Abstract
Pollution (e.g. by chemicals, noise, light, heat) is an insidious consequence of anthropogenic activity that affects environments worldwide. Exposure of wildlife to pollutants has the capacity to adversely affect animal communication and behaviour across a wide range of sensory modalities—by not only impacting the signalling environment, but also the way in which animals produce, perceive and interpret signals and cues. Such disturbances, particularly when it comes to sex, can drastically alter fitness. Here, we consider how pollutants disrupt communication and behaviour during mate choice, and the ecological and evolutionary changes such disturbances can engender. We explain how the different stages of mate choice can be affected by pollution, from encountering mates to the final choice, and how changes to these stages can influence individual fitness, population dynamics and community structure. We end with discussing how an understanding of these disturbances can help inform better conservation and management practices and highlight important considerations and avenues for future research.
This article is part of the theme issue ‘Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation’.
Keywords: environmental change, communication, courtship, mate preferences, sexual selection, signals
1. Pollution and mate choice
Environmental pollution is a serious and growing problem. In a human-dominated world, habitats everywhere are increasingly being drenched by chemicals, disturbed by anthropogenic noise, illuminated by artificial light or thermally altered by human activities. Such pervasive pollutants not only have the capacity to drastically change the environment, but can also interfere with key sensory and physiological processes of exposed organisms [1–3]. In so doing, pollutants can influence the ability of animals to receive and perceive information about their environment and potentially impinge on their ability to mount an adaptive response [4–6]. In this regard, altered communication, especially when it comes to sex, can have important fitness consequences [7,8].
For many species, mate choice plays a fundamental role in determining which individuals are able to successfully reproduce [9]. Typically, males compete vigorously for fertilization opportunities, while females make careful choices among potential mates (although large variation in this pattern is found among species). Indeed, the elaborate male ornaments and conspicuous courtship displays that evolve in response to female mate preferences can reflect a whole suite of direct and indirect fitness benefits for choosy individuals, from access to mates that deliver superior parental care to the inheritance of superior genes that increase offspring viability [10]. Display traits can also be non-informative, or even deceptive, and evolve because signallers take advantage of pre-existing sensory biases in mate choosers [10].
As an important fitness determinant that can influence both the quantity and quality of offspring produced, mate choice relies on the capacity of individuals to exercise their reproductive decisions prudently among the pool of suitors available to mate. For this to occur, choosy individuals must accurately perceive and obtain reliable information about the quality of potential mates, as well as process this information to make adaptive mating decisions [9]. In this regard, pollution-induced changes to the environment—by altering these fundamental processes—can have a direct bearing on individual mating decisions and mate choice.
Altered mate choice can have repercussions not only for individuals, but for the viability of populations and the survival of species [11]. Changes in the number and quality of offspring can affect population dynamics by influencing key demographic parameters resulting in population declines [12]. Such changes, in turn, can affect species interactions and impact the structure and function of the ecological communities they inhabit [13]. Disturbance to mate choice can also influence vital evolutionary processes and the strength and direction of selection [14]. It can affect premating reproductive isolation, which may promote population differentiation and speciation on the one hand [15], or lead to interspecific matings and the loss of biodiversity, on the other [16].
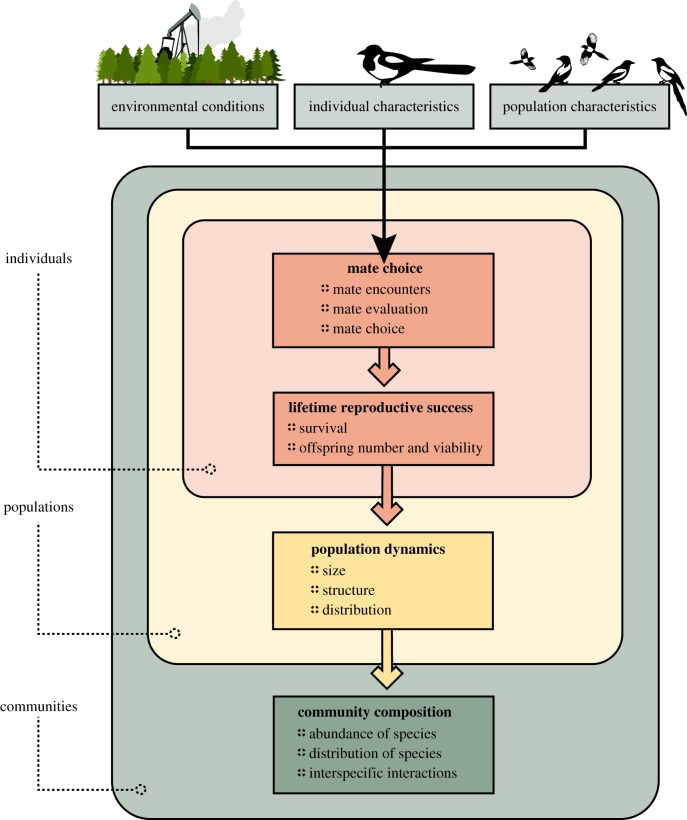
Here, we discuss the effects that pollution has on communication and behaviours in a mate choice context, and how these changes influence the dynamics of populations and, hence, the structure and function of communities (figure 1). We begin by explaining how pollution affects the different stages of the mate choice process. We then discuss how changes in mate choice can impact individual fitness and, in so doing, population dynamics and species characteristics. We continue by reflecting on the effect that changes in population characteristics can have on species interactions and community structure. Finally, we consider how an improved understanding of the effects of pollution on animal communication and mate choice can inform more effective conservation and management outcomes.
Figure 1.
Impact of altered mate choice on individuals, populations and communities.
