Abstract
Protecting wild places is conservation's most pressing task given rapid contemporary declines in biodiversity and massive land use changes. We suggest that behavioural ecology has a valuable, albeit limited, role to play in this agenda. Behaviourally based empiricism and modelling, especially of animal movements and habitat preferences have enjoyed wide applicability in delineating reserve boundaries. In protected areas that sanction exploitation, it may also be important to understand individuals' behavioural and life-history responses to management decisions. We also argue, however, that the in-depth studies of behavioural ecologists may have an important role in conservation by elevating species’ status from mundane to charismatic and often sparking public empathy, and their mere presence in field generates local (or broader) intrigue. More generally behavioural ecologists will only be listened to, and their contributions considered of conservation importance, if they become more involved in decision-making processes as witnessed by several prominent examples that have supported the establishment of protected areas.
This article is part of the theme issue ‘Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation’.
Keywords: flagship species, habitat selection, hunting, movement ecology, participation, reserve design
1. The critical import of in situ conservation
It is widely accepted that the principal biological challenge this century is to conserve biodiversity and functioning ecosystems [1,2]. Generally, it is agreed that this involves protecting wild nature effectively in its natural state and in the absence of invasive species, rather than in anthropogenically altered habitats [3,4]. This goal is becoming increasingly difficult to achieve, however, because remaining wild places are dwindling rapidly as they are absorbed by humans [5,6]. To make headway, we need to establish and effectively manage protected areas quickly because species richness declines so precipitously with anthropogenic disturbance [7,8]. Here we ask whether behavioural ecologists can contribute to saving wild habitats and the biodiversity that lives there, and thus help bridge the time until circumstances hopefully become more benign [9].
Several aspects of behavioural ecology have applicability to conservation. Sometimes the study of kin selection, sexual selection, social networks, sensory ecology, mating systems, decision making, altruism, antipredator strategies and animal personalities can provide information for captive breeding programmes of endangered species, increase the probability of reintroductions being successful [10], or help animals survive in anthropogenically altered landscapes [11]. Moreover, animal behaviour studies can predict responses of species to anthropogenic disturbance [12] such as noise (e.g. [13]), habitat fragmentation (e.g. [14]) and chemical or light pollution (e.g. [15,16]) although this, in essence, is a reactive paradigm documenting and predicting behavioural responses to environmental change [17] rather than being a proactive approach to protect species better.
These behaviour–conservation strategies are not the focus of this article, however. Instead we want to explore a different and ambitious question concerning the extent to which behavioural ecology study can facilitate the protection of large numbers of species and ecological processes in the wild, specifically through the establishment and maintenance of protected areas. Not only is this question germane to protecting natural ecosystems but professional societies that concentrate on behavioural ecology and animal behaviour now have a strong practical interest in how their disciplines contribute to in situ conservation [18]. In addition to examining the discipline of behavioural ecology, we will argue that it may be that the motivation and long-term presence of its practitioners in the field has as much chance of promoting in situ conservation as their scientific findings per se. Such a supposition may seem counterintuitive. However, conservation is not simply concerned with amassing good data but critically relies on people working together for a common cause and developing policy that promotes biodiversity. Often this is through the establishment of effective protected areas [19,20].
Here we divide the establishment of protected areas into three categories: (i) generating initial interest and political will, (ii) delineating protected area boundaries, and (iii) maintaining protection once the reserve is recognized. If behavioural ecologists can become involved in these activities they can potentially transform conservation landscapes [21].
2. Generating interest in setting up protected areas
Despite conservation biologists' best efforts in systematic conservation planning using metrics steeped in assessments of species richness, endemism and threatened status [22], politicians wanting to lend support to protected area establishment still gravitate towards particularly compelling species, especially those that resonate with the public (table 1). Often flagship species are large charismatic mammals or perhaps birds [23], and animal behaviourists working on other species sometimes contend that their study organism is inappropriate for promoting the conservation agenda [24]. We would argue, however, that charismatic species are not a unitary category; the criteria by which flagship species are chosen differ subtly according to objectives that include raising conservation awareness, influencing policy, protection of species or habitats, or fund raising. In regards to choosing flagship species to protect habitats and other species, Barua et al. [25] suggest the most important factors are geographical location and range, conservation status and population size, and their ability to act as umbrella species [26] rather than being large homeotherms. Indeed there are now plenty of examples of scientists advancing poikilothermic vertebrates as flagship species (e.g. [27]). As illustrations, high-profile species such as marine mammals, seabirds, predatory fishes, sea turtles and sharks congregate in productive areas [28] where protection of these areas could result in conserving much marine biodiversity. Similarly, in freshwater environments, aquatic mammals, birds, fishes, mussels and dragonflies have all been suggested as offering protection to additional species [29]. Other authors have proposed insects [30], plants [31] and fungi [32] as flagship species. Kitulo National Park in Tanzania was set up to conserve orchids. Moreover, charismatic status is not a universal quality; it varies by location and over time. For instance, we found that contrary to western expectations, jaguars Panthera onca, giant anteaters Myrmecophaga tridactyla and tapirs Tapirus terrestris were less popular for Guyanese schoolchildren, but aripaima fish Aripaima aripaima, macaws and toucans were more popular, cautioning against western non-government organizations (NGOs) using their ‘own’ flagship species to generate local conservation interest [33]. Similarly, rarity is a critical aspect for conservation effort and as populations dwindle over time, new species gain prominence. Pangolins and sharks are now important foci of protection owing to intense exploitation. Difficult as it may be, behavioural ecologists interested in in situ conservation could do well to choose to work on rare species, although the route is tricky because small sample sizes may prevent publication in top journals.
Table 1.
