Abstract
Pathogen spillover from managed bees is increasingly considered as a possible cause of pollinator decline. Though spillover has been frequently documented, evidence of the pathogen's virulence in the new host or mechanism of transmission is rare. Stingless bees (Apocrita: Meliponini) are crucial pollinators pan-tropically and overlap with managed honeybees (Apis mellifera) in much of their range. Nosema ceranae is the most prevalent disease of adult A. mellifera. We used laboratory experiments and field surveys to investigate the susceptibility of stingless bees (Tetragonula hockingsi) to N. ceranae, infection prevalence and transmissibility via flowers. We found that 67% of T. hockingsi fed sucrose with N. ceranae had detectable spores in their ventriculus, and they died at 2.96 times the rate of sucrose-only fed bees. Five of six field hives harboured bees with N. ceranae present at least once during our five-month survey, with prevalence up to 20%. In our floral transmission experiment, 67% of inflorescences exposed to infected A. mellifera yielded N. ceranae spores, and all resulted in T. hockingsi with N. ceranae spores in their guts. We conclude that N. ceranae is virulent in T. hockingsi under laboratory conditions, is common in the local T. hockingsi population and is transmissible via flowers.
Keywords: Apis mellifera, floral transmission, Nosema ceranae, pathogen spillover, pollinators, stingless bees
1. Background
Species distributions are changing rapidly due to habitat loss, climate change and anthropogenic species introductions leading to novel combinations of interacting species that share no evolutionary history. Species that are introduced to novel habitats may bring with them their pathogens and parasites, and thereby provide an opportunity for these natural enemies to spill over to novel hosts [1]. For example, squirrel pox virus and crayfish plague were both introduced into Europe with their invasive squirrel and crayfish hosts, respectively, and spilled over into native counterparts, resulting in substantial population declines of the native species [2,3]. Organisms that host an array of parasites and pathogens and are frequently relocated by humans would be likely to have the greatest opportunity to be vectors of pathogens for spillover.
Pathogen spillover represents an opportunity to gain insights into pathogen–host coevolution and disease dynamics, which are broadly relevant to conservation and human health. Novel hosts will not have had the opportunity to develop specific defences against new pathogens and may be particularly susceptible. For example, human immunodeficiency virus and severe acute respiratory syndrome jumped from chimpanzees and bats, respectively, to humans, and have resulted in millions of deaths [4]. Likewise, rabies jumped from domestic dogs to African wild dogs and Ethiopian wolves, further reducing the populations of these already endangered species [5]. Pathogens that are highly virulent to their novel hosts may decrease their opportunity for transmission [6]. However, the presence of multiple suitable hosts in the community and mechanisms for transmission may increase spread even when virulence is high [5].
The European or western honeybee (Apis mellifera) (Apoidea: Apini) is globally widespread and hosts a range of pathogens, making it an ideal possible source of pathogen spillover [7,8]. Whereas some diseases of A. mellifera are host species-specific, most are able to infect different Apoidea species [8–10]. Eusocial bees are particularly vulnerable to diseases because of close social contact, high densities within their colonies and high relatedness among individuals within the colony [11,12]. Due to its reputation as an important pollinator, A. mellifera is now present in every region worldwide except Antarctica [13]. Many of its diseases have followed. The spillover of honeybee parasites and pathogens to wild bee populations has been reported in many places, including Argentina, Brazil, Europe, Japan and North America [14–18]. However, we know little about the effect of these pathogens on wild bees [8,19].
Flowers are an obvious site for possible pathogen transfer among bees [14,20–22]. They are frequently visited by multiple bee species, and their morphology has evolved to engender contact, thereby providing opportunity for vectoring among flowers and bee species. Flower visitors that are not susceptible to the disease may still act as vectors by transferring disease-causing agents from one flower to another [22]. However, flowers can be a hostile environment for pathogens that are adapted to internal environments of animals [20]. Pathogens that can persist in harsh conditions in a resting state or as spores may, therefore, be at an advantage [20].
Nosema ceranae is a spore-forming microsporidian that has been experimentally shown to be transferable from A. mellifera and Bombus terrestris via flowers [22]. Its congeners, N. apis and N. bombi, have long been known to infect Apis spp. and Bombus spp. with adverse consequences for their hosts [23,24]. Ingested N. ceranae spores germinate in the bee midgut, penetrate host cells and produce additional spores within 48–96 h that either infect adjacent cells or are passed in the faeces [25]. Viable spores may then be consumed during cleaning or grooming, and some are left behind on the flower after an infected bee visits [21,22]. In addition to its spore-forming, other characteristics of N. ceranae increase the likelihood of it spilling over to wild bee species and having detrimental effects. It has already demonstrated the ability to switch hosts. Nosema ceranae is thought to have originated in A. cerana, where it was first described in 1996 [26]. By 2005, it was detected in A. mellifera, and within 10 years it had also been detected in multiple Bombus species [27,28]. Nosemosis has become the most prevalent disease of adult honeybees globally [29]. Individual A. mellifera infected with N. ceranae have decreased longevity, changed foraging habits, and are less willing to share food with other individuals of their colony [26,29–31]. Thus, N. ceranae is an excellent model pathogen in which to investigate effects on, and transmission to, other bees.
Stingless bees (Apoidea: Meliponini) encompass over 500 species, are crucial generalist pollinators in tropical and subtropical regions, and, like A. mellifera, live in dense colonies of closely related individuals [32,33]. Despite the similarities to honeybees, it is generally believed that stingless bees have low levels of disease, possibly because they line their hives with propolis, a mixture of plant resins and bee secretions that has antifungal properties [32–34]. However, compared with honeybees, our knowledge of stingless bee diseases is limited to a few published studies [33,34]. Reports of a decrease in the stingless bee population in Brazil led researchers to the discovery of acute bee paralysis, a disease of A. mellifera. However, further research is required to ascertain whether the infection is pathogenic to those bees [16]. Other surveys have detected pathogens including deformed wing virus, black queen cell virus and N. ceranae in stingless bees [15,16,35]. However, these studies stopped short of demonstrating that these disease agents have any effect on stingless bees. Conversely, a recent study noted an absence of protozoan and microsporidian pathogens in six stingless bee species in southern Brazil, a surprising result considering the prevalence of these pathogens within Apoidea generally [9,36,37].
