Abstract
Sexually selected weapons are assumed to trade off with traits related to ejaculates, such as testes. However, remarkably little is known about what governs resource allocation and why trade-offs are found in some cases and not others. Often-used models depict competitive allocation occurring within the functional grouping of traits (e.g. reproduction); however, other factors including tissue expense and developmental timing may influence allocation. Experimental comparisons of investment across the sexes have the potential to illuminate allocation rules, because the sexes do not always use traits for the same functions. Here, we capitalize upon a species where females have weapons–testes homologues. We report that a documented trade-off in investment between hind-limb weapons and testes in leaf-footed cactus bugs, Narnia femorata, is even more pronounced in female hind limbs and ovaries. Female hind limbs in this species do not share the clear reproductive function of male hind limbs; therefore, this trade-off spans trait functional groups. Such patterns of investment suggest that future studies of reproductive trade-offs should consider factors such as tissue expense and developmental timing.
Keywords: sexual selection, pre-copulatory, post-copulatory, resource allocation, Y-model
1. Introduction
Trade-offs between costly fitness traits are a fundamental tenet in evolutionary biology [1–3]. An organism cannot optimally invest in all life-history traits associated with reproduction, development and survival, but must balance these competing functions. Decades of research have focused on documenting and understanding life-history trade-offs, and yet empirical findings continue to produce surprising results, including positive associations among traits that are expected to trade off [4,5]. One reason for the surprising results is that the functional and mechanistic relationships among traits remain quite uncertain [3,6].
Hierarchical Y-models of investment can be a useful heuristic to explain allocation decisions [7,8]. They often depict resources being allocated according to major life-history functions, such as reproduction, development and survival [6,8–12]. According to some depictions, resources ‘flow’ from the trunk to branches. Each branch represents a major functional group (e.g. reproduction) with allocation to certain branches prioritized over others. Additionally, traits compete for resources within each branch. The pool of resources that enters the reproductive branch, for example, is expected to be divided among traits associated with reproduction, including pre- and post-copulatory sexually selected traits. Resource allocation trade-offs are the result [6]. Indeed, a fundamental assumption of sperm competition theory is that a single pool of resources is set aside for competitive investment in pre-copulatory traits used to achieve access to females, such as sexually selected weapons, and post-copulatory traits used to maximize fertilization success, such as testes [13–15].
Although hierarchical models can be a useful heuristic, they might also be misleading because much remains unknown about the nature of allocation and investment. For example, trait proximity, tissue expense and developmental timing can influence trait investment, leading to trade-offs that are not purely based on functional grouping [16–21]. Our lack of knowledge on the principles that govern resource allocation and investment is a critical obstacle in our understanding of reproductive trait evolution. One way to improve knowledge in this area is to use experiments comparing male and female patterns of investment.
Male–female comparisons have been useful throughout the history of biology [22–24]. However, until now, studies of trade-offs in pre- and post-copulatory sexual traits have focused predominately on males [6,19,25–27]. The extent to which homologues in females follow similar allocation patterns is relatively unknown. In many species, females have a homologue to the male weapon, such as the female horns in some African ungulates, and in many cases, such homologues are not used in pre-copulatory sexual selection and do not serve any other direct reproductive function [28]. These homologues are often made of the same tissues and can develop at the same time as male weapons. As a result, studies that examine investment in testes, weapons and their female equivalents have the potential to yield insights into reproductive allocation patterns, with implications for trade-offs more generally.
Our goal in this study was to compare male and female patterns of investment using phenotypic engineering, a powerful experimental approach for understanding functional elements of life-history trade-offs [3]. The benefits of experimental manipulation for understanding trait allocation patterns is conceptually and empirically well established [3,29,30]. Our approach was simple: we limited investment in a weapon and its female homologue to determine the extent to which an established negative correlation between a male weapon and the testes was paralleled in females. Specifically, we determined if reduced investment in a weapon homologue in females led to larger ovaries. We chose a species where the weapon homologue does not play a direct role in reproduction and is instead involved in survival and dispersal. If resource allocation patterns are based on the functional group of traits (e.g. reproduction), then a negative correlation between weapons and testes in males should be seen at a reduced level in female homologous traits or not at all. If resource allocation patterns are based on the expense of tissues, developmental timing or a factor other than the functional group of the traits, then female homologous tissues should negatively associate as they do in their male equivalents.
We used the leaf-footed cactus bug, Narnia femorata (Hemiptera: Coreidae; figure 1; electronic supplementary material, figure S1), to compare allocation patterns in males and females after inducing them to drop a hind-leg weapon (males) or its homologue (females). Males of N. femorata use their enlarged hind limbs for locomotion, for signalling to other males, and in aggressive male–male contests [31,32]. When contests escalate, males will turn around end-to-end and squeeze each other with their spiny hind limbs. Males with larger limbs are more likely to win contests overall [31,33], and those missing a hind limb have reduced male dominance and mating success in a competitive context [34]. By contrast, female hind limbs are used primarily for locomotion, and no female signalling or fighting has been observed in this well-studied species. Whereas male hind limbs serve a key function in reproduction, the same is not true for female hind limbs. Males and females overlap substantially in size, though females are typically larger in body size (electronic supplementary material, figure S1) [32,35,36]. It is common for invertebrate females to be larger than males in overall body size because large size is often linked to higher fecundity [10,37].
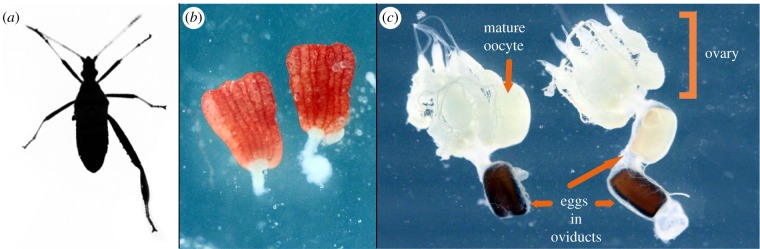
Figure 1.
(a) Silhouette of Narnia femorata with an autotomized left hind limb, (b) male testes and (c) female reproductive system. (Online version in colour.)
