Abstract
Background
A clinical large anterior vessel occlusion (LAVO)-prediction scale could reduce treatment delays by allocating intra-arterial thrombectomy (IAT)-eligible patients directly to a comprehensive stroke center.
Aim
To subtract, validate and compare existing LAVO-prediction scales, and develop a straightforward decision support tool to assess IAT-eligibility.
Methods
We performed a systematic literature search to identify LAVO-prediction scales. Performance was compared in a prospective, multicenter validation cohort of the Dutch acute Stroke study (DUST) by calculating area under the receiver operating curves (AUROC). With group lasso regression analysis, we constructed a prediction model, incorporating patient characteristics next to National Institutes of Health Stroke Scale (NIHSS) items. Finally, we developed a decision tree algorithm based on dichotomized NIHSS items.
Results
We identified seven LAVO-prediction scales. From DUST, 1316 patients (35.8% LAVO-rate) from 14 centers were available for validation. FAST-ED and RACE had the highest AUROC (both >0.81, p < 0.01 for comparison with other scales). Group lasso analysis revealed a LAVO-prediction model containing seven NIHSS items (AUROC 0.84). With the GACE (Gaze, facial Asymmetry, level of Consciousness, Extinction/inattention) decision tree, LAVO is predicted (AUROC 0.76) for 61% of patients with assessment of only two dichotomized NIHSS items, and for all patients with four items.
Conclusion
External validation of seven LAVO-prediction scales showed AUROCs between 0.75 and 0.83. Most scales, however, appear too complex for Emergency Medical Services use with prehospital validation generally lacking. GACE is the first LAVO-prediction scale using a simple decision tree as such increasing feasibility, while maintaining high accuracy. Prehospital prospective validation is planned.
Keywords: Acute ischemic stroke, clinical scale, endovascular thrombectomy, intra-arterial thrombectomy, large vessel occlusion, prehospital
Introduction
Time is the most crucial factor limiting clinical effectiveness of intra-arterial thrombectomy (IAT) in stroke due to large anterior vessel occlusion (LAVO).1,2 With every minute of delay, 4.2 days of disability-free life are lost, and chances of undergoing IAT are reduced by 2.5%.3,4 A clinical scale to identify LAVO in the prehospital Emergency Medical Services (EMS) setting could reduce treatment delays by allocating IAT-eligible patients directly to a comprehensive stroke center (CSC).5,6 Ideally, such a scale should be straightforward, widely applicable, have high interrater reliability and high accuracy in terms of LAVO-prediction.7 Various scales have been designed, but it is unclear which performs best in clinical practice.8–15 The National Institutes of Health Stroke Score (NIHSS) retains the highest overall accuracy predicting LAVO,16,17 but is too extensive for EMS personnel. The Face-arm-speech-time (FAST) score is widely used by EMS personnel but was primarily developed to distinguish stroke from non-stroke rather than stroke subtype.18
LAVO-prediction scales were compared before, but never systematically, and in different datasets with radiological endpoints not reflecting current clinical practice.14,19,20
Patient characteristics may improve a LAVO-prediction model but were not included in previous scales.21,22
Aims
We aimed to (i) systematically identify published LAVO-prediction scales designed for prehospital use, (ii) assess these scales in terms of feasibility, (iii) assess predictive value in a large, multicenter, prospective dataset with IAT-eligible LAVO as a well-defined radiological outcome measure, (iv) compare these scales to NIHSS and FAST, and finally, (v) develop a prediction model assessing both NIHSS items and patient characteristics associated with LAVO.
Methods
Systematic literature search
A computerized literature search was performed in the following databases: MEDLINE, EMBASE, EMCARE and Web of Science from October 1991 to June 2017 using the following search terms: “stroke,” “cerebrovascular accident,” “scales,” “scores,” “large vessel occlusion,” “large artery occlusion,” “Emergency Medical Services,” “prehospital” and “triage.” Two reviewers (GTK and TTMN) independently screened titles and abstracts for eligibility. Full-text versions were obtained from all studies that were considered to be potentially relevant by one or both reviewers. After a first selection, bibliographies of all relevant studies were searched manually for additional studies and this method of crosschecking was continued until no further publications were found. Authors of relevant articles were contacted for supplementary information.
Cohort studies were reviewed with the STROBE (Strengthening the Reporting of OBservational studies in Epidemiology) statement and had to comply with the following inclusion criteria: (1) original data report on an inception cohort or a clinical trial; (2) a clinical score had to be assessed within 6 h from stroke onset; (3) it had to be clear from the paper at what moment and by whom (e.g. EMS personnel, neurologist) a clinical score was assessed; (4) assessment of LAVO had to be done with either CT angiography (CTA), magnetic resonance angiography, or digital subtraction angiography; (5) data available on the performance of clinical score(s) used had to be expressed as: area under the receiver operating characteristics curve (AUROC), sensitivity/specificity or likelihood ratio, and (6) the clinical score had to be retrievable from NIHSS. Because studies had to fulfill these strict inclusion criteria, no further formal quality assessment was undertaken.