We conducted this study to investigate (1) the susceptibility of an Australian stingless bee species to infection by N. ceranae and the consequences of infection in terms of host survival, (2) whether wild populations of stingless bees are currently infected with N. ceranae and (3) whether N. ceranae can be transmitted from A. mellifera to stingless bees via flowers. We used Tetragonula hockingsi Cockerell, 1929 as our experimental stingless bee species because it has many typical stingless bee characteristics: it is active year round, nests in a variety of places and constantly builds new brood cells, rather than reusing them [32,33]. Additionally, T. hockingsi occurs in a variety of tropical and sub-tropical habitats, is locally widespread and is in a widely dispersed genus. Along with other native stingless bees, it is an important pollinator of crops such as macadamia, avocado, mango and lychee [33].
2. Methods
(a). Susceptibility to infection and effects on longevity
(i). Inoculum preparation
We obtained and purified the N. ceranae spores for the inoculum as per Ferguson et al. [38]. We used PCR to confirm that spores were N. ceranae and not N. apis, which has spores very similar in appearance [39] (see ‘PCR’ under ‘Spore counting and identification’ below). We diluted the solution with 50% w : v sucrose to obtain spore concentrations specified below.
(ii). Tetragonula hockingsi inoculation
We collected T. hockingsi from six hives located at different sites (electronic supplementary material, table S1). We captured 120 returning forager bees from each hive directly into plastic bags. We then randomly assigned groups of 15 bees to one of eight cages (rectangular 500 ml polypropylene containers) per hive. We randomly assigned four cages from each hive to the inoculated group with the remaining four assigned to the non-inoculated (control) group.
Cages were maintained in a dark incubator at 27°C and 40% relative humidity throughout the experiment. Since there are no standard methods for infecting stingless bees, we adapted the method of Fries et al. [39], who recommend mass-infecting 100 A. mellifera by providing 4 ml of a 50% sucrose solution with 1 × 106 spores and fresh additional solution over several hours. We considered the smaller body size of T. hockingsi (approx. 1/15th the size of an A. mellifera) and reduced amount and the concentration provided. Each N. ceranae-inoculated group received 15 µl of a 50% w : v sucrose solution containing approximately 7.5 × 103 N. ceranae spores, which was provided on the cage floor hourly for 5 h as six 0.5 µl drops. Cages in the sham-inoculated group received 15 µl of a 50% w : v sucrose solution without spores by the same method. We switched both groups to 25% sucrose solution ad libitum thereafter. Bees did not have access to pollen or propolis. We removed dead bees daily and counted their spores and ran PCR to confirm spore species (see Spore counting and identification). We rotated all the cages within the incubator on a daily basis, ensuring that any desiccation by fans and heating elements would affect all cages equally. In total, we caught 720 T. hockingsi for our experiment, of which six escaped during feeding. All the escaped T. hockingsi were from different cages, leaving 357 bees in both N. ceranae-inoculated and sham-inoculated groups. We continued the experiment until all bees were dead.
(b). Prevalence of Nosema ceranae spores in wild populations of Tetragonula hockingsi
We investigated the prevalence of N. ceranae in T. hockingsi in the field by monthly surveys of six T. hockingsi hives from April to August 2016. The hives were located across a diverse range of habitats in the study area (Queensland, Australia). With the exception of one hive (which had been removed), these were the same hives used in the first experiment (electronic supplementary material, table S1). We collected 15 returning foragers from each colony and examined them for N. ceranae spores (see ‘Spore counting and identification’ below). April–May samples were tested by grouping the 15 individual bees collected from a colony together, yielding a total spore count for the hive. Bees collected from June to August were tested individually, thus yielding individual and group spore counts, plus the number of infected individual bees.
(c). Transmission of Nosema ceranae via flowers
(i). Apis mellifera inoculation
We fed newly eclosed A. mellifera from a single hive 1.5 ml of inoculum containing 3 × 106 N. ceranae spores in 50% sucrose solution. After 3 h, we provided them with 50% sucrose solution ad libitum and maintained them in an incubator until they were 14 days old, the age at which bees from this hive had been observed to begin foraging in a pilot study.
(ii). Nosema ceranae transmission via flowers
We placed five of the 14-day-old N. ceranae-infected A. mellifera into each of 15 cages containing a single Sphagneticola trilobata (Singapore daisy) inflorescence. The inflorescence had been picked as a closed bud the previous day and placed into an insect-proof cage to open, thus preventing any contamination of the inflorescence prior to opening. The five bees were allowed to forage for 3 h [21], after which they were removed, euthanized and tested for spore presence (see ‘Spore counting and identification’ below). The inflorescence was then removed and placed into a new cage, thus removing the cage as a possible source of transmission. Meanwhile, we collected 150 T. hockingsi foragers from a colony found to be free of N. ceranae during the survey (KOA1; see ‘Results’). We randomly assigned half of these, in groups of five, to the 15 cages containing an inflorescence previously exposed to the infected A. mellifera, and the other half, in groups of five, to 15 identical cages containing an inflorescence that had not been exposed to infected A. mellifera. All T. hockingsi were left in the cages for 3 h during which they foraged on the inflorescences. Afterwards they were transferred to an incubator and fed 25% sucrose solution ad libitum. Eight days post-exposure, we euthanized all T. hockingsi and tested for N. ceranae spore presence. All inflorescences were also tested for N. ceranae spore presence (see ‘Spore counting and identification’).
(d). Spore counting and identification
For each of the three parts of the study (susceptibility experiment, field survey and transmission experiment), we determined stingless bee spore load using microscopy and confirmed N. ceranae identification for all positive samples with PCR. For the floral transmission experiment, we did spore counts and PCR for all inflorescence samples. Spore counting and PCR were conducted blind to treatment.
(i). Spore counts for stingless bees and inflorescences
For individual stingless bees (all bees except those collected in April and May in the field survey), we washed each bee in distilled water to remove any external spores and removed the intestinal tract including the crop from the abdomen taking care not to contact the external parts of the bee. We placed the intestinal tract into a 0.5 ml microtube containing a 2 mm steel ball and 20 µl of distilled water. We vortexed the microtube for 90 s at 3300 r.p.m. and then pipetted 5 µl of the solution under each side of a Neubauer improved haemocytometer. For bees collected in April and May in the field survey, we conducted the above steps on the group of 15 bees. We counted the spores at 400× magnification using the method described by Cantwell [40]. The remaining solution was used to confirm spore species identity with PCR.