Although hind-leg weapons are important for pre-copulatory contests, they are also readily dropped to escape life-threatening situations, such as predation attempts and entrapment due to bad moults (i.e. autotomy [38]). Twelve per cent of wild adult N. femorata are missing one or more limbs [38], and weapon loss in the late juvenile stages has no detectable impact on survival to adulthood [39]. We capitalized upon this natural phenomenon to phenotypically engineer young males to have reduced weapon expression (i.e. one hind-leg weapon instead of two, as in [39]). The methods here resemble ablation experiments that have been used in developmental biology for almost two centuries [40], though in this case with minimal trauma to the study subjects.
Across animal taxa, not all weapons have detectable developmental or maintenance costs. When costs are low, resource allocation trade-offs may be small. However, weapons in this family of insects are metabolically expensive [41], and trade-offs should be more pronounced among costly traits [16]. Loss of a hind leg may ‘free up’ energy and resources for other traits, in part because N. femorata do not regenerate their autotomized limbs [42]. Indeed, hind-limb weapon loss during development results in larger testes by adulthood in this species and relatives [39,43], and males with larger testes sire more offspring in non-competitive scenarios [44,45]. Additional evidence for this trade-off comes from a closely related insect where a negative correlation between testes and weapons is found in wild-living individuals [46]. The trade-off appears to be concentrated on the testes and weapons dyad; the loss of a hind-limb weapon does not result in any notable increases in the size of other investigated traits, including overall body size, the size of other limbs, the length of the antennae and the length of sperm [39,43].
In a new experiment, we induced autotomy of the hind limb in juveniles of both sexes, then examined adult investment in the female ovaries and male testes of N. femorata. We discovered that, following autotomy, female ovaries increased in size more than the testes of males. We obtained these results using single-sex and combined-sex statistical analyses. These surprising and consistent results yield insights into female trait allocation, suggesting trade-offs that span major functions (e.g. reproduction and locomotion). Further, the similarity in response in males and females in this species hints at the possibility that weapons–testes trade-offs in males across taxa may be tied to factors such as tissue expense and/or developmental timing, factors that should be considered in greater detail in the future.
2. Methods
(a). Rearing of young insects
We collected adult Narnia femorata from Starke, FL (29.9804° N, 81.9848° W) in October 2016. We randomly paired males and females in plastic containers with a single Opuntia mesacantha spp. lata cladode planted in soil and provided ripe cactus fruit. Pairs were placed in a greenhouse with 14 h of natural and fluorescent lighting provided per day. Each day, we collected second-instar nymphs and pooled them from across families into groups of eight to ten. These groups of young insects were provided a potted cactus cladode and three to four ripe fruits to facilitate ad libitum feeding. Young nymphs were kept in these cups until they moulted into fourth-instar nymphs.
(b). Experimental design and rearing of older insects
Hemipteran testes growth increases at the fourth (penultimate) instar [47,48], so we induced hind-limb autotomy at this stage as done previously [39]. We sequentially divided 379 fourth-instar juveniles into one of two autotomy treatments: induced autotomy of the left hind limb (experimental treatment) or no autotomy (intact insects; control treatment). A previous study on testes investment following autotomy in this species incorporated one experimental treatment and three control treatments, including a baseline control (similar to the control treatment here), a handling procedural control and a mid-limb removal control [39]. No difference in testes investment was found across the three control treatments in this previous study, thus we used only one control treatment here. To induce autotomy, we gripped the left hind limb with reverse-action forceps and gently brushed the body with a small paintbrush until the individual performed a characteristic manoeuvre, disconnecting the limb from its body at the trochanter-femoral joint [38]. To keep track of our treatment groups effectively, we raised autotomized individuals with other autotomized individuals and intact individuals with other intact individuals during these final two instars. We took this approach because keeping track of individual juveniles (and their assigned treatments) can be difficult due shedding of ID markings during moults.
Groups of N. femorata were provided a cactus cladode planted in topsoil with four ripe cactus fruits. Males and females were kept together during the juvenile stages, because the sexes are indistinguishable until adulthood. We placed fourth-instar nymphs in groups of 3–11, reflecting the range of group sizes commonly seen in nature. Upon reaching adulthood, the average group size was 5.11 with a median of 3. New adults were separated into individual containers until they reached fourteen days after eclosion; they were then individually frozen for later measurements. Approximately 85% of juveniles survived to adulthood, consistent with previous studies [39]. Our rearing design produced a total of 160 females and 202 males. Fifty-eight containers produced one or more adult females, and 71 containers produced one or more adult males. We randomly selected one adult male and one adult female from each cup for measurement and analyses. Our final sample size included 71 males (37 autotomized and 34 intact) and 58 females (26 autotomized and 32 intact).
(c). Testes, ovaries and egg counts
We removed testes and ovaries from previously frozen individuals and submerged them in 70% EtOH. We dried gonads at 60°C for 24 h, then measured the mass in milligrams with accuracy to the nearest microgram (Mettler Toledo XP6: Columbus, OH, USA) [49,50]. Females in our experiment were not mated. In the Hemiptera, mating is not required to produce oocytes, and mature, unmated females commonly lay infertile eggs [51,52]. We counted the number of visible oocytes in the ovaries because they contributed to ovarian mass (see below). We also counted the unfertilized eggs in the oviducts and laid in the container to determine if effects of juvenile autotomy may translate into greater egg production early in life, even without a male present.
(d). Length and area measurements
We took photographs of previously frozen insects under a dissecting microscope (Leica M165C) with a Canon EOS 50D digital camera. We measured pronotal width to the nearest millimetre. Pronotal width has been shown to be an excellent metric for overall body size in this species [33,53]. All measurements were made using ImageJ software (v1.46r [54]).
