Abstract
Understanding what affects population growth in novel environments is fundamental to forecast organisms' responses to global change, including biological invasions and land use intensification. Novel environments are challenging because they can cause maladaptation, increasing the risk of extinction by negative population growth. Animals can avoid extinction by improving the phenotype–environment match through behavioural responses, notably matching habitat choice and learning. However, the demographic consequences of these responses remain insufficiently understood in part because they have not been analysed within a life-history context. By means of an individual-based model, we show here that matching habitat choice and learning interact with life history to influence persistence in novel environments. In maladaptive contexts, the likelihood of persisting is higher for life-history strategies that increase the value of adults over the value of offspring, even at the cost of decreasing reproduction. Such a strategy facilitates persistence in novel environments by reducing the costs of a reproductive failure while increasing the benefits of behavioural responses. Our results reinforce the view that a more predictive theory for extinction risk under rapid environmental changes requires considering behavioural responses and life history as part of a common adaptive strategy to cope with environmental changes.
This article is part of the theme issue ‘Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation’.
Keywords: biological invasions, extinction risk, demographic stochasticity, cognitive ecology, habitat selection, urbanization
1. Introduction
Most organisms experience serious difficulties when exposed to novel environments. Novel contexts often generate mismatches between the phenotype and the environment, leading to maladaptation and extinction through negative population growth [1]. Maladaptation is one of the reasons why translocations of species from their native ranges to novel environments generally fail to establish self-sustaining populations [2,3], and it is also a primary cause of extinction by land use intensification [4]. Given that biotic exchanges and land use intensification are becoming increasingly frequent as a result of human activities, there is an urgent need to understand the mechanisms that influence population persistence in novel environments.
Several processes can allow organisms to improve the matching of their phenotypes to new contexts and hence facilitate persistence in novel environments. Natural selection—the most obvious process—can contribute to reconstitute the phenotype–environment match through genetic changes, a process known as evolutionary rescue [1]. However, an evolutionary rescue is less effective in animals with long generation time, such as many birds and mammals, which exhibit slow evolutionary responses to selection. In these animals, behavioural responses are an alternative to reduce the phenotype–environment mismatch [4–8]. Individuals may, for instance, improve fitness in novel environments by choosing the habitats where they live and reproduce that best fit their phenotype, a process known as matching habitat choice [9–12]. Animals can also decide when is best to reproduce, and skip reproduction when conditions are unfavourable [13].
The choice of where and when to live and reproduce can express activational plasticity, that is, an innate response to environmental cues [14,15]. In a novel environment, however, individuals must often take decisions with insufficient information and using cues that may have changed relative to those from the old environment, which can lead them to settle in poor-quality habitats (ecological traps) [8]. Yet, animals can improve decision-making, and hence avoid extinction, through learning [16,17]. Learning can modify decision-making based on previous experiences of the individual [18,19]—for example, changing habitat after a reproductive failure—or by using public information generated by more experienced conspecific or heterospecifics [20]. Evidence is accumulating that species which readily adjust behaviours to novel contexts are better able to survive and reproduce in a novel environment than species that persist with the behaviours of their old environments [17,21].
While the importance of behaviour in the response to environmental changes is widely recognized, we still lack a general theory regarding how such processes influence population growth in novel environments [2]. One important reason is that behavioural responses have rarely been investigated within a life-history context [2,22]. Life history—defined as the way organisms distribute their limited time and energy into growth, reproduction and survival [23]—is relevant because it affects how populations increase and fluctuate over time. The demography of the organism is particularly influenced by its position in the fast–slow continuum of life-history variation [24]. Species at the fast side of the continuum have short life expectancy but mature early and show high fecundity, which give them a high potential for rapid population growth under favourable conditions. Growing fast may confer advantages during the invasion of novel environments by reducing the period that the population remains small and hence vulnerable to extinction by demographic stochasticity. Species at the slow side of the continuum have delayed maturity and low fecundity, and hence cannot increase in number so fast when the population is small. Yet, their long life expectancy (and long generation time) buffers their populations from fluctuations driven by demographic and environmental stochasticity that can lead to extinction [25,26]. A slow strategy also reduces the fitness costs of a reproductive failure, as individuals have higher chances of breeding again in the future. This offers advantages in novel environments by spreading the risk of reproductive failure over several breeding attempts (a type of bet-hedging) and by allowing individuals to skip a reproductive event (and hence improve their survival) when conditions are unfavourable [27].
Thus, when we analyse how behavioural responses affect the demography of animals in novel, stochastic environments, we need to be aware that these responses will be affected by the organism's life history. This is relevant because the position of the animal in the fast–slow continuum can alter the benefits and costs of gathering environmental information and constructing appropriate behavioural responses [28–32]. The net benefit should generally be higher in slow animals, which are less constrained by time to explore and learn, and can use the learned behaviours for longer periods. The costs of delaying reproduction when conditions are unfavourable should also decrease in slow species, as individuals can reproduce again in the future, increasing the opportunities for acquiring environmental information and, through learning, improve the match of the phenotype to the novel conditions [27]. The demographic consequences of behavioural responses in novel, stochastic environments are also expected to vary depending on whether the phenotype–environment mismatch mainly affects offspring or adult survival. This is because fast and slow strategies differ in their sensitivity to changes in the demographic parameters, with fast strategies being highly sensitive to changes in fecundity and slow strategies to changes in adult survival [33]. Thus, understanding how behavioural responses contribute to population persistence in novel, stochastic environments requires us to consider the position of the animal in the fast–slow continuum [2].