Use of flagship species in establishing reserves (from [23]).
| species | reserve |
|---|---|
| arthropods | |
| monarch butterflies | El Rosario Monarch Butterfly Sanctuary, Michoacan, Mexico |
| birds | |
| pelicans | Pelican National Wildlife Reserve, Florida, USA |
| flamingoes | Lake Nakuru National Park, Kenya |
| ivory-billed woodpecker | expansion of National Wildlife Refuge system in SE USA |
| mammals | |
| jaguar | Cockscomb Jaguar Nature Reserve, Belize |
| tiger | 15 tiger reserves in India |
| Javan rhinoceros and Javan tiger | Ujung Kulon National Park, Indonesia |
| African elephant | Addo National Park, South Africa |
| Baird's tapir | Tapir Mountain Nature Reserve, Belize |
| plants | |
| redwoods | Avenue of Giants Redwood State and National Parks, California, USA |
In summary, we think that behavioural ecologists can use their findings about almost any species to enthuse governmental bodies and local politicians [34]. Perhaps this is where knowledge of behavioural ecology can be most effective in advancing interest in conserving species by making innocuous (at least to the public) species ‘exciting’. Fascinating discoveries about even small species create public intrigue, for example rodents dismissed as vermin or just rats and mice (table 2). Peculiar behavioural findings have often been instrumental in making species infamous. Antechinus was viewed as simply a small brown marsupial until discoveries about its life history made it unique among mammals in that all males die after mating once, giving insights into the reasons that animals show natal dispersal [41].
Table 2.
Examples of rodents that have extraordinary traits.
| arctic ground squirrel Spermophilus parryii
survives core body temperatures less than 0°C without freezing |
[35] |
| California ground squirrel Otospermophilus beecheyi
populations sympatric with Pacific rattlesnakes exhibit venom resistance |
[36] |
| crested rat Lophiomys imhausi chews, masticates and licks poisonous cardenolide from the plant Acokanthera on to its fur and advertises this aposematically |
[37] |
| North American porcupine Erethizon dorsatum has detachable quills |
[38] |
| Norway lemming Lemmus lemmus migrates at high densities |
[39] |
| Laotian rock rat Laonastes aenigmamus is the sole surviving member of a long-extinct family (Diatomyidae) |
[40] |
An example with which we are familiar is Caro's current study of the adaptive significance of red/blue coloration in coconut crabs on Pemba Island, Tanzania that is generating support for conserving this species. To investigate this colour polymorphism, it was necessary to determine where coconut crab populations were greatest and this led to an island-wide survey (figure 1). Next, repeated counting of crab morphs in promising areas established population monitoring programmes. Then working with the Department of Forestry and Non-Renewable Natural Resources, crab conservation posters were distributed to all schools across the island, visits were made to every coastal ward to assess crab exploitation by children, and village education meetings were set up in those wards where crab populations are robust. The Department then featured live coconut crabs in an island-wide exhibition of its conservation activities. Such collaborative actions (see below) were initiated as a consequence of a basic research study [42].
Figure 1.
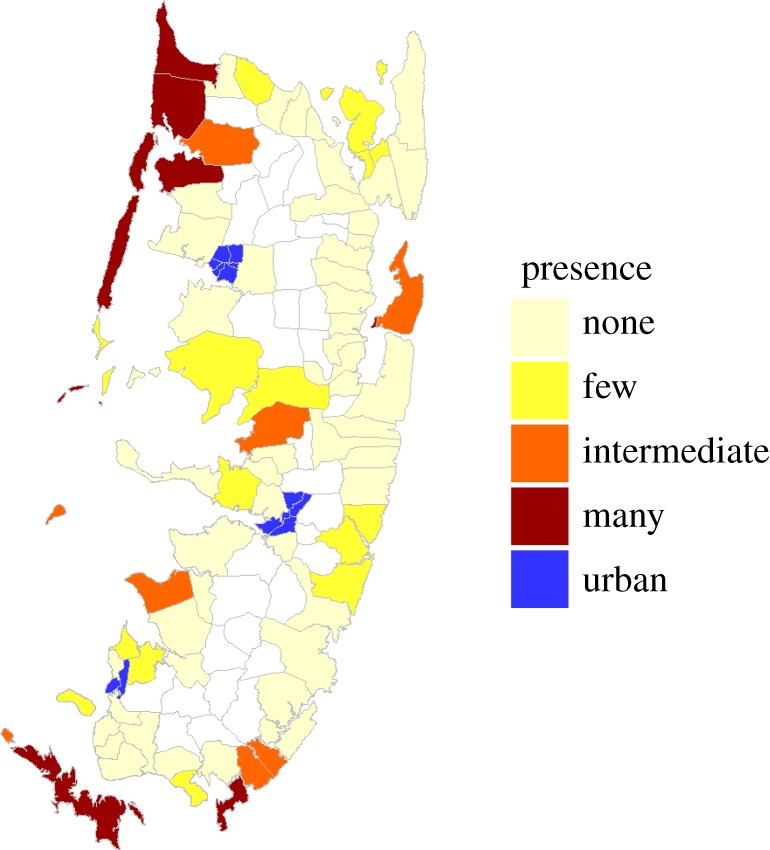
Map of coconut crab distribution on Pemba 2017.
Finally, wildlife films that feature all types of species' behaviour and ecology are regarded as having enormously increased the public's affiliation with the natural world and wish to conserve it [43]. Ideally local engagement in film production should be increased and shown as part of a conservation education programme that incorporates other education materials and group discussion so that the desired conservation message is reinforced. For example, in western Tanzania, the WASIMA (Watu, Simba na Masingira; people, lions and the environment) campaign to stop lion killing by Sukuma pastoralists uses wildlife films in villages to sensitize Sukuma warriors to the wonders of nature and attracts hundreds of people at film sittings [44] (http://www.lcmo.or.tz/wasima/).