We estimated and/or retrieved the following characteristics from identified studies: feasibility for EMS use, interrater reliability and external validity (i.e. applicability to the unselected population of suspected acute stroke patients).
Validation cohort
To assess validity, we used the Dutch acute Stroke study (DUST) cohort.23 DUST is a multicenter, prospective, observational cohort study conducted in six university and eight non-university hospitals in the Netherlands. From May 2009 to August 2013, consecutive patients >18 years presented at the emergency department with a suspicion of acute (<9 h) ischemic stroke (based on clinical assessment and non-contrast CT (NCCT) imaging) and NIHSS >1 and/or considered eligible for intravenous thrombolysis (IVT) were included. All patients received CTA within 9 h after symptom onset as part of the CT stroke work-up including NCCT, CT perfusion and CTA. The DUST imaging protocol has been described before.24
Analysis
Patients with CTA yielding insufficient diagnostic quality to assess LAVO were excluded. We defined LAVO according to current IAT-eligible criteria: proximal middle cerebral artery (MCA: M1- and/or M2-segment), proximal anterior cerebral artery (ACA: A1- and/or A2-segment), intracranial carotid artery (ICA) or tandem (ICA plus MCA) occlusion.25 Patients with incomplete admission NIHSS were excluded from analyses related to validation of existing scales, since NIHSS was required to reconstruct these.
Descriptive statistics were used to determine baseline characteristics of the validation cohort. Categorical variables were compared with the χ2 test and presented as number (percentage). Continuous variables are compared using the t test or Mann–Whitney U test, and are presented as mean ± standard deviation or median (interquartile range, IQR) if appropriate.
To assess predictive value, we computed AUROC and respective 95% confidence intervals (CIs) per identified LAVO-prediction scale, and for the NIHSS and FAST score.
Having data from 14 sites participating in DUST, we performed external validation by excluding one site at a time (cross-validation) for every scale. This is an important advantage, because external validation gives a better indication of the generalization error. We performed all pairwise comparisons of the cross-validated AUROCs of the various scales using the DeLong’s test.26
For the development of a new prediction model, we did not exclude patients with NIHSS items that could not be assessed since this reflects clinical practice. In addition, we introduced (combinations of) patient characteristics into the model that we considered to be predictive of LAVO provided that these also differed on baseline between LAVO and non-LAVO patients. These include: history of atrial fibrillation (AF), AF without the use of anticoagulation, and AF without diabetes mellitus and/or hyperlipidemia.
Group lasso regression analysis was used to reveal (a combination of) NIHSS items and patient characteristics yielding the highest predictive value for LAVO.27 The lasso is a popular method for penalized regression and classification that also performs variable selection.28 The group lasso is a variant where the user can specify groups of variables (e.g. all variables within one NIHSS item) that are either all in or all out of the model.29 We used the R package “grpreg” with default settings to fit the group lasso.30
In addition, a decision tree algorithm and diagram based on dichotomized NIHSS items ((1) ‘yes/present/abnormal’, or (0) ‘no/absent/normal’) was developed. A decision tree works by consecutively assessing the item with the highest predictive value in the (remaining) cohort, as such leading to a minimum amount of items to be assessed to reach an outcome (i.e. LAVO or non-LAVO), with the highest possible predictive value. Cross-validation (as described before) will determine the number of knots in the decision tree. The decision tree was fitted using the R-package “rpart” using default settings. In particular, this means that the default priors are proportional to the data counts, the losses default to 1, and the split defaults to the Gini index.
Statistical analysis was performed using SPSS software (version 23, IBM, New York, USA), and R software (version 3.4.1).