We extracted material from each inflorescence following the procedure outlined in Graystock et al. [22] (see electronic supplemental material, ‘Methods: inflorescence processing for spore counts and PCR’). We conducted spore counts and PCR on the resulting supernatant as described for bees to yield a spore count for each entire inflorescence.
(ii). PCR
We conducted PCR to confirm that spores were N. ceranae for inocula in the susceptibility and transmission experiments, all inflorescences in the transmission experiment and for all stingless bee samples from all three parts of the study in which spores were observed. We performed DNA extraction and PCR as described by Peng et al. [41] and modified by Ferguson et al. [38] (see electronic supplementary material, ‘Methods: PCR’). To minimize the possibility of contamination, DNA extraction, PCR mixture preparation and post-PCR analysis were performed in separate rooms using equipment designated for each area.
(e). Statistical analysis
We performed all analyses using R v. 3.5 [42]. We analysed data from the susceptibility experiment with three models (probability of infection, spore load and survival). To determine whether N. ceranae-inoculated bees were more likely to be infected, we used a generalized linear mixed model (glmer; lme4 v. 1.1-18) [43] with a binomial distribution (logistic regression). The response variable was the proportion of T. hockingsi that had N. ceranae spores present within their intestinal tract at death (n = 714 bees). Inoculation status and hive were fixed effects and cage was a random effect. We could not test the interaction between hive and treatment due to singularities (some hives did not have bees with spores in the sham-inoculated treatment). To determine whether spore load varied with days post-inoculation, we ran a generalized linear mixed model with a Poisson error distribution on bees with spore counts greater than zero with treatment, day post-inoculation at time of death, hive and all possible interactions as fixed effects, and cage and observation number (to eliminate overdispersion) as random effects. We removed the three-way interaction and the interaction between day post-inoculation and hive because doing so lowered the AIC. For both of these models, we tested the proportion of variance explained by fixed and random effects using ‘piecewiseSEM' v. 2.0.2) to generate the marginal and conditional R2 [44,45].
To investigate whether N. ceranae infection decreases longevity of T. hockingsi, we compared survival between the N. ceranae and sham-inoculated groups (n = 357 per group). We excluded bees that died within the first 24 h post-infection because these were likely to be due to handling effects [46]. We included bees that escaped during the experiment in the survival analysis as ‘censored'; this allowed them to be taken into account up to the point of escape. We initially ran a mixed-effects Cox model (coxme) [47] that included treatment, hive and their interaction as fixed effects and cage as a random effect, but removing the interaction improved the model (lower AIC). For bees that were infected upon death (spore count greater than 0), we initially tested the effects of spore load, hive and treatment as fixed effects on survival, with cage as a random effect, but removing hive and treatment lowered the AIC. To analyse whether the overall number of N. ceranae spores detected during the survey varied, we used a generalized linear model with a negative binomial error distribution. The total number of N. ceranae spores detected monthly for each hive (n = 6 hives per month) was the response variable and explanatory variables were month and hive. In our floral transmission experiment, we encountered ‘complete separation' (all of our positive values were only in one treatment group). We therefore tested whether stingless bee infection was dependent on treatment with a Bayesian analysis with non-informative priors using ‘bglmer' from the blme package [48,49]. We checked for overdispersion in Poisson and negative binomial models, and produced diagnostic plots, including residuals versus leverage (Cook's distance), residuals versus fitted and scale−location, using plot(lm) [42] to confirm that there were no outlying data points affecting the models.
3. Results
(a). Susceptibility to infection and effects on longevity
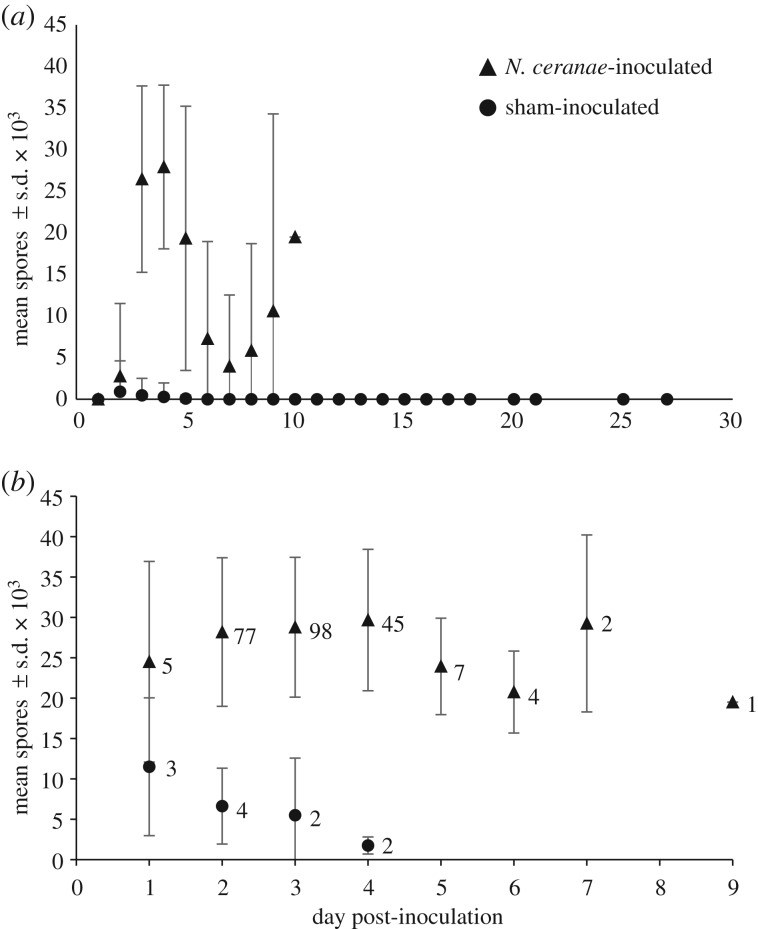
Over the course of the 26-day experiment, 240 of 357 (67.2%) N. ceranae-inoculated T. hockingsi had detectable numbers of N. ceranae spores in their digestive tract compared to 11 of 357 (3.08%) sham-inoculated T. hockingsi (Z = 12.412; p < 0.001), indicating that individual T. hockingsi are susceptible to N. ceranae infection. Of the 11 sham-inoculated T. hockingsi that were infected, seven came from the same hive, but had been distributed across four different cages, and the remaining four came from two different hives and had been distributed across three different cages. Nosema ceranae inoculation accounted for 50.0% of the variation in the proportion of T. hockingsi that had N. ceranae spores present within their intestinal tract at death (marginal R2 = 0.500); little additional variation was accounted for by the random effect of cage (conditional R2 = 0.508). Among bees that had spores, spore loads slightly increased for the N. ceranae-inoculated bees and decreased for the sham-inoculated bees that had spores with day post-inoculation (table 1 and figure 1; electronic supplementary material, table S3). The treatment effect on spore load also varied significantly by hive (table 1). Fixed effects explained 55.6% (marginal R2 = 0.556) of the variation in spore load; including the random effect of cage increased the explained variation to 77.8 (conditional R2 = 0.778).