While the demographic consequences of behaviour have been previously modelled by several authors [8,34–37], it remains to be seen to what extent behavioural responses influence population growth in novel, stochastic environments as a function of the position of the animal in the fast–slow continuum. Here, we use an individual-based simulation model to address this issue. The behavioural responses that we investigate include innate preferences for habitats that better matches the organism's phenotype, learning rules to reduce the preference for inadequate habitats and decisions about skipping a reproductive event when individuals stay in a habitat that does not match their phenotype. We use the model to illustrate how considering life-history variation refines predictions of classic theory regarding the role of behaviour in facilitating population persistence in novel environments.
2. Model description
Building on previous studies [34,36], we envision a species that is introduced in a novel region with two habitats. Individuals are allowed to survive, reproduce and move between habitats, and the likelihood that the population persists in the novel region (establishment success) is estimated through simulations (electronic supplementary material, figure S1). Establishment success is estimated through a stage-structured population-based model (which allows us to compare the outcome for species differing in life history), in scenarios varying in the degree of phenotype–environment mismatch (causing negative population growth) and demographic stochasticity (causing extinction by demographic accidents). The introduced species has a particular life-history strategy that positions it along the fast–slow continuum, fixing the values of its onset of first reproduction, average fecundity and age-specific survival of individuals (see details below). Behavioural responses are studied by assessing how modifying the probabilities of changing habitat and skipping reproduction affects establishment success. Below, we briefly summarize the main features of the model. For further details about specific parts of the model and about its inner workings, we refer the reader to the electronic supplementary material. The model was built using the R language [38], and an accompanying R package implementing the model, with its corresponding tutorial, is also offered as the electronic supplementary material.
(a). Stage-classified population
We chose a stage-classification approach to account for the complex life cycle of our simulated populations. Based on pre-breeding census, we classify the population into three individual classes: juveniles, subadults (only for strategies with age at first reproduction greater than 1 year) and adults. In turn, adults are divided into non-breeder (i.e. adults that decide not to breed in a given year) and breeders. Finally, breeding individuals are split at each brooding step into successful or failed breeders, distinguishing whether breeding yields viable juveniles or not, respectively. Only females are considered.
(b). Demographic model set-up
Our population model includes the main processes that must be considered when evaluating the life cycle of a stage-classified population, namely survival, growth and reproduction (table 1):
(i) survival: each stage-class (juveniles, subadults, non-breeding adults and breeding adults) is defined by an annual survival rate. In addition, juvenile survival is decomposed into individual survival and brood survival, the latter affecting all individuals in the same brood (e.g. as a result of nest predation). Data about the sources of juvenile mortality are scarce, and hence, we fixed the brood level mortality to account for 50% of the juvenile mortality;
(ii) growth: individuals can be promoted to the next stage if they survive to the next year. Individuals only remain 1 year in the juvenile class, after which they move up to the subadult or adult class. After they reach adulthood, they remain in that condition until they die; and
(iii) reproduction: each year, the algorithm determines which proportion of adults becomes non-breeders or breeders, and also which proportion of the latter may successfully breed. Only adults that are classified at each step as breeders can reproduce during a year.
Table 1.
Symbols and descriptions of the model parameters.
| symbol | definition |
|---|---|
| q | number of offspring per brood in habitat h |
| m | number of broods per year |
| nSa | number of subadult stages |
| x | labels for adult breeder type, x = {nb, b, bs, bf}. Label nb identifies adults that skip breeding and label b indicates adult individuals that try to breed. In turn, the latter can be divided into those which breed successfully (labelled bs) or those which fail to do so (labelled bf) |
| y | labels for survival, y = {j, sa, nb, b}, where labels refer to juveniles, subadults, non-breeder and breeder adults, respectively |
| h | index for habitat type, h = {1, 2} |
| r | label for subadult stage, |
| t | subindex for time steps, measured in years, t = {1 · · · 50} |
| probability for an individual to become a breeder (successful or not) in habitat h | |
| probability of complete brood failure for a breeder in habitat h | |
| probability of survival in habitat h for individuals of type Sy. | |
| probability for an adult to move from habitat type 1 to 2, or vice versa | |
| probability for a stage-r subadult to move from habitat type 1 to 2, or vice versa |
(c). Implementation of the demographic model
Each simulation starts with the introduction of a particular number of adults with an evolved life-history strategy along the fast–slow continuum. This cohort of adults is equally distributed between both habitats (labelled h). After the introduction phase, the growth of the population from year t to t + 1 is determined by the number of births and deaths within each habitat. The cohort of adults in each habitat is first divided into non-breeder and breeder adults with a probability . Then the model enters the breeding phase, which consists of a loop within which m breeding episodes take place. At each step within that loop, breeder adults are randomly split between failed and successful breeders (), and only the latter give rise to viable juveniles. The number of juveniles per successful breeding attempt is the product of the clutch size (q) and probability for a juvenile to survive (). After each reproductive event, breeders (failed or successful) may change habitat with a probability (if they move from habitat 1 to habitat 2) or (if they move from habitat 2 to habitat 1), with . Once the breeding loop has finished, non-breeder adults and subadults are allowed to change habitats and, finally, all individuals are promoted to the next class after their survival is evaluated (table 1).
(d). Demographic stochasticity
Demographic stochasticity is implemented both in the survival probability of each age class and in the probability of a brood failure (table 1) by means of binomial distributions defined by each probability and population size, obtaining random deviates from the mean value. The number of individuals introduced defines the extent to which the population is exposed to demographic stochasticity. We consider population growth to be density-independent (i.e. we assume that during the establishment phase, the population is far from its carrying capacity) and little influenced by Allee effects [36].
(e). Environmental scenarios to simulate maladaptation
The degree of match between the phenotype and the environment is modelled by varying the costs of selecting a habitat where the species can be viable but maladapted [35], defined by the following scenarios:
(i) high phenotype–environment match, simulated by defining the two habitats as identical and without penalties (scenario 1). Therefore, fecundity and survival rates attain their maximum values, as defined by the species' life history;
(ii) insufficient phenotype–environment match penalizing adult survival (), simulated by imposing an increase in adult mortality of either 50% (scenario 2.1) or 100% (scenario 2.2) in habitat 2 (low-quality habitat, hereafter); and
(iii) insufficient phenotype–environment match penalizing offspring survival, simulated by increasing the probability of a brood failure () by either 50% (scenario 3.1) or 100% (scenario 3.2) in habitat 2 (low-quality habitat).