2. How does pollution influence mate choice?
Mate choice is a multi-staged process that requires individuals to encounter potential suitors, acquire accurate information about the quality of these individuals, process the information gathered and make an informed choice. At each step, pollution has the potential to impinge on the mate choice process, and it can do so in three key ways: (i) by altering environmental conditions, (ii) by affecting the intrinsic properties of potential mates and the individuals performing the mate choice, and (iii) by impacting key population parameters (figure 1). Pollution may influence one or several stages of the mate choice process, and the changes it causes at one stage can alter its effects at other stages.
(a). Mate encounter rate
(i). Environmental conditions
Pollution can influence the ability of individuals to detect, attract and search for mates. For instance, in glow-worms (Lampyris noctiluca), light pollution (artificial light at night) hinders the ability of males to detect the bioluminescent glow of signalling females [17]. Similarly, in Lusitanian toadfish (Halobatrachus didactylus), exposure to noise pollution from shipping activity affects the ability of individuals to detect the courtship sounds of conspecifics [18]. Apart from these direct effects, pollution can also affect mate encounter rates indirectly by altering species interactions (e.g. risk of actual predation) that influence the cost of attracting and searching for mates.
(ii). Individual characteristics
Pollution that influences behavioural, morphological and physiological traits of individuals can alter mate encounter rates. For instance, several herbicides influence the synthesis of pheromones in moths and, hence, their ability to attract mates [19]. Stress-inducing pollutants, such as noise, can disturb behaviours essential for maximizing mate encounters, such as general activity and responsiveness to cues of mates [20], or cause neurobiological changes that affect the perception or production of cues [21]. Pollution can also influence investment into mate searching through effects on food intake, metabolism, body condition and the motivation to search for mates [22].
(iii). Population characteristics
Pollution that alters the size, structure or distribution of populations can have a direct bearing on mate encounter rates. For instance, toxic compounds that increase mortality and reduce population density, or those that inhibit reproductive maturation, can reduce the number of individuals available to mate, as well as the probability of encountering mates. Similarly, avoidance of pollutants, such as urban noise or light, can severely reduce the mate encounter rate of those that remain in polluted areas [23].
Pollution that alters sex ratio can affect the intensity of competition for mates and, in so doing, the benefit of investing in mate attraction and mate searching [24]. This can arise, for example, if pollution-induced mortality is sex-dependent, or if sex determination is disrupted. In regard to the latter, species with environmental sex determination may be particularly sensitive to pollutants that can alter key environmental parameters, such as temperature [25]. Pollution-mediated changes in sex ratio can also occur in species with primarily genetic sex determination, especially in the context of so-called endocrine-disrupting chemicals that disturb the normal hormone function of exposed organisms [26]. For instance, the synthetic hormone oestrogen, EE2, skews sex ratios towards females. Such changes can relax competition among males for females, while increasing investment of females into mate searching [27].
Pollution can also influence the expression of alternative reproductive strategies and, hence, the mates that are encountered. For instance, light pollution that affects sleeping patterns of songbirds can influence the possibility of cuckoldry, as individuals that delay the onset of daily activity are more easily cuckolded [28].
Changes in the variation among individuals in mate quality can similarly alter the benefit of mate attraction and mate search. In this respect, an increase in variation among individuals raises the benefit of mate choice and, hence, may increase investment into mate searching, while reduced variation may have the opposite effect [29].
(b). Information reliability
(i). Environmental conditions
Sexual signals are often finely attuned to the environment in which they have evolved. Pollution that alters the physical characteristics of the landscape, including its visual, acoustic and olfactory properties, can therefore affect both the quantity and quality of the information being emitted and transmitted through the signalling environment. This, in turn, can influence the information these signals are purported to encode and, hence, their reliability. The low-frequency din of urban noise, for instance, can mask the low-frequency components of the songs of birds, which alters their information content [30]. Similarly, chemical compounds are known to interfere with the transmission of olfactory signals by destroying or degrading them [31], while temperature changes owing to global warming reduces detectability and persistence of olfactory signals, as in the scent markings of the mountain lizard (Iberolacerta cyreni) [32].
Pollution can also impact the amount of resources available to individuals for investing into signals used for advertising quality. If competition for limited resources intensifies, the reliability of signals as indicators of resource-holding potential may improve [33]. However, pollution can also reduce signal reliability by creating ecological traps [34]. Such a possibility can arise through the emergence of novel cues that mimic those that individuals traditionally rely upon to guide their behavioural decisions. Artificial light, for instance, attracts night-active insects, such as glow-worms and fireflies that locate mates based on light emission [35].
(ii). Individual characteristics
It is well documented that exposure to certain pollutants can have a direct bearing on the expression of sexual signals. Exposure of fishes to municipal wastewater treatment effluent, in particular, the various pharmaceutical pollutants in the wastewater, is known to reduce male courtship behaviours [36]. Exposures of tree frogs (Hyla arborea) to noise pollution elevate their stress hormone levels, which reduces the colour of their vocal sacs used to attract females [21].
Changes in either the assessed trait, or in the quality of the assessed individuals, can disrupt the relationship between the trait and the honesty of the information it is purported to convey. However, while evidence exists of pollution altering signal and cue expression, much less is known about the impact of altered signals on their reliability in guiding adaptive mating decisions. For example, in the context of noise pollution, there is ample evidence documenting how animals, such as frogs, birds and insects, are able to adjust their acoustic signals to avoid vocal masking by, for example, calling louder [37] or at higher frequencies [38,39]. Yet, despite such changes, it remains unclear how signal modification might affect the content of the signal and, hence, its reliability as an indicator of mate quality. For instance, in frogs, females often prefer males that produce lower-pitched calls as these advertise body size [40]. Hence, if males are forced to produce higher pitched calls in noisy environments, such adjustments could potentially result in a conflict between signal audibility on the one hand, and signal reliability, on the other [30]. In this regard, the use of the signal will depend on whether all signalling individuals are similarly affected by the pollutant, and whether signal expression changes concomitantly with the quality of these individuals so that the signal continues to function as an honest indicator of mate quality.
