Abstract
Establishing the cues or constraints that influence avian timing of breeding is the key to accurate prediction of future phenology. This study aims to identify the aspects of the environment that predict the timing of two measures of breeding phenology (nest initiation and egg laying date) in an insectivorous woodland passerine, the blue tit (Cyanistes caeruleus). We analyse data collected from a 220 km, 40-site transect over 3 years and consider spring temperatures, tree leafing phenology, invertebrate availability and photoperiod as predictors of breeding phenology. We find that mean night-time temperature in early spring is the strongest predictor of both nest initiation and lay date and suggest this finding is most consistent with temperature acting as a constraint on breeding activity. Birch budburst phenology significantly predicts lay date additionally to temperature, either as a direct cue or indirectly via a correlated variable. We use cross-validation to show that our model accurately predicts lay date in two further years and find that similar variables predict lay date well across the UK national nest record scheme. This work refines our understanding of the principal factors influencing the timing of tit reproductive phenology and suggests that temperature may have both a direct and indirect effect.
Keywords: blue tit Cyanistes caeruleus, constraints, cues, laying date, nest-box hole-nesting, trophic mismatch
1. Introduction
Global climate change is leading to increased ambient air temperatures and causing an advance of spring phenological events (seasonal natural phenomena) [1,2] across the Northern Hemisphere, by an average of 2.6 days per °C in the UK [3]. The timing of phenological events is often critical to the organisms involved, influencing whether key life-history stages (e.g. reproduction) coincide with favourable environmental conditions. These conditions could be purely abiotic, such as temperature, but often involve temporal synchrony with organisms at other trophic levels, be they resources or consumers [4,5]. Individuals that mistime such phenological events may incur considerable fitness costs [6,7]. Not all organisms or trophic levels are advancing their phenologies at the same pace in relation to climate change, however, as each may respond to different environmental cues or to similar cues dissimilarly [3,8,9]. This variation in response can cause trophic mismatch, whereby consumer phenology becomes asynchronous with an important resource [4,5].
Predicting how phenology will affect populations in the future requires detailed knowledge of the aspect(s) of the environment that species use to schedule their phenological events, and the magnitude of their responses to these environmental variables [10]. These environmental predictors might act as cues, signalling favourable future conditions, or constraints, prohibiting advancing phenology until certain conditions are met. A model terrestrial system for studying phenology and trophic mismatch is the deciduous tree—folivorous caterpillar—insectivorous passerine bird (e.g. tits Paridae) food chain [4,11,12], hereafter referred to as the focal system. In this system, there is an ephemeral superabundance of caterpillars in late spring, which consume young leaves before the trees impart defensive chemicals [13]. Adult birds that synchronize the peak demand of their offspring to coincide with this caterpillar peak fledge more young of higher quality [7,12]. Initiation of nest building occurs over a month before peak offspring resource demand; in the intervening period a clutch is laid, incubated and the chicks are partially reared [4,14]. Birds may, therefore, determine the timing of egg-laying in response to aspects of the environment that are informative of the timing of the future resource peak [15].
Despite the popularity of the focal system among researchers, the environmental variables that affect the reproductive phenology of the birds are only partially understood. One contributing predictor is photoperiod, whereby increasing daylight hours indicate approaching favourable breeding conditions [16]. The role of photoperiod has been demonstrated experimentally, as sustained exposure of blue tits (Cyanistes caeruleus) to artificially inflated photostimulation caused them to breed three months early when supplied with unlimited food [17]. Photostimulation operates through rapidly stimulating gonadal and follicular growth and signalling song production [18,19]. While there is an interval of approximately eight weeks between the onset of gonadal development and egg laying in wild tits, this can be reduced to five weeks under artificial photostimulation [17,19]. Such plasticity indicates that, while photostimulation is necessary to initiate reproduction, it is not in itself sufficient, and other stimuli act to fine-tune timing [20]. In addition, while variable laying dates among populations can be explained by locally adapted photoperiodic responses [21], photoperiod is consistent inter-annually and therefore cannot be responsible for substantial in situ variation in phenology (which can be several weeks) [22].
The average temperature during a period of spring has been shown to be a strong negative correlate of clutch initiation in woodland passerines [10,11,23]. For tit species, a rise of 1°C elicits a 3.5–5 day advancement in clutch initiation [4,22,24], but the mechanism whereby average temperature affects birds is unknown [25]. A direct effect of temperature on breeding phenology is often interpreted as being a cue that predicts the timing of the peak caterpillar resource several weeks later [26]. Alternatively, low temperatures might act as a constraint, limiting the onset of energetically costly processes such as egg production and incubation [27], although cue and constraint scenarios need not be mutually exclusive. In the space of about two weeks, a female blue tit can lay a clutch of eggs weighing in excess of 150% of her body weight [14]. In support of the temperature constraint hypothesis, cooling nest-boxes delays egg formation in starlings (Sturnus vulgaris) [28] and reduces egg volume in blue tits [29,30]. All previous observational studies have used daily average temperatures, but it is possible that temperatures at different times of day may act via different mechanisms. For instance, rising day-time temperatures may provide a cue of advancing conditions, whereas thermoregulation costs associated with low night-time temperatures may act as a constraint on egg-laying or a short-term cue of the predicted costs of incubation.
Whether temperature acts directly or via an indirect pathway, such as tree phenology or invertebrate abundance, is yet to be fully established. Tree leafing phenology, most frequently oak (Quercus sp.) or birch (Betula sp.), correlates positively with forest passerine lay date over time [31,32] and across space at the site [33] and UK-wide level [34]. As some of these studies omitted temperature as a predictor, it is possible that such phenological correlations arise because plants, invertebrates and birds all respond directly to temperature. A clear mechanism whereby vegetation phenology would affect bird breeding phenology has not been established, although it is possible that birds derive chemical cues from buds or visually assess tree phenology. Bud consumption is minimal and temporally consistent however [35], and inserting leafing branches into aviaries has no effect on lay date [36]. Artificial supplementary feeding of passerines has been found to advance lay dates by a few days to a week [37,38], including in woodland insectivores [39]. Manipulation of resources has been found to elicit greater responses in years [39] and territories [40] with lower food resource levels, indicating a possible alleviation of an environmental nutrient/energy constraint [41]. As far as we are aware, no previous analysis has tested the role of natural food resource availability as a phenological driver of breeding phenology in the focal system.