(f). Behavioural responses
To investigate how behavioural responses influence persistence in the different environmental scenarios, we first explore what happens when individuals are not allowed to take decisions (i.e. their behaviour is ‘neutral’). Thus, we assume that the probability of changing from one habitat to the other is the same ( and ) and all individuals reproduce after achieving adulthood (). To incorporate behavioural responses, we modify these parameters as follows:
(i) matching habitat choice (abbreviated GoodChoice) is an innate preference for the habitat that better matches the organism's phenotype (i.e. the high-quality habitat), which reduces either adult or offspring mortality depending on the environmental scenarios previously defined. To do so, the preference for habitat 1 is either doubled (moderate response) or quadrupled (strong response) in each simulation;
(ii) habitat mismatching choice (WrongChoice) describes an innate preference for the habitat that does not match the organism's phenotype (low-quality habitat), thereby increasing either adult or offspring mortality depending on the environmental scenario. Habitat mismatching choice simulates ecological traps [8]. To do so, is either doubled (moderate response) or quadrupled (strong response) in each simulation;
(iii) reproductive skipping (ReprSkip) refers to the decision about skipping or not a reproductive event when the individual is in the low-quality habitat. This simulates the storage effect [39] by which adults improve survival by skipping reproduction when conditions are inadequate. To achieve it, the probability to breed in habitat 2 is reduced to either 0.5 (moderate response) or 0.25 (strong response) in each simulation. Non-breeding adults are given a 50% increase relative to breeding adults in the probability to survive from t to t + 1;
(iv) learning through exploration (LearnExpl) refers to a decreased preference for the low-quality habitat after exploring any of the two habitats. This describes the process of gathering information to make more informed decisions [40]. To do so, the preference for the high-quality habitat once the individual has explored the low-quality habitat is either doubled (moderate response) or quadrupled in each simulation (strong response), while the probability of moving from the best to the worse habitat () is set to zero, except for breeders that failed to reproduce. In this latter case, is doubled or quadrupled; and
(v) learning from a breeding experience (LearnBreed) is the decision about changing habitat or not according to the result of the past breeding attempt. Regardless of the habitat, a reproductive failure in the habitat makes it more likely that individuals change the habitat in the next breeding attempt. Thus, and is 0 when the reproduction is successful (i.e. at least one offspring is produced), and the probability of shifting habitat in each simulation is either doubled (moderate response) or quadrupled (strong response) after a failed reproduction.
(g). Simulations
The probability of persisting in the novel environment was estimated for different initial population sizes (N0 from 2 to 100) as the proportion of populations that avoid extinction after 50 years, based on 10 000 replicates. This allowed us to describe the curves relating the likelihood of establishment with N0 for each possible combination of life-history strategy, behavioural response and environmental scenario (see details below). As an integrative measure of the likelihood of population persistence, we used the initial population size that allows 50% of the populations to persist during the 50 years (N0P50%). The value of each N0P50% was estimated through a lineal search testing different initial population size.
(h). Exploration of the parameters
The exploration of the parameters was carried out by crossing all combinations of life-history traits with the behavioural responses and environmental scenarios. To obtain all combinations of life-history traits, we first defined regular sequences for each life-history trait within the ranges found in birds, based on published information [27,41]. The traits and ranges included adult survival (0.1–0.95), number of broods per year (1–2), number of offspring per brood (1–20) and age at first reproduction (1–4). For subadult stages, we used the same survival as for the adults. Next, we created all the possible combinations of life-history traits and fixed the deterministic growth rate λ from 1.05 to 1.2 by adjusting juvenile mortality rate, solving the Euler–Lotka equation (see the electronic supplementary material for details). Strategies with juvenile survival lower than 0.1 or higher than adult survival were discarded. The total number of life-history strategies resulting from the combination of life-history traits was 3612.
To evaluate the impact of these life-history strategies on the persistence of the populations in the novel environment, we first tested the sensitivity of N0P50% to λ, fecundity, age at first reproduction and age-specific survival by means of partial rank correlation coefficients (PRCC) [42]. This method measures the association between two variables while accounting for the effect of other variables, and has the advantage of being little affected by collinearity and nonlinear relationships. In addition, we also compared how N0P50% varies between fast and slow strategies as a function of behavioural responses and maladaptive scenarios. The position of each life-history strategy along the fast–slow continuum was assessed as the relative sensitivity (i.e. elasticity) of population growth to changes in fecundity. Given that the fast–slow continuum describes a fecundity–survival trade-off [23], any combination of life-history traits characterizing slow species should be related to high elasticities for adult survival and low elasticities for fecundity, the contrary being true for fast species. We classified life-history strategies as slow when their elasticities for fecundity were in the first quartile and as fast when their elasticities for fecundity were in the uppermost quartile (using elasticities for adult survival gives qualitatively similar results).