Results
Systematic literature search
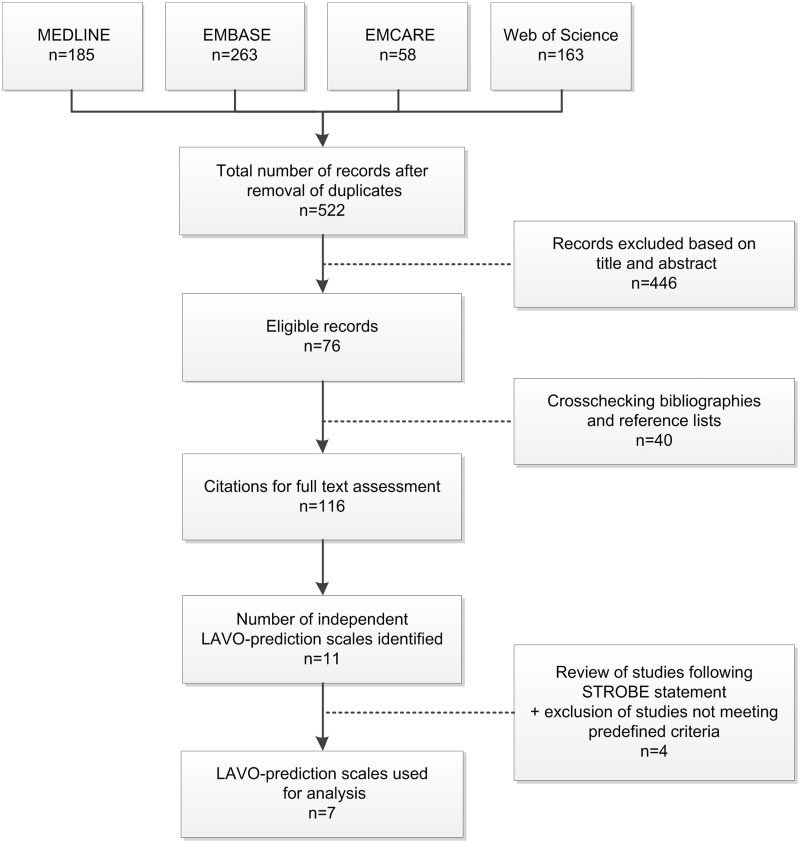
The MEDLINE search yielded 185 citations, the EMBASE search 263 citations, the EMCARE search 58 citations, and the Web of Science search 163 citations. After removal of duplicates, 522 records remained; 446 records were excluded based on title and abstract; 28 additional relevant studies were found by searching the bibliographies. Screening reference lists and a search of the Science Citation Index yielded 12 additional studies. One-hundred-and-sixteen citations remained for full text assessment. A total of seven clinical scales meeting pre-defined criteria were identified (see Figure 1). Clinical scale characteristics and methods of validation are shown in Supplementary Material I. Except for the RACE scale, all validations were performed retrospectively and/or in-hospital and validation cohorts ranged between 62 and 3505 patients. Generally, patients with intracerebral hemorrhage (ICH) were excluded. Definition of LAVO varied substantially between studies (ranging from ‘MCA occlusion’ to ‘anterior or posterior circulation occlusion’), and LAVO-rate ranged between 21 and 73%.
Figure 1.
Flowchart systematic literature search. LAVO: large anterior vessel occlusion; STROBE: strengthening the reporting of observational studies in epidemiology.
Validation cohort
A total of 1393 patients were included in DUST. Of these, 59 (4%) were excluded because of incomplete NIHSS and 18 (1%) because CTA was of insufficient diagnostic quality to assess LAVO. This left 1316 patients for analysis.
LAVO was present in 471 patients (35.8%). Demographic details of the validation cohort, stratified by presence of LAVO, are presented in Table 1. LAVO-patients were similar in age and sex compared to non-LAVO patients. AF was more prevalent in LAVO-patients, whereas other cardiovascular risk factors (previous stroke, hyperlipidemia) and antiplatelet therapy were more prevalent in non-LAVO patients. LAVO-patients had higher baseline NIHSS compared to non-LAVO patients; NIHSS 12 [IQR 7–17] versus NIHSS 4 [2–7] were more frequently treated with IVT, and onset-to-needle time was shorter; 97 [72–140] min in LAVO-patients versus 115 [85–170] in non-LAVO patients. Median systolic blood pressure was lower in LAVO-patients: 150 mmHg [133–167] versus 157 mmHg [140–180] in non-LAVO patients.
Table 1.
