Abstract
Cervical insufficiency is a medical condition of pregnancy which causes the cervix to soften, shorten and dilate before full term, typically between 18 and 22 weeks of gestation, such that a preterm birth occurs. It is a common cause of second trimester pregnancy loss. Before meeting the diagnostic criteria, a patient that experiences early cervical remodelling or the development of a short cervix may receive surgical intervention. Once detected, the typical treatment is a cerclage procedure (a purse string suture to close the cervix) and progesterone medication. There are conflicting studies on the efficacy of the cerclage procedure, with conclusions drawn from clinical evidence as opposed to mechanical properties. The purpose of this study is to understand the mechanical limitations of the cerclage procedure. Working with physicians at George Washington University School of Medicine and Health Sciences, we created generalized three dimensional models of the cervix from ultrasound images. To fabricate the synthetic cervices, we used a silicone rubber to mimic the qualitative feel of the cervix according to collaborating physicians. Using this qualitative information, we performed material testing for quantitative analysis. The synthetic cervices were then sutured using clinical techniques. The maximum force required for the synthetic tissue to rupture due to the cerclage stitch was recorded. The impact of material softness on the integrity of the cerclage is investigated.
Keywords: cervical insufficiency, reproductive biomechanics, human reproduction, biomechanics
1. Introduction
The cervix is a dynamic organ, drastically changing from the beginning to the end of pregnancy. During pregnancy, the cervix must remain closed and firm while the fetus develops (figure 1). Towards the end of pregnancy [1], the cervix softens, shortens and dilates for a successful delivery. Cervical insufficiency is used to describe the inability of the uterine cervix to retain a pregnancy in the absence of the signs and symptoms of clinical contractions, or labour, or both in the second trimester [2]. Between 9 and 12% [3,4] of births worldwide are preterm, defined as delivery before 37 weeks gestation. Often these deliveries result in significant neonatal complications [5] and prolonged hospital stays. Understanding the causes of preterm birth is an important step to minimizing the rate of incidence. While the contribution of cervical insufficiency to preterm birth is not known [6], some studies estimate the rate of incidence to be 1% of all births [7].
Figure 1.
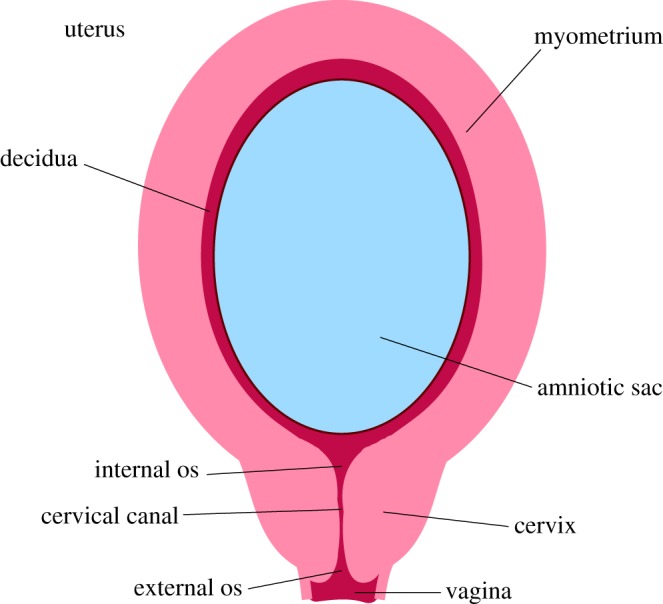
This figure highlights important anatomical features of a uterus and cervix during pregnancy. (Online version in colour.)
This condition can be detected by a combination of transvaginal ultrasound, clinical examination and patient history [8]. Even though cervical insufficiency is a leading cause of premature delivery, no cure for the condition exists and the standard treatment procedure yields varying results. Prolonging the pregnancy is the most important aspect of managing this condition [9] as the survival rate of preterm birth increases dramatically between 23 weeks (23%) and 25 weeks (54%) [10]. Therefore, understanding the cases in which a cerclage will be mechanically more successful could improve treatment decisions.