The aim of this study is to separate the effects of different putative predictors of breeding phenology (temperature, tree phenology, food availability and photoperiod), establishing which factors are most important in generating spatio-temporal variation in blue tit reproductive phenology. We analyse data collected from a 220 km transect of 40 woodlands across Scotland [42]. In contrast to typical single-site approaches to studying woodland bird phenology, by considering spatial and temporal variation this study design somewhat uncouples covariation between the putative predictors. In addition, while previous studies primarily focused solely on lay date as a measure of avian reproductive phenology, we also examine the predictors of an earlier phenological phase, nest building initiation date, as different environmental aspects may control the timing of each and permit fine-tuning of phenology throughout the breeding season [43,44]. We then assess the robustness of our predictions in two ways. Firstly, we conduct a cross-validation in which we test the performance of our model in predicting lay dates in two subsequent years. Secondly, we examine the generality of our predictions by combining three national datasets to test the performance of two key predictors with respect to blue tit lay dates across a long-term (47-year) UK-wide dataset incorporating 36 839 records.
2. Methods
(a). Study system
This study was conducted along a 220 km transect from Edinburgh (55°98′ N, 3°40′ W) to Dornoch (57°89′ N, 4°08′ W) in Scotland, incorporating 40 deciduous woodland sites (electronic supplementary material, figure A1) which varied in elevation (8–440 m above sea level) [42]. Each site had six nest-boxes (26 mm hole Schwegler 1B) used by breeding blue tits during 2014–2018. All dates used in this study are ordinal dates counted from 1 January. Temperature was monitored by two Thermachron iButtons (DS1922 L-F5), which were installed at opposite ends of each site from mid-February until mid-June every year. They were secured 1.5 m high on the north side of a tree, to avoid direct sunlight, in a waterproof white plastic film cartridge with a 20 mm diameter hole in the bottom to allow ambient air circulation and temperatures were recorded every hour on the hour to a sensitivity of 0.0625°C. Invertebrate availability was monitored over 4 day intervals using two caged, double-sided yellow sticky traps (245 × 100 mm) at each site, hung at ca 1.75 m [42]. Invertebrates over 3 mm in length were counted [42] and flying invertebrates captured using this technique are important dietary items during early spring [45,46].
Habitat surveys were conducted at all 40 sites as detailed in [42]. Tree phenology was studied on 6–10 locally representative focal trees per site per year, with the focal tree selection protocol detailed in the electronic supplementary material, appendix A, and focal tree taxa and coverage in the electronic supplementary material, table A1. On each visit (every other day), each focal tree was visually inspected using binoculars. The phenology of each focal tree was tracked, recording the dates of: (i) budburst—when the green leaf first emerges from the earliest bud on any part of the tree, and (ii) leafing—when the first leaf on any part of the tree is fully unfurled and looks to be the correct shape, if not eventual full size, for the leaf of that tree species [33].
All nest-boxes at intensively studied sites were checked every other day throughout the breeding season. The nest initiation date reflected the earliest day on which either the entire floor of the nest-box was covered with nesting material, or the nesting material had built up to greater than or equal to 45 mm depth at the front of the nest-box (measured from the bottom of the exterior of the nest-box to the top of the nesting material bulk). Lay date was defined as the date at which the first egg was laid in a lined nest, calculated as the previous day if two eggs were found as blue tits lay one egg per day, generally early morning [14]. One second brood occurred and was excluded from analyses.
(b). Statistical analyses
(i). Individual predictor models
To establish the best predictor belonging to each putative predictor block (temperature, tree phenology, invertebrate availability) of blue tit reproductive phenology, each measure of each predictor (detailed below) was first modelled individually in a linear mixed model (LMM) [47], with site and year as random effects, using maximum-likelihood. We assume that the effects of all variables on phenology are similar across space and time [22], meaning that we interpret the slope as indicative of plasticity with respect to the environmental predictor. Akaike information criteria (AIC) were then used for model comparison [48], and the model with the lowest AIC within each predictor block was selected. All models were also compared with a null model which included all random terms but only the intercept as a fixed effect, and marginal R2 values (representing the variance explained by fixed factors) and conditional R2 values (representing the variance explained by the entire model) were calculated for each model [49].
We considered five measures of temperature as predictors of blue tit phenology (24 h, day-time, night-time, daily maximum and daily minimum) to examine whether bird phenology is sensitive to temperatures at particular times of the day or temperature extremes. Each temperature predictor was calculated as a mean over a thermal sensitivity period, which was different for nest initiation and lay date. The use of a sliding window [10,22] to identify this thermal sensitivity period proved to be ineffective with our dataset owing to the very high among-day correlation between mean temperatures estimated over different sliding windows, a consequence of most of our replication being spatial rather than temporal (i.e. high elevation sites are typically colder than low elevation sites). We, therefore, used the sensitivity period for lay date (days 75–128) estimated by an earlier study for blue tits across the UK [22]. As there are no published estimates of the sensitivity period available for nest initiation, we subtracted the mean lag between nest initiation and lay date in our dataset (n = 20 days) from the period used for lay date (days 55–108). Day-time was defined as those hours after sunrise and before sunset throughout the entire sensitivity period (0800–1700 h for nest initiation, 0700–1800 h for lay date), with night-time the hours always after sunset and prior to sunrise (2000–0500 h for nest initiation, 2100–0400 h for lay date). In a post hoc test of the importance of day-time versus night-time temperature, we included both fixed terms in a single LMM and report these results in the electronic supplementary material, figure A2.