3. Delineating appropriate protected area boundaries
(a). Animal movement
Studies of animal movement can delineate the size and shape of a proposed reserve (figure 2). While individuals of most species live in circumscribed areas sometimes defending territories, some individuals, particularly those of large-bodied organisms may range widely. Such species can be useful in setting reserve boundaries because they may encompass viable populations of other taxa (act as umbrella species, [23]). The shape of the boundaries of the Serengeti National Park and Ngorongoro Conservation Area, Tanzania and Masai Mara National Reserve, Kenya were established to incorporate the annual migration of wildebeest Connochaetes taurinus, for example [45]. In marine environments, protected areas are being created to encompass movements of high profile species such as sharks [46] and migratory sea turtles [47]. Choosing a wide ranging species on which to conduct behavioural ecological research therefore has considerable conservation merit, although may be difficult to study.
Figure 2.
Examples where knowledge of species' movement affects protected area policy. (a) Annual migration patterns of wildebeest delineated the boundaries of the Serengeti National Park, Tanzania (photo Tim Caro); (b) understanding movements of large carnivores outside protected areas predict human-wildlife conflict and pressures on carnivore populations inside reserves (photo Joel Berger); (c) path of the annual trek of pronghorn antelope in Wyoming USA shaped protection of a wildlife corridor (photo Joel Berger); (d) knowledge of behaviour of large mammals near roads formulates road crossing construction (photo Tim Caro).
Ranging data show the size of a reserve necessary to support a viable population of a particular species, and can also indicate the proportion of individuals that spend time outside a reserve. This is an important consideration for dangerous predators that may be persecuted by people [48], for edible species that may be eaten [49], or for species that can be infected by diseases of domestic animals [50]. Also, natal dispersal, crucial to maintaining genetic variation, needs documentation and requires following known individuals over time, or using non-invasive genetic methods for assessing individual identities and population structure. Reserve size can potentially be enlarged on the basis of these types of behavioural data [51]. Behavioural ecological study of the underlying causes of movement patterns can shed light on an area's suitability and how diseases spread [52].
To provide functional connectivity between protected areas, we need to know about animal movement patterns across anthropogenically altered landscapes using remote sensing, GPS tracking and high-resolution data (e.g. [53–55]). Berger [56] used movement information to pinpoint bottlenecks in a long distance migration of pronghorn antelope Antilocapra americana so as to delimit a corridor through areas under heavy petroleum development in Wyoming, USA. This effort led to the nation's only federally protected migration pathway: Path of the Pronghorn. It was not science alone that brought about this conservation policy success but a prolonged foray in social and human dimensions that involved multiple governmental agencies, other stakeholders including petroleum and livestock interests, and the public [57].
Human footprints are expanding in many ways including the construction and upgrading of roads through protected areas [58,59]. To facilitate compromises between development and conservation, crossing structures are being used to maintain animal movements. Behavioural observations have been valuable in determining whether animals use bridges, underpasses and culverts to cross these road barriers and hence how they should be constructed [60]. Observations have shown that location of crossing points, structural dimensions, approaches, fencing and traffic noise are important factors influencing the probability of individuals traversing [61,62]; behavioural knowledge can be used to construct environmental cues that will attract animals to crossing structures.
(b). Habitat selection
Animals spend disproportionate amounts of time in certain places. These may be areas of food abundance, where mating opportunities exist, where they can give birth or where predators and parasites are less prevalent. Such places need formal protection and effective policing. Therefore, pinpointing the extent to which individuals use clumped resources such as ephemeral green flushes of grass, or salt licks and why they use them is important. Further, the timing of the appearance of food such as masting trees or vernal pools needs documentation. Similarly, the places and times at which males collect on leks to attract females need to be known if protection is to be effective. In Wyoming, locations of sage grouse lekking sites have been incorporated into an order from the State Governor's office to enlarge the area of protection and safe guard 1300 more males on leks. As a consequence, another 150 000 acres of land have been protected, limiting oil and gas development in 24% of the State: 82% of the State's sage grouse population is now protected (https://www.wyoleg.gov/InterimCommittee/2015/06-0810APPENDIX18.pdf).
Such behavioural knowledge is akin to knowing about meta-population structure (where ephemeral subpopulations occupy temporary habitats and send out dispersers to other empty habitats). Thus the reasons that animals move, the cues they use during movement and the environmental factors promoting settling are all important in understanding the extent to which subpopulations are interconnected [63]. Indeed, such landscape spatial arrays may go undetected in the absence of behavioural ecological knowledge.
Understanding cues that individuals use in selecting habitats is also important in fragmented surroundings where individuals need to move between protected areas across anthropogenically altered landscapes [64,65]. This knowledge opens up the possibility of using these cues to attract animals to breeding sites or safe areas [66,67].
4. Furthering protection
Better management of certain target species within a protected area can be informed by behavioural data. Examples include understanding flight distances and behavioural changes caused by recreational tourism. Set-back zones can then be initiated near feeding stations or waterholes where animals collect, or else movable buffer areas can be designated around the animals themselves (e.g. [68]). Species management understandably falls under the purview of sovereign countries, and the rules that are implemented vary geographically for the same species. In Norway where people have been injured or killed by muskoxen Ovibos moschatus, minimal viewing distances are mandated at 200 m. In Alaska, restrictions vary from 50 m in protected federal parks to none on state land [69].
In multiple-use protected areas where legal exploitation of target species occurs, monogamous and weakly polygynous species are much more susceptible to culling of males than highly polygynous species [70,71] as are species with male parental care or infanticide [72]. Knowledge about infanticide in lions Panthera leo has led to rules being established and booklets being produced for hunters using multiple-use protected areas [73]. This is because removal of breeding adult males encourages new males to enter the pride and commit infanticide, a behaviour which in turn reduces juvenile recruitment (see also [74]).