3. Results
(a). Behavioural responses in stochastic, maladaptive scenarios
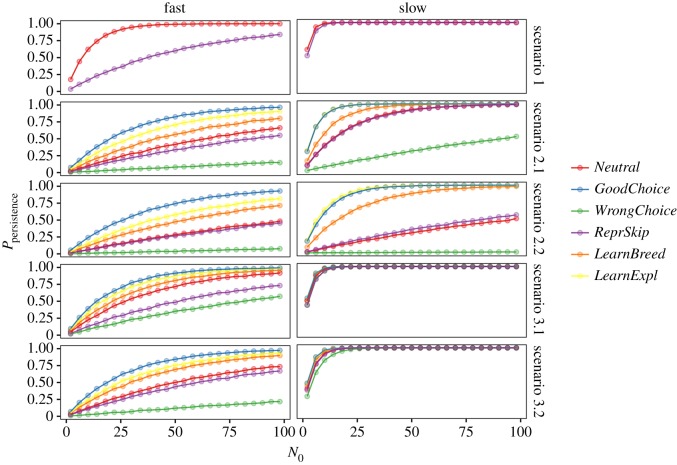
We first illustrate the results of the model by presenting the simulations for two species with the same maximum deterministic growth rate (λ = 1.05) but striking differences in life history, one being at the fast extreme of the fast–slow continuum and the other at the slow extreme. Figure 1 presents the simulated probability that these species thrive in a novel environment as a function of initial population size (N0), according to different behavioural responses and scenarios of maladaptation (see also the electronic supplementary material, figure S2). In all the scenarios, the likelihood of establishment increases with N0 until reaching a threshold above which the probability of population persistence is 1 (i.e. all simulated populations become established). This pattern, which has also been found empirically [43,44], reflects the pervasive effect of demographic stochasticity at small population sizes.
Figure 1.
Simulations of probability of population persistence for 10 000 replicates as a function of behavioural responses (Neutral, random behavioural responses; GoodChoice, matching habitat choice; BadChoice, habitat mismatching choice; ReprSkip, reproductive skipping; LearnExpl, learning through exploration; LearnBreed, learning from breeding experience) for different initial population sizes according to different life histories (fast and slow). Simulations have been run with the same deterministic growth rate (λ) of 1.05 and moderate behavioural responses, under the five different scenarios: phenotype–environmental matching (scenario 1) and phenotype–environmental mismatch causing moderate increases of adult mortality (scenario 2.1), extremely high adult mortality (scenario 2.2), moderate increases of juvenile mortality (scenario 3.1) and extremely high juvenile mortality (scenario 3.2). Simulations with strong behavioural responses are shown in the electronic supplementary material, figure S2. The fast strategy is characterized by early onset of first reproduction (1 year old), high annual fecundity (q = 8) and low adult survival (), while the slow strategy exhibits delayed onset of reproduction (3 years old), low fecundity (q = 8) and delayed onset of first reproduction but high adult survival ( ). Note that in scenario 1, the two habitats are the same, and therefore, all behavioural responses except reproductive skip are equivalent to the neutral behaviour.
In the absence of behavioural responses (red line), the curve relating the probability of persistence and N0 becomes flatter under maladaptation (figure 1, scenarios 2.1, 2.2, 3.1 and 3.2) relative to scenarios where there is phenotype–environment match. This is because the population not only suffers from demographic stochasticity but also from the negative population growth of the fraction of the population settled in the low-quality habitat. The new route towards extinction largely reduces population persistence, notably in scenarios where the phenotype–environment mismatch is higher (electronic supplementary material, figure S2, scenarios 2.2. and 3.2).
When individuals are allowed to take decisions, either based on inherited or learned preferences, the probability of persistence experiences substantial changes relative to the situation where their behavioural responses are neutral (figure 1; electronic supplementary material, figure S2). Matching habitat choice and learning both contribute substantially to increase the likelihood of persistence in a context of maladaptation. Learning is generally not so efficient as an innate choice based on perfect knowledge. When knowledge is imperfect, however, innate responses can increase extinction risk by leading individuals to choose an inappropriate habitat. Likewise, the decision of skipping a reproductive event when conditions are unfavourable often entails important fitness costs, reducing the probability of establishment.
(b). Integrating behavioural responses and life-history strategies
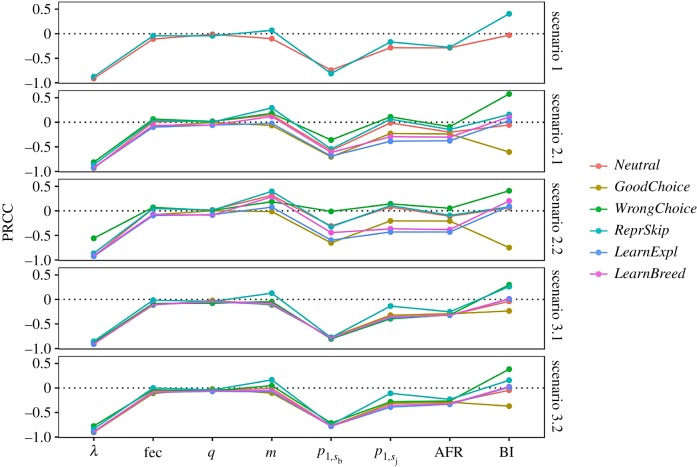
Figure 1 suggests that the way behavioural responses influence persistence in the novel environment differ according to the position of the species in the fast–slow continuum. To formally explore this, we repeated the simulations for the 3612 life-history strategies resulting from all combinations of life-history traits with λ between 1.05 and 1.2 (see the section Exploration of the parameters for details). For each life-history strategy, we then estimated N0P50% to describe the likelihood that the species persists in the novel scenario as a function of their behaviour. Sensitive analyses across all scenarios and behavioural strategies show that λ is the most important factor facilitating population persistence in the novel environments (figure 2). Life-history strategies with higher λ show lower N0P50%, implying that they need fewer individuals to become established. However, adult survival is the life-history trait with greater influence in population persistence, suggesting that slow strategies have generally higher chances than fast strategies to persist in novel environments (figure 2). The high persistence of slow species in novel environments does not merely result from the individuals initially introduced being able to survive the entire simulation period. The explored life-history trait combinations rarely allow individuals to survive 50 years, and in most cases, the final population is higher than the initial one (electronic supplementary material, figure S3).
Figure 2.