When pollution influences only one component of a multicomponent signal (e.g. ornament colour, but not size), or only one sensory modality of a multimodal signal (e.g. colour, but not the intensity of courtship), the different components may convey contradictory information that reduces signal reliability [41]. Similarly when different components change in different directions, the resultant signal may yield contradictory information.
(iii). Population characteristics
Investment into signals depends on the intensity of competition for mates [10]. If pollution relaxes mate competition by altering the density or structure of populations, investment into signals may decrease [42]. This, in turn, can reduce the reliability of signals as indicators of mate quality. For instance, a reduced density of males can relax the social control over the expression of sexual signals and allow subdominant males in poor physical condition to signal dishonestly [43,44]. An example of this seen in the electric signals produced by the fish Brachyhypopomus gauderio, where a lower population density reduces social interactions and, hence, decreases the honesty of electric discharges as indicators of body size [45]. Pollution that influences the perceived intensity of competition for mates can similarly influence signal reliability without altering population size or structure. For instance, increased water turbidity in eutrophied environments reduces visibility and the detection of rival males in three-spined sticklebacks (Gasterosteus aculeatus). This relaxes the social control of signals and, hence, their reliability as indicators of male condition and offspring viability [46,47].
(c). Information processing and choice
(i). Environmental conditions
Pollution that alters food availability or predation risk can influence the costs and benefits of engaging in mate choice. For instance, a reduced ability to find food may force individuals to spend more time and energy on foraging and less on mate choice [48]. Similarly, a hampered ability to detect predators can increase the perception of risk, resulting in individuals becoming less choosy to mitigate the chances of being eaten [49]. An impaired ability to detect mates can, in turn, reduce the opportunity for choice [50]. Grim future reproductive opportunities may cause individuals to prioritize mating and become less choosy in order to maximize their chances of securing a mate [51]. Such changes can also induce individuals to switch from the use of signals in one sensory modality to another, such as paying less attention to acoustic signals in favour of visual signals in noisy environments.
(ii). Individual characteristics
The ability of choosy individuals to receive and process the information that reaches them depends on a range of intrinsic factors, including sensory and cognitive function, decision rules (e.g. mate acceptance thresholds), hormonal levels and body condition—all of which can potentially be disturbed by pollution [52]. This is especially true of pollutants that interfere with the endocrine system and alter sexual motivation and behaviour, as well as impinge on sensory systems and the reception of information [31]. For instance, the insecticide endosulfan resulted in male red-spotted newts (Notophthalmus viridescens) taking longer to detect female pheromones, which in turn reduced mate encounter rates [53]. This illustrates how the impact of pollutants may influence several mate choice stages, including the processing of signals as well as encounters with mates.
Pollution can also alter the body condition of choosy individuals and, hence, the amount of resources they can invest into mate choice [54]. For instance, female wolf spiders (Schizocosa stridulans) are less selective for males in good condition when food is limited [55]. Considering the profound effects that pollutants often have on body functions, changes to the intrinsic properties of choosers is probably a common pathway through which various pollutants can influence mate choice.
(iii). Population characteristics
Changes in the density and structure of populations can alter investment into mate assessment and choice in a manner similar to the effects described earlier for other components of the mate choice process. For instance, pollution that decimates a population increases the cost of choosiness by increasing the prospects of remaining unmated [56].
Pollution that alters aggression and negative interactions among individuals can also impact the costs of choice. For example, decreased population density may lower the frequency and intensity of male sexual harassment and, hence, reduce the cost to females from having to fend off undesirable mates [4]. It is becoming increasingly apparent that males, in attempting to maximize their own reproductive payoffs, can also behave in ways that override or impinge on female mate choice [57]. An example of this is seen in guppies (Poecilia reticulata), with exposure to the agricultural pollutant 17β-trenbolone, a powerful synthetic steroid, increasing male coercive matings and, in so doing, circumventing female choice [58,59].
3. Adaptive or maladaptive mate choice?
Whether the response of an individual to pollution is adaptive or not depends on its genetically determined reaction norm, and how the response can be altered through environmental effects, learning and evolutionary (genetic) changes. Reaction norms have evolved under past conditions and, hence, their adaptive value largely depends on the resemblance of the polluted conditions to earlier encountered conditions [5,60]. When the difference is large, the reaction norms are likely to be maladaptive. For instance, individuals may lack the sensory and neuroendocrine functions required to perceive changes in mate quality in a polluted environment, or they may not be able to overcome the challenges that the pollutant imposes on mate detection and evaluation.
When polluted conditions resemble earlier encountered conditions, animals may be more adept at plastically adjusting to pollution. For instance, individuals from environments with fluctuating noise levels may have evolved the flexibility to pay more attention to visual cues when noise levels are high. In general, species that can switch among cues may be better predisposed to deal with human-induced pollution when the pollution reduces the efficiency of signals and cues in certain sensory modalities, but not others [41]. However, when pollution alters the information content of different signals, and animals continue to pay attention to them, this could lead to contradictory information being acquired, which can render mate choice more difficult.