We considered six measures of tree phenology (mean budburst/leafing, foliage-weighted budburst/leafing, birch budburst/leafing). Firstly, the mean budburst of all focal trees was calculated for each site in each year. Secondly, a weighted budburst was calculated using electronic supplementary material, equation A1 that considered the composition of the habitat at each site given the coverage offered by the focal trees. Thirdly, mean birch budburst was calculated for each site containing birch in each year, as birch is the commonest tree genus on the transect [42], has early phenology, and has been previously linked to bird phenology [32]. Where we lacked birch phenology data (n = 4), birch budburst was taken from the geographically nearest site. Identical measures as detailed above were also taken to create mean leafing, weighted leafing and birch leafing per site per year. Leafing was not considered as a predictor of nest initiation as it occurred on average 19 days later.
To establish the measure of invertebrate availability, total invertebrate numbers were logged (log x + 1) for each sticky trap owing to the lognormal distribution of abundances, and mean totals per site collection day were calculated. To obtain a number per day, the exponent (exp x − 1) of these totals was then divided by four (as sticky traps were collected every 4 days) and logged again (log x + 1). A sliding window approach [10,22] was then used to identify the time period during which mean invertebrate availability best predicted nest initiation and lay date across all sites and years. For the sliding window, starting dates 82–100 and durations of 10–60 days were considered, with a cut-off end date representing the mean of the respective blue tit phenology.
(ii). Combined predictor models
A full model (lmer) was generated [47] to analyse the predictors of blue tit reproductive phenology simultaneously. Nest initiation and lay date were the responses, in separate models, with the best temperature measure predictor, the best tree phenology predictor, the best invertebrate availability predictor (all respective for each response) and latitude (as a proxy for photoperiod) included as fixed effects, and site and year as random effects. The same models were run using the spaMM package [50], with the inclusion of a Matern spatial autocorrelation term to (i) determine the extent of spatial autocorrelation and (ii) assess the sensitivity of results to the effects of spatial autocorrelation, allowing for an exponential decay (nu = 0.5). A null model, containing no fixed predictors of each response and site and year as random effects, was also created for comparison.
(iii). Robustness of predictions
The predictive performance of the significant terms from the full lay date model was assessed in two ways (nest initiation predictions were not assessed owing to poor model performance). First, we employed a cross-validation approach and tested the ability of our estimated model coefficients to predict lay date in two subsequent years (2017–2018) at the same sites. For this, a new full model was created identical to that described above (lmer), but without invertebrate availability, as these data were not collected in 2017–2018. Based on latitude, mean night-time temperature (days 75–128) and mean birch budburst, this model predicted lay date for each nest-box in 2017–2018. This prediction was then compared with the observed lay date at each nest-box during each year and the root-mean-square-error and out of sample cross-validated R2 were calculated.
To assess whether the drivers we identified are able to predict phenology on a considerably larger spatial and temporal scale, we combined three national databases. We used blue tit lay date from the British Trust for Ornithology nest record scheme [51], including records from the period 1970–2016 for which the uncertainty in lay date was less than or equal to 10 days (n = 36 839). Our temperature measure was mean 24 h temperature for days 75–128 for each matched 5 km grid square in each year, derived from daily interpolations from UK weather stations [52]. We used birch leafing dates from across the UK as recorded by the Woodland Trust's Nature's Calendar citizen science scheme for the period 1998–2014 (n = 14 892), using leafing rather than budburst as these are subject to less measurement error by citizen scientists [53]. We analysed these data as a trivariate response in a Bayesian generalized LMM (GLMM) [54], treating lay date as censored Gaussian [55] and the other variables as Gaussian. We included 50 km grid cell, year, 50 km grid cell : year interaction, 5 km grid cell and residual as random terms, using parameter expanded priors except for the residual (inverse Wishart, nu = 0.002) [56]. For each random term other than the residual, we can estimate the variance-covariance of lay date, temperature and birch phenology (electronic supplementary material, appendix A: trivariate model matrix) and from this coefficients of bird phenology regressed on tree phenology and temperature can be calculated (see the electronic supplementary material, appendix A [56]); for the residual we only estimated the variance of each of the response terms. Model convergence was assessed via inspection of trace files and all effective sample sizes for focal parameters exceeded 1000.
3. Results
(a). Individual predictor models
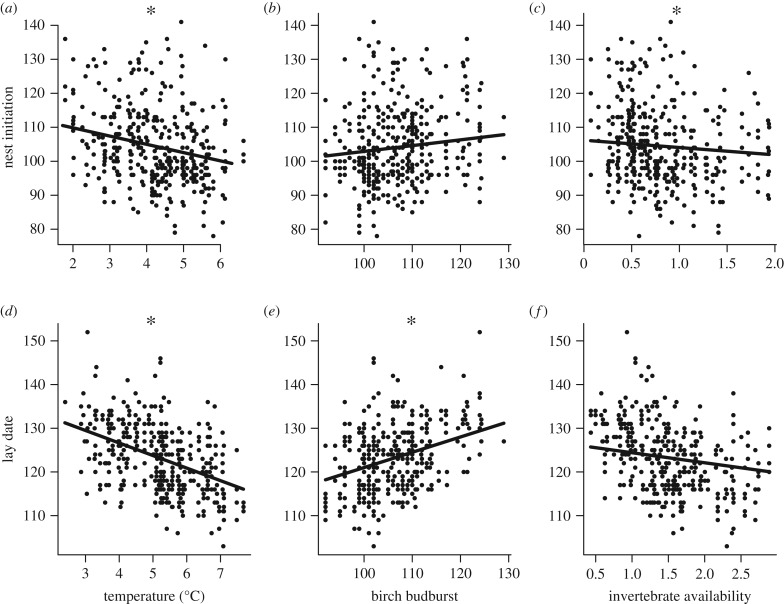
All temperature predictors for blue tit reproductive phenology returned a negative slope, and all but one were a significant improvement on their respective null models (ΔAIC > 2, electronic supplementary material, table A2). The best temperature predictor for both nest initiation and lay date was mean night-time temperature over their respective time sensitivity periods, which significantly outperformed all other temperature predictors (electronic supplementary material, table A2) and showed similar responses for both nest initiation (−2.43 ± 0.83 days °C−1) and lay date (−2.87 ± 0.56 days °C−1). In a post hoc test that included both mean day-time and mean night-time temperate predictors, the slope for mean night-time temperature was consistent with the slope in the original model, whereas the slope for mean day-time temperature was far shallower, consistent with night-time temperature being the stronger predictor (electronic supplementary material, figure A2). Temperature predictor models for lay date consistently captured more variance (electronic supplementary material, table A2, marginal R2 = 0.19) than those for nest initiation (electronic supplementary material, table A2, marginal R2 = 0.05). For nest initiation, site variance was much more pronounced than year variance, and mean night-time temperature explained approximately a third of each (electronic supplementary material, table A2). For lay date, site and year variance were more similar in magnitude and mean night-time temperature explained more than four-fifths of inter-annual variance, and over a third of site variance (electronic supplementary material, table A2).