Table 1.
Summary of generalized linear model results and survival analysis for the susceptibility experiment, field survey and floral transmission experiment.
| response and explanatory variables | d.f. | χ2 or z | p-values |
|---|---|---|---|
| susceptibility experiment | |||
| probability of infection (n = 714 bees) | |||
| treatment | 1 | 148.9 | <0.0001 |
| hive | 1 | 2.44 | 0.79 |
| spore load (n = 251 bees) | |||
| treatment | 1 | 3.05 | 0.0809 |
| days post-inoculation | 1 | 0.37 | 0.54 |
| hive | 5 | 1.37 | 0.93 |
| treatment × days post-inoculation | 1 | 8.55 | 0.0035 |
| treatment × hive | 2 | 6.47 | 0.0394 |
| survival (n = 714 bees) | |||
| treatment | 1 | 87.0 | <0.0001 |
| hive | 5 | 21.6 | 0.0006 |
| survival (n = 251 bees) | |||
| spore load | 1 | 0.18 | 0.67 |
| field survey | |||
| spore load (n = 6 hives, five months) | |||
| hive | 5 | 18.5 | 0.0023 |
| month | 1 | 3.99 | 0.0457 |
| floral transmission experiment | |||
| infection of bees (n = 150) | |||
| treatment | 1 | 3.04 | 0.00235 |
Figure 1.
Mean spore count ± s.d. on day of death of (a) all dead T. hockingsi and (b) T. hockingsi that tested positive for N. ceranae spores by day post-inoculation and treatment group. In (b), the number of bees that died each day in each treatment is shown to the right of the data point. Three per cent (n = 11/357) of sham-inoculated and 67% (n = 240/357) of N. ceranae-inoculated T. hockingsi had N. ceranae spores in their guts upon death.
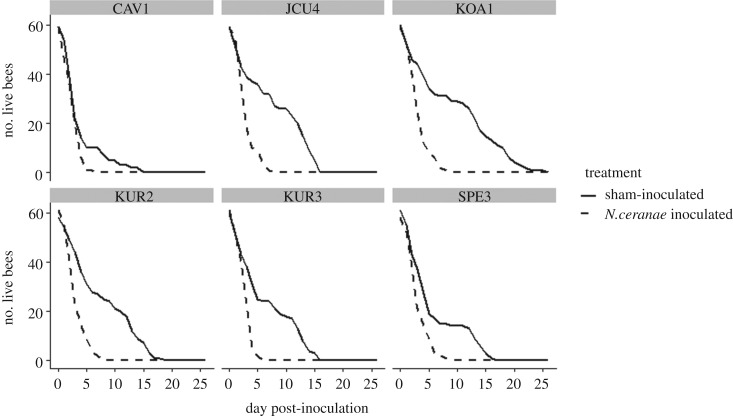
Treatment and hive were significant predictors of survival (table 1 and figure 2). Across all hives, N. ceranae-inoculated bees died at about three times the rate of non-inoculated bees (hazard ratio = 2.96, 95% CI = 2.64–3.33). All N. ceranae-inoculated stingless bees died within 9 days of inoculation, whereas the longest-lived sham-inoculated bee survived for 26 days post-inoculation (figure 2). Spore load was not a significant predictor of survival among bees that had spores in their gut upon death (table 1). NanoDrop values for the quantity and purity of DNA were within the recommended range in all cases. PCR analysis confirmed the spores were N. ceranae and corresponded with the light microscopy results in all cases.
Figure 2.
The number of surviving T. hockingsi by day post-inoculation by treatment for each of the six hives.
(b). Prevalence of Nosema ceranae spores in wild populations of Tetragonula hockingsi
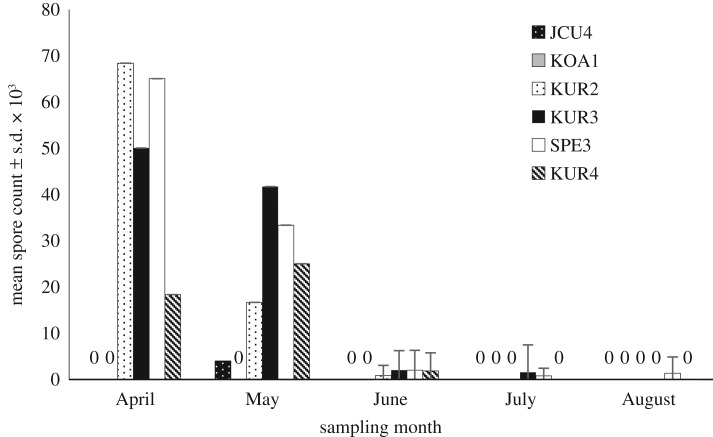
Our survey results reveal that N. ceranae was present in five out of six sampled colonies during one or more months (figure 3). Over the period of the survey, the total number of spores detected monthly declined significantly from April to August, with August having the lowest overall count (table 1 and figure 3). From June to August, when the 90 collected T. hockingsi were individually tested, 11, 4 and 2 bees, respectively, had N. ceranae spores present (electronic supplementary material, table S4).