Learning may also improve the ability of individuals to assess signals and cues and make favourable choices. For instance, white-crowned sparrows (Zonotrichia leucophyrs) learn to adjust theirs song to noise from tutor songs through cultural selection [61]. Individuals may also learn to pay less attention to cues that are unreliable indicators of mate quality, or to adjust the timing of their reproductive activities. For instance, birds living near airports advance the timing of their chorus to avoid overlap with periods of intense aircraft noise [62]. It is important to point out, however, that plastic adjustments are not always possible [63] or may simply not be enough to counter the effects of pollution [64]. Under such circumstances, evolutionary changes may be required.
4. Consequences of altered mate choice
(a). Individual level
Maladaptive mate choice may reduce the number of offspring that individuals produce if the chooser selects a mate that has a low fertilization success or fecundity, has less resources to provide, or is a poor parent. Maladaptive mate choice can also influence the quality of the offspring produced, particularly if the selected mate is of low genetic quality. For instance, three-spined stickleback females are more likely to choose a mate that sires offspring of low viability when visibility is reduced owing to algal blooms [46].
When individuals increase their investment into mate choice in polluted habitats to compensate for a compromised ability to evaluate mates, this may reduce the amount of resources available to invest in other reproductive components, such as fecundity, parental care and future reproductive opportunities [65]. Similarly, elevated costs of searching for, and evaluating, mates can reduce survival and fecundity and, hence, lifetime reproductive success.
When individuals reduce their investment into mate choice, maladaptive choices may follow that lower the number and quality of offspring they produce. For instance, canaries (Serinus canaria) produce smaller clutch sizes when choosing a mate in a noisy environment, probably because hampered male–female vocal communication reduces female motivation to reproduce [66]. Such reduced investment can be adaptive under natural, fluctuating conditions if conditions improve with time. However, in human-modified habitats, conditions may not improve and the reduction in investment may, instead, reduce fitness.
Pollution can, in some instances, facilitate mate choice or reduce the cost of choosing a mate, and improve reproductive success. For instance, the disappearance of predators from polluted environments can allow prey species to spend more time searching for and evaluating mates [2]. Pollution that increases the randomness in mate choice may, in turn, improve the reproductive success of individuals that may otherwise have low mating prospects [46]. In this regard, altered distribution of mating success among individuals could have important population-level consequences.
(b). Population level
Altered reproductive success of individuals can influence population dynamics and demographics. If a large proportion of the population makes maladaptive mate choices and produces fewer offspring or offspring of lower viability, the population may decline [67].
Altered mate choice can also influence the evolution of traits. Maladaptive preferences and signals may be lost, while new traits may evolve [68]. However, the evolution of signals and preferences is generally a slow process, as it depends on generation time and the presence of suitable genetic variation [69]. Thus, evolution may frequently not be fast enough to rescue mate choice systems in rapidly changing environments.
Altered mate choice that influences selection on traits can, in turn, influence selection on correlated traits. It can also influence selection later in life. For instance, relaxed selection at the mate choice stage can strengthen selection at other life-history stages, such as among juveniles if more offspring of low viability are born into the population when mate choice becomes more random [70]. There is also evidence suggesting that mate choice and sexual selection may promote the evolution of mechanisms that can allow animals to better cope with pollutants. An example of this is seen in flour beetles (Tribolium castaneum), which evolved resistance to a pyrethroid pesticide faster under sexual selection [71].
(c). Community level
Changes in population dynamics can influence community composition. Species able to adapt their mate choice system to pollution may thrive, while those that cannot may flounder. For instance, the composition of a community of nesting birds in New Mexico changed with increasing noise levels. Species that adjusted their vocalizations during reproduction to the noise flourished, while those that did not declined [13]. Such changes may in turn influence species interactions. For instance, a declining predator population may release its prey population from predation, or its competitors from competition and, hence, influence the population dynamics of these species [72]. However, little is currently known about such community-wide consequences of altered mate choice.
Pollution that impairs species recognition can increase the frequency of interspecific matings. This can result in unviable offspring, or in hybrids that have a lower viability than their parental species. Such maladaptive matings may use up valuable time and energy and, hence, decrease offspring production. On the other hand, pollution that increases interspecific matings also have the potential to select for traits that contribute to population divergence. This may promote species differentiation and possible speciation [73]. Alternatively, interspecific matings because of pollution may result in hybrids that are more adept at succeeding under altered conditions. This can lead to the loss of biodiversity through the breakdown of species isolation mechanisms, as demonstrated, for example, in African cichlids [16].
5. How can the knowledge be of use in conservation management?
Studies of wildlife behavioural responses to human-altered conditions, including altered reproductive responses, such as mate choice, are crucial in understanding the harmful effects of pollution on species. Behavioural responses can be used as first indicators of changes to ecosystems, as well as reveal mechanisms and pathways through which pollution influences population dynamics and, further, how the effects spread through the species community [74].
Because behaviour is the manifestation of numerous complex developmental and physiological processes, it is an exceptionally powerful and biologically relevant indicator of environmental impacts. Hence, in the context of environmental monitoring, behaviour can be a much more comprehensive and sensitive biomarker than standard laboratory assays used to test for pollutants in the environment (e.g. chemicals), which typically target only one or a few biochemical or physiological parameters [75]. Given the central role of mate choice in determining fitness and population dynamics, it is a particularly important indicator of impacts of environmental pollution on species.
Indeed, from a practical management and conservation perspective, there are many lessons that can be gleaned from knowledge of how pollution affects mate choice. For instance, the finding that birds and anurans differ in their capacity to shift vocal frequencies [76] suggests that different approaches may be required to effectively manage anthropogenic noise pollution in different kinds of habitats. In the context of noise pollution, mitigation strategies that are already widely used to limit the impact of anthropogenic noise on humans, such as sound barriers and noise curfews, may also be effective in managing the impact of noise disturbance on wildlife [77].