Baseline characteristics of DUST validation cohort
| Total patients n = 1316 | LAVO n = 471 (36%) | non-LAVO n = 845 (64%) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 69 [57–78] | 68 [55–77] | 69 [58–78] | 0.14 |
| Male sex | 752 (57) | 264 (56) | 488 (58) | 0.55 |
| Medical history | ||||
| Atrial fibrillation, 18 missings | 168 (13) | 72 (15) | 96 (11) | 0.04 |
| Atrial fibrillation without anticoagulation, 27 missings | 88 (7) | 44 (9) | 44 (5) | <0.01 |
| Diabetes mellitus, 11 missings | 198 (15) | 59 (13) | 139 (16) | 0.05 |
| Previous stroke, 12 missings | 316 (24) | 82 (17) | 234 (28) | <0.01 |
| Hypertension, 16 missings | 680 (52) | 238 (51) | 442 (52) | 0.43 |
| Hyperlipidemia, 41 missings | 433 (33) | 135 (29) | 298 (35) | 0.01 |
| Coronary artery disease, 42 missings | 242 (18) | 79 (17) | 163 (19) | 0.48 |
| Medication on admission | ||||
| Anticoagulation, 9 missings | 154 (12) | 49 (10) | 105 (12) | 0.54 |
| Antiplatelet therapy, 10 missings | 443 (34) | 141 (30) | 302 (36) | 0.07 |
| Clinical parameters on admission | ||||
| Systolic blood pressure (mmHg), 11 missings | 154 [138–177] | 150 [133–167] | 157 [140–180] | <0.01 |
| Diastolic blood pressure (mmHg), 11 missings | 85 [75–95] | 82 [72–96] | 85 [75–95] | 0.02 |
| Glucose (mmol/L), 20 missings | 6.6 [5.8–8.1] | 6.6 [5.9–7.8] | 6.6 [5.7–8.1] | 0.43 |
| NIHSS at admission | 6 [3–12] | 12 [7–17] | 4 [2–7] | <0.01 |
| Reperfusion therapy | ||||
| Intravenous thrombolysis | 815 (62) | 331 (70) | 484 (57) | <0.01 |
Note: Values are expressed as median [interquartile range] for continuous variables unless stated otherwise and as absolute counts (percentage) for categorical variables.
LAVO: large anterior vessel occlusion; NIHSS: National Institutes of Health Stroke Scale.
Comparison of clinical scales
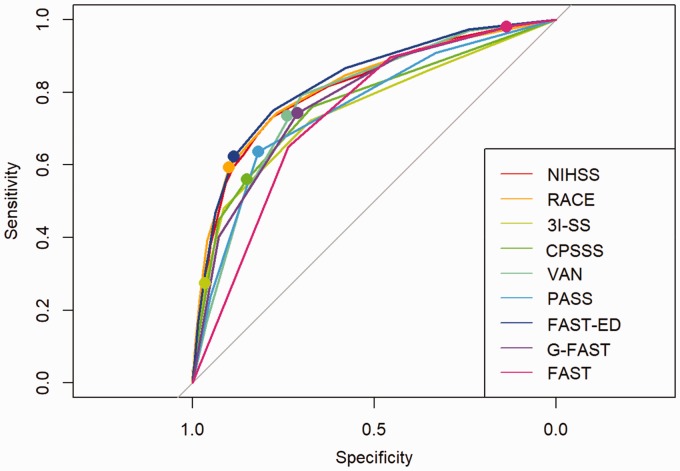
The FAST-ED (AUROC 0.83, 95% CI 0.80–0.85), RACE scale (AUROC 0.82, 95% CI 0.79–0.84) and NIHSS (AUROC 0.81, 95% CI 0.79–0.84) showed the highest AUROC for detecting LAVO in comparison with other scales (p < 0.01). FAST-ED showed a comparable AUROC to RACE but a significantly higher AUROC than NIHSS (p < 0.01). The FAST score showed the lowest specificity, and the 3I-SS scale showed the lowest sensitivity (see Figure 2 and Table 2).
Figure 2.
Receiver operating characteristics (ROC) curves of identified LAVO-prediction scales, and the NIHSS and FAST score. For every LAVO-prediction scale, the marked point in the ROC indicates the combination of sensitivity and specificity at the original authors’ recommended cut-off point. 3I-SS: 3-item stroke scale; CPSSS: Cincinnati prehospital stroke severity scale; FAST: Face-arm-speech-time; FAST-ED: Face-arm-speech-time-eye deviation-denial/neglect; G-FAST: Gaze-face-arm-speech-time; NIHSS: National institutes of health stroke scale; PASS: Prehospital acute stroke severity; RACE: Rapid arterial occlusion evaluation; VAN: Vision aphasia neglect.
Table 2.
AUROCs and respective 95%-CIs with corresponding p-values comparing identified LAVO-prediction scales, NIHSS and FAST
| Clinical scale | AUC (95% CI) | FAST | 3I-SS | PASS | CPSSS | G-FAST | VAN | NIHSS | RACE | FAST-ED |
|---|---|---|---|---|---|---|---|---|---|---|
| FAST | 0.74 (0.71–0.76) | X | ||||||||
| 3I-SS | 0.75 (0.72–0.78) | 0.25 | X | |||||||
| PASS | 0.76 (0.73–0.78) | 0.10 | 0.55 | X | ||||||
| CPSSS | 0.76 (0.74–0.79) | 0.04 | 0.08 | 0.31 | X | |||||
| G-FAST | 0.78 (0.76–0.81) | <0.01 | <0.01 | <0.01 | 0.12 | X | ||||
| VAN | 0.78 (0.76–0.81) | <0.01 | <0.01 | <0.01 | 0.09 | 0.79 | X | |||
| NIHSS | 0.81 (0.79–0.84) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | X | ||
| RACE | 0.82 (0.79–0.84) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.34 | X | |
| FAST-ED | 0.83 (0.80–0.85) | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.17 | X |
3I-SS: 3-item stroke scale; CPSSS: Cincinnati prehospital stroke severity scale; FAST: Face-arm-speech-time; FAST-ED: Face-arm-speech-time-eye deviation-denial/neglect; G-FAST: Gaze-face-arm-speech-time; NIHSS: National institutes of health stroke scale; PASS: Prehospital acute stroke severity; RACE: Rapid arterial occlusion evaluation; VAN: Vision aphasia neglect.