Figure 3.
Mean number of N. ceranae spores ± s.d. × 103 from 15 T. hockingsi collected monthly from six different hives located across the study area (Cairns region, Queensland, Australia). Nosema ceranae was present in five out of six sampled colonies during one or more months. For April and May, the total number of spores in the combined 15-bee sample was divided by 15 to achieve the mean count, hence no standard deviations are shown for those months. For all other months, the means were calculated from 15 individual bees per hive.
(c). Transmission of Nosema ceranae via flowers
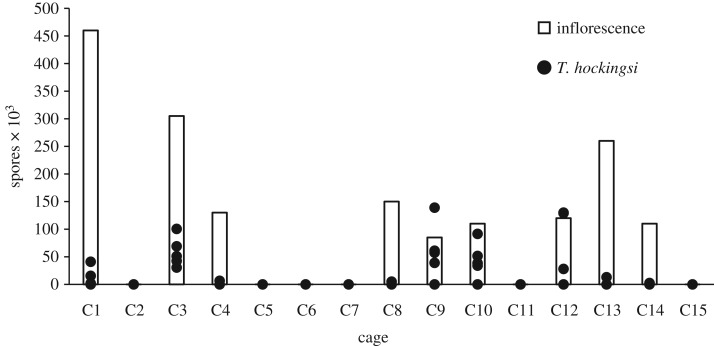
Nine of the 15 inflorescences exposed to infected A. mellifera had N. ceranae spores on them. Spore counts on these inflorescences ranged from 8.5 × 104 to 4.6 × 105 spores (figure 4). In all nine of those cages, there was at least one T. hockingsi with N. ceranae spores in its gut, whereas neither inflorescences nor T. hockingsi from the cages that were not exposed to infected A. mellifera had any detectable spores (table 1 and figure 4). The number of T. hockingsi with spores per cage varied from one to all five (figure 4), and overall we detected N. ceranae spores in 23 of the 45 T. hockingsi (51.1%) in cages with spore-laden inflorescences. Spore counts in the 23 bees ranged from 2 × 103 to 13.9 × 104 (electronic supplementary material, table S5) and all of these bees survived the 8 days post-exposure. PCR analysis confirmed the light microscopy results in all cases.
Figure 4.
Number of N. ceranae spores on inflorescences and in the five sacrificed T. hockingsi from each of the 15 cages exposed to N. ceranae infected A. mellifera. All cages containing inflorescences in which N. ceranae spores were detected yielded infected T. hockingsi. None of the cages (n = 15) that were not exposed to infected A. mellifera had N. ceranae spores on their inflorescences or in any of the T. hockingsi.
4. Discussion
To the best of our knowledge, our findings are the first report of pathogen spillover from A. mellifera to an Australian stingless bee species. Although other studies have reported the presence of honeybee diseases in stingless bees, none of them attempted to test whether the pathogens identified were detrimental to the bees, nor did they evaluate possible transmission mechanisms [15,16,35]. We found that under laboratory conditions, inoculation with N. ceranae reduces T. hockingsi longevity. Additionally, we demonstrated that wild populations of T. hockingsi harbour N. ceranae and that flowers are an effective means of transmission of N. ceranae spores between A. mellifera and T. hockingsi.
The greatly reduced life expectancy among experimentally infected T. hockingsi suggests N. ceranae may be a highly virulent parasite in T. hockingsi [50]. In our susceptibility experiment, N. ceranae-inoculated bees died three times as fast as sham-inoculated bees and were all dead within 9 days of inoculation. An important caveat to our study is that we demonstrated decreased longevity in a laboratory setting, depriving the bees of access to honey, pollen and propolis in their hive, which would have provided additional nutrition and possibly antimicrobial defence. [32,51–53]. It may be that in the wild T. hockingsi can harbour N. ceranae but not suffer adverse effects because of their access to propolis or other high-quality food, as has been observed for N. ceranae-infected A. cerana and A. mellifera [38,54]. The lack of mortality for up to 8 days following infection in our floral transmission experiment further suggests that there may be environmental factors that temper N. ceranae virulence. Spores on flowers may encounter conditions that diminish their eventual ability to germinate in the bee gut. Under laboratory conditions, N. ceranae spores lose viability over a period of months [25], but it is unknown how exposure to sunlight, floral chemistry and the floral microbiome may affect N. ceranae spore viability. Floral morphology and nectar chemistry affect transmission and persistence of Crithidia bombi among B. impatiens [55–57], and the possibility of similar effects on N. ceranae is worth investigation. Alternatively, or in addition, hive health may play a role. All of the T. hockingsi we used in the transmission experiment came from the one hive (KOA1) in which we never detected N. ceranae during our survey, so as to minimize the risk of using bees that were already infected. Our survey and transmission experiment mortality results together suggest that workers in this hive may be less susceptible to N. ceranae infection. However, we did not observe a reduced mortality rate for N. ceranae-inoculated workers from this hive in the susceptibility experiment, which suggests that where and how exposure occurs may affect ultimate virulence.
Though N. ceranae may be virulent, our laboratory and survey results suggest it may have low infectivity in, and transmissibility among, T. hockingsi. In the laboratory experiment, a third of bees provided sucrose with N. ceranae spores did not have spores in their guts when they died. It is possible that these bees did not feed on the spore-laden sucrose and therefore had no opportunity for direct infection. Even so, they occurred in the same cages as infected bees, indicating limited transmission among the bees. Similarly, the 11 infected bees in the control group were distributed among seven cages. At most, 20% of the bees in a single control cage were infected. Our survey data, though limited, revealed a low natural prevalence of N. ceranae, with at most 20% of the 15 captured bees from a single hive each month having N. ceranae present, despite spore counts within the range of dead inoculated bees in the laboratory experiment. We did not formally assess colony vigour during our survey, but our casual observations while collecting bees revealed no changes in hive health. In the hive, stingless bees maintain a latrine and eventually remove the waste as pellets with their mandibles, unlike A. mellifera, which does not defecate in the hive [33]. Keeping faecal matter in the hive and handling it during removal would, in theory, increase opportunity for transmission, unless spores became non-viable in the latrine conditions. Trophallaxis might also be a means of spreading spores around the hive. We did not observe a build-up of faecal matter in the cages (perhaps because of the lack of solid food) and the bees would have had little reason to trophallax when food was provided ad libitum, so there may have been limited opportunity for infection to spread in our experiment if viable spores were being produced by infected bees. Very high virulence, such that bees die before they shed viable spores, could also explain low transmission within the cages and low prevalence in field hives. Vespula germanica (German wasps) that become infected with N. apis die from the infection before the parasite is able to complete its life cycle [23]. Parasites typically trade-off virulence and transmission [6], and we could expect that a parasite in a novel host would not have evolved to have lower virulence [58]. If within-hive transmission was high (and virulence low), we would have expected higher prevalence and a possible increase in prevalence over time, effects of propolis and season notwithstanding. The lack of transmission cannot be attributed to propolis in the hives, considering the similarly low transmission rate in the laboratory.