Sensitivity of the probability of population persistence to life-history traits for different behavioural responses and maladaptive scenarios, based on PRCC. Population persistence is measured as N0P50%, the initial population that give a 50% chance of persistence. Notation not shown in table 1 is as follows: λ is the deterministic grow rate; fec is fecundity expressed as the number of offspring produced annually (m*q); AFR, is the age at first reproduction; BI is the intensity of the behavioural responses, i.e. either moderate or strong. Analyses are based on 3612 combinations of life-history traits distributing species along the fast–slow continuum.
(c). Costs and benefits of behavioural responses in fast and slow strategies
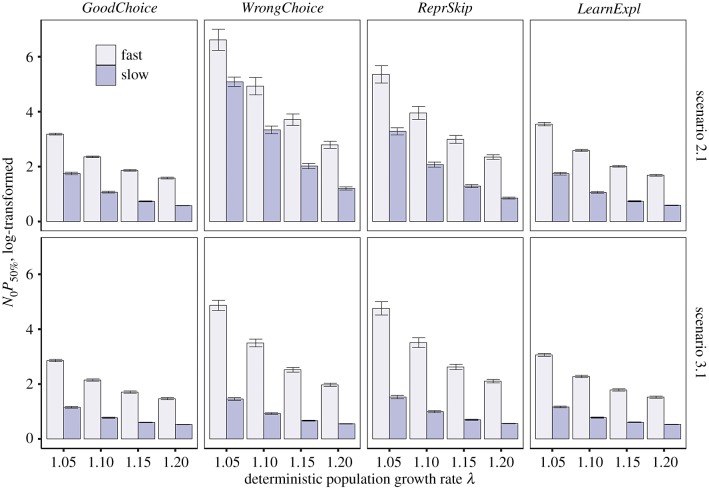
To further investigate the interaction between behaviour and life history, we compared life-history strategies positioned either at the fast or slow extremes of the fast–slow continuum (see the section Exploration of the parameters for details). The results confirm that slow strategies generally need a lower N0P50% than fast strategies to persist in the novel environments (figure 3). To reach a success similar to that of slow strategies, fast strategies must have values of λ substantially higher (often more than 15% higher) than those of slow strategies.
Figure 3.
Effects of behavioural responses on population persistence in novel environments as a function of the position of the animal along the fast–slow continuum. Population persistence is estimated as N0P50% and behavioural responses are moderate (for strong responses, see the electronic supplementary material, figure S4). For details on abbreviations, see figure 1. (Online version in colour.)
Under maladaptive scenarios, the probability of persistence depends on whether the phenotype–environment mismatch mainly affects offspring or adults, as fast and slow strategies differ in their sensitivity to changes in fecundity and adult mortality. Thus, although the general tendency of slow species to be superior invaders is consistent across environmental scenarios, slow species are particularly affected by scenarios increasing adult mortality and fast species by those affecting offspring mortality.
The benefits and costs of the behavioural responses are also contingent to the position of the species along the fast–slow continuum (figure 3; electronic supplementary material, figures S6–S10). In slow species, the gains of learning are substantial when maladaptation increases adult mortality, while the gains are almost negligible when maladaptation affects offspring because they are already well protected for their life history. Because slow strategies have more opportunities to reproduce in the future, they are less penalized than fast species by mistakenly choosing an inappropriate habitat to reproduce. Likewise, the decision of skipping a reproductive event when conditions are unfavourable, which is generally costly (figure 1), has a negligible impact on the demography of slow species when the risk of reproductive failure is high.
For fast species, learning through exploration and an innate preference for the high-quality habitat tend to improve population persistence in all scenarios, although the gains are modest and rapidly decrease at higher λ values (figure 3; electronic supplementary material, figures S6 and S8). Learning from a reproductive failure is marginally beneficial only when phenotype–environment match increases offspring survival, even though the risk of extinction remains high (electronic supplementary material, figure S9). The costs of preferring a low-quality habitat or skipping a reproductive event are also generally high in most scenarios, compared to those of slow species, and generally cannot be compensated by increasing λ (electronic supplementary material, figure S7).
4. Discussion
Our results show strong support for the notion that behavioural responses interact with life history to influence persistence in novel environments. Under maladaptive scenarios, where the match of the phenotype to the environment is insufficient, the simulations suggest that it pays to have a slow life history that increase the value of adults over the value of offspring even at the cost of decreasing reproduction. This is in part owing to the demographic consequences of the life-history strategy itself and in part owing to the added benefits of behavioural responses. Thus, a slow strategy represents a strong buffer against maladaptation causing high offspring mortality, indirectly affecting adult survival and hence the opportunities for future reproduction. Instead, behavioural responses primarily buffer individuals against maladaptation causing high adult mortality. As novel environments are likely to increase both adult and offspring survival, the complementary effects of behavioural responses and life history make slow animals particularly well equipped to cope with sudden changes in the environment.
The notion that slow animals exposed to novel environments generally gain greater benefits from behavioural responses has been suggested in previous studies. Animals at the ‘slow’ extreme of the fast–slow continuum are generally believed to explore more accurately the environment and exhibit better performance in learning than those at the ‘fast’ extreme (reviewed in [2]). Eliassen et al. [19], for instance, developed a model to investigate how foragers benefit from using a simple learning rule to update estimates of temporal changes in resource levels; the model showed that as lifetime expectancy decreases, learners invest less in information acquisition and show lower foraging performance when resource level changes through time. Our simulations generally align with these studies, even though we did not explicitly consider cognitive differences in learning between fast and slow animals. Although it is likely that including these differences accentuate the superiority of slow species in contexts of maladaptation, this will depend on costs that are difficult to estimate. Our model assumes some costs of behavioural responses, such as imperfect information leading to choose a low-quality habitat and a loss of breeding opportunities. However, there are other costs not considered, such as those related to the need to invest time and energy to produce and maintain the neural and cognitive functions needed to acquire and respond to environmental information.