Group lasso LAVO-prediction model
Group lasso analysis showed a prediction model containing a combination of the following NIHSS items (AUROC 0.84, 95% CI 0.81–0.87): (1) level of consciousness (LOC) questions, (2) gaze, (3) visual fields, (4) facial asymmetry, (5) arm motor function, (6) aphasia, and (7) extinction/inattention. Whereas AF was more prevalent in LAVO-patients, it did not contribute to the prediction model as a separate variable or in combination with other patient characteristics as outlined before.
Decision tree
Figure 3 displays the GACE (Gaze, facial Asymmetry, level of Consciousness, Extinction/inattention) decision tree. The GACE decision tree enables prediction of LAVO by assessment of a maximum of only four predefined dichotomized NIHSS items with an AUROC of 0.76 (95% CI 0.68–0.83). ‘Gaze’, the item with the highest predictive value for LAVO in our cohort, is the first item to be assessed. For both the group of patients with an abnormal gaze (27%, left side of the diagram) as for the group of patients with a normal gaze (73%, right side of diagram), the following item with the highest predictive value for LAVO is determined. For both subgroups, this item is ‘facial asymmetry’. Assessment of this second item leads directly to an outcome (i.e. LAVO or non-LAVO) in 61% of all patients (scoring (a) gaze ‘yes’ plus facial asymmetry ‘yes’, (b) gaze ‘yes’ plus facial asymmetry ‘no’, or (c) gaze ‘no’ plus facial asymmetry ‘no’). Only for the remaining 39% of patients, the full 4-item decision tree (adding ‘LOC questions’, followed by ‘LOC commands’ or ‘extinction/inattention’) has to be completed (see Figure 3 and Table 3).
Figure 3.
GACE decision tree diagram based on dichotomized NIHSS items (assessed in DUST). Numbers on the left side of each bottom box indicate the number of patients (percentage) with a LAVO outcome, whereas numbers on the right side of each bottom box indicate the number of patients (percentage) with a non-LAVO outcome. The color of each box indicates the ratio of LAVO (green) and non-LAVO patients (blue): the higher intensity of the color, the higher the ratio. 830 patients (61%, group a, b and c) reach an outcome (i.e. LAVO or non-LAVO) after assessment of only 2 items. LAVO: large anterior vessel occlusion; LOC: level of consciousness.
Table 3.
Number of patients (%) reaching a LAVO/non-LAVO outcome per number of completed items within GACE
| Number of completed items | Total patients (n = 1370) |
|---|---|
| 2 items i.e. (i) gaze,(ii) facial asymmetry | 830 (60.6%) |
| 4 items i.e. (i) gaze, (ii) facial asymmetry, (iii) LOC questions, (iv) LOC commands or extinction/inattention a | 540 (39.4%) |
LOC: level of consciousness.
Dependent on result of assessment of item iii.
Discussion
Our systematic search revealed seven LAVO-prediction scales designed for use in the prehospital phase. However, the majority was retrospectively validated in (small) monocenter cohorts in an in-hospital setting, making it difficult to determine which scale outperforms the other in prehospital clinical practice.
In our large multicenter validation cohort, we found that FAST-ED and RACE had the highest AUROC for prediction of LAVO. A seemingly important advantage of RACE over FAST-ED is that it was validated in the prehospital setting. Nevertheless, RACE appears too complex for prehospital EMS use, comprising a 5-item, 9-point assessment in which the decision to use or omit certain scale items (i.e. agnosia, aphasia) depends on the assumed involved hemisphere.9 Indeed, during the validation phase, the scale was not performed in 40% of suspected stroke patients.9 Although FAST-ED is based on the widely used FAST score and outperforms the NIHSS for prediction of LAVO in our database, it has potential drawbacks as it (a) imposes scoring a ‘partial’, ‘mild’ or ‘moderate’ deficit in most items, hampering interrater reliability; and (b) uses complex items (e.g. extinction/inattention) which are difficult for EMS personnel to assess.13,31
G-FAST seems more feasible for EMS use. However, in the original G-FAST study (i) vessel imaging modality to detect LAVO is unclear, and more importantly, (ii) definition of LAVO does not meet current clinical IAT-criteria (excluding ACA and M2 occlusion).14
In our cohort, as expected, AF was more common in LAVO-patients. Although neither this nor other patient characteristics improved the group lasso model, the model including seven NIHSS-items had a higher AUROC (0.84) than the scales derived from the literature.