Measuring mate choice in nature, however, can often be difficult, and what is measured in the laboratory may not reflect processes in nature. Thus, care needs to be taken when planning how to investigate the impact of pollutants on mate choice.
6. Future research directions
Much information exists on the effects of pollutants on mate choice behaviour, while less is known about the consequences of altered mate choice for individual fitness, population dynamics, species interactions and community structure [11]. Because mate choice is an important fitness determinant, disruptions to the behaviour can have far reaching consequences for both ecological and evolutionary processes, and need to be considered in studies on the effects of pollution on ecosystems.
The response of wildlife to pollutants often depends on the enormity of the disturbance. Thus, researchers should be cognizant of employing exposure levels that are ecologically relevant [75]. Here, it is important to realize that the relationship between the magnitude of the response and the extent of the disturbance may not necessarily be linear. For instance, several studies examining the behavioural responses of wildlife to chemical pollutants have reported non-monotonic dose responses, whereby exposure to lower concentrations can induce effects not seen at higher exposure levels [78]. Such findings underscore the importance of testing responses across multiple levels of disturbance.
A better understanding of the longer-term impacts of pollutants is also needed. Many pollutants are highly pervasive in the environment. Yet, there has been a tendency for experimental studies to employ extremely short exposure times (in some cases, only a matter of hours) [2]. This is true even though the impacts of pollutants, such as chemical contaminants, can take time to manifest. Moreover, there is now good evidence to suggest that exposure to pollutants can induce effects that transcend generations by causing developmental changes that are epigenetic [79]. For example, in laboratory mice, exposure to an endocrine disruptor affects female mating preferences three generations removed from the actual exposure [80]. Such studies underscore the fact that exposure to pollutants need not even be permanent to exert long-lasting effects on the mate choice process.
In addition, greater emphasis needs to be given to understanding the impact of pollutants in interaction with other environmental stressors. In the wild, animals are typically confronted with a myriad of environmental challenges simultaneously (from both natural and anthropogenic sources). Yet, despite this, there has been a tendency for researchers to examine the wildlife impacts of pollution in a vacuum, isolated from the influence of other environmental factors. Predicting the response of wildlife to pollutants in the presence of other kinds of environmental stressors cannot be achieved by studying these different disturbances in isolation, as multiple stressors can interact to induce effects that can be either greater (synergistic) or less (antagonistic) than the sum of their independent effects [81]. Multifactorial studies, in this regard, could be useful in disentangling the underlying mechanisms behind wildlife responses to pollutants under more realistic, multi-stressor environments.
Both within and between species differences are also important. Within species, responses can vary among individuals, depending on a range of factors, such as life-history stage, sex, age and body size. For instance, Bertram et al. [58] reported sex-specific differences in the response of guppies to a widespread agricultural contaminant, 17β-trenbolone, with altered reproductive behaviour in males, but not females. Among species, the bulk of research effort focusing on the impacts of pollution on mate choice have tended to focus on only a handful of taxa, even though the response of wildlife to pollutants can vary. The effects of noise pollution provide a good case in point. Here, most studies exploring the impacts of anthropogenic noise on acoustic signals have centred on terrestrial environments, with a heavy emphasis on the mating calls of birds and frogs, while impacts of noise in aquatic habitats have largely focused on marine mammals (mostly in a non-reproductive context). By contrast, far less attention has been given to understanding impacts of noise pollution on other acoustically communicating taxa, such as fishes, where the use of sound as a form of communication, including in mate choice, appears to be underappreciated [3,82]. Here, taxonomic differences in the mechanisms of sound production and detection, as well as differences in the transmission properties of sound in water and air, underscore the necessity for more direct testing of anthropogenic impacts in taxa that have, to date, been largely neglected.
In advancing the field, an important challenge will be to overcome our own sensory biases. To date, understanding of how pollution disrupts animal communication and mate choice has tended to focus almost exclusively on visual, acoustic and olfactory communication [7]. Yet, non-human animals can employ an extraordinarily diverse range of sensory channels for conspecific communication, many of which are very different from our own. Moreover, even in cases where the same sensory modalities are employed, perceptual abilities are often strikingly different. For example, some species, in contrast to humans, are able to see ultraviolet signals or hear infrasound. Yet, despite this, our current understanding of how pollutants affect these systems remains rudimentary. A related issue is the multimodality of animal communication systems. In this regard, impairment of any one (or combination) of different sensory modalities can have implications that are likely to depend on a range of factors, including environmental context, the relative importance of the different sensory modalities, and the information being conveyed [7,11]. Important insights will no doubt come from research that is less encumbered by our own sensory tendencies and better informed by sensory ecology [83].
Finally, more information is needed on the relative importance of plastic responses and genetic changes in coping with polluted environments. In particular, more attention needs to be paid to the possibility of mate choice behaviour evolving to be better suited to polluted conditions: when is evolutionary rescue likely and when is it not, and which factors determine whether a species will be able to adapt to pollution [60]? Insights into these questions will be pivotal in understanding the longer-term consequences of altered mate choice in an increasingly human-dominated world.
Acknowledgements
We thank Jakob Bro-Jørgensen for inviting us to contribute to this special issue, and Jake Martin for the design and editing of the figure.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed to the development and write up of the article's content, and gave final approval for its publication.
Competing interests
The authors have no competing interests.
Funding
Funding support was provided by the Academy of Finland (no. 277667) (to U.C.) and a Discovery Grant from the Australian Research Council (no. DP160100372) (to B.B.M.W.).