(e). Hind-limb mass measurements
We removed the remaining (right) hind limbs from our previously frozen individuals at the trochanter-femoral joint. We then used a single-blade razor to make a horizontal slit across the femora from the trochanter-femoral to the femoral-tibial joints. Each limb was then placed into 70% EtOH. The hind limbs were later dried at 60°C for 72 h. We weighed the dried hind limbs in units of milligrams with accuracy to the nearest microgram. To determine the mass of the cuticle versus the soft tissue, we placed the hind limbs in a 10% KOH solution (0.5 ml at 90°C) for 1 h to dissolve the soft tissue. Hind-limb cuticles were then re-dried at 60°C for 72 h and massed. We calculated an individual's hind-limb muscle mass by subtracting the cuticle mass from the total mass. Dried muscle mass of males and females was on average 47% of the mass of the entire limb.
(f). Statistical analyses
We investigated both male and female trait allocation for those that autotomized (dropped) a left hind limb during development relative to those that did not. One adult male and one adult female were selected randomly from each rearing container for analysis. Measurements were loge transformed to improve linearity, normality, and homoscedasticity, unless it is mentioned that raw values were used. We assumed a normal distribution for all the analyses of morphology, a negative binomial distribution for the analysis of egg count, and a binomial distribution for the analysis of the presence/absence of oocytes in females. We used IBM SPSS v. 24 for all analyses.
(i). What are the effects of developmental autotomy on males and females (sexes considered separately)?
We examined whether autotomy during development (as a binary explanatory variable) affected adult body size (measured as pronotal width) separately by sex. We found no effect on male or female body size (see below for statistical results). Thus, we proceeded to use body size as a covariate in subsequent GLMs (generalized linear models) to examine the effects of autotomy on the remaining (right) hind-leg muscle mass and gonadal mass. All these initial analyses were run separately by sex. The full models for both the remaining leg muscle mass and male testicular mass included body size as a continuous covariate, autotomy as a binary factor, and their interaction. The full model for female ovarian mass also included oocyte number (see next paragraph).
Twenty-one females (36%) had visible oocytes within their ovaries, with a total number ranging from one to eight (mean = 2.9 and median = 3 for those with oocytes). We suspected that these oocytes added to ovarian mass (figure 1c) and indeed found that they did (Spearman rank correlation = 0.844, p < 0.001; electronic supplementary material, figure S2). The mean mass for a single visible oocyte was approximately 0.550 mg, calculated from the raw data, which is sizable relative to the mean mass of the ovaries without oocytes, 0.183 mg. For these reasons, we included the number of oocytes as an additional covariate in the GLM for female ovarian mass, as mentioned above. Our initial model included body size and oocyte number as continuous covariates, autotomy (yes or no) as a binary factor and the interaction of body size with autotomy. The pairwise interaction was not statistically significant, thus we ran our final model using only main effects. Next, we examined whether juvenile autotomy led to greater egg production early in life prior to mating. For this analysis, our response variable was the combined number early adulthood (infertile) eggs present in the oviducts and those laid in the container. We included body size as a continuous covariate and autotomy as a binary explanatory factor. Because the count data was overdispersed for a Poisson distribution, we used the negative binomial distribution.
(ii). Do males exhibit a greater gain in gonadal mass than females following developmental autotomy?
A further objective was to compare gonadal allocation in males and in females. We expected that males might increase allocation to gonads more than females following autotomy given the common reproductive function of testes and weapons. We approached this problem in two ways. First, we quantitatively compared males and females in the same analysis, adjusting for body size and the amount of limb muscle tissue that was never grown (i.e. resource investment that was likely reallocated or ‘saved’ for another purpose). Tremendous growth happens in the hind limbs between the fourth instar and adulthood. The average dry mass of a single hind limb of a fourth instar N. femorata is 0.108 mg. The average dry mass of an adult hind limb at 14 days post-eclosion is 1.800 mg, a 16.66-fold increase. Thus, autotomy at the fourth instar reduces further allocation to the autotomized limb and, because these organisms cannot regenerate, resources may be reallocated to other structures. Yet autotomy of one hind limb does not appear to lead to an increase in the overall external size of the other hind limb [39], a result similar to our findings here (below). Thus, we treated the muscle mass in the remaining hind limb as a proxy for the muscle mass that was not grown in the missing limb. With this first approach, we constructed a GLM including both sexes with body size and adult muscle mass of the remaining limb as continuous covariates and gonadal mass as the response variable (see table 1 for full model structure). Our main interest in this analysis was to test if one sex reallocated more of the ‘saved’ muscle tissue to their gonads than the other sex, which would be revealed with a statistically significant sex × autotomy interaction and/or a three-way interaction of sex × autotomy × muscle mass.
Table 1.
Results of GLMs to examine the relative effects of hind-limb autotomy on male and female gonadal mass. The model on the left combined the sexes into the same analysis for a quantitative comparison, while the others focused on a single sex for a qualitative comparison. Italics indicate statistical significance (p < 0.05).
| loge gonadal mass (both sexes) |
loge ovarian mass (females only) |
loge testicular mass (males only) |
||||
|---|---|---|---|---|---|---|
| source | Wald χ2 | p | Wald χ2 | p | Wald χ2 | p |
| body size | 1.002 | 0.317 | 3.018 | 0.082 | 29.188 | <0.001 |
| limb muscle mass | 105.688 | <0.001 | 74.802 | <0.001 | 9.266 | 0.002 |
| autotomy | 15.766 | <0.001 | 4.556 | 0.033 | 8.368 | 0.004 |
| oocyte number | 193.077 | <0.001 | 118.637 | <0.001 | ||
| sex | 17.665 | <0.001 | ||||
| autotomy × sex | 4.944 | 0.026 | ||||
| muscle × sex | 66.871 | <0.001 | ||||
| autotomy × muscle | 0.892 | 0.345 | ||||
| auto × muscle × sex | 8.698 | 0.003 | ||||
Our final step was to take a simpler and complementary approach to compare the sexes that did not require males to be assigned a value for oocyte number. In this case, we built two GLMs, one for each sex (table 1). The model for females considered autotomy, body size, muscle mass and oocyte count as covariates and gonadal mass as the response variable. The model for males was identical but did not include oocyte count. We then back-transformed the output from the models to examine the relative increase in gonadal mass following autotomy for the average-sized individual of each sex.