The slopes of all models using tree phenology as a predictor of blue tit reproductive phenology reveal that later tree phenology predicts later reproductive phenology (electronic supplementary material, table A3). The best tree phenology predictor of both nest initiation and lay date was birch budburst (electronic supplementary material, table A3). While birch budburst was not a significant predictor of nest initiation (b = 0.17 ± 0.11, ΔAIC = 0.4, marginal R2 = 0.01), it was a significant predictor of lay date (b = 0.35 ± 0.07, ΔAIC = 18.6, marginal R2 = 0.11).
Using sliding windows, we found the best mean invertebrate availability predictors of blue tit phenology were between days 82–95 for nest initiation and days 93–123 for lay date. Invertebrate availability significantly predicted nest initiation (electronic supplementary material, table A4), but captured very little variance in either nest initiation or lay date (marginal R2 = 0.01–0.03), and the effect sizes were small, such that nest initiation and lay date were predicted to occur just 4 and 5 days earlier, respectively, when invertebrate availability was at its highest value compared to its lowest (figure 1c,f).
Figure 1.
Relationship between the best individual environmental predictor variables and two measures of blue tit reproductive phenology (a–c: nest initiation, d–f: lay date). (a) Mean night-time (2000–0500 h) temperature during the period 24 February–18 April; (b) mean birch budburst date; (c) mean invertebrate availability during the period 23 March–5 April; (d) mean night-time (2100–0400 h) temperature during the period 16 March–8 May; (e) mean birch budburst date; and (f) mean invertebrate availability during the period 3 April–3 May. All slopes shown are taken from the best predicting models summarized in the electronic supplementary material, tables A2–A4 and significant slopes are marked with an asterisk.
(b). Combined predictor models
In the full models, that included the best predictor from each single predictor model and latitude as a proxy for photoperiod, nest initiation was not significantly predicted by any single predictor variable and the full model performs rather poorly in explaining the variance (table 1, marginal R2 = 0.06, conditional R2 = 0.25). In comparison, lay date was significantly predicted by both night-time temperature (b = −1.65 ± 0.69) and birch budburst (b = 0.22 ± 0.09), explaining a substantial proportion of the variance (marginal R2 = 0.20, conditional R2 = 0.44), capturing approximately 39% of site variance and 93% of inter-annual variance (table 1). Latitude was a non-significant predictor of both responses. Models that estimated spatial autocorrelation returned very similar results and revealed spatial autocorrelation to be negligible, with the range at which autocorrelation drops to 0.1 being less than 0.01° for both nest initiation and lay date, equating to distances within a site [42].
Table 1.
Summary of model outputs from LMM's incorporating all predictors of nest initiation and lay date. (Significance asterisks show p-values (*0.05). Temperature shows the slope for the best temperature predictor found for each response in the electronic supplementary material, table A2 (mean night-time temperature for both responses), tree phenology shows the slope for the best tree phenology predictor for each response in the electronic supplementary material, table A3 (birch budburst for both responses), invertebrate availability shows the slope for the best invertebrate availability predictor for each response in the electronic supplementary material, table A4 (mean availability between days 82–95 for nest initiation, days 93–123 for lay date), and photoperiod shows the slope for latitude as a proxy for photoperiod. Random effect variances for each model are also shown (site, year and residual). In spaMM models, nu was fixed at 0.5 to constrain the spatial autocorrelation to follow an exponential decay.)
| response | model | intercept | temperature | tree phenology | invertebrate availability | photoperiod proxy | site variance | year variance | residual variance | R2 marginal | R2 conditional |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nest initiation | null | 104.5 ± 1.5 | 28.2 | 4.1 | 97.9 | 0.00 | 0.25 | ||||
| lmer | 139.8 ± 102.7 | −2.00 ± 1.27 | 0.07 ± 0.14 | −1.18 ± 1.63 | −0.59 ± 1.74 | 22.9 | 3.1 | 98.3 | 0.06 | 0.25 | |
| spaMM | 127.6 ± 99.6 | −1.86 ± 1.16 | 0.07 ± 0.14 | −1.25 ± 1.57 | −0.39 ± 1.69 | 28.7 | 1.7 | 89.8 | rho = 283.5 | ||
| lay date | null | 123.2 ± 2.4 | 17.2 | 16.2 | 34.2 | 0.00 | 0.49 | ||||
| lmer | 139.7 ± 67.2 | −1.65 ± 0.69* | 0.22 ± 0.09* | −1.50 ± 1.07 | −0.50 ± 1.15 | 10.5 | 1.2 | 33.6 | 0.20 | 0.44 | |
| spaMM | 129.0 ± 67.9 | −1.48 ± 0.69* | 0.23 ± 0.08* | −1.29 ± 1.04 | −0.37 ± 1.16 | 14.3 | 1.4 | 29.7 | rho = 267.3 |
(c). Robustness of predictions
The cross-validation model using data collected in the subsequent 2 years was found to provide an accurate (root-mean-square-error = 6.05 days) and unbiased (figure 2) prediction of lay date, with the explanatory power very similar to that of the original model (out-of-sample cross-validated R2 = 0.21).