Though N. ceranae may have limited infectivity among T. hockingsi, our results show high transmissibility from A. mellifera via flowers. To the best of our knowledge, ours is the first study to experimentally demonstrate pathogen transmission to stingless bees via flowers. Transmission of pathogens via the shared use of flowers has been demonstrated in several studies of bumblebees [21,22,55,56] as well as honeybees [22]. However, whether the transmitted pathogens can become detrimental to their novel hosts has not been extensively studied. Research that builds on recent work demonstrating variation in pathogen deposition, persistence and transmission among flower parts and species [55,56], as well as the role of pollen and nectar as antimicrobial defences (e.g. [38,57]), will be particularly useful in ascertaining and mitigating the threat of pathogens to managed and wild bees.
Our survival analysis and comparison of spore counts in T. hockingsi across our three datasets indicate that spore load alone is not a reliable predictor of mortality. Bees that we inoculated with spores were all dead within 9 days, and had spore counts ranging from 7.5 × 103 to 5.8 × 104 upon death, which were similar to spore counts we observed in sacrificed bees in the field (2.5 × 103 to 2.3 × 104) and the floral transmission experiment (2.0 × 103 to 1.4 × 105). In A. mellifera, spore load is also not a strong predictor of infection or mortality [59]. Infected A. mellifera that consume high-quality pollen have increased spore loads as well as increased longevity [38]. Nutritional status of the host, the presence of other stressors, host age and immune response, as well as spore viability and haplotype, may be among the many factors moderating the relationship between spore load and virulence for any host [25].
Our field survey revealed a pattern of reduced spore load coincident with the onset of drier weather. Rainfall records from a local weather station indicate 155 and 178 mm fell in April and May, respectively, whereas 68, 83 and 41 mm fell in June, July and August, respectively [60]. Wetter weather may curtail foraging, thereby increasing social contact within the hive and reducing food availability. In managed A. mellifera hives in the region, N. ceranae does not show any seasonal prevalence patterns (L.L. 2016, unpublished data). Nosema ceranae is likely to be present in most, if not all, of the geographical range of T. hockingsi [61]. Further surveys over a longer time frame and a greater geographical area would be helpful in determining how much prevalence is influenced by environmental conditions or other factors.
Our results add to those of other studies that demonstrate the threat of pathogens from managed bees to wild bee populations [14,19,28,62]. Stingless bees should be a high priority group for further elucidating the threats posed by emergent diseases and their possible mitigation given the bees' importance as pollinators, broad geographical overlap with A. mellifera, harbouring of A. mellifera viruses [15,16] and now demonstrated susceptibility to N. ceranae. Decreased longevity is only part of the risk; N. ceranae induces behavioural changes in A. mellifera [25] and reduces learning in B. terrestris [63]. Reducing risk of pathogen transmission from managed to wild bees presents multiple challenges and must involve the beekeeping community for any real change to occur [28]. Development of rapid effective diagnostic tools and reliable means of preventing and treating infection will be important advances. Our results should provide further incentive for elucidating the effect of bee pathogens within the broader ecological community and for overcoming some of these challenges.
Supplementary Material
Supplementary Material
Acknowledgements
We thank four anonymous reviewers who provided constructive and insightful comments. We thank property owners for access to hives on their property, Jade Ferguson for assistance with PCR and Peter Yeeles for assistance with R.
Data accessibility
Data are available in the Tropical Data Hub [64].
Authors' contributions
T.P. and L.L. conceived and designed experiments. T.P. conducted all fieldwork and experiments. T.P. prepared the first draft of the manuscript and figures, and T.P. and L.L. revised the manuscript.
Competing interests
We declare no competing interests.
Funding
This work was supported by student grants from the Skyrail Rainforest Foundation and the Wet Tropics Management Authority (grant no. 937) to T.P. This work was also supported by an Australian Research Council Discovery Early Career Research Award to L.L.