Beyond infanticide, additional conservation insights are emerging by comparing animals' behaviour in protected areas where hunting-mediated tourism is allowed or precluded. On Russia's Wrangel Island—the Arctic's only UNESCO designated Biosphere's Reserve—the hunting of muskoxen is forbidden, but in Arctic Alaska it is permissible. Consequently, adult sex ratios are highly skewed in Alaska. More than 97% of muskoxen groups on Wrangel contain females with at least one male, whereas almost a third of the Alaskan groups lack males. Because female-only groups are more likely to flee in simulated interactions with brown bears Ursus arctos, the probability of predatory pursuit is likely to increase in the presence of hunting and this may result in poorer juvenile survival [69]. Protected areas have important roles in providing behavioural baselines.
In long-lived species, certain older individuals have great knowledge so that removal of these individuals by hunting or live-capture can have disproportionately negative effects on the groups [75]. For example, older African elephant Loxodonta africana matriachs are better able to discriminate calls of close and distant associates [76] and are more adept at discriminating roars of dangerous male lions from less troublesome female lions [77], even if network analysis suggests that the loss of knowledge is partly compensated for by daughters in the wake of poaching [78].
In marine protected areas where fishing offtake is permitted, knowledge of fish breeding systems can set more appropriate fishing rules. For example, in species with size-dependent hermaphroditism, offtake of larger individuals has differential effects on population. For instance in gag Mycteroperca microlepis, a hermaphroditic grouper, high fishing mortality results in highly skewed female sex ratios necessitating changes in fishing practices (e.g. [79]).
5. Assessing the impact of management and policy decisions
Management decisions made in situ sometimes require behavioural study to assess conservation consequences. Zimbabwe and Namibia were the first countries to remove horns from live black rhinoceroses Diceros bicornis in an attempt to reduce poacher incentives yet the biological outcomes of the manipulation were unknown. Behavioural ecological study eventually revealed that mothers without weapons were poor at defending their calves against spotted hyaenas Crocuta crocuta [80]. In another example, individual monitoring of moose Alces alces juveniles showed that the practice of hunting their mothers reduced overwinter survival [81]. By contrast, juvenile body size and maternal dominance did not. These findings from protected areas in the Greater Yellowstone ecosystem led the states of Wyoming and Idaho to alter human harvest regulations and led ultimately to policy change that helped to protect mothers and indirectly help their offspring [81]. In summary, study of behavioural and life-history components embedded in an analytical framework exploring juvenile survival had an unintended conservation consequence and netted a change in harvest policy.
6. Researcher presence
The presence of behavioural ecologists at field sites may also help in reserve protection, at least in some instances. Laurance [82] pieced together more than 10 anecdotal reports of researchers reducing poaching pressure, ranging from unconventional anti-poaching patrols conducted by Dian Fossey, to using camera traps to detect poachers, to funding aerial surveys to detect reserve encroachment. Most often, researcher influence is passive with poachers avoiding areas used for study. For example, in the Greater Mahale ecosystem in western Tanzania, the rate at which snares were encountered rose with distance from the researcher base and overall mammal encounter rates decreased. Critically, encounter rates of nine edible mammals rose every year after the arrival of researchers in this remote area with minimal governmental surveillance [83].
Sometimes behavioural ecologists working in the field conduct conservation projects at their field site while separately carrying out basic research. For example, Caro documented biodiversity under different forms of land use and monitored large mammal population changes over time in Katavi National Park at the same time as trying to discover why zebra species have stripes. To a conservation manager wanting advice in making protected area decisions, the fact that a researcher is carrying out two biological projects may be of little consequence.
‘Volunteer’ researchers now often pay considerable sums of money to work for short periods on ecological or behavioural research projects in protected areas, usually to organizations that arrange their logistics. Arrangements could be made for some of that money to be used to help fund the upkeep of reserve facilities [84]. Behavioural ecologists are well placed to catalyse research and conservation funding in this way.
Seminars and workshops given in villages adjacent to reserves can help to change local opinion about natural resources on their doorstep. At the very least, this sort of researcher engagement raises interest [85] and is an approach regularly used by NGOs such as the Wildlife Conservation Society.
7. The need to engage
There is still immense enthusiasm for gazetting new protected areas, in part to meet the Aichi biodiversity targets of the Convention on Biological Diversity in setting aside at least 17% of terrestrial and inland water, and 10% of coastal and marine areas by 2020. Sometimes interest in new reserves is initiated by charismatic biologists with great drive, some of whom are now legends of the conservation movement. John Muir facilitated the establishment of Yosemite National Park, and Bernard Grzimek the Serengeti National Park. Other important contributions made by individuals who studied the behaviour and ecology of mammals include George Schaller who was pivotal in forming both the Wolong giant panda reserve in western China and the Chang Tang nature reserve in northern Tibet, Alan Rabinowitz who helped set up the Cockscomb jaguar reserve in Belize, and Jane Goodall and Toshisada Nishida who were instrumental in helping to establish, respectively, Gombe Stream and Mahale Mountains national parks in Tanzania (figure 3). More recently, John Weaver's efforts with caribou Rangifer tarandus and grizzly bears Ursus arctos [51] led to a sixfold increase in the size of Nahanni National Park Reserve, a 2017 UNESCO-recognized enclave in northern Canada (see [86] for other examples). Such fieldworkers with interests in animal behaviour worked with an array of stakeholders that included NGOs, donors, politicians, governments and other scientists to raise a groundswell of support for setting up a protected area. These were committed scientists willing to engage in the political process.
Figure 3.
Portraits of people whose behavioural and ecological studies led to the establishment of a protected area. (a) Bernard Grzimek (right), photographed with Alan Root (wildlife photographer, centre) and Sammy Mankoto, former Director General of the Institut Congolais pour la Conservation de la Nature (photo Markus Borner), (b) George Schaller (photo Billy Karesh), (c) Alan Rabinowitz (photo WCS), (d) Jane Goodall (photo Richard Wrangham), (e) Toshisada Nishida (photo Richard Wrangham).