Figure 2.
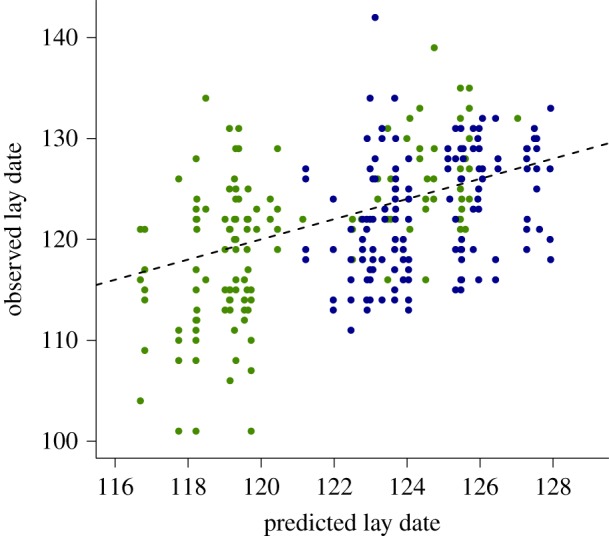
The relationship between predicted and observed lay dates during the validation years 2017 (green points) and 2018 (blue points) on the Scottish transect. The dashed line is the 1 : 1 relationship. Note that observed lay date varies more than predicted lay date because predictions are made for site means. (Online version in colour.)
Across the UK (50 × 50 km grid cells), the regression coefficients for mean 24 h temperature as a predictor of lay date were negative but non-significant (b = −2.070, 95% credible interval (CI) = −7.186–3.550), whereas over time the equivalent slope was significant (b = −2.059, 95% CI = −3.370−0.858) (electronic supplementary material, figure A3). Similarly, birch leafing was a positive but non-significant predictor of lay date across the UK but significant across years (b = 0.311, CI = 0.092–0.516), with the slope similar to that obtained for our transect (electronic supplementary material, figure A3). On average, birch leafing occurred 11.7 days (95% CI = 11.08–12.33) before blue tit lay date in the UK. The slope estimates obtained for temperature and birch as predictors of lay date do not differ significantly over space versus time and are similar to those obtained for our transect.
4. Discussion
In this study, we aimed to gain a clearer understanding of the proximate environmental drivers of the breeding phenology of a passerine bird by testing multiple putative drivers (temperature, tree phenology, prey abundance and photoperiod) both independently and then together. Mean night-time temperature in early spring and the budburst phenology of birch trees are the most important predictors of blue tit breeding phenology, with elevated night-time temperatures and earlier birch budburst significantly predicting earlier lay dates across sites and years. These predictors performed well in cross-validation using data for two additional years, and using variants on these predictors we found that they generalize to a considerably larger spatial scale (UK) and over a much longer time scale. These results concur with previous studies suggesting that temperature is a strong causal predictor of lay dates in woodland passerines [22,23], but advance our understanding by identifying night-time temperatures as most predictive. From this, we infer that warmer night-time conditions may remove a constraint on breeding rather than providing a cue [27]. A striking result emerging from our work is that birch phenology outperformed both mean tree phenology, and mean tree phenology weighted for local tree abundance, indicating that blue tits may be sensitive to the seasonality of particular tree species within the landscape.
Spring temperatures are well known to be a strong negative correlate of woodland passerine laying dates, though the mechanism through which it acts is unknown [25]. The multiple regression slope we estimate is shallower than that we obtain in the single predictor models and estimates from other blue tit studies [22,24] and this discrepancy arises because analyses that consider temperature as the sole driver of breeding phenology will estimate a slope that combines both direct and indirect effects of temperature, whereas our analyses include variables that represent proximate drivers arising via two indirect pathways (birch phenology and invertebrate availability). This is, to our knowledge, the first study to identify night-time temperatures as the most important temperature predictor and we suggest that increasing night-time temperatures may lift a thermal energetic constraint on producing and incubating eggs [27,57]. This would also explain why female yolk development [58]—but not male gonadal development [59]—correlates with laying dates. It remains possible that our finding that night-time temperatures are more important than day-time temperatures arises owing to instances of direct sunlight contributing to measurement error of the latter. Nonetheless we suggest that the hypothesis that night-time temperatures are a constraint warrants further exploration.
Tree phenology was a poor predictor of nest initiation, both in individual and combined predictor models, but birch budburst was a strong and significant predictor of lay date in all models. This is consistent with birds responding to certain tree genera more than others, as has been suggested for birch in northern Europe previously [32]. In the UK national dataset used in this study, birch leafing is strongly positively correlated with the more widely reported and relatable oak leafing across both space (r = 0.973) and time (r = 0.909) but occurs on average 13.8 days earlier (see the electronic supplementary material, appendix A for further details). We suggest that this early phenology of birch provides an indicator of future environments earlier in the year than other genera, coinciding with the bird's requirement for information; this is supported by budburst predicting lay date better than later leafing. As tree phenology was a very poor predictor of nest initiation but a significant predictor of the first egg date, this could indicate that it provides a supplementary cue between the two phenological phases allowing for fine-tuning of the timing of egg laying after nest building. Such a cue could be visual or chemical [35], or possibly indirect through invertebrate availability on, or in, birch buds, food resources shown via faecal metabarcoding to be heavily used by blue tits in Scotland in early spring but not captured by the sticky traps [45]. In addition, if the effect of temperature proves to be indirect via tree phenology or invertebrate availability, then the reliability of assuming that temperature has a direct causal effect [22,60] will depend on the linearity of temperature effects on tree and invertebrate phenology. Birch, for instance, is delayed by warmer conditions during a chilling period in the early winter [53], such that a focus only on the spring period may overestimate the advance that this species will show.