References
- 1.Swaddle JP, et al. 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. ( 10.1016/j.tree.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 2.Saaristo M, et al. 2018. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. R. Soc. B 285, 10 ( 10.1098/rspb.2018.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Sluijs I, et al. 2011. Communication in troubled waters: responses of fish communication systems to changing environments. Evol. Ecol. 25, 623–640. ( 10.1007/s10682-010-9450-x) [DOI] [Google Scholar]
- 4.Wong BBM, Candolin U. 2015. Behavioral responses to changing environments. Behav. Ecol. 26, 665–673. ( 10.1093/beheco/aru183) [DOI] [Google Scholar]
- 5.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 6.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 7.Rosenthal GG, Stuart-Fox D. 2012. Environmental disturbance and animal communication. In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BBM), pp. 16–31. Oxford, UK: Oxford Unviersity Press. [Google Scholar]
- 8.Candolin U. In press Mate choice in a changing world. Biol. Rev. Camb. Philos Soc. 94, 1246–1260. ( 10.1111/brv.12501) [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal GG. 2017. Mate choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 10.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.Candolin U, Wong BBM. 2012. Sexual selection in changing environments: consequences for individuals and populations. In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BBM), pp. 201–215. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Holman L, Kokko H. 2013. The consequences of polyandry for population viability, extinction risk and conservation. Phil. Trans. R. Soc. B. 368, 20150053 ( 10.1098/rstb.2012.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis CD, Ortega CP, Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419. ( 10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 14.Candolin U, Vlieger L. 2013. Estimating the dynamics of sexual selection in changing environments. Evol. Biol. 40, 589–600. ( 10.1007/s11692-013-9234-7) [DOI] [Google Scholar]
- 15.Pfennig KS. 2016. Reinforcement as an initiator of population divergence and speciation. Curr. Zool. 62, 145–154. ( 10.1093/cz/zow033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seehausen O, Alphen JJM, Witte F. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. ( 10.1126/science.277.5333.1808) [DOI] [Google Scholar]
- 17.Bird S, Parker J. 2014. Low levels of light pollution may block the ability of male glow-worms (Lampyris noctiluca L.) to locate females. J. Insect Conserv. 18, 737–743. ( 10.1007/s10841-014-9664-2) [DOI] [Google Scholar]
- 18.Vasconcelos RO, Amorim MCP, Ladich F. 2007. Effects of ship noise on the detectability of communication signals in the Lusitanian toadfish. J. Exp. Biol. 210, 2104–2112. ( 10.1242/jeb.004317) [DOI] [PubMed] [Google Scholar]
- 19.Eliyahu D, Applebaum S, Rafaeli A. 2003. Moth sex-pheromone biosynthesis is inhibited by the herbicide diclofop. Pest. Biochem. Physiol. 77, 75–81. ( 10.1016/s0048-3575(03)00101-9) [DOI] [Google Scholar]
- 20.Wingfield JC. 2015. Coping with change: a framework for environmental signals and how neuroendocrine pathways might respond. Front. Neuroendocrinol. 37, 89–96. ( 10.1016/j.yfrne.2014.11.005) [DOI] [PubMed] [Google Scholar]
- 21.Troianowski M, Mondy N, Dumet A, Arcanjo C, Lengagne T. 2017. Effects of traffic noise on tree frog stress levels, immunity, and color signaling. Conserv. Biol. 31, 1132–1140. ( 10.1111/cobi.12893) [DOI] [PubMed] [Google Scholar]
- 22.Heuschele J, Salminen T, Candolin U. 2012. Habitat change influences mate search behaviour in three-spined sticklebacks. Anim. Behav. 83, 1505–1510. ( 10.1016/j.anbehav.2012.03.027) [DOI] [Google Scholar]
- 23.Blickley JL, Blackwood D, Patricelli GL. 2012. Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv. Biol. 26, 461–471. ( 10.1111/j.1523-1739.2012.01840.x) [DOI] [PubMed] [Google Scholar]
- 24.Weir LK, Grant JWA, Hutchings JA. 2011. The influence of operational sex ratio on the intensity of competition for mates. Am. Nat. 177, 167–176. ( 10.1086/657918) [DOI] [PubMed] [Google Scholar]
- 25.Sandra GE, Norma MM. 2010. Sexual determination and differentiation in teleost fish. Rev. Fish. Biol. Fish. 20, 101–121. ( 10.1007/s11160-009-9123-4) [DOI] [Google Scholar]
- 26.Wedekind C. 2017. Demographic and genetic consequences of disturbed sex determination. Phil. Trans. R. Soc. B. 372, 20160326 ( 10.1098/rstb.2016.0326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orton F, Tyler CR. 2015. Do hormone-modulating chemicals impact on reproduction and development of wild amphibians? Biol. Rev. 90, 1100–1117. ( 10.1111/brv.12147) [DOI] [PubMed] [Google Scholar]
- 28.Greives TJ, et al. 2015. Costs of sleeping in: circadian rhythms influence cuckoldry risk in a songbird. Funct. Ecol. 29, 1300–1307. ( 10.1111/1365-2435.12440) [DOI] [Google Scholar]
- 29.Owens IPF, Thompson DBA. 1994. Sex-differences, sex-ratios and sex-roles. Proc. R. Soc. Lond. B 258, 93–99. ( 10.1098/rspb.1994.0148) [DOI] [PubMed] [Google Scholar]