A particularly intriguing question is to what extent innate preferences and learning interact to influence the realized preferences for habitats. Kawecki [17] argued that an individual with no clear innate preference will be more amenable to changing its preference as a result of experience than an individual that already shows a strong innate preference, even when it means choosing a low-quality resource. Thus, it may be that some species primarily rely on matching the environment to the phenotype through habitat matching choice, while others rely more on improving the match of the phenotype to the new environment through learning. Several factors might contribute to favour one strategy over the other. Natural selection on heritable variation in habitat preferences should be more efficient in fast species, whose short generation times increase mutation rates and changes in allele frequency. Instead, in slow species that respond more slowly to selection, learned preferences would outperform genetically determined preferences (present study, see also [8]). Learning might also be particularly favoured in ecological generalists. A generalist strategy selects against local adaptation [35], and frequently exposes individuals to new challenges that require learned responses [45,46]. Our simulations suggest an additional factor that might contribute to favour learning over phenotype matching choice: the degree of novelty in the environment. We find that learning does not avoid extinctions as efficiently as perfect knowledge, but in terms of population persistence, it avoids the risk of falling into an ecological trap. Learning seems thus to be a better strategy than matching habitat choice to thrive in environments that are very different from the ancestral environments or that change too fast to provide reliable cues for habitat choice. One example could be urban environments. These environments expose animals to a variety of challenges that are drastically different from those found in nature, such as the need to confront frequent disturbances by people or avoid risks associated with traffic and buildings. Growing evidence indicate that urban animals tend to be more proficient in learning than non-urban animals [15].
Our results contribute to the debate over whether successful invaders should be characterized as fast or slow, an issue of high relevance to predict and prevent the spread and impact of biological invasions. Although life history has long been deemed essential to understanding the success of invaders [30], confidence in theoretical arguments has been undermined by a perceived lack of empirical support [27]. The dissociation between theoretical and empirical work has in part been attributed to the excessive focus on the ‘small population paradigm’ [2], which assumes that demographic stochasticity is the main driver of extinction in introduced populations. This has led to the widespread belief that successful invaders are characterized by high fecundity that reduces the risk of stochastic extinctions by facilitating rapid population growth from small initial populations. While this process has received some empirical support [47,48], our results align with theoretical and empirical work suggesting that it mainly applies when the organism's phenotype matches well with the environment [27,49]. Yet, under maladaptive scenarios our simulations indicate that fast strategies are more affected by ecological traps and are only superior to slow strategies when their population growth rate is substantially higher. Moreover, this superiority is only noticeable when the phenotype mismatch with the environment increases adult mortality, reflecting that population growth of fast species is less sensitive to changes in adult mortality than in fecundity. Given the importance of parental care in many animals, however, it is unrealistic to assume that a high adult mortality will not be accompanied by increased offspring mortality [50]. The crucial question is therefore to what extent fast animals can maintain high population growth rates in a context of maladaptation. Current evidence in birds and mammals does not indicate that fast species have higher population growth rates in the wild than slow-lived species (electronic supplementary material, figure S11). To properly clarify this issue on empirical grounds, however, we would need field estimations of population growth rate for fast and slow populations exposed to different degrees of phenotype–environment mismatch. Unfortunately, this type of information is currently unavailable.
As any model, ours is a simplified representation of the reality. An issue that remains insufficiently resolved is how different behavioural responses affect establishment success when acting in concert. In our simulations, we have investigated behavioural mechanisms separately, to be able to disentangle their effects, but in reality, it is likely that they act in concert, either synergically or antagonistically. The challenge here is to parametrize the models in a way that is realistic enough to avoid biasing the simulations, but this requires a better understanding of mechanisms. Another issue that will need further attention in the future is the possibility that other mechanisms in addition of those analysed here also influence the response to environmental changes. We have previously suggested that producing several broods in the same breeding season can afford high benefits when the chances of a reproductive failure are high, as it provides the advantage of a high annual fecundity while reducing the costs of a reproductive failure [27]. Future models will also have to consider Allee effects, that is, the decline in the rates of reproduction and/or survival at low population densities. These effects are not only highly relevant during the early stages of the invasion process, but may also be tied to the life history and behavioural strategies of the species [51]. A preference for a low-quality habitat is indeed a type of Allee effect, as it slows population growth at low densities [8], but other types of Allee effects could also be relevant [52]. Allee effects are expected to be particularly relevant in highly social animals that rely more on social and public information to take decisions and learn. Advancing in all these themes will offer a more complete picture of how animals cope with environmental changes.
Although organisms that are slow-lived relative to the rate of environmental fluctuations often exhibit enhanced learning abilities [45], the evolutionary causes are less well understood. It has been suggested that the causal link between learning and longevity could be bi-directional [19,53,54]. The possibility of constructing behavioural responses to ecological challenges might directly affect the evolution of life histories by buffering individuals from extrinsic mortality. The evolved combination of life-history traits might in turn alter the fitness benefits and costs of behavioural responses, as suggested here. However, the covariation between learning and life history can also result from correlated evolution [45]. Our results reinforce this latter view, suggesting that the environments which favour slow life-history strategies are similar to those favouring learning. Thus, behavioural plasticity and slow life histories might be dimensions of a same pace-of-life syndrome to cope with sudden environmental changes [45].
We have shown that considering variation in life-history species is relevant when predicting the influence of behaviour on the probability of persisting in novel environments. Although the interplay between behaviour and life history is still insufficiently understood, our results highlight that to continue advancing, we need to acknowledge that both may be part of a broader adaptive system of organisms to cope with rapid environmental changes.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Jakob Bro-Jørgensen, Dan Franks and Kristine Meise for the invitation to participate in the ZSL symposium and to contribute to this special issue, Hanna Kokko for helping to develop the model during the stay of J.M. at the University of Zurich, three anonymous reviewers for insightful comments and all Sol laboratory members for fruitful discussions.