3. Results
(a). Males grew larger testes following developmental autotomy
Our first question was whether the previously detected negative relationship between weapons and testes in males [39] was confirmed in this study. Indeed, we found that juvenile males that autotomized a hind limb during development, relative to intact males, grew larger testes (Wald χ2 = 10.270, d.f. = 1, p = 0.001), an effect also seen when we adjusted for body size (autotomy: Wald χ2 = 5.304, d.f. = 1, p = 0.021; autotomy × body size: Wald χ2 = 4.472, d.f. = 1, p = 0.034; electronic supplementary material, figure S3). The males missing a hind limb did not grow larger in absolute body size (Wald χ2 = 2.227, d.f. = 1, p = 0.136) nor in the muscle mass of the remaining hind limb (Wald χ2 = 0.449, d.f. = 1, p = 0.503).
(b). Females grew larger ovaries following developmental autotomy
Our second question was whether female allocation patterns resembled male patterns. Indeed, we found that juvenile females that autotomized a hind limb during development, relative to intact females, grew larger ovaries (adjusting for oocyte number; Wald χ2 = 10.353, d.f. = 1, p = 0.001), an effect also seen when we also adjusted for body size (Wald χ2 = 8.996, d.f. = 1, p = 0.003). Fifty per cent of autotomized females had oocytes in their ovaries, while only 25% of intact females had oocytes (Wald χ2 = 3.491, d.f. = 1, p = 0.062). The number of oocytes had a large impact on ovarian mass, such that the effect of autotomy on ovarian mass was not detectable when we removed oocyte number from the model (Wald χ2 = 1.501, d.f. = 1, p = 0.220). Female ovaries without oocytes in our study had a mean of 0.165 mg and a range from 0.20 to 0.797 mg, a 3.985-fold difference. The female with eight oocytes had an ovarian mass of 3.725 mg, an 18.625-fold increase over the smallest ovarian mass (electronic supplementary material, figure S2). For comparison, male testes in our study had a mean mass of 0.222 mg and a range from 0.075 to 0.408 mg, a 5.44-fold difference (electronic supplementary material, figure S3).
The females that experienced juvenile autotomy did not grow larger in absolute body size (Wald χ2 = 0.694, d.f. = 1, p = 0.405) nor the mass of the muscle in the remaining hind limb (Wald χ2 = 2.849, d.f. = 1, p = 0.091). During this period of early adulthood, they produced 53% more unfertilized eggs than intact females, a difference that was not statistically significant (5.79 versus 3.79 eggs; Wald χ2 = 2.055, d.f. = 1, p = 0.152).
(c). Females exhibited a greater gain in gonadal mass than males
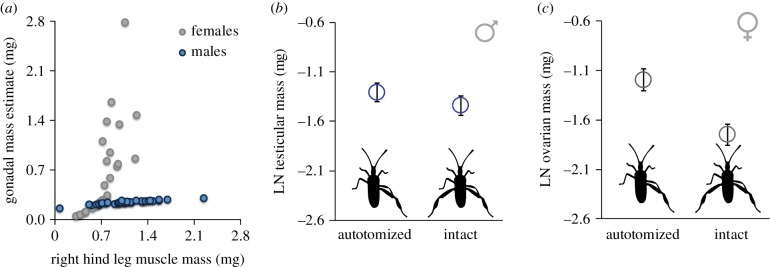
Finally, we examined in more detail whether the magnitude of the muscle–gonad trade-off differed between males and females. We approached this problem in two ways. Our first approach was to combine males and females in the same analyses, which allowed a more direct comparison. Body size in females of this species is larger than in males (Wald χ2 = 23.93, d.f. = 1, p < 0.001) and muscle mass of male hind limbs is greater than that of female limbs (Wald χ2 = 15.268, d.f. = 1, p < 0.001). In this first approach, we constructed a GLM that included both sexes, considering body size, muscle mass and oocyte number as continuous covariates and gonadal mass as the response variable with the full model structure shown in table 1. Males received a zero for oocyte number. This model revealed that those individuals with greater hind-limb muscle mass also have larger gonads (table 1 and figure 2a). The scaling relationship between hind-leg muscle mass and the size of gonads was sex-specific, with females growing larger gonads for a given amount of hind-leg muscle than males (sex * muscle interaction; table 1 and figure 2a). Importantly, following autotomy, the boost that females received in gonadal mass exceeded that of males (sex * autotomy interaction; table 1 and figure 2b,c), with the greatest difference found between the males and females that had the most resources to reallocate (sex * muscle * autotomy interaction; table 1). We back-transformed the estimates produced from our first model (estimates shown in figure 2b,c; calculated at the overall mean body size and hind-leg muscle mass) to discover that at these values females received a 73% gain in ovarian mass from autotomy (0.302 mg with autotomy versus 0.174 mg intact) while males received a 14% gain in testicular mass from autotomy (0.270 mg with autotomy versus 0.236 mg intact).
Figure 2.
Both males and females with autotomy showed a positive relationship between the muscle mass of the remaining (right) hind leg and gonadal mass (a), though the slope of this scaling relationship was steeper for females. At the mean overall body size, females (c) received a greater boost in gonadal mass following autotomy than did males (b). In (a), back-transformed raw data (x-axis) and model estimates (y-axis) are displayed, produced from the full (both sexes) model in table 1. We used model estimates of gonadal mass in (a) to adjust for differences in oocyte number within the ovaries which contributed substantially to their mass. In (b) and (c) hollow circles denote estimated marginal means (EMM) ± standard error bars that were generated directly from the same model without back-transformation. EMM produced at the mean body size (loge pronotal width = 1.4215 mg). Simpler models (right columns in table 1) produced qualitatively the same results as depicted here. (Online version in colour.)
Our second approach to this problem was simpler and a more qualitative comparison that did not require males to be assigned a value for oocyte number. In this case, males and females were analysed separately (right columns in table 1). We back-transformed output from these models, revealing that, at the mean female body size and hind-leg muscle mass, those females with autotomy had a 37% increase in ovarian mass (0.341 mg with autotomy versus 0.249 mg intact). Males at mean male body size and hind-leg muscle mass had a 20% increase in testicular mass (0.225 mg with autotomy versus 0.188 mg intact). Though the magnitude of the estimates differ due the specifics of the models, our two approaches for comparing males and females produced the same clear finding: females received a greater gain in gonadal mass following autotomy than did males.