- 30.Halfwerk W, Bot S, Buikx J, van der Velde M, Komdeur J, ten Cate C, Slabbekoorn H. 2011. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554. ( 10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lurling M, Scheffer M. 2007. Info-disruption: pollution and the transfer of chemical information between organisms. Trends Ecol. Evol. 22, 374–379. ( 10.1016/j.tree.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 32.Martin J, Lopez P. 2013. Effects of global warming on sensory ecology of rock lizards: increased temperatures alter the efficacy of sexual chemical signals. Funct. Ecol. 27, 1332–1340. ( 10.1111/1365-2435.12128) [DOI] [Google Scholar]
- 33.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 34.Schlaepfer MA, Runge MC, Sherman PW. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. ( 10.1016/s0169-5347(02)02580-6) [DOI] [Google Scholar]
- 35.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.2307/3868314) [DOI] [Google Scholar]
- 36.Soffker M, Tyler CR. 2012. Endocrine disrupting chemicals and sexual behaviors in fish: a critical review on effects and possible consequences. Crit. Rev. Toxicol. 42, 653–668. ( 10.3109/10408444.2012.692114) [DOI] [PubMed] [Google Scholar]
- 37.Brumm H, Todt D. 2002. Noise-dependent song amplitude regulation in a territorial songbird. Anim. Behav. 63, 891–897. ( 10.1006/anbe.2001.1968) [DOI] [Google Scholar]
- 38.Parris KM, Velik-Lord M, North JMA. 2009. Frogs call at a higher pitch in traffic noise. Ecol. Soc. 14, 24 ( 10.5751/es-02687-140125) [DOI] [Google Scholar]
- 39.Lampe U, Schmoll T, Franzke A, Reinhold K. 2012. Staying tuned: grasshoppers from noisy roadside habitats produce courtship signals with elevated frequency components. Funct. Ecol. 26, 1348–1354. ( 10.1111/1365-2435.12000) [DOI] [Google Scholar]
- 40.Ryan MJ. 1980. Female mate choice in a neotropical frog. Science 209, 523–525. ( 10.1126/science.209.4455.523) [DOI] [PubMed] [Google Scholar]
- 41.Partan SR. 2017. Multimodal shifts in noise: switching channels to communicate through rapid environmental change. Anim. Behav. 124, 325–337. ( 10.1016/j.anbehav.2016.08.003) [DOI] [Google Scholar]
- 42.Candolin U, Heuschele J. 2008. Is sexual selection beneficial during adaptation to environmental change? Trends Ecol. Evol. 23, 446–452. ( 10.1016/j.tree.2008.04.008) [DOI] [PubMed] [Google Scholar]
- 43.Candolin U. 1999. Male-male competition facilitates female choice in sticklebacks. Proc. R. Soc. Lond. B 266, 785–789. ( 10.1098/rspb.1999.0706) [DOI] [Google Scholar]
- 44.Candolin U. 2000. Increased signalling effort when survival prospects decrease: male-male competition ensures honesty. Anim. Behav. 60, 417–422. ( 10.1006/anbe.2000.1481) [DOI] [PubMed] [Google Scholar]
- 45.Gavassa S, Goldina A, Silva AC, Stoddard PK. 2013. Behavioral ecology, endocrinology and signal reliability of electric communication. J. Exp. Biol. 216, 2403–2411. ( 10.1242/jeb.082255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Candolin U, Tukiainen I, Bertell E. 2016. Environmental change disrupts communication and sexual selection in a stickleback population. Ecology 97, 969–979. ( 10.1890/15-1090) [DOI] [PubMed] [Google Scholar]
- 47.Wong BBM, Candolin U, Lindström K. 2007. Environmental deterioration compromises socially-enforced signals of male quality in three-spined sticklebacks. Am. Nat. 170, 184–189. ( 10.1086/519398) [DOI] [PubMed] [Google Scholar]
- 48.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 49.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends Ecol. Evol. 6, 183–186. ( 10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 50.Engström-Öst J, Candolin U. 2007. Human-induced water turbidity alters selection on sexual displays in sticklebacks. Behav. Ecol. 18, 393–398. ( 10.1093/beheco/arl097) [DOI] [Google Scholar]
- 51.Backwell PY, Passmore NI. 1996. Time constraints and multiple choice criteria in the sampling behaviour and mate choice of the fiddler crab, Uca annulipes. Behav. Ecol. Sociobiol. 38, 407–416. ( 10.1007/s002650050258) [DOI] [Google Scholar]
- 52.Buchanan KL, Partecke J. 2012. The endocrine system: can homeostasis be maintained in a changing world? In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BBM), pp. 32–45. Oxford, UK: Oxford University Press. [Google Scholar]
- 53.Park D, Propper CR. 2002. Endosulfan affects pheromonal detection and glands in the male red-spotted newt, Notophthalmus viridescens. Bull. Environ. Contam. Toxicol. 69, 609–616. ( 10.1007/s00128-002-0104-8) [DOI] [PubMed] [Google Scholar]
- 54.Cotton S, Small J, Pomiankowski A. 2006. Sexual selection and condition-dependent mate preferences. Curr. Biol. 16, R755–R765. ( 10.1016/j.cub.2006.08.022) [DOI] [PubMed] [Google Scholar]
- 55.Hebets EA, Wesson J, Shamble PS. 2008. Diet influences mate choice selectivity in adult female wolf spiders. Anim. Behav. 76, 355–363. ( 10.1016/j.anbehav.2007.12.021) [DOI] [Google Scholar]
- 56.Willis PM, Ryan MJ, Rosenthal GG. 2011. Encounter rates with conspecific males influence female mate choice in a naturally hybridizing fish. Behav. Ecol. 22, 1234–1240. ( 10.1093/beheco/arr119) [DOI] [Google Scholar]
