.1344) [DOI] [Google Scholar]
- 31.Herborn KA, Macleod R, Miles WTS, Schofield ANB, Alexander L, Arnold KE. 2010. Personality in captivity reflects personality in the wild. Anim. Behav. 79, 835–843. ( 10.1016/j.anbehav.2009.12.026) [DOI] [Google Scholar]
- 32.Armstrong DP, Castro I, Griffiths R. 2007. Using adaptive management to determine requirements of re-introduced populations: the case of the New Zealand hihi. J. Appl. Ecol. 44, 953–962. ( 10.1111/j.1365-2664.2007.01320.x) [DOI] [Google Scholar]
- 33.Ewen JG, Surai P, Stradi R, Møller AP, Vittorio B, Griffiths R, Armstrong DP. 2006. Carotenoids, colour and conservation in an endangered passerine, the hihi or stitchbird (Notiomystis cincta). Anim. Conserv. 9, 229–235. ( 10.1111/j.1469-1795.2006.00028.x) [DOI] [Google Scholar]
- 34.Page D. 2008. Hand rearing attempt on a 20 day old hihi (Notiomystis cincta) nestling. Internal Report to the New Zealand Department of Conservation.
- 35.Spiegelhalter D, Thomas A, Best N, Lunn D. 2014. OpenBUGS user manual, version 3.2.3. Cambridge, UK: Biostatistics Unit. [Google Scholar]
- 36.Nakagawa S, Freckleton RP. 2008. Missing inaction: the dangers of ignoring missing data. Trends Ecol. Evol. 23, 592–596. ( 10.1016/j.tree.2008.06.014) [DOI] [PubMed] [Google Scholar]
- 37.Kéry M, Schaub M. 2012. Bayesian population analysis using WinBUGS: a hierarchical perspective. Waltham, MA: Academic Press. [Google Scholar]
- 38.Schofield MR, Barker RJ, MacKenzie DI. 2009. Flexible hierarchical mark-recapture modeling for open populations using WinBUGS. Environ. Ecol. Stat. 16, 369–387. ( 10.1007/s10651-007-0069-1) [DOI] [Google Scholar]
- 39.Groothuis TG, Trillmich F. 2011. Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641–655. ( 10.1002/dev.20574) [DOI] [PubMed] [Google Scholar]
- 40.Krause ET, Krüger O, Schielzeth H. 2017. Long-term effects of early nutrition and environmental matching on developmental and personality traits in zebra finches. Anim. Behav. 128, 103–115. ( 10.1016/j.anbehav.2017.04.003) [DOI] [Google Scholar]
- 41.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 42.Cavigelli SA, McClintock MK. 2003. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc. Natl Acad. Sci. USA 100, 16 131–16 136. ( 10.1073/pnas.2535721100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piquet JC, López-Darias M, van der Marel A, Nogales M, Waterman J. 2018. Unraveling behavioral and pace-of-life syndromes in a reduced parasite and predation pressure context: personality and survival of the Barbary ground squirrel. Behav. Ecol. Sociobiol. 72, 147 ( 10.1007/s00265-018-2549-8) [DOI] [Google Scholar]
- 44.Costantini D, Carere C, Caramaschi D, Koolhaas JM. 2008. Aggressive and non-aggressive personalities differ in oxidative status in selected lines of mice (Mus musculus). Biol. Lett. 4, 119–122. ( 10.1098/rsbl.2007.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herborn KA, Coffey J, Larcombe SD, Alexander L, Arnold KE. 2011. Oxidative profile varies with personality in European greenfinches. J. Exp. Biol. 214, 1732–1739. ( 10.1242/jeb.051383) [DOI] [PubMed] [Google Scholar]
- 46.Arnold KE, Herborn KA, Adam A, Alexander L. 2015. Individual variation in the oxidative costs of personality traits. Funct. Ecol. 29, 522–530. ( 10.1111/1365-2435.12375) [DOI] [Google Scholar]
- 47.Constantini D. 2014. Oxidative stress and hormesis in evolutionary ecology and physiology: a marriage between mechanistic and evolutionary approaches, 348 p Berlin, Germany: Springer. [Google Scholar]
- 48.Ballew NG, Mittelbach GG, Scribner KT. 2017. Fitness consequences of boldness in juvenile and adult largemouth bass. Am. Nat. 189, 396–406. ( 10.1086/690909) [DOI] [PubMed] [Google Scholar]
- 49.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamps J, Groothuis TG. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. Camb. Philos. Soc. 85, 301–325. ( 10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 51.Groothuis TGG, Maestripieri D. 2013. Parental influences on offspring personality traits in oviparous and placental vertebrates. In Animal personalities: behavior, physiology and evolution (eds Carere C, Maestripieri D), pp. 317–352. Chicago, IL: University of Chicago Press. [Google Scholar]
- 52.McGraw KJ, Adkins-Regan E, Parker RS. 2005. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92, 375–380. ( 10.1007/s00114-005-0003-z) [DOI] [PubMed] [Google Scholar]
- 53.Cucco M, Guasco B, Malacarne G, Ottonelli R. 2006. Effects of β-carotene supplementation on chick growth, immune status and behaviour in the grey partridge, Perdix perdix. Behav. Processes 73, 325–332. ( 10.1016/j.beproc.2006.08.002) [DOI] [PubMed] [Google Scholar]
- 54.Converse SJ, Moore CT, Armstrong DP. 2013. Demographics of reintroduced populations: estimation, modeling, and decision analysis. J. Wildlife Manage. 77, 1081–1093. ( 10.1002/jwmg.590) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Dataset is provided in the electronic supplementary material, table S1.