4. Discussion
We found that a negative association between testes and weapons can extend to the homologous traits in females. In fact, the female ovaries grew more following loss of the weapon homologue than did the male testes following loss of the weapon (table 1 and figure 2b,c). These unexpected results suggest life-history trade-offs in females across traits that span major functions, reminiscent of the reproduction–dispersal and signalling–longevity trade-offs documented in crickets [3,55]. The increased gonadal growth in both males and females following limb loss suggests that factors other than the functional groupings of traits must be considered in studies of testes and weapons investment in males and in resource allocation more generally.
The hind limbs of male and female N. femorata are enlarged (electronic supplementary material, figure S1) and filled with muscle [41]. Female N. femorata use their slimmer hind limbs for walking, while males use their thicker hind limbs for locomotion, and for signalling and fighting with other males. When male N. femorata lose a hind limb during development, they grow larger testes [39]. Such males have increased fertilization success in non-competitive scenarios [45]. We found that hind-limb loss in females resulted in a surprisingly pronounced increase in gonadal mass (figure 2b,c). Even in these early days of adulthood, hind-limb loss was accompanied by a mean increase in the number of oocytes and eggs produced, though the increases were not statistically significant. It would be useful to follow females for longer periods of time and provide them with opportunities to mate to determine the lifelong reproductive consequences of juvenile autotomy.
Resource allocation patterns are hypothesized to follow a series of dichotomous decisions, leading in aggregate to a hierarchical allocation tree and a series of positive and negative trait covariances [7]. Such Y-models are a helpful and often-used heuristic for explaining the concept of trade-offs. Major splitting points of the tree, and thus resource flow, are often depicted as based on fundamental functions including growth, maintenance, dispersal and reproduction [6,8–12]. Indeed, sperm competition theory assumes that suites of traits associated with reproduction compete for a single pool of resources [13–15]. The growth of male testes after limb loss in N. femorata is consistent with these assumptions; however, the amplified patterns in female homologues suggests trade-offs that span major functional groupings. While males and females may follow distinct allocation rules, the results suggest a common phenomenon that has little to do with trait function. Investment patterns across traits are poorly understood in general [3], and much more work needs to be done.
Empirical and theoretical work has suggested that numerous factors may impact trait allocation, including the (1) proximity of traits, (2) expense of tissues and (3) developmental timing. In onthophagine horned beetles, for example, trait allocation can be influenced by trait proximity. An across species comparative study revealed that traits developing near to beetle horns are relatively smaller (e.g. taxa with head horns have relatively smaller eyes [18]). By contrast, previous work in N. femorata did not find an effect of juvenile hind-limb loss on adult body size or the growth of nearby traits, thus providing no support in this species, to date, for a role of trait proximity in investment patterns [39].
Expensive traits, such as the brain, gut, muscle, gonads, liver and immune system can have pronounced trade-offs with each other [17,56]. These traits have vastly distinct forms and functions, and yet they share a high demand for energy and/or growth factors. Across species of primates, those with big muscles may have small brains and vice versa [57]. Guppies (Poecilia reticulata) artificially selected for large brains evolve smaller guts [58] and have weakened innate immune responses [59]. Finally, many insects display a trade-off between wing musculature and both female and male reproductive capacity [60–63]. These examples suggest the possibility that the expense of tissues may better predict which traits trade off rather than trait functional groupings. Indeed, hind legs and gonads in N. femorata are probably expensive tissues. Hind legs in this species and many relatives are enlarged and filled with metabolically expensive muscle tissue [41]. By removing a single hind limb during development, we were able to cut total hind-leg muscle mass at adulthood by approximately 50% in both males and females. Considering the size and the expense of the hind legs in these insects, it is perhaps no surprise to find evidence that hind-leg weapons and their homologues trade off with the gonads. We would not expect such a pronounced trade-off in those cases where weapons and their homologues are composed of relatively inexpensive tissues.
Developmental timing is another factor hypothesized to play a role in the presence and magnitude of trade-offs [20,21]. For example, Moczek & Nijhout [19] discovered that testes ablation in male beetles led to horn growth in medium-sized individuals, but only when those testes were ablated during the period of horn growth. Males of this size did not grow sizeable horns if ablation occurred earlier in development. In N. femorata hind limbs experience drastic growth in the final two moults, and this is same ontogenetic window where gonads develop across the Hemiptera [47,48]. We stimulated insects to drop a single hind leg prior to the final two moults, and the testes grew. Future work should compare gonadal growth when a hind limb is dropped other developmental stages or during adulthood; we might expect reduced gonadal growth in these cases.
Our results suggest that tissue expense and developmental timing may play a part in determining which traits trade off. Further work in this area should examine more trait correlations in males and females, including traits that vary in location, expense and developmental timing. Experimentation is a powerful approach to get at the mechanisms behind trade-offs [3]. Yet experimental techniques involving phenotypic engineering, the general approach we used here, can be quite invasive [19,25,27]. By contrast, the dropping of a hind limb to escape entrapment is routine in many leaf-footed bugs (Family: Coreidae [38]), providing an excellent context for the experimental investigation of trade-offs. Here in this laboratory-based study, survivorship of autotomized individuals did not differ from those left intact. While our study system provides unique opportunities, using a natural phenomenon to remove limbs from males and females comes with its own set of uncertainties. Each empirical approach to investigating trade-offs, including experimental, genetic and observational, has strengths and weaknesses, and thus the use of multiple complementary approaches would be ideal [3,29,64–68].
Autotomy is common in wild populations in this insect family [38], and thus a history of selection following autotomy may be responsible for shifts in allocation. For example, we cannot exclude the possibility that the loss of a limb is, for both males and females, a cue of a high-predation environment or, more generally, a cue of a reduced lifespan. It is possible that wild individuals have historically done better to shift to early reproduction following autotomy, contributing to evolutionary change and the patterns witnessed here. While such a history of selection may contribute to the documented patterns, an observational study of another leaf-footed bug (Leptoscelis tricolor) provides evidence that a trade-off between testes and weapons exists naturally, even in the absence of limb loss [46]. Together, the results of these studies support our conclusion that the negative correlation between gonad and leg investment reflects, at least in part, an allocation trade-off.