Flying invertebrate abundance was a significant predictor of nest initiation when tested in isolation, but captured relatively little of the variation and was not a significant predictor of either phase of blue tit reproductive phenology in the combined models. We note that the predicted effect size of a few days difference in lay dates between high and low prey availability is of similar magnitude to the responses to artificial feeding observed in other studies [39,40] and could reflect the maximum amount that females can plastically shift laying owing to food availability, which would presumably alleviate energetic constraints like increasing night-time temperatures. However, sticky trap derived estimates of food availability may provide an incomplete estimate of the resource available to blue tits, owing to the variability inherent in catching insects on sticky traps and not recording non-flying taxa. Thus, we cannot exclude the possibility that average nightly temperature and birch phenology provide a better predictor of the true available prey abundance than our sampling yields.
Previous research has demonstrated that photostimulation is fundamental in commencing temperate passerine reproductive phenology [17,18], but we found no evidence that it explains the spatial variation observed on the scale of our study. This supports the idea that photostimulation opens a ‘window' for possible breeding beyond which other supplementary cues refine the exact timing, and these processes give rise to the observed variation.
The breeding phenology of many avian species across the temperate Northern Hemisphere is advancing at a similar rate to that noted in this study in response to warming temperatures [24,61] and it is possible that other species in this region use a similar set of environmental predictors. In the temperate Southern Hemisphere avian breeding phenology is also associated with vegetation productivity and food resources, but the productive period extends for longer and its timing is less predictable [62]. Moreover, conversely to the north, physiological stress from high temperatures rather than low appears to constrain breeding, suggesting that our insights may not generalize here [63].
In summary, mean night-time temperatures and birch budburst phenology are significant predictors of lay date in Scottish blue tits, consistent with temperature having both a direct and indirect effect and acting as a thermal constraint rather than a cue. Our models performed well in cross-validation and as the effects we estimated in Scotland could be generalized to the national scale over a longer time period this gives a degree of confidence in the robustness and generality of our inferences, and highlights their value for predicting future variation in blue tit breeding phenology. This will enable more accurate prediction of the effects of trophic mismatch in this focal system [10,22].
Supplementary Material
Acknowledgements
We thank all of the landowners and managers who allowed us access to their land, a number of fieldworkers and BTO/WT volunteers who helped collect the data, the BTO/JNCC partnership for supporting the Nest Record Scheme, Jarrod Hadfield who assisted in conceiving the transect design, and Sophie Bell for advice about temperature loggers.
Ethics
All birds were handled and ringed by fieldworkers with appropriate British Trust for Ornithology permits.
Data accessibility
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.814vb1b [64].
Authors' contributions
J.D.S. participated in the design of the study, collected the transect field data, performed the statistical analyses and drafted the manuscript. I.B.C., K.K. and J.M.S. helped collect transect field data. D.L. and L.W. provided national datasets. M.D.B. participated in the design of the study. A.B.P. conceived, designed and supervised the study and assisted in collecting field data and performing statistical analyses. All authors contributed to manuscript comments and gave final approval for publication.
Competing interests
We declare no competing interests.
Funding
This work was funded by a NERC DTG PhD award to J.D.S., a NERC advanced fellowship (NE/I020598/1O) and NERC grant no. (NE/P011802/1) to A.B.P.
References
- 1.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 3.Thackeray SJ, et al. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535, 241–245. ( 10.1038/nature18608) [DOI] [PubMed] [Google Scholar]
- 4.Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870. ( 10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 5.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B. 365, 3177–3186. ( 10.1098/rstb.2010.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winder M, Schindler DE. 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85, 2100–2106. ( 10.1890/04-0151) [DOI] [Google Scholar]
- 7.Reed TE, Jenouvrier S, Visser ME. 2013. Phenological mismatch strongly affects individual fitness but not population demography in a woodland passerine. J. Anim. Ecol. 82, 131–144. ( 10.1111/j.1365-2656.2012.02020.x) [DOI] [PubMed] [Google Scholar]
- 8.Durant JM, Hjermann DØ, Ottersen G, Stenseth NC. 2007. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283. ( 10.3354/cr033271) [DOI] [Google Scholar]
- 9.Gienapp P, Reed TE, Visser ME. 2014. Why climate change will invariably alter selection pressures on phenology. Proc. R. Soc. B 281, 20141611 ( 10.1098/rspb.2014.1611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmonds EG, Cole EF, Sheldon BC. In press. Cue identification in phenology: a case study of the predictive performance of current statistical tools. J. Anim. Ecol. ( 10.1111/1365-2656.13038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charmantier A, McCleery RH, Cole LR, Perrins CM, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 12.Burger C, et al. 2012. Climate change, breeding date and nestling diet: how temperature differentially affects seasonal changes in pied flycatcher diet depending on habitat variation. J. Anim. Ecol. 81, 926–936. ( 10.1111/j.1365-2656.2012.01968.x) [DOI] [PubMed] [Google Scholar]
- 13.Feeny P. 1970. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51, 565–581. ( 10.1017/CBO9781107415324.004) [DOI] [Google Scholar]
- 14.Perrins CM. 1979. British tits. London, UK: Collins. [Google Scholar]
- 15.Chevin L-M, Lande R. 2015. Evolution of environmental cues for phenotypic plasticity. Evolution 69, 2767–2775. ( 10.1111/evo.12755) [DOI] [PubMed] [Google Scholar]