Despite significant differences in performance of the scales, it should be pointed out that many of these differences are associated with small absolute differences in AUROC (i.e. 0.02). We accepted a small reduction in AUROC for the GACE decision tree (compared with the group lasso model), as we estimate that the prehospital feasibility is high since EMS personnel only has to take two steps to rule out transportation to a CSC for a substantial proportion of patients (61%, see Table 3), and only four for the remainder.
From a clinical perspective, it seems remarkable that facial asymmetry is such an important scoring item for GACE since it appears to have little localizing value.32 It is important, however, to bear in mind that it is not this separate item, but the combination with gaze assessment that leads to a high predictive value for LAVO in our cohort.
In addition to LAVO prediction, allocation decision also depends on (1) the impact of delay on clinical efficacy of both IVT and IAT, (2) patient characteristics (e.g. medical history, time from symptom onset, course of the disease) and (3) logistic factors (e.g. urban/rural area, number of comprehensive and primary stroke centers (PSC) and distance to scene of stroke, inter-hospital distance, in-hospital door-to-needle and door-to-groin times).33 Therefore, we chose to display ROCs, enabling determination of a clinically relevant cut-off point considering local circumstances.
Moreover, allocation decision highly depends on what kind of error one is willing to allow: (a) having more patients come to a CSC accepting that some of these may not have LAVO and incorrectly bypass a PSC delaying IVT (false-positives); or (b) being focused on only allocating LAVO-patients to a CSC accepting that some LAVO-patients will primarily be transported to a PSC without IAT-facilities (false-negatives).
For example, a 75-year-old patient presenting with a partial gaze palsy, facial asymmetry, dysarthria and moderate left hemiparesis is assessed by EMS personnel 2.5 h after symptom onset. Scores for this patient on the best performing LAVO-prediction scales in our validation cohort are: RACE 4/9, FAST-ED 2/13 and G-FAST 4/4. When applying the original authors’ cut-off point, the patient has a moderate to high chance of LAVO, advising direct transport to a CSC with G-FAST, and a transport to the nearest PSC with RACE and FAST-ED. These scales, however, do not take into account local circumstances.
Consider that a PSC is located 10 min- and a CSC 20 min from scene of stroke (with equal door-to-needle times). Bypassing the PSC is associated with a 10-min delay to IVT but a more substantial time delay to IAT is avoided by preventing inter-hospital transfer. Keeping in mind that IVT has limited efficacy in LAVO-patients,25,34–39 a scale with a high sensitivity (such as G-FAST) seems the more desirable for this specific situation.
However, when transport time to a PSC is only 10 and to a CSC is 50 min, a scale with a high sensitivity is less desirable. Most patients (including false-positives) will then be transported to the CSC with a more substantial time delay to IVT (40 min) for non-LAVO patients and, in addition, overloading the CSC with a large volume of patients. Therefore, in this situation, a scale with a high specificity (such as RACE or FAST-ED) would probably be the more desired choice.
Overall, time is brain, but since LAVO-patients appear to clinically benefit more substantially from earlier IAT than overall stroke patients benefit from earlier IVT,3,40 a moderate to high likelihood of LAVO seems to allow a fair time delay to IVT. How much delay exactly, however, remains complex, as logistics (which are dynamic as resources shift over time) determine the amount of accepted time delay at expense of the number of false-positively referred patients. To what extent implementation of a clinical LAVO-prediction scale affects local logistics and health care-related costs has to be estimated, since no formal cost-effectiveness analysis was performed.
Our study has several limitations. First, our study was performed retrospectively which could have led to selection bias. Data, however, were collected prospectively minimizing such an effect. Second, our cohort does not represent an unselected prehospital cohort. For example, ICHs were not included, leading to an artificially high prevalence of LAVO and IVT-treated patients, which could result in an overestimation of the prediction scales. To what extent this influences a decision to use a scale depends very much on local circumstances since ICHs are often concentrated in CSCs.
Of note, the retrospective nature and lack of an unselected cohort account for all LAVO-prediction scales included in our analysis and therefore do not diminish validity of between scale comparisons.