References
- 1.Dunn AM, et al. 2012. Indirect effects of parasites in invasions. Funct. Ecol. 26, 1262–1274. ( 10.1111/j.1365-2435.2012.02041.x) [DOI] [Google Scholar]
- 2.Tompkins DM, White AR, Boots M. 2003. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196. ( 10.1046/j.1461-0248.2003.00417.x) [DOI] [Google Scholar]
- 3.Reynolds JD. 1988. Crayfish extinctions and crayfish plague in central Ireland. Biol. Conserv. 45, 279–285. ( 10.1016/0006-3207(88)90059-6) [DOI] [Google Scholar]
- 4.Morens DM, Fauci AS. 2013. Emerging infectious diseases: threats to human health and global stability. PLoS Pathog. 9, e1003467 ( 10.1371/journal.ppat.1003467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power AG, Mitchell CE. 2004. Pathogen spillover in disease epidemics. Am. Nat. 164(Suppl 5), S79–S89. ( 10.1086/424610). [DOI] [PubMed] [Google Scholar]
- 6.Frank SA. 1996. Models of parasite virulence. Q. Rev. Biol. 71, 37–78. ( 10.1086/419267) [DOI] [PubMed] [Google Scholar]
- 7.Goulson D. 2003. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 34, 1–26. ( 10.1146/annurev.ecolsys.34.011802.132355) [DOI] [Google Scholar]
- 8.Goulson D, Hughes WOH. 2015. Mitigating the anthropogenic spread of bee parasites to protect wild pollinators. Biol. Conserv. 191, 10–19. ( 10.1016/j.biocon.2015.06.023) [DOI] [Google Scholar]
- 9.Ravoet J, De Smet L, Meeus I, Smagghe G, Wenseleers T, de Graaf DC. 2014. Widespread occurrence of honey bee pathogens in solitary bees. J. Invertebr. Pathol. 122, 55–58. ( 10.1016/j.jip.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 10.Manley R, Boots M, Wilfert L. 2015. Review: emerging viral disease risk to pollinating insects: ecological, evolutionary and anthropogenic factors. J. Appl. Ecol. 52, 331–340. ( 10.1111/1365-2664.12385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naug D, Camazine S. 2002. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 215, 427–439. ( 10.1006/jtbi.2001.2524) [DOI] [PubMed] [Google Scholar]
- 12.Shykoff JA, Schmid-Hempel P. 1991. Parasites and the advantage of genetic variability within social insect colonies. Proc. R. Soc. Lond. B 243, 55–58. ( 10.1098/rspb.1991.0009) [DOI] [Google Scholar]
- 13.Whitfield CW, et al. 2006. Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science 314, 642–645. ( 10.1126/science.1132772) [DOI] [PubMed] [Google Scholar]
- 14.Graystock P, Blane EJ, McFrederick QS, Goulson D, Hughes WOH. 2016. Do managed bees drive parasite spread and emergence in wild bees? Int. J. Parasitol. Parasites Wildlife 5, 64–75. ( 10.1016/j.ijppaw.2015.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman-Novoa E, Hamiduzzaman MM, Anguiano-Baez R, Correa-Benítez A, Castañeda-Cervantes E, Arnold NI. 2015. First detection of honey bee viruses in stingless bees in North America. J. Apicultural Res. 54, 93–95. ( 10.1080/00218839.2015.1100154) [DOI] [Google Scholar]
- 16.Ueira-Vieira C, Almeida LO, de Almeida FC, Amaral IMR, Brandeburgo MAM, Bonetti AM. 2015. Scientific note on the first molecular detection of the acute bee paralysis virus in Brazilian stingless bees. Apidologie 46, 628–630. ( 10.1007/s13592-015-0353-2) [DOI] [Google Scholar]
- 17.Plischuk S, Martín-Hernández R, Prieto L, Lucía M, Botías C, Meana A, Abrahamovich AH, Lange C, Higes M. 2009. South American native bumblebees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honeybees (Apis mellifera). Environ. Microbiol. Rep. 1, 131–135. ( 10.1111/j.1758-2229.2009.00018.x) [DOI] [PubMed] [Google Scholar]
- 18.Kojima Y, Toki T, Morimoto T, Yoshiyama M, Kimura K, Kadowaki T. 2011. Infestation of Japanese native honey bees by tracheal mite and virus from non-native European honey bees in Japan. Microb. Ecol. 62, 895–906. ( 10.1007/s00248-011-9947-z) [DOI] [PubMed] [Google Scholar]
- 19.Tehel A, Brown MJF, Paxton RJ. 2016. Impact of managed honey bee viruses on wild bees. Curr. Opin. Virol. 19, 16–22. ( 10.1016/j.coviro.2016.06.006) [DOI] [PubMed] [Google Scholar]
- 20.McArt SH, Koch H, Irwin RE, Adler LS, Gurevitch J. 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624–636. ( 10.1111/ele.12257) [DOI] [PubMed] [Google Scholar]
- 21.Durrer S, Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302. ( 10.1098/rspb.1994.0176) [DOI] [Google Scholar]
- 22.Graystock P, Goulson D, Hughes WOH. 2015. Parasites in bloom: flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 282, 20151371 ( 10.1098/rspb.2015.1371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fantham HB, Porter A. 1913. The pathogenicity of Nosema apis to insects other than hive bees. Ann. Trop. Med. Parasitol. 7, 569–582. ( 10.1080/00034983.1913.11687627) [DOI] [Google Scholar]
- 24.Fantham HB, Porter A. 1914. The morphology, biology, and economic importance of Nosema bombi, n. sp., parasitic in various humble bees (Bombus spp.). Ann. Trop. Med. Parasitol. 8, 623–628. ( 10.1080/00034983.1914.11687667) [DOI] [Google Scholar]
- 25.Goblirsch M. 2018. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 49, 131–150. ( 10.1007/s13592-017-0535-1) [DOI] [Google Scholar]
- 26.Fries I, Feng F, da Silva A, Slemenda SB, Pieniazek NJ. 1996. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 32, 356–365. ( 10.1016/S0932-4739(96)80059-9) [DOI] [Google Scholar]
- 27.Graystock P, Yates K, Darvill B, Goulson D, Hughes WO. 2013. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. J. Invertebr. Pathol. 114, 114–119. ( 10.1016/j.jip.2013.06.005) [DOI] [PubMed] [Google Scholar]
- 28.Furst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364 ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fries I. 2010. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 103, S73–S79. ( 10.1016/j.jip.2009.06.017) [DOI] [PubMed] [Google Scholar]
- 30.Fries I, Martín R, Meana A, García-Palencia P, Higes M. 2006. Natural infections of Nosema ceranae in European honey bees. J. Apicultural Res. 47, 230–233. ( 10.3896/IBRA.1.45.4.13) [DOI] [Google Scholar]
- 31.Martín-Hernández R, Botías C, Barrios L, Martínez-Salvador A, Meana A, Mayack C, Higes M. 2011. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 109, 605–612. ( 10.1007/s00436-011-2292-9) [DOI] [PubMed] [Google Scholar]