- 57.Wong BBM, Candolin U. 2005. How is female mate choice affected by male competition? Biol. Rev. 80, 559–571. ( 10.1017/S1464793105006809) [DOI] [PubMed] [Google Scholar]
- 58.Bertram MG, Saaristo M, Baumgartner JB, Johnstone CP, Allinson M, Allinson G, Wong BBM. 2015. Sex in troubled waters: widespread agricultural contaminant disrupts reproductive behaviour in fish. Horm. Behav. 70, 85–91. ( 10.1016/j.yhbeh.2015.03.002) [DOI] [PubMed] [Google Scholar]
- 59.Tomkins P, Saaristo M, Bertram MG, Tomkins RB, Allinson M, Wong BBM. 2017. The agricultural contaminant 17 beta-trenbolone disrupts male-male competition in the guppy (Poecilia reticulata). Chemosphere 187, 286–293. ( 10.1016/j.chemosphere.2017.08.125) [DOI] [PubMed] [Google Scholar]
- 60.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moseley DL, Derryberry GE, Phillips JN, Danner JE, Danner RM, Luther DA, Derryberry EP. 2018. Acoustic adaptation to city noise through vocal learning by a songbird. Proc. R. Soc. B 285, 20181356 ( 10.1098/rspb.2018.1356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gil D, Honarmand M, Pascual J, Perez-Mena E, Garcia CM. 2015. Birds living near airports advance their dawn chorus and reduce overlap with aircraft noise. Behav. Ecol. 26, 435–443. ( 10.1093/beheco/aru207) [DOI] [Google Scholar]
- 63.Lengagne T. 2008. Traffic noise affects communication behaviour in a breeding anuran. Hyla arborea. Biol. Conser. 141, 2023–2031. ( 10.1016/j.biocon.2008.05.017) [DOI] [Google Scholar]
- 64.Nemeth E, Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am. Nat. 176, 465–475. ( 10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 65.Roff D. 1992. The evolution of life-histories. New York, NY: Chapman and Hall. [Google Scholar]
- 66.des Aunay GH, Grenna M, Slabbekoorn H, Nicolas P, Nagle L, Leboucher G, Malacarne G, Draganoiu TI. 2017. Negative impact of urban noise on sexual receptivity and clutch size in female domestic canaries. Ethology 123, 843–853. ( 10.1111/eth.12659) [DOI] [Google Scholar]
- 67.Kokko H, Brooks R. 2003. Sexy to die for? Sexual selection and the risk of extinction. Ann. Zool. Fennici 40, 207–219. [Google Scholar]
- 68.McNair A, Nakagawa S, Grimm V. 2014. The evolutionary consequences of disrupted male mating signals: an agent-based modelling exploration of endocrine disrupting chemicals in the guppy. PLoS ONE 9, 9 ( 10.1371/journal.pone.0103100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barrett RDH, Hendry AP. 2012. Evolutionary rescue under environmental change? In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U, Wong BBM), pp. 216–233. Oxford, UK: Oxford University Press. [Google Scholar]
- 70.Winemiller KO. 1992. Life-history strategies and the effectiveness of sexual selection. Oikos 63, 318–327. ( 10.2307/3545395) [DOI] [Google Scholar]
- 71.Jacomb F, Marsh J, Holman L. 2016. Sexual selection expedites the evolution of pesticide resistance. Evolution 70, 2746–2751. ( 10.1111/evo.13074) [DOI] [PubMed] [Google Scholar]
- 72.Hoover SER, Tylianakis JM. 2012. Species interactions. In Behavioural responses to a changing world: mechanisms and consequences (eds Candolin U., Wong B.B.M.), pp. 129–142. Oxford, UK: Oxford University Press. [Google Scholar]
- 73.Boughman JW. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571–577. ( 10.1016/S0169-5347(02)02595-8) [DOI] [Google Scholar]
- 74.Candolin U, Wong BBM. 2012. Behavioural responses to a changing world: mechanisms and consequences; 280 p Oxford, UK: Oxford University Press. [Google Scholar]
- 75.Zala SM, Penn DJ. 2004. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim. Behav. 68, 649–664. ( 10.1016/j.anbehav.2004.01.005) [DOI] [Google Scholar]
- 76.Roca IT, et al. 2016. Shifting song frequencies in response to anthropogenic noise: a meta-analysis on birds and anurans. Behav. Ecol. 27, 1269–1274. ( 10.1093/beheco/arw060) [DOI] [Google Scholar]
- 77.Slabbekoorn H, Ripmeester EAP. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83. ( 10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 78.Martin JM, Saaristo M, Bertram MG, Lewis PJ, Coggan TL, Clarke BO, Wong BBM. 2017. The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish. Environ. Pollut. 222, 592–599. ( 10.1016/j.envpol.2016.10.010) [DOI] [PubMed] [Google Scholar]
- 79.Walker DM, Gore AC. 2011. Transgenerational neuroendocrine disruption of reproduction. Nat. Rev. Endocrinol. 7, 197–207. ( 10.1038/nrendo.2010.215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. 2007. Transgenerational epigenetic imprints on mate preference. Proc. Natl Acad. Sci. USA 104, 5942–5946. ( 10.1073/pnas.0610410104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Folt CL, Chen CY, Moore MV, Burnaford J. 1999. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877. ( 10.4319/lo.1999.44.3_part_2.0864) [DOI] [Google Scholar]
- 82.Radford AN, Kerridge E, Simpson SD. 2014. Acoustic communication in a noisy world: can fish compete with anthropogenic noise? Behav. Ecol. 25, 1022–1030. ( 10.1093/beheco/aru029) [DOI] [Google Scholar]
- 83.Lim MLM, Sodhi NS, Endler JA. 2008. Conservation with sense. Science 319, 281 ( 10.1126/science.319.5861.281b) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.