In this study, the trade-off between the gonads and the legs was, interestingly, paired with a positive association between these traits, even when adjusting for body size differences (table 1 and figure 2a). Insects with larger legs had larger gonads on average. While such results might initially seem contradictory, they are to be expected for traits that have heightened condition dependence [32,69,70]. Here, we provided all insects a cactus pad with a ripe cactus fruit, typically an excellent diet [32]. In general, negative correlations among traits can shift to positive under good conditions [5]. It is probable that a purely observational study in this context would have only detected a positive association between these traits. If we had not taken an experimental approach (etiam [39,43]), we may have wrongly concluded that a trade-off between the hind limbs and the gonads did not exist.
Not only did female ovaries grow following hind-limb loss, but they grew more than did male testes. The bigger the limb lost, the greater the increase in female ovaries. Female limbs in N. femorata are slimmer than male limbs but still enlarged relative to the hind limbs in some related species (electronic supplementary material, figure S1) [71]. The enlargement in female limbs and the associated developmental trade-off may be a result of sexual antagonism. However, other factors beyond sexual antagonism may contribute to the sex-specific responses observed in this study (figure 2b,c). For example, the growth of ovarian tissue may be less expensive than the growth of testicular tissue, leading to an increased female response. Additionally, males and females may differ in their allocation priorities due to the history of selection experienced. Males may benefit less from an increase in gonadal tissue than females and instead reallocate resources to the machinery used to produce sex pheromones, to higher activity levels or to components of the sensory system, for example.
This study is the first of our knowledge to experimentally examine ovarian investment using the perspectives and approaches used to study testes-weapons investment. We found that a documented negative correlation between weapons and testes in male leaf-footed cactus bugs is more pronounced in the female homologous traits, hind limbs and ovaries (figure 2). Our results are consistent with both males and females experiencing a resource allocation trade-off between their gonads and their legs. The allocation patterns suggest the possibility of a common mechanism influencing trait allocation, potentially influenced by tissue expense and/or developmental timing. Many more studies are needed to investigate the functional and mechanistic relationship between pre- and post-copulatory traits, and male–female comparisons may continue to play a valuable role in this pursuit.
Supplementary Material
Acknowledgements
We thank R. Shepherd for meticulous measurements and to the UF/IFAS internship programme for her support. Many thanks also to L. A. Cirino and U. Somjee for providing comments on a previous version of this manuscript and to the entire Miller Lab for thoughtful critiques along the way.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8g104tc [72].
Authors' contributions
P.N.J. and C.W.M. conceived the study. P.N.J. performed the experiment and provided a first draft of the manuscript. C.W.M. performed the statistical analyses, structured and wrote the final draft of the manuscript. R.M.K., Z.E. and P.N.J. contributed critical ideas throughout the analysis and writing stages. All authors helped with the editing of the manuscript, gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We have no competing interests.
Funding
This work was funded by NSF grant IOS-1552100 and REEport Project no. FLA-ENY-005691. The collaboration between universities was made possible by Sidney-Sussex College at the University of Cambridge where C.W.M. was a visiting fellow for the Easter Term of 2018 in the Department of Zoology.
References
- 1.Roff D. 1993. Evolution of life histories: theory and analysis. New York, NY: Springer US. [Google Scholar]
- 2.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 3.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 4.Reznick D, Nunney L, Tessier A. 2000. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol. Evol. 15, 421–425. ( 10.1016/S0169-5347(00)01941-8) [DOI] [PubMed] [Google Scholar]
- 5.Van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 6.Simmons LW, Lüpold S, Fitzpatrick JL. 2017. Evolutionary trade-off between secondary sexual traits and ejaculates. Trends Ecol. Evol. 32, 964–976. ( 10.1016/j.tree.2017.09.011) [DOI] [PubMed] [Google Scholar]
- 7.De Jong G. 1993. Covariances between traits deriving from successive allocations of a resource. Funct. Ecol. 7, 75–83. ( 10.2307/2389869) [DOI] [Google Scholar]
- 8.De Laguérie P, Olivieri I, Atlan A, Gouyon P-H. 1991. Analytic and simulation models predicting positive genetic correlations between traits linked by trade-offs. Evol. Ecol. 5, 361–369. ( 10.1007/BF02214153) [DOI] [Google Scholar]
- 9.Márquez-García A, Canales-Lazcano J, Rantala MJ, Contreras-Garduño J. 2016. Is juvenile hormone a potential mechanism that underlay the ‘branched Y-model’? Gen. Comp. Endocrinol. 230, 170–176. ( 10.1016/j.ygcen.2016.03.027) [DOI] [PubMed] [Google Scholar]
- 10.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Tomimatsu H, Ohara M. 2006. Evolution of hierarchical floral resource allocation associated with mating system in an animal-pollinated hermaphroditic herb, Trillium camschatcense (Trilliaceae). Am. J. Bot. 93, 134–141. ( 10.3732/ajb.93.1.134) [DOI] [Google Scholar]
- 12.Worley AC, Houle D, Barrett SC. 2003. Consequences of hierarchical allocation for the evolution of life-history traits. Am. Nat. 161, 153–167. ( 10.1086/345461) [DOI] [PubMed] [Google Scholar]
- 13.Ball MA, Parker GA. 2000. Sperm competition games: a comparison of loaded raffle models and their biological implications. J. Theor. Biol. 206, 487–506. ( 10.1006/jtbi.2000.2142) [DOI] [PubMed] [Google Scholar]