Finally, clinical scale assessment was performed in the in-hospital setting, rendering translation to the prehospital setting limited. Indeed, prospective validation in this setting is much warranted and our results should primarily be considered an important step towards a large prehospital prospective validation study which we planned to embed in the ongoing ‘A Reduction in Time with Electronic Monitoring in Stroke’ (ARTEMIS) trial conducted within three EMS regions, which allows patients to be electronically tracked from the first moment the dispatch office is alarmed up until start of reperfusion therapy (clinicaltrials.gov identifier: NCT02808806).41
Nevertheless, clinical LAVO-detection could also be very helpful in order to optimize in-hospital logistics of potential IAT-eligible patients (e.g. pre-notification of neuro-interventional team and preparation of the angio suite can reduce door-to-groin times).42
Conclusion
We identified seven LAVO-prediction scales of which FAST-ED and RACE performed best and comparable to the NIHSS. An important limitation remains; however, that prospective validation in the prehospital EMS setting is lacking.
We developed a practical and easy-to-use decision tree that utilizes only two dichotomized NIHSS items for LAVO prediction for 61% of patients, and four items for the remaining patients in our cohort. Prospective validation of GACE in the prehospital setting is planned.
Supplemental Material
Supplemental material for Clinical prediction of thrombectomy eligibility: A systematic review and 4-item decision tree by Gaia T Koster, T Truc My Nguyen, Erik W van Zwet, Bjarty L Garcia, Hannah R Rowling, J Bosch, Wouter J Schonewille, Birgitta K Velthuis, Ido R van den Wijngaard, Heleen M den Hertog, Yvo BWEM Roos, Marianne AA van Walderveen, Marieke JH Wermer and Nyika D Kruyt in International Journal of Stroke
Acknowledgements
The authors would like to thank the Dutch acute Stroke study (DUST) investigators for acquisition and provision of the DUST data.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by The Netherlands Brain Foundation (project number HA2015.01.02) and The Dutch Health Care Insurers Innovation Foundation (project number 3240). The original study (DUST) was funded by The Netherlands Heart Foundation (grant numbers 2008 T034 and 2012 T061) and The Nuts Ohra Foundation (grant number 0903–012).
Authors’ contributions
GTK, EWvZ, MJHW and NDK created the study concept and design. GTK and TTMN performed literature search. GTK, HRR, WJS, BKV, YBWEMR, MAAvW, MJHW, NDK acquired data. GTK, TTMN, EWvZ, MJHW and NDK analysed and interpreted data. GTK and NDK drafted the original manuscript. GTK and TTMN prepared figures and tables. All authors critically revised the manuscript for intellectual content.
References
- 1.Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to reperfusion and treatment effect for acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2016; 73: 190–196. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Jadhav AP, Bonafe A, et al. Analysis of Workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology 2016; 279: 888–897. [DOI] [PubMed] [Google Scholar]
- 3.Meretoja A, Keshtkaran M, Tatlisumak T, Donnan GA, Churilov L. Endovascular therapy for ischemic stroke: save a minute-save a week. Neurology 2017; 88: 2123–2127. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S, Ward E, John S, et al. Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke 2011; 42: 1626–1630. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 7.Michel P. Prehospital Scales for large vessel occlusion: closing in on a moving target. Stroke 2017; 48: 247–249. [DOI] [PubMed] [Google Scholar]
- 8.Singer OC, Dvorak F, du Mesnil de Rochemont R, Lanfermann H, Sitzer M, Neumann-Haefelin T. A simple 3-item stroke scale: comparison with the National Institutes of Health Stroke Scale and prediction of middle cerebral artery occlusion. Stroke 2005; 36: 773–776. [DOI] [PubMed] [Google Scholar]
- 9.Perez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke 2014; 45: 87–91. [DOI] [PubMed] [Google Scholar]
- 10.Katz BS, McMullan JT, Sucharew H, Adeoye O, Broderick JP. Design and validation of a prehospital scale to predict stroke severity: Cincinnati prehospital stroke severity scale. Stroke 2015; 46: 1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teleb MS, Ver Hage A, Carter J, Jayaraman MV, McTaggart RA. Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. J Neurointerv Surg 2017; 9: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastrup S, Damgaard D, Johnsen SP, Andersen G. Prehospital Acute stroke severity scale to predict large artery occlusion: design and comparison with other scales. Stroke 2016; 47: 1772–1776. [DOI] [PubMed] [Google Scholar]