As we have intimated through our own work (see above), engagement is key to behavioural ecologists helping to establish protected areas [87]. While much continues to be written about the relationships between behaviour and conservation (e.g. [10,65,88]) and the scholarly nature of many of these contributions is indisputable, they may fall short of field conservation targets because these important findings simply sit in the literature [89]; they have yet to find firm grounding when it comes to protected area management [90]. The mismatch stems in part from a failure to frame behavioural ecological studies to specific conservation goals even though they may carry relevance to metrics of population viability [91], and because behavioural ecologists engage with management authorities far less than with academics [92]. One-on-one conversations and workshops are critical in enabling stakeholders to understand how specific components of behavioural ecology can offer information about mechanisms that affect individuals and populations on a case by case basis [93]. Caro & Sherman [24] provide a checklist of practical research, education, political and activism avenues for engagement.
8. Conclusion
We have briefly examined the relationship between the discipline of behavioural ecology, that is study of the adaptive significance of behaviour in ecological context, and its relevance to protecting species in natural and minimally anthropogenically altered habitats. Understanding movement patterns and habitat requirements and the mechanisms underlying these behaviours can help in reserve establishment; and behavioural ecological knowledge can be useful in bolstering populations.
Here we have also argued that an equally important benefit of behavioural ecologists working in the field is their love of nature, desire to maintain it and at times to communicate it to an audience beyond scientists. This can help to protect their study animal in the wild and protect their study site although this involves setting time aside to interact with non-academic stakeholders, and maintaining a long-term presence in the field. It necessitates returning to study sites again and again [94]. We believe that biologists studying behaviour could have greater impact if they moved further into the policy arena surrounding protected area establishment [57,69,87] not only because of their science but also in their role as scientists sitting at the conservation table.
Acknowledgements
We thank the University of California, Davis, and Colorado State University for support, Ilaria Pretelli and Haji Hamad for help with figure 2, and Damien Caillaud, Douglas Kelt and Gail Patricelli for discussion, and anonymous reviewers and Jakob Bro-Jorgensen for comments.
Data accessibility
This article has no additional data.
Authors' contributions
T.C. wrote the first draft, then both authors reworked drafts together.
Competing interests
We declare we have no competing interests.
References
- 1.Watson JEM, Shanahan DF, Di Marco M, Allan J, Laurance WF, Sanderson EW, Mackey B, Venter O. 2016. Catastrophic declines in wilderness areas undermine global environment targets. Curr. Biol. 26, 2929–2934. ( 10.1016/j.cub.2016.08.049) [DOI] [PubMed] [Google Scholar]
- 2.Jones KR, et al. 2018. The location and protection status of Earth's diminishing marine wilderness. Curr. Biol. 28, 2506–2512. ( 10.1016/j.cub.2018.06.010) [DOI] [PubMed] [Google Scholar]
- 3.Wuerthner G, Crist E, Butler T. 2015. Protecting the wild: parks and wilderness, the foundation for conservation. Washington, DC: Island Press. [Google Scholar]
- 4.Wilson EO. 2017. Half-earth: our planet‘s fight for life. New York, NY: W.W. Norton. [Google Scholar]
- 5.Thomas CD. 2017. Inheritors of the earth: how nature is thriving in an age of extinction. London, UK: Penguin Random House. [Google Scholar]
- 6.Watson JEM, Venter O, Lee J, Jones KR, Robison JG, Possingham HP, Allan JR. 2018. Protect the last of the wild. Nature 563, 28–30. ( 10.1038/s41586-018-0572-6) [DOI] [PubMed] [Google Scholar]
- 7.Newbold T, et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45 ( 10.1038/nature14324) [DOI] [PubMed] [Google Scholar]
- 8.Barlow J, et al. 2016. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147. ( 10.1038/nature18326) [DOI] [PubMed] [Google Scholar]
- 9.Sanderson EW, Walston J, Robinson JG. 2018. From bottleneck to breakthrough: urbanization and the future of biodiversity conservation. Bioscience 68, 412–426. ( 10.1093/biosci/biy039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumstein DT, Fernandez-Juricic E. 2010. A primer of conservation behavior. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 11.Berger-Tal O, Saltz D. 2016. Conservation behavior: applying behavioral ecology to wildlife conservation and management. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Wong BBM, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 13.Hu Y, Cardoso GC. 2010. Which birds adjust the frequency of vocalizations in urban noise? Anim. Behav. 79, 863–867. ( 10.1016/j.anbehav.2009.12.036) [DOI] [Google Scholar]
- 14.Tellería JL, Virgós E, Carbonell R, Pérez-Tris J, Santos T. 2001. Behavioural responses to changing landscapes: flock structure and anti-predator strategies of tits wintering in fragmented forests. Oikos 95, 253–264. ( 10.1034/j.1600-0706.2001.950207.x) [DOI] [Google Scholar]
- 15.Zala SM, Penn DJ. 2004. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 68, 649–664. ( 10.1016/j.anbehav.2004.01.005) [DOI] [Google Scholar]