- 14.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. Camb. Philos. Soc. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 15.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126. ( 10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 16.Aiello LC. 1997. Brains and guts in human evolution: the expensive tissue hypothesis. Braz. J. Genet. 20, 141–148. ( 10.1590/S0100-84551997000100023) [DOI] [Google Scholar]
- 17.Aiello LC, Wheeler P. 1995. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 36, 199–221. ( 10.1086/204350) [DOI] [Google Scholar]
- 18.Emlen DJ. 2001. Costs and the diversification of exaggerated animal structures. Science 291, 1534–1536. ( 10.1126/science.291.5508.1534) [DOI] [PubMed] [Google Scholar]
- 19.Moczek AP, Nijhout HF. 2004. Trade-offs during the development of primary and secondary sexual traits in a horned beetle. Am. Nat. 163, 184–191. ( 10.1086/381741) [DOI] [PubMed] [Google Scholar]
- 20.Nijhout HF, Emlen DJ. 1998. Competition among body parts in the development and evolution of insect morphology. Proc. Natl Acad. Sci. USA 95, 3685–3689. ( 10.1073/pnas.95.7.3685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowicki S, Searcy WA, Peters S. 2002. Brain development, song learning and mate choice in birds: a review and experimental test of the ‘nutritional stress hypothesis'. J. Comp. Physiol. A Neuroethol. Sensory Neural Behav. Physiol. 188, 1003–1014. ( 10.1007/s00359-002-0361-3). [DOI] [PubMed] [Google Scholar]
- 22.Darwin C. 1888. The descent of man and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 23.Mayhew R. 2010. The female in Aristotle's biology: reason or rationalization. Chicago, IL: University of Chicago Press. [Google Scholar]
- 24.Pagel W. 1968. Galen and the usefulness of the parts of the body. Ithaca, NY: Cornell University Press. [Google Scholar]
- 25.Fry CL. 2006. Juvenile hormone mediates a trade-off between primary and secondary sexual traits in stalk-eyed flies. Evol. Dev. 8, 191–201. ( 10.1111/j.1525-142X.2006.00089.x) [DOI] [PubMed] [Google Scholar]
- 26.Lupold S, Tomkins JL, Simmons LW, Fitzpatrick JL. 2014. Female monopolization mediates the relationship between pre- and postcopulatory sexual traits. Nat. Commun. 5, 3184 ( 10.1038/ncomms4184) [DOI] [PubMed] [Google Scholar]
- 27.Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc. Natl Acad. Sci. USA 103, 16 346–16 351. ( 10.1073/pnas.0603474103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 29.Partridge L, Sibly R. 1991. Constraints in the evolution of life histories. Phil. Trans. R. Soc. Lond. B 332, 3–13. ( 10.1098/rstb.1991.0027) [DOI] [Google Scholar]
- 30.Salmon AB, Marx DB, Harshman LG. 2001. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution 55, 1600–1608. ( 10.1111/j.0014-3820.2001.tb00679.x) [DOI] [PubMed] [Google Scholar]
- 31.Nolen ZJ, Allen PE, Miller CW. 2017. Seasonal resource value and male size influence male aggressive interactions in the leaf footed cactus bug, Narnia femorata. Behav. Processes 138, 1–6. ( 10.1016/j.beproc.2017.01.020) [DOI] [PubMed] [Google Scholar]
- 32.Miller CW, McDonald GC, Moore AJ. 2016. The tale of the shrinking weapon: seasonal changes in nutrition affect weapon size and sexual dimorphism, but not contemporary evolution. J. Evol. Biol. 29, 2266–2275. ( 10.1111/jeb.12954) [DOI] [PubMed] [Google Scholar]
- 33.Procter D, Moore A, Miller C. 2012. The form of sexual selection arising from male–male competition depends on the presence of females in the social environment. J. Evol. Biol. 25, 803–812. ( 10.1111/j.1420-9101.2012.02485.x) [DOI] [PubMed] [Google Scholar]
- 34.Emberts Z, St Mary CM, Herrington TJ, Miller CW. 2018. Males missing their sexually selected weapon have decreased fighting ability and mating success in a competitive environment. Behav. Ecol. Sociobiol. 72, 81 ( 10.1007/s00265-018-2494-6) [DOI] [Google Scholar]
- 35.Cirino LA, Miller CW. 2017. Seasonal effects on the population, morphology and reproductive behavior of Narnia femorata (Hemiptera: Coreidae). Insects 8, 16 ( 10.3390/insects8010013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CW, McDonald GC, Moore AJ. 2016. Data from: The tale of the shrinking weapon: seasonal changes in nutrition affect weapon size and sexual dimorphism, but not contemporary evolution. Dryad Digital Repository. ( 10.5061/dryad.t2g67) [DOI] [PubMed] [Google Scholar]
- 37.Blanckenhorn WU. 2000. The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407. ( 10.1086/393620) [DOI] [PubMed] [Google Scholar]
- 38.Emberts Z, St Mary CM, Miller CW. 2016. Coreidae (Insecta: Hemiptera) limb loss and autotomy. Ann. Entomol. Soc. Amer. 109, 678–683. ( 10.1093/aesa/saw037) [DOI] [Google Scholar]
- 39.Joseph PN, Emberts Z, Sasson DA, Miller CW. 2018. Males that drop a sexually selected weapon grow larger testes. Evolution 72, 113–122. ( 10.1111/evo.13387) [DOI] [PubMed] [Google Scholar]
- 40.Gilbert SF. 2014. Developmental biology, 10th edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 41.Somjee U, Woods HA, Duell M, Miller CW. 2018. The hidden cost of sexually selected traits: the metabolic expense of maintaining a sexually selected weapon. Proc. R. Soc. B 285, 20181685 ( 10.1098/rspb.2018.1685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emberts Z, Miller CW, Kiehl D, St Mary CM. 2017. Cut your losses: self-amputation of injured limbs increases survival. Behav. Ecol. 28, 1047–1054. ( 10.1093/beheco/arx063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somjee U, Miller C, Tatarnic N, Simmons L. 2018. Experimental manipulation reveals a trade-off between weapons and testes. J. Evol. Biol. 31, 57–65. ( 10.1111/jeb.13193) [DOI] [PubMed] [Google Scholar]
- 44.Greenway EV, Cirino LA, Wilner D, Somjee W, Miller CW.Extreme variation in testes size in an insect is linked to recent mating activity. In preparation. [DOI] [PubMed]
- 45.Cirino LA, Lenga SH, Miller CW.Weapon damage can boost reproductive success. In preparation.