- 16.Dawson A. 2007. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Phil. Trans. R. Soc. B 363, 1621–1633. ( 10.1098/rstb.2007.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambrechts MM, Perret P. 2000. A long photoperiod overrides non-photoperiodic factors in blue tits' timing of reproduction. Proc. R. Soc. B 267, 585–588. ( 10.1098/rspb.2000.1041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D.. 2013. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. B 280, 20130016 ( 10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverin B, Viebke P, Westin J. 1989. An artificial simulation of the vernal increase in day length and its effects on the reproductive system in three species of tits (Parus spp). Condor 91, 598–608. ( 10.2307/1368110) [DOI] [Google Scholar]
- 20.Caro SP, Lambrechts MM, Balthazart J, Perret P. 2007. Non-photoperiodic factors and timing of breeding in blue tits: impact of environmental and social influences in semi-natural conditions. Behav. Process. 75, 1–7. ( 10.1016/j.beproc.2007.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perfito N, Jeong SY, Silverin B, Calisi RM, Bentley GE, Hau M. 2012. Anticipating spring: wild populations of great tits (Parus major) differ in expression of key genes for photoperiodic time measurement. PLoS ONE 7, e34997 ( 10.1371/journal.pone.0034997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillimore AB, Leech DI, Pearce-Higgins JW, Hadfield JD. 2016. Passerines may be sufficiently plastic to track temperature-mediated shifts in optimum lay date. Glob. Chang. Biol. 22, 3259–3272. ( 10.1111/gcb.13302) [DOI] [PubMed] [Google Scholar]
- 23.Visser ME, Holleman LJM, Caro SP. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331. ( 10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean N, Lawson CR, Leech DI, van de Pol M. 2016. Predicting when climate-driven phenotypic change affects population dynamics. Ecol. Lett. 19, 595–608. ( 10.1111/ele.12599) [DOI] [PubMed] [Google Scholar]
- 25.Caro SP, Schaper SV, Hut RA, Ball GF, Visser ME. 2013. The case of the missing mechanism: how does temperature influence seasonal timing in endotherms? PLoS Biol. 11, e1001517 ( 10.1371/journal.pbio.1001517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lof ME, Reed TE, McNamara JM, Visser ME. 2012. Timing in a fluctuating environment: environmental variability and asymmetric fitness curves can lead to adaptively mismatched avian reproduction. Proc. Biol. Sci. 279, 3161–3169. ( 10.1098/rspb.2012.0431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson IR, Bryant DM. 2000. Climate change and constraints on breeding. Nature 406, 366–367. ( 10.1038/35019151) [DOI] [PubMed] [Google Scholar]
- 28.Meijer T, Neinaber U, Langer U, Trillmich F. 1999. Temperature and timing of egg-laying of European starlings. Condor 101, 124–132. ( 10.2307/1370453) [DOI] [Google Scholar]
- 29.Nager RG, van Noordwijk AJ.. 1992. Energetic limitation in the egg-laying period of great tits. Proc. R. Soc. B 249, 259–263. ( 10.1098/rspb.1992.0112) [DOI] [Google Scholar]
- 30.Simmonds EG, Sheldon BC, Coulson T, Cole EF. 2018. Experimental manipulation of nocturnal nest cavity temperature in wild blue tits. bioRxiv, 279455.
- 31.Thomas DW, Bourgault P, Shipley B, Perret P, Blondel J. 2010. Context-dependent changes in the weighting of environmental cues that initiate breeding in a temperate passerine, the Corsican blue tit (Cyanistes caeruleus). Auk 127, 129–139. ( 10.1525/auk.2009.09141) [DOI] [Google Scholar]
- 32.Nilsson J-Å, Källander H. 2006. Leafing phenology and timing of egg laying in great tits Parus major and blue tits P. caeruleus. J. Avian Biol. 37, 357–363. ( 10.1111/j.2006.0908-8857.03604.x) [DOI] [Google Scholar]
- 33.Hinks AE, Cole EF, Daniels KJ, Wilkin TA, Nakagawa S, Sheldon BC. 2015. Scale-dependent phenological synchrony between songbirds and their caterpillar food source. Am. Nat. 186, 84–97. ( 10.1086/681572) [DOI] [PubMed] [Google Scholar]
- 34.Burgess MD, et al. 2018. Tritrophic phenological match–mismatch in space and time. Nat. Ecol. Evol. 2, 970–975. ( 10.1038/s41559-018-0543-1 [DOI] [PubMed] [Google Scholar]
- 35.Bourgault P, Caro SP, Perret P. 2006. Do blue tits time their breeding based on cues obtained by consuming buds? J. F. Ornithol. 77, 399–403. ( 10.1111/j.1557-9263.2006.00070.x) [DOI] [Google Scholar]
- 36.Schaper SV, Rueda C, Sharp PJ, Dawson A, Visser ME. 2011. Spring phenology does not affect timing of reproduction in the great tit (Parus major). J. Exp. Biol. 214, 3664–3671. ( 10.1242/jeb.059543) [DOI] [PubMed] [Google Scholar]
- 37.Seward AM, Beale CM, Gilbert L, Jones TH, Thomas RJ. 2014. The impact of increased food availability on reproduction in a long-distance migratory songbird: implications for environmental change? PLoS ONE 9, e111180 ( 10.1371/journal.pone.0111180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robb GN, McDonald RA, Chamberlain DE, Bearhop S. 2008. Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. ( 10.1890/060152) [DOI] [Google Scholar]
- 39.Nager RG, Ruegger C, van Noordwijk AJ. 1997. Nutrient or energy limitation on egg formation: a feeding experiment in great tits. J. Anim. Ecol. 66, 495–507. ( 10.2307/5944) [DOI] [Google Scholar]
- 40.Svensson E, Nilsson J-Å. 1995. Food supply, territory quality, and reproductive timing in the blue tit (Parus caeruleus). Ecology 76, 1804–1812. ( 10.2307/1940712) [DOI] [Google Scholar]
- 41.Allander K, Bennett GF. 1995. Retardation of breeding onset in great tits (Parus major) by blood parasites. Funct. Ecol. 9, 677–682. ( 10.2307/2390160) [DOI] [Google Scholar]
- 42.Shutt JD, Bolton M, Cabello IB, Burgess MD, Phillimore AB. 2018. The effects of woodland habitat and biogeography on blue tit (Cyanistes caeruleus) territory occupancy and productivity along a 220 km transect. Ecography (Cop.) 41, 1967–1978. ( 10.1111/ecog.03573) [DOI] [Google Scholar]
- 43.Cresswell W, McCleery RH. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72, 356–366. ( 10.1046/j.1365-2656.2003.00701.x) [DOI] [Google Scholar]
- 44.Simmonds EG, Sheldon BC, Coulson T, Cole EF. 2017. Incubation behavior adjustments, driven by ambient temperature variation, improve synchrony between hatch dates and caterpillar peak in a wild bird population. Ecol. Evol. 7, 9415–9425. ( 10.1002/ece3.3446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shutt JD.2018. Expanding a classic woodland food chain into a geographically variable food web. PhD thesis, University of Edinburgh, Edinburgh, UK.