- 16.Van Doren BM, Horton KG, Dokter AM, Klinck H, Elbin SB, Farnsworth A. 2017. High-intensity urban light installation dramatically alters nocturnal bird migration. Proc. Natl Acad. Sci. USA 114, 11 175–11 180. ( 10.1073/pnas.1708574114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angeloni L, Schlaepfer MA, Lawler JJ, Crooks KR. 2008. A reassessment of the interface between conservation and behaviour. Anim. Behav. 75, 731–737. ( 10.1016/j.anbehav.2007.08.007) [DOI] [Google Scholar]
- 19.Weber WA, Vedder A. 2001. In the kingdom of gorillas: fragile species in a dangerous land. New York, NY: Simon and Shuster. [Google Scholar]
- 20.Wilkie DS, Morelli GA, Demmer J, Starkey M, Telfer P, Steil M. 2006. Parks and people: assessing the human welfare effects of establishing protected areas for biodiversity conservation. Conserv. Biol. 20, 247–249. [DOI] [PubMed] [Google Scholar]
- 21.Tilman D, Clark M, Williams DR, Kimmel K, Polasky S, Packer C. 2017. Future threats to biodiversity and pathways to their prevention. Nature 546, 73–81. ( 10.1038/nature22900) [DOI] [PubMed] [Google Scholar]
- 22.Margules CR, Pressey RL. 2000. Systematic conservation planning. Nature 405, 243–253. ( 10.1038/35012251) [DOI] [PubMed] [Google Scholar]
- 23.Caro T. 2010. Conservation by proxy: indicator, umbrella, keystone, flagship and other surrogate species. Washington, DC: Island Press. [Google Scholar]
- 24.Caro T, Sherman PW. 2011. Endangered species and a threatened discipline: behavioural ecology. Trends Ecol. Evol. 26, 111–118. ( 10.1016/j.tree.2010.12.008) [DOI] [PubMed] [Google Scholar]
- 25.Barua M, Root-Bernstein M, Ladie TJ, Jepson P. 2011. Defining flagship uses is critical for flagship selection: a critique of the IUCN climate change flagship fleet. Ambio 40, 431–435. ( 10.1007/s13280-010-0116-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberge JM, Angelstam PER. 2004. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 18, 76–85. ( 10.1111/j.1523-1739.2004.00450.x) [DOI] [Google Scholar]
- 27.Welton LJ, Siler CD, Bennett D, Diesmos A, Duya MR, Dugay R, Rico ELB, Van Weerd M, Brown RM. 2010. A spectacular new Philippine monitor lizard reveals a hidden biogeographic boundary and a novel flagship species for conservation. Biol. Lett. 6, 654–658. ( 10.1098/rsbl.2010.0119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worm B, Lotze HK, Myers RA. 2003. Predator diversity hotspots in the blue ocean. Proc. Natl Acad. Sci. USA 100, 9884–9888. ( 10.1073/pnas.1333941100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalinkat G, et al. 2017. Flagship umbrella species needed for the conservation of overlooked aquatic biodiversity. Conserv. Biol. 31, 481–485. ( 10.1111/cobi.12813) [DOI] [PubMed] [Google Scholar]
- 30.Guiney MS, Oberhauser KS. 2009. Insects as flagship conservation species. Terr. Arthropod. Rev. 1, 111–123. ( 10.1163/187498308X414733) [DOI] [Google Scholar]
- 31.Ball SMJ. 2004. Stocks and exploitation of East African blackwood: a flagship species for Tanzania's miombo woodlands? Oryx 38, 266–272. ( 10.1017/S0030605304000493) [DOI] [Google Scholar]
- 32.Cannon PF. 2011. The caterpillar fungus, a flagship species for conservation of fungi. Fungal Conserv. 1, 35–39. [Google Scholar]
- 33.Borgerhoff Mulder M, Schacht R, Caro T, Schacht J, Caro B. 2009. Knowledge and attitudes of children of the Rupunini: implications for conservation in Guyana. Biol. Conserv. 142, 879–887. ( 10.1016/j.biocon.2008.12.021) [DOI] [Google Scholar]
- 34.Smith AM, Sutton SG. 2008. The role of flagship species in the formation of conservation intentions. Hum. Dimen. Wildlife 13, 127–140. ( 10.1080/10871200701883408) [DOI] [Google Scholar]
- 35.Barnes BM. 1989. Freeze avoidance in a mammal: body temperatures below 0°C in an Arctic hibernator. Science 244, 1593–1595. ( 10.1126/science.2740905) [DOI] [PubMed] [Google Scholar]
- 36.Poran NS, Coss RG, Benjamini ELI. 1987. Resistance of California ground squirrels (Spermophilus beecheyi) to the venom of the northern Pacific rattlesnake (Crotalus viridis oreganus): a study of adaptive variation. Toxicon 25, 767–777. ( 10.1016/0041-0101(87)90127-9) [DOI] [PubMed] [Google Scholar]
- 37.Kingdon J, Agwanda B, Kinnaird M, O'Brien T, Holland C, Gheysens T, Boulet-Audet M, Vollrath F. 2012. A poisonous surprise under the coat of the African crested rat. Proc. R. Soc. B 279, 675–680. ( 10.1098/rspb.2011.1169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stankowich T, Campbell LA. 2016. Living in the danger zone: exposure to predators and the evolution of spines and body armor in mammals. Evolution 70, 1501–1511. ( 10.1111/evo.12961) [DOI] [PubMed] [Google Scholar]
- 39.Aho J, Kalela O. 1966. January. The spring migration of 1961 in the Norwegian lemming, Lemmus lemmus (L.), at Kilpisjärvi, Finnish Lapland. Annal. Zool. Fenn. 3, 53–65. [Google Scholar]
- 40.Le M, Nguyen HM, Duong HT, Nguyen TV, Dinh LD, Nguyen NX, Nguyen LD, Dinh TH, Nguyen DX. 2015. Phylogeography of the Laotian rock rat (Diatomyidae: Laonastes): implications for Lazarus taxa. Mammal Study 40, 109–114. ( 10.3106/041.040.0206) [DOI] [Google Scholar]
- 41.Cockburn A, Scott MP, Scotts DJ. 1985. Inbreeding avoidance and male-biased natal dispersal in Antechinus spp. (Marsupialia: Dasyuridae). Anim. Behav. 33, 908–915. ( 10.1016/S0003-3472(85)80025-7) [DOI] [Google Scholar]
- 42.Caro T, et al. In press. A case study of coconut crabs on Zanzibar highlights global threats and conservation solutions. Oryx.
- 43.Wright JH. 2010. Use of film for community conservation education in primate habitat countries. Am. J. Primatol. 72, 462–466. ( 10.1002/ajp.20749) [DOI] [PubMed] [Google Scholar]
- 44.Genda P, Borgerhoff Mulder M, Caro T, Fitzherbert E, Ballard H. 2012. Launching WATU, SIMBA NA MAZINGIRA project in Katavi-Rukwa. Kakakuona Magazine Oct-Dec, 55–56.