- 13.Lima FO, Silva GS, Furie KL, et al. Field assessment stroke triage for emergency destination: a simple and accurate prehospital scale to detect large vessel occlusion strokes. Stroke 2016; 47: 1997–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheitz JF, Abdul-Rahim AH, MacIsaac RL, et al. Clinical selection strategies to identify ischemic stroke patients with large anterior vessel occlusion: results from SITS-ISTR (safe implementation of thrombolysis in stroke international stroke thrombolysis registry). Stroke 2017; 48: 290–297. [DOI] [PubMed] [Google Scholar]
- 15.Nazliel B, Starkman S, Liebeskind DS, et al. A brief prehospital stroke severity scale identifies ischemic stroke patients harboring persisting large arterial occlusions. Stroke 2008; 39: 2264–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke 2013; 44: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 17.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005; 36: 2121–2125. [DOI] [PubMed] [Google Scholar]
- 18.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke 2003; 34: 71–76. [DOI] [PubMed] [Google Scholar]
- 19.Turc G, Maier B, Naggara O, et al. Clinical Scales do not reliably identify acute ischemic stroke patients with large-artery occlusion. Stroke 2016; 47: 1466–1472. [DOI] [PubMed] [Google Scholar]
- 20.Heldner MR, Hsieh K, Broeg-Morvay A, et al. Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible? J Neurol 2016; 263: 1633–1640. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto Y, Sato S, Kuronuma Y, Nagatsuka K, Minematsu K, Toyoda K. Factors associated with proximal carotid axis occlusion in patients with acute stroke and atrial fibrillation. J Stroke Cerebrovasc Dis 2014; 23: 799–804. [DOI] [PubMed] [Google Scholar]
- 22.Vanacker P, Heldner MR, Amiguet M, et al. Prediction of Large vessel occlusions in acute stroke: National Institute of Health Stroke Scale is hard to beat. Crit Care Med 2016; 44: e336–e343. [DOI] [PubMed] [Google Scholar]
- 23.van Seeters T, Biessels GJ, Kappelle LJ, et al. The prognostic value of CT angiography and CT Perfusion in acute ischemic stroke. Cerebrovasc Dis 2015; 40: 258–269. [DOI] [PubMed] [Google Scholar]
- 24.van Seeters T, Biessels GJ, van der Schaaf IC, et al. Prediction of outcome in patients with suspected acute ischaemic stroke with CT perfusion and CT angiography: the Dutch acute stroke trial (DUST) study protocol. BMC Neurol 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 27.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 2008; 9: 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996; 58: 267–288. [Google Scholar]
- 29.Meier L, Geer SVD, Bühlmann P. The group lasso for logistic regression. J R Stat Soc Series B Stat Methodol 2008; 70: 53–71. [Google Scholar]
- 30.Breheny P, Huang J. Group descent algorithms for nonconvex penalized linear and logistic regression models with grouped predictors. Stat Comput 2015; 25: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesinger MR, Sequeira DJ, Buffalini S, Guyette FX. Comparing National Institutes of Health Stroke Scale among a stroke team and helicopter emergency medical service providers. Stroke 2015; 46: 575–578. [DOI] [PubMed] [Google Scholar]
- 32.Brazis PW, Masdeu JC, Biller J. Cranial nerve VII (the facial nerve). In: Brazis PW, Masdeu JC, Biller J. (eds). Localization in clinical neurology, 6th ed The Netherlands: Wolters Kluwer Health, 2012, pp. 321–340. [Google Scholar]
- 33.Milne MS, Holodinsky JK, Hill MD, et al. Drip ‘n Ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke 2017; 48: 791–794. [DOI] [PubMed] [Google Scholar]
- 34.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 35.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 36.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 37.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 38.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 39.Badhiwala JH, Nassiri F, Alhazzani W, et al. Endovascular thrombectomy for acute ischemic stroke: a meta-analysis. JAMA 2015; 314: 1832–1843. [DOI] [PubMed] [Google Scholar]
- 40.Meretoja A, Keshtkaran M, Saver JL, et al. Stroke thrombolysis: save a minute, save a day. Stroke 2014; 45: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 41.Koster GT, Nguyen TTM, Groot AED, et al. A Reduction in Time with Electronic Monitoring In Stroke (ARTEMIS): study protocol for a randomised multicentre trial. BMJ Open 2018; 8: e020844. [DOI] [PMC free article] [PubMed]
- 42.Mehta BP, Leslie-Mazwi TM, Chandra RV, et al. Reducing door-to-puncture times for intra-arterial stroke therapy: a pilot quality improvement project. J Am Heart Assoc 2014; 3: e000963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Clinical prediction of thrombectomy eligibility: A systematic review and 4-item decision tree by Gaia T Koster, T Truc My Nguyen, Erik W van Zwet, Bjarty L Garcia, Hannah R Rowling, J Bosch, Wouter J Schonewille, Birgitta K Velthuis, Ido R van den Wijngaard, Heleen M den Hertog, Yvo BWEM Roos, Marianne AA van Walderveen, Marieke JH Wermer and Nyika D Kruyt in International Journal of Stroke