- 46.Betts MM. 1955. The food of titmice in oak Woodland. J. Anim. Ecol. 24, 282–323. ( 10.2307/1715) [DOI] [Google Scholar]
- 47.Bates D, Maechler M, Bolker BM, Walker S. 2015. lme4: linear mixed-effects models using Eigen and S4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 48.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 49.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 50.Rousset F, Ferdy JB. 2014. Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography (Cop.) 37, 781–790. ( 10.1111/ecog.00566) [DOI] [Google Scholar]
- 51.Crick HQP, Baillie SR, Leech DI. 2003. The UK nest record scheme: its value for science and conservation. Bird Study 50, 254–270. ( 10.1080/00063650309461318) [DOI] [Google Scholar]
- 52.Perry M, Hollis D. 2005. The generation of monthly gridded datasets for a range of climatic variables over the UK. Int. J. Climatol. 25, 1041–1054. ( 10.1002/joc.1161) [DOI] [Google Scholar]
- 53.Tansey CJ, Hadfield JD, Phillimore AB. 2017. Estimating the ability of plants to plastically track temperature-mediated shifts in the spring phenological optimum. Glob. Chang. Biol. 23, 3321–3334. ( 10.1111/gcb.13624) [DOI] [PubMed] [Google Scholar]
- 54.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.1002/ana.22635)20808728 [DOI] [Google Scholar]
- 55.Hadfield JD, Heap EA, Bayer F, Mittell EA, Crouch NMA. 2013. Intraclutch differences in egg characteristics mitigate the consequences of age-related hierarchies in a wild passerine. Evolution (NY) . 67, 2688–2700. ( 10.1111/evo.12143) [DOI] [PubMed] [Google Scholar]
- 56.Phillimore AB, Stålhandske S, Smithers RJ, Bernard R. 2012. Dissecting the contributions of plasticity and local adaptation to the phenology of a butterfly and its host plants. Am. Nat. 180, 655–670. ( 10.1086/667893) [DOI] [PubMed] [Google Scholar]
- 57.Yom-Tov Y, Wright J. 1993. Effect of heating nest boxes on egg laying in the blue tit (Parus caeruleus). Auk 110, 95–99. [Google Scholar]
- 58.Caro SP, Charmantier A, Lambrechts MM, Blondel J, Balthazart J, Williams TD. 2009. Local adaptation of timing of reproduction: females are in the driver's seat. Funct. Ecol. 23, 172–179. ( 10.1111/j.1365-2435.2008.01486.x) [DOI] [Google Scholar]
- 59.Caro SP, Lambrechts MM, Chastel O, Sharp PJ, Thomas DW, Balthazart J. 2006. Simultaneous pituitary-gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm. Behav. 50, 347–360. ( 10.1016/j.yhbeh.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 60.Vedder O, Bouwhuis S, Sheldon BC. 2013. Quantitative assessment of the importance of phenotypic plasticity in adaptation to climate change in wild bird populations. PLoS Biol. 11, e1001605 ( 10.1371/journal.pbio.1001605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDermott ME, DeGroote LW. 2016. Long-term climate impacts on breeding bird phenology in Pennsylvania, USA. Glob. Chang. Biol. 22, 3304–3319. ( 10.1111/gcb.13363) [DOI] [PubMed] [Google Scholar]
- 62.Englert Duursma D, Gallagher RV, & Griffith SC. 2017. Characterizing opportunistic breeding at a continental scale using all available sources of phenological data: an assessment of 337 species across the Australian continent. Auk 134, 509–519. ( 10.1642/AUK-16-243.1) [DOI] [Google Scholar]
- 63.Englert Duursma D, Gallagher RV, Griffith SC. 2019. Variation in the timing of avian egg-laying in relation to climate. Ecography (Cop.) 42, 535–548. ( 10.1111/ecog.03602) [DOI] [Google Scholar]
- 64.Shutt JD, Cabello IB, Keogan K, Leech DI, Samplonius JM, Whittle L, Burgess MD, Phillimore AB.. 2019. Data from: The environmental predictors of spatiotemporal variation in the breeding phenology of a passerine bird Dryad Digital Repository. ( 10.5061/dryad.814vb1b) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Shutt JD, Cabello IB, Keogan K, Leech DI, Samplonius JM, Whittle L, Burgess MD, Phillimore AB.. 2019. Data from: The environmental predictors of spatiotemporal variation in the breeding phenology of a passerine bird Dryad Digital Repository. ( 10.5061/dryad.814vb1b) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.814vb1b [64].