- 45.Pennycuick L. 1975. Movements of the migratory wildebeest population in the Serengeti area between 1960 and 1973. Afr. J. Ecol. 13, 65–87. ( 10.1111/j.1365-2028.1975.tb00124.x) [DOI] [Google Scholar]
- 46.Chapman DD, Pikitch EK, Babcock E, Shivji MS. 2005. Marine reserve design and evaluation using automated acoustic telemetry: a case-study involving coral reef-associated sharks in the Mesoamerican Caribbean. Mar. Tech. Soc. J. 39, 42–55. ( 10.4031/002533205787521640) [DOI] [Google Scholar]
- 47.Salm RV, Clark JR, Siirila E. 2000. Marine and coastal protected areas: a guide for planners and managers Gland, Switzerland: IUCN Monographs. [Google Scholar]
- 48.Woodroffe R, Ginsberg JR. 1998. Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128. ( 10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]
- 49.Holmern T, Muya J, Røskaft E. 2007. Local law enforcement and illegal bushmeat hunting outside the Serengeti National Park, Tanzania. Environ. Conserv. 34, 55–63. ( 10.1017/S0376892907003712) [DOI] [Google Scholar]
- 50.Cleaveland S, Mlengeya T, Kaare M, Haydon DAN, Lembo T, Laurenson MK, Packer C. 2007. The conservation relevance of epidemiological research into carnivore viral diseases in the Serengeti. Conserv. Biol. 21, 612–622. ( 10.1111/j.1523-1739.2007.00701.x) [DOI] [PubMed] [Google Scholar]
- 51.Weaver JL. 2006. Big animals and small parks: implications of wildlife distribution and movements for expansion of Nahanni National Park Reserve. Toronto, Canada: Wildlife Conservation Society. [Google Scholar]
- 52.Vicente J, Delahay RJ, Walker NJ, Cheeseman CL. 2007. Social organization and movement influence the incidence of bovine tuberculosis in an undisturbed high-density badger Meles meles population. J. Anim. Ecol. 76, 348–360. ( 10.1111/j.1365-2656.2006.01199.x) [DOI] [PubMed] [Google Scholar]
- 53.Riggio J, Mbwilo F, Van de Perre F, Caro T. 2018. The forgotten link between northern and southern Tanzania. Afr. J. Ecol. 56, 1012–1016. ( 10.1111/aje.12533) [DOI] [Google Scholar]
- 54.Smith JA, Duane TP, Wilmers CC. 2019. Moving through the matrix: promoting permeability for large carnivores in a human-dominated landscape. Landscape and Urban Planning 183, 50–58. ( 10.1016/j.landurbplan.2018.11.003) [DOI] [Google Scholar]
- 55.Wittemyer G, Northrup JM, Bastille-Rousseau G. 2019. Behavioural valuation of landscapes using movement data. Phil. Trans. R. Soc. B 374, 20180046 ( 10.1098/rstb.2018.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger J. 2004. The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331. ( 10.1111/j.1523-1739.2004.00548.x) [DOI] [Google Scholar]
- 57.Berger J, Cain SL. 2014. Moving beyond science to protect a mammalian migration corridor. Conserv. Biol. 28, 1142–1150. ( 10.1111/cobi.12327) [DOI] [PubMed] [Google Scholar]
- 58.Caro T, Dobson A, Marshall AJ, Peres CA. 2014. Compromise solutions between conservation and road building in the tropics. Curr. Biol. 24, R722–R724. ( 10.1016/j.cub.2014.07.007) [DOI] [PubMed] [Google Scholar]
- 59.Laurance WF, et al. 2014. A global strategy for road building. Nature 513, 229 ( 10.1038/nature13717) [DOI] [PubMed] [Google Scholar]
- 60.Seidler RG, Green DS, Beckmann JP. 2018. Highways, crossing structures and risk: behaviors of greater Yellowstone pronghorn elucidate efficacy of road mitigation. Global Ecol. Conserv. 15, e00416 ( 10.1016/j.gecco.2018.e00416) [DOI] [Google Scholar]
- 61.Glista DJ, DeVault TL, DeWoody JA. 2009. A review of mitigation measures for reducing wildlife mortality on roadways. Landscape Urban Planning 91, 1–7. ( 10.1016/j.landurbplan.2008.11.001) [DOI] [Google Scholar]
- 62.St. Clair CC, Backs J, Friesen A, Gangadharan A, Gilhooly P, Murray M, Pollock S. 2019. Animal learning may contribute to both problems and solutions for wildlife–train collisions. Phil. Trans. R. Soc. B 374, 20180050 ( 10.1098/rstb.2018.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanski I, Gilpin M. 1991. Metapopulation dynamics: brief history and conceptual domain. Biol. J. Linn. Soc. 42, 3–16. ( 10.1111/j.1095-8312.1991.tb00548.x) [DOI] [Google Scholar]
- 64.Lima SL, Zollner PA. 1996. Towards a behavioral ecology of ecological landscapes. Trends Ecol. Evol. 11, 131–135. ( 10.1016/0169-5347(96)81094-9) [DOI] [PubMed] [Google Scholar]
- 65.Berger-Tal O, Saltz D. 2019. Invisible barriers: anthropogenic impacts on inter- and intra-specific interactions as drivers of landscape-independent fragmentation. Phil. Trans. R. Soc. B 374, 20180049 ( 10.1098/rstb.2018.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stamps JA, Swaisgood RR. 2007. Someplace like home: experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 102, 392–409. ( 10.1016/j.applanim.2006.05.038) [DOI] [Google Scholar]
- 67.Jones HP, Kress SW. 2012. A review of the world's active seabird restoration projects. J. Wildl. Manag. 76, 2–9. ( 10.1002/jwmg.240) [DOI] [Google Scholar]