- 46.Somjee U, Allen PE, Miller CW. 2015. Different environments lead to a reversal in the expression of weapons and testes in the heliconia bug, Leptoscelis tricolor (Hemiptera: Coreidae). Biol. J. Linnean Soc. 115, 802–809. ( 10.1111/bij.12544) [DOI] [Google Scholar]
- 47.Dumser JB, Davey KG. 1974. Endocrinological and other factors influencing testis development in Rhodnius prolixus. Can. J. Zool. 52, 1011–1022. ( 10.1139/z74-135) [DOI] [PubMed] [Google Scholar]
- 48.Economopoulos AP, Gordon HT. 1971. Growth and differentiation of the testes in the large milkweed bug, Oncopeltus fasciatus (Dallas). J. Exp. Zool. 177, 391–405. ( 10.1002/jez.1401770402) [DOI] [PubMed] [Google Scholar]
- 49.Joseph P, Sasson D, Allen P, Somjee U, Miller C. 2016. Adult nutrition, but not inbreeding, affects male primary sexual traits in the leaf-footed cactus bug Narnia femorata (Hemiptera: Coreidae). Ecol. Evol. 6, 4792–4799. ( 10.1002/ece3.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sasson DA, Munoz PR, Gezan SA, Miller CW. 2016. Resource quality affects weapon and testis size and the ability of these traits to respond to selection in the leaf-footed cactus bug, Narnia femorata. Ecol. Evol. 6, 2098–2108. ( 10.1002/ece3.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon HT, Bandal SK. 1967. Effect of mating on egg production by large milkweed bug Oncopeltus Fasciatus (Hemiptera—Lygaeidae). Ann. Entomol. Soc. Amer. 60, 1099 ( 10.1093/aesa/60.5.1099) [DOI] [Google Scholar]
- 52.Adams TS. 2000. Effect of diet and mating status on ovarian development in a predaceous stink bug Perillus bioculatus (Hemiptera: Pentatomidae). Ann. Entomol. Soc. Amer. 93, 529–535. ( 10.1603/0013-8746(2000)093[0529:EODAMS]2.0.CO;2) [DOI] [Google Scholar]
- 53.Gillespie SR, Tudor MS, Moore AJ, Miller CW. 2014. Sexual selection is influenced by both developmental and adult environments. Evolution 68, 3421–3432. ( 10.1111/evo.12526) [DOI] [PubMed] [Google Scholar]
- 54.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussiére LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027. ( 10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 56.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 57.Muchlinski MN, Hemingway HW, Pastor J, Omstead KM, Burrows AM. 2018. How the brain may have shaped muscle anatomy and physiology: a preliminary study. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 301, 528–537. ( 10.1002/ar.23746) [DOI] [PubMed] [Google Scholar]
- 58.Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brännström I, Immler S, Maklakov AA, Kolm N. 2013. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr. Biol. 23, 168–171. ( 10.1016/j.cub.2012.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kotrschal A, Kolm N, Penn DJ. 2016. Selection for brain size impairs innate, but not adaptive immune responses. Proc. R. Soc. B 283, 20152857 ( 10.1098/rspb.2015.2857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dingle H. 1996. Migration: the biology of life on the move. New York, NY: Oxford University Press. [Google Scholar]
- 61.Zera AJ, Denno RF. 1997. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 42, 207–230. ( 10.1146/annurev.ento.42.1.207) [DOI] [PubMed] [Google Scholar]
- 62.Roff D, Gelinas M. 2003. Phenotypic plasticity and the evolution of trade-offs: the quantitative genetics of resource allocation in the wing dimorphic cricket, Gryllus firmus. J. Evol. Biol. 16, 55–63. ( 10.1046/j.1420-9101.2003.00480.x) [DOI] [PubMed] [Google Scholar]
- 63.Saglam IK, Roff DA, Fairbairn DJ. 2008. Male sand crickets trade-off flight capability for reproductive potential. J. Evol. Biol. 21, 997–1004. ( 10.1111/j.1420-9101.2008.01548.x) [DOI] [PubMed] [Google Scholar]
- 64.Linden M, Moller AP. 1989. Cost of reproduction and covariation of life history traits in birds. Trends Ecol. Evol. 4, 367–371. ( 10.1016/0169-5347(89)90101-8) [DOI] [PubMed] [Google Scholar]
- 65.Rose MR, Bradley TJ. 1998. Evolutionary physiology of the cost of reproduction. Oikos 83, 443–451. ( 10.2307/3546672) [DOI] [Google Scholar]
- 66.Sinervo B, Basolo AL. 1996. Testing adaptation using phenotypic manipulations. In Adaptation (eds Rose MR, Lauder GV), pp. 149–185. San Diego, CA: Academic Press. [Google Scholar]
- 67.Zera AJ, Cisper G. 2001. Genetic and diurnal variation in the juvenile hormone titer in a wing-polymorphic cricket: implications for the evolution of life histories and dispersal. Physiol. Biochem. Zool. 74, 293–306. ( 10.1086/319664) [DOI] [PubMed] [Google Scholar]
- 68.Zera AJ, Potts J, Kobus K. 1998. The physiology of life-history trade-offs: experimental analysis of a hormonally induced life-history trade-off in Gryllus assimilis. Am. Nat. 152, 7–23. ( 10.1086/286146) [DOI] [PubMed] [Google Scholar]
- 69.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 70.Bonduriansky R. 2007. The evolution of condition-dependent sexual dimorphism. Am. Nat. 169, 9–19. ( 10.1086/510214) [DOI] [PubMed] [Google Scholar]
- 71.CoreoideaSF Team. 2019. Coreoidea species file online. Version 5.0. See http://coreoidea.speciesfile.org.
- 72.Miller CW, Joseph PN, Kilner RM, Emberts Z. 2019. Data from: A weapons–testes trade-off in males is amplified in female traits Dryad Digital Repository. ( 10.5061/dryad.8g104tc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Miller CW, Joseph PN, Kilner RM, Emberts Z. 2019. Data from: A weapons–testes trade-off in males is amplified in female traits Dryad Digital Repository. ( 10.5061/dryad.8g104tc) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8g104tc [72].