- 32.Vit P, Pedro SRM, Roubik DW. 2013. Pot-honey: a legacy of stingless bees. New York, NY: Springer. [Google Scholar]
- 33.Heard T. 2016. The Australian native bee book: keeping stingless bee hives for pets, pollination and sugarbag honey. West End, Australia: Sugarbag Bees. [Google Scholar]
- 34.Farnesi AP, Aquino-Ferreira R, De Jong D, Bastos JK, Soares AEE. 2009. Effects of stingless bee and honey bee propolis on four species of bacteria. Genet. Mol. Res. 8, 635–640. ( 10.4238/vol8-2kerr023) [DOI] [PubMed] [Google Scholar]
- 35.Porrini MP, Porrini LP, Garrido PM, Porrini DP, Muller F, Nuñez LA, Alvarez L, Iriarte PF, Eguaras MJ. 2017. Nosema ceranae in South American native stingless bees and social wasp. Microb. Ecol. 74, 761–764. ( 10.1007/s00248-017-0975-1 ) [DOI] [PubMed] [Google Scholar]
- 36.Nunes-Silva P, Piot N, Meeus I, Blochtein B, Smagghe G. 2016. Absence of Leishmaniinae and Nosematidae in stingless bees. Sci. Rep. 6, 32547 ( 10.1038/srep32547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sophie EFE, Roberts KE, Laurenson L, Pietravalle S, Hui J, Biesmeijer JC, Smith JE, Budge G, William OHH. 2012. Pervasiveness of parasites in pollinators. PLoS ONE 7, e30641 ( 10.1371/journal.pone.0030641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson JA, Northfield TD, Lach L. 2018. Honey bee (Apis mellifera) pollen foraging reflects benefits dependent on individual infection status. Microb. Ecol. 76, 482–491. ( 10.1007/s00248-018-1147-7) [DOI] [PubMed] [Google Scholar]
- 39.Fries I, et al. 2013. Standard methods for Nosema research. J. Apicultural Res. 52, 1–28. ( 10.3896/IBRA.1.52.1.14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantwell GE. 1970. Standard methods for counting nosema spores. Am. Bee J. 110, 222–223. [Google Scholar]
- 41.Peng Y, Baer-Imhoof B, Millar AH, Baer B. 2015. Consequences of Nosema apis infection for male honey bees and their fertility. Sci. Rep. 5, 10565 ( 10.1038/srep10565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Bates D, et al. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 44.Lefcheck JS, Freckleton R. 2016. piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 45.Nakagawa S, Schielzeth H, O'Hara RB. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 46.Milbrath MO, van Tran T, Huang W-F, Solter LF, Tarpy DR, Lawrence F, Huang ZY. 2015. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera). J. Invertebr. Pathol. 125, 9–15. ( 10.1016/j.jip.2014.12.006) [DOI] [PubMed] [Google Scholar]
- 47.Therneau T. 2018. Mixed effects cox models. R package v. 2.2-10.
- 48.Bolker B. 2018. GLMM worked examples digression: complete separation. See https://bbolker.github.io/mixedmodels-misc/ecostats_chap.html.
- 49.Chung Y, Rabe-Hesketh S, Dorie V, Gelman A, Liu J. 2013. A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika 78, 685–709. ( 10.1007/s11336-013-9328-2) [DOI] [PubMed] [Google Scholar]
- 50.Higes M, García-Palencia P, Martín-Hernández R, Meana A. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94, 211–217. ( 10.1016/j.jip.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 51.Simone-Finstrom M, Spivak M. 2010. Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie 41, 295–311. ( 10.1051/apido/2010016) [DOI] [Google Scholar]
- 52.Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. 1999. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 64, 235–240. ( 10.1016/S0378-8741(98)00131-7) [DOI] [PubMed] [Google Scholar]
- 53.Bankova VS, de Castro SL, Marcucci MC. 2000. Propolis: recent advances in chemistry and plant origin. Apidologie 31, 3–15. ( 10.1051/apido:2000102) [DOI] [Google Scholar]
- 54.Yemor T, Phiancharoen M, Eric Benbow M, Suwannapong G. 2015. Effects of stingless bee propolis on Nosema ceranae infected Asian honey bees, Apis cerana. J. Apicultural Res. 54, 468–473. ( 10.1080/00218839.2016.1162447) [DOI] [Google Scholar]
- 55.Figueroa LL, et al. 2019. Bee pathogen transmission dynamics: deposition, persistence and acquisition on flowers. Proc. R. Soc. B 286, 20190603 ( 10.1098/rspb.2019.0603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adler LS, Michaud KM, Ellner SP, McArt SH, Stevenson PC, Irwin RE. 2018. Disease where you dine: plant species and floral traits associated with pathogen transmission in bumble bees. Ecology 99, 2535–2545. ( 10.1002/ecy.2503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, Manson JS, Irwin RE. 2015. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 282, 20142471 ( 10.1098/rspb.2014.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenski RE, May RM. 1994. The evolution of virulence in parasites and pathogens: reconciliation between 2 competing hypotheses. J. Theor. Biol. 169, 253–265. ( 10.1006/jtbi.1994.1146) [DOI] [PubMed] [Google Scholar]
- 59.Zheng HQ, Lin ZG, Huang SK, Sohr A, Wu L, Chen YP. 2014. Spore loads may not be used alone as a direct indicator of the severity of Nosema ceranae infection in honey bees Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 107, 2037–2044. ( 10.1603/ec13520) [DOI] [PubMed] [Google Scholar]
- 60.Bureau of Meteorology. 2016. Climate statistics for Australian locations. See http://www.bom.gov.au/climate/dwo/IDCJDW4024.latest.shtml (accessed 15 December 2016).
- 61.Roberts J, Anderson D, Durr P. 2015. Upgrading knowledge on pathogens (particularly viruses) of Australian honey bees. Barton, Australia: Rural Industries Research and Development Corporation; See https://www.agrifutures.com.au/wp-content/uploads/publications/15-095.pdf. [Google Scholar]
- 62.Arbetman MP, Meeus I, Morales CL, Aizen MA, Smagghe G. 2013. Alien parasite hitchhikes to Patagonia on invasive bumblebee. Biol. Invasions 15, 489–494. ( 10.1007/s10530-012-0311-0) [DOI] [Google Scholar]
- 63.Piiroinen S, Goulson D. 2016. Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. Proc. R. Soc. B 283, 20160246 ( 10.1098/rspb.2016.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purkiss T, Lach L. 2018. Nosema ceranae spillover from Apis mellifera to an Australian stingless bee; Tetragonula hockingsi, Far North Queensland, Australia, 2016. Dataset. Cairns, Australia: James Cook University; ( 10.25903/5c006f9370a22) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the Tropical Data Hub [64].