Data accessibility
This article has no additional data.
Authors' contributions
J.M. and D.S. designed the study, J.M. implemented the model and the simulations under the supervision of D.S. and R.M.-H., D.S. led manuscript writing assisted by J.M. and R.M.-H., and R.M.-H. developed the formal notation of the model.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Spanish government (CGL2017-90033-P to D.S.).
References
- 1.Bell G. 2017. Evolutionary rescue. Annu. Rev. Ecol. Evol. Syst. 48, 605–627. ( 10.1146/annurev-ecolsys-110316) [DOI] [Google Scholar]
- 2.Sol D, Maspons J. 2016. Life history, behaviour and invasion success. In Biological invasions and animal behaviour (eds Weis JS, Sol D), pp. 63–81. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Sakaia K, et al. 2001. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332. ( 10.1146/annurev.ecolsys.32.081501.114037) [DOI] [Google Scholar]
- 4.Sih A, Ferrari MCO, Harris DJ. 2011. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. ( 10.1111/j.1752-4571.2010.00166.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klopfer PH. 1981. Behavioral mechanisms in ecology. Ecology 62, 286 ( 10.2307/1936697) [DOI] [Google Scholar]
- 6.Sol D. 2003. Behavioural innovation: a neglected issue in the ecological and evolutionary literature? In Animal innovation (eds Reader SM, Laland KN), pp. 63–82. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Tuomainen U, Candolin U. 2011. Behavioural responses to human-induced environmental change. Biol. Rev. 86, 640–657. ( 10.1111/j.1469-185X.2010.00164.x) [DOI] [PubMed] [Google Scholar]
- 8.Kokko H, Sutherland WJ. 2001. Ecological traps in changing environments: ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol. Ecol. Res. 3, 537–551. [Google Scholar]
- 9.Nicolaus M, Edelaar P. 2018. Comparing the consequences of natural selection, adaptive phenotypic plasticity, and matching habitat choice for phenotype-environment matching, population genetic structure, and reproductive isolation in meta-populations. Ecol. Evol. 8, 3815–3827. ( 10.1002/ece3.3816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamps JA, Swaisgood RR. 2007. Someplace like home: experience, habitat selection and conservation biology. Appl. Anim. Behav. Sci. 102, 392–409. ( 10.1016/j.applanim.2006.05.038) [DOI] [Google Scholar]
- 11.Greene CM, Stamps JA. 2009. Habitat selection at low population densities. Ecology 82, 2091–2100. [Google Scholar]
- 12.Schmidt KA, Dall SRX, van Gils JA.. 2010. The ecology of information: an overview on the ecological significance of making informed decisions. Oikos 119, 304–316. ( 10.1111/j.1600-0706.2009.17573.x) [DOI] [Google Scholar]
- 13.Williams G. 1966. Adaptation and natural selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Snell-Rood EC. 2013. An overview of the evolutionary causes and consequences of behavioural plasticity. Anim. Behav. 85, 1004–1011. ( 10.1016/j.anbehav.2012.12.031) [DOI] [Google Scholar]
- 15.Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101–1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 16.Grieco F. 2002. Evidence for the effect of learning on timing of reproduction in blue tits. Science 296, 136–138. ( 10.1126/science.1068287) [DOI] [PubMed] [Google Scholar]
- 17.Kawecki TJ. 2010. Evolutionary ecology of learning: insights from fruit flies. Popul. Ecol. 52, 15–25. ( 10.1007/s10144-009-0174-0) [DOI] [Google Scholar]
- 18.Baudains TP, Lloyd P. 2007. Habituation and habitat changes can moderate the impacts of human disturbance on shorebird breeding performance. Anim. Conserv. 10, 400–407. ( 10.1111/j.1469-1795.2007.00126.x) [DOI] [Google Scholar]
- 19.Eliassen S, Jørgensen C, Mangel M, Giske J. 2007. Exploration or exploitation: life expectancy changes the value of learning in foraging strategies. Oikos 116, 513–523. ( 10.1111/j.2006.0030-1299.15462.x) [DOI] [Google Scholar]
- 20.Doligez B, Danchin E, Clobert J.. 2002. Public information and breeding habitat selection in a wild bird population. Science 297, 1168–1170. [DOI] [PubMed] [Google Scholar]
- 21.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 22.Ricklefs RE. 2004. The cognitive face of life histories. Wilson Bull. 116, 119–133. ( 10.1676/04-054) [DOI] [Google Scholar]
- 23.Stearns S. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 24.Stearns SC. 1983. The influence of size and phylogeny on patterns of covariation among life-history traits in the mammals. Oikos 41, 173–187. ( 10.2307/3544261) [DOI] [Google Scholar]
- 25.Sæther B-E, et al. 2013. How life history influences population dynamics in fluctuating environments. Am. Nat. 182, 743–759. ( 10.1086/673497) [DOI] [PubMed] [Google Scholar]
- 26.Sæther B, Engen S, Pape Møller A, Weimerskirch H, Visser ME, Fiedler W, Matthysen E. 2004. Life-history variation predicts the effects of demographic stochasticity on avian population dynamics. Am. Nat. 164, 793–802. ( 10.1086/425371) [DOI] [PubMed] [Google Scholar]
- 27.Sol D, Maspons J, Vall-llosera M, Bartomeus I, Garcia-Pena GE, Pinol J, Freckleton RP. 2012. Unraveling the life history of successful invaders. Science 337, 580–583. ( 10.1126/science.1221523) [DOI] [PubMed] [Google Scholar]