- 68.Fernández-Juricic E, Venier MP, Renison D, Blumstein DT. 2005. Sensitivity of wildlife to spatial patterns of recreationist behavior: a critical assessment of minimum approaching distances and buffer areas for grassland birds. Biol. Conserv. 125, 225–235. ( 10.1016/j.biocon.2005.03.020) [DOI] [Google Scholar]
- 69.Berger J. 2018. Extreme conservation: life at the edges of the world. Chicago, IL: University of Chicago Press. [Google Scholar]
- 70.Greene C, Umbanhowar J, Mangel M, Caro T. 1998. Animal breeding systems, hunter selectivity, and consumptive use in wildlife conservation. In Behavioral ecology and conservation biology (ed. Caro T.), pp. 271–305. New York, NY: Oxford University Press. [Google Scholar]
- 71.Sæther B-E, Engen S. 2019. Towards a predictive conservation biology: the devil is in the behaviour. Phil. Trans. R. Soc. B 374, 20190013 ( 10.1098/rstb.2019.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caro TM, Young CR, Cauldwell AE, Brown DDE. 2009. Animal breeding systems and big game hunting: models and application. Biol. Conserv. 142, 909–929. ( 10.1016/j.biocon.2008.12.018) [DOI] [Google Scholar]
- 73.Whitman KL, Packer C. 2006. A hunter's guide to aging lions in eastern and southern Africa. Huntington Beach, CA: Conservation Force. [Google Scholar]
- 74.Swenson J. 2003. Implications of sexually selected infanticide for the hunting of large carnivores. In Animal behavior and wildlife conservation (eds Festa-Bianchet M, Apollonio M), pp. 171–189. Washington, DC: Island Press. [Google Scholar]
- 75.Williams R, Lusseau D. 2006. A killer whale social network is vulnerable to targeted removals. Biol. Lett. 2, 497–500. ( 10.1098/rsbl.2006.0510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 77.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: the adaptive value of age. Proc. R. Soc. B 278, 3270–3276. ( 10.1098/rspb.2011.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldenberg SZ, Douglas-Hamilton I, Wittemyer G. 2016. Vertical transmission of social roles drives resilience to poaching in elephant networks. Curr. Biol. 26, 75–79. ( 10.1016/j.cub.2015.11.005) [DOI] [PubMed] [Google Scholar]
- 79.Heppell SS, Heppell SA, Coleman FC, Koenig CC. 2006. Models to compare management options for a protogynous fish. Ecol. Appl. 16, 238–249. ( 10.1890/04-1113) [DOI] [PubMed] [Google Scholar]
- 80.Berger J, Cunningham C. 1994. Phenotypic alterations, evolutionarily significant structures, and rhino conservation. Conserv. Biol. 8, 833–840. ( 10.1046/j.1523-1739.1994.08030833.x) [DOI] [Google Scholar]
- 81.Berger J. 2012. Estimation of bodysize traits by photogrammetry in large mammals to inform conservation. Conserv. Biol. 26, 769–777. ( 10.1111/j.1523-1739.2012.01896.x) [DOI] [PubMed] [Google Scholar]
- 82.Laurance WF. 2013. Does research help to safeguard protected areas? Trends Ecol. Evol. 28, 261–266. ( 10.1016/j.tree.2013.01.017) [DOI] [PubMed] [Google Scholar]
- 83.Piel AK, Lenoel A, Johnson C, Stewart FA. 2015. Deterring poaching in western Tanzania: the presence of wildlife researchers. Glob. Ecol. Conserv. 3, 188–199. ( 10.1016/j.gecco.2014.11.014) [DOI] [Google Scholar]
- 84.Brightsmith DJ, Stronza A, Holle K. 2008. Ecotourism, conservation biology, and volunteer tourism: a mutually beneficial triumvirate. Biol. Conserv. 141, 2832–2842. ( 10.1016/j.biocon.2008.08.020) [DOI] [Google Scholar]
- 85.Packer C. 2015. Lions in the balance: man-eaters, manes, and men with guns. Chicago, IL: University of Chicago Press. [Google Scholar]
- 86.Balmford A. 2012. Wild hope. Chicago, IL: University of Chicago Press. [Google Scholar]
- 87.Durant SM, et al. 2019. Bridging the divide between scientists and decision-makers: how behavioural ecologists can increase the conservation impact of their research? Phil. Trans. R. Soc. B 374, 20190011 ( 10.1098/rstb.2019.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berger-Tal O, Polak A, Lubin Y, Kotler B, Saltz D. 2011. Integrating animal behavior and conservation biology: a conceptual framework. Behav. Ecol. 22, 236–239. ( 10.1093/beheco/arq224) [DOI] [Google Scholar]
- 89.Clark JA. 1994. The endangered species act: its history, provisions, and effectiveness. In Endangered species recovery (eds Clark TW, Reading RP, Clarke AL), pp. 19–46. Washington, DC: Island Press. [Google Scholar]
- 90.Aycrigg JL, et al. 2016. Completing the system: opportunities and challenges for a national habitat conservation system. BioScience 66, 774–784. ( 10.1093/biosci/biw090) [DOI] [Google Scholar]
- 91.Berger J. 1996. Animal behaviour and plundered mammals: is the study of mating systems a scientific luxury or a conservation necessity? Oikos 77, 207–216. ( 10.2307/3546059) [DOI] [Google Scholar]
- 92.Festa-Bianchet M, Apollonio M. (eds). 2003. Animal behaviour and wildlife conservation. Washington, DC: Island Press. [Google Scholar]
- 93.Caro T. 2018. Who reads nowadays?: comment on Berger-Tal et al. Behav. Ecol. 30, 11–12. ( 10.1093/beheco/ary136) [DOI] [Google Scholar]
- 94.Willyard C, Scudellari M, Nordling L. 2018. Partners in science. Nature 562, 24–28. ( 10.1038/d41586-018-06858-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.