- 28.Forcada J, Trathan PN, Murphy EJ. 2008. Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Glob. Change Biol. 14, 2473–2488. ( 10.1111/j.1365-2486.2008.01678.x) [DOI] [Google Scholar]
- 29.Sætther B-E, Bakke O. 2014. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81, 642–653. ( 10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2) [DOI] [Google Scholar]
- 30.Lewontin RC, Cohen D. 1969. On population growth in a randomly varying environment. Proc. Natl Acad. Sci. USA 62, 1056–1060. ( 10.1073/pnas.62.4.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sæther B-E, et al. 2005. Generation time and temporal scaling of bird population dynamics. Nature 436, 99–102. ( 10.1038/nature03666) [DOI] [PubMed] [Google Scholar]
- 32.Starrfelt J, Kokko H. 2012. Bet-hedging—a triple trade-off between means, variances and correlations. Biol. Rev. 87, 742–755. ( 10.1111/j.1469-185X.2012.00225.x) [DOI] [PubMed] [Google Scholar]
- 33.Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C. 2000. Temporal variation in fitness components and population dynamics of large herbivores. Annu. Rev. Ecol. Syst. 31, 367–393. ( 10.1146/annurev.ecolsys.31.1.367) [DOI] [Google Scholar]
- 34.Cressman R, Křivan V. 2013. Two-patch population models with adaptive dispersal: the effects of varying dispersal speeds. J. Math. Biol. 67, 329–358. ( 10.1007/s00285-012-0548-3) [DOI] [PubMed] [Google Scholar]
- 35.Kisdi É. 2002. Dispersal: risk spreading versus local adaptation. Am. Nat. 159, 579–596. ( 10.1086/339989) [DOI] [PubMed] [Google Scholar]
- 36.Kawecki TJ. 1995. Demography of source–sink populations and the evolution of ecological niches. Evol. Ecol. 9, 38–44. ( 10.1007/BF01237695) [DOI] [Google Scholar]
- 37.Strasser CA, Neubert MG, Caswell H, Hunter CM. 2012. Contributions of high- and low-quality patches to a metapopulation with stochastic disturbance. Theor. Ecol. 5, 167–179. ( 10.1007/s12080-010-0106-9) [DOI] [Google Scholar]
- 38.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 39.Warner RR, Chesson PL. 1985. Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am. Nat. 125, 769–787. ( 10.1086/284379) [DOI] [Google Scholar]
- 40.Eliassen S, Jørgensen C, Mangel M, Giske J. 2009. Quantifying the adaptive value of learning in foraging behavior. Am. Nat. 174, 478–489. ( 10.1086/605370) [DOI] [PubMed] [Google Scholar]
- 41.Sol D, Maspons J, Gonzalez-Voyer A, Morales-Castilla I, Garamszegi LZ, Møller AP. 2018. Risk-taking behavior, urbanization and the pace of life in birds. Behav. Ecol. Sociobiol. 72, 59 ( 10.1007/s00265-018-2463-0) [DOI] [Google Scholar]
- 42.Saltelli A, Tarantola S, Campolongo F, Ratto M. 2002. Sensitivity analysis in practice. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 43.Blackburn TM, Prowse TAA, Lockwood JL, Cassey P. 2013. Propagule pressure as a driver of establishment success in deliberately introduced exotic species: fact or artefact? Biol. Invasions 15, 1459–1469. ( 10.1007/s10530-013-0451-x) [DOI] [Google Scholar]
- 44.Sol D, González-Lagos C, Moreira D, Maspons J. 2013. Measuring tolerance to urbanization for comparative analyses. Ardeola 60, 3–13. ( 10.13157/arla.60.1.2012.3) [DOI] [Google Scholar]
- 45.Sol D, Sayol F, Ducatez S, Lefebvre L. 2016. The life-history basis of behavioural innovations. Phil. Trans. R. Soc. B 371, 20150187 ( 10.1098/rstb.2015.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ducatez S, Clavel J, Lefebvre L. 2015. Ecological generalism and behavioural innovation in birds: technical intelligence or the simple incorporation of new foods? J. Anim. Ecol. 84, 79–89. ( 10.1111/1365-2656.12255) [DOI] [PubMed] [Google Scholar]
- 47.Allen WL, Street SE, Capellini I. 2017. Fast life history traits promote invasion success in amphibians and reptiles. Ecol. Lett. 20, 222–230. ( 10.1111/ele.12728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capellini I, Baker J, Allen WL, Street SE, Venditti C. 2015. The role of life history traits in mammalian invasion success. Ecol. Lett. 18, 1099–1107. ( 10.1111/ele.12493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeppsson T, Forslund P. 2012. Can life history predict the effect of demographic stochasticity on extinction risk? Am. Nat. 179, 706–720. ( 10.1086/665696) [DOI] [PubMed] [Google Scholar]
- 50.Santema P, Kempenaers B. 2018. Complete brood failure in an altricial bird is almost always associated with the sudden and permanent disappearance of a parent. J. Anim. Ecol. 87, 1239–1250. ( 10.1111/1365-2656.12848) [DOI] [PubMed] [Google Scholar]
- 51.Leung B, Drake JM, Lodge DM. 2013. Predicting invasions: propagule pressure and the gravity of Allee effects. Ecology 85, 1651–1660. ( 10.1890/02-0571) [DOI] [Google Scholar]
- 52.Reznick D, Bryant MJ, Bashey F. 2002. r- and K-selection revisited: the role of population regulation in life-history evolution. Ecology 83, 1509–1520. ( 10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2) [DOI] [Google Scholar]
- 53.Ratikainen II, Kokko H. 2019. The coevolution of lifespan and reversible plasticity. Nat. Commun. 10, 538 ( 10.1038/s41467-019-08502-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sol D. 2009. The cognitive-buffer hypothesis for the evolution of large brains. In Cognitive ecology II (eds Dukas R, Ratcliffe JM), pp. 111–134. Chicago, IL: University of Chicago Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.