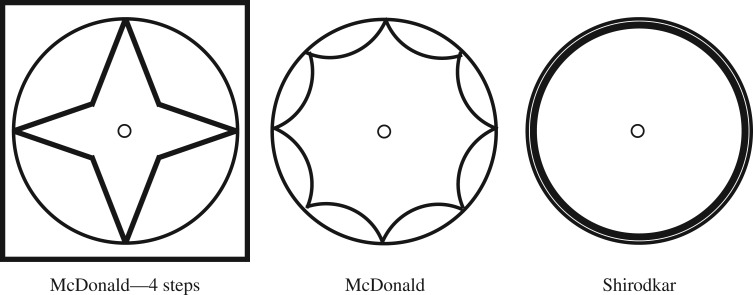
Once cervical insufficiency is recognized, the most common treatment plan is to give progesterone supplementation (hydroxyprogesterone caproate) and to perform a cervical cerclage. The cervical cerclage involves stitching the cervix with a purse string suture to pull the cervix closed. There are different techniques used for performing the cervical cerclage; the two primary are the McDonald and Shirodkar, diagramed in figure 2.
Figure 2.
This figure diagrams the suture method for three cerclage protocols. In this study, the physician used a 4 step McDonald method (far left and boxed).
The McDonald cerclage is begun by grasping the anterior and posterior lips of the cervix with ring forceps. Next, a curved needle loaded with a large calibre or absorbable synthetic suture is inserted at 12 o’clock, at the junction of the rugated vaginal epithelium and the cervix distal to the vesicocervical reflection and at least 2 cm above the external orifice.
Around four to six passes of a purse string suture are taken circumferentially around the entire cervix, avoiding the bladder, rectum and uterine vessels. Each pass extends midway into the cervical stroma to reduce the risk that the suture will pull out over time, but should not enter the endocervical canal. The two ends of the suture are then tied securely and cut, leaving the ends long enough to grasp with a clamp when it is time to remove it.
The Shirodkar procedure is more complicated because it requires incisions and dissection of the paracervical area. Similarly to the McDonald, the cervix is pulled toward the surgeon with ring forceps. A scalpel is used to make a 1–3 cm incision on the posterior cervix at the junction of the rugated vaginal epithelium and the smooth cervix. After the posterior incision is made, a similar transverse incision is made anteriorly.
The rectum is then bluntly dissected off the posterior cervix and the bladder is bluntly dissected off of the anterior cervix. Two blunt needles premounted with a single 5 mm Mersilene tape for the cerclage are used. The tip of one needle is introduced anteriorly at the lateral edge of the incision at the level of the internal os and threaded submucosally adjacent to the cervical stroma to emerge at the lateral edge of the posterior incision at the level of the internal os. The procedure is then repeated on the opposite side and the two ends are tied tightly using four to seven square knots.
Many studies have been done to examine the impact of suture techniques, treatment options and condition progression (i.e. how dilated the cervix is at the time the cerclage is performed) on the success of the pregnancy [11–15]. However, all of these studies rely on clinical investigations to draw conclusions. Biomechanical investigations into cervical tissue, the role of the cervix throughout pregnancy and mechanical aspects of the cerclage stitch are limited. Some work has been done on the mechanical and biochemical properties of cervical tissue ex vivo [16,17] providing valuable insight into the tissue complexity. The dynamic nature of the cervix means ex vivo specimens and tissue samples of hysterectomy patients may not have the same material properties as a softening, pregnant cervix. Recently, in vivo evaluations of a pregnant cervix have had success in determining uterine cervical stiffness [1,18].
In this study, we develop a synthetic model of the cervix to investigate the role of material softness on cerclage integrity. Working with physicians at George Washington University School of Medicine and Health Sciences, we move from ultrasound images to a generalized model of the cervix and incorporate qualitative analysis in designing three geometrically identical cervices with different material softness. After fabricating the synthetic cervices, cerclage operations are performed by trained physicians. Force is applied to the stitched samples until the suture ruptures and a relationship between maximum force and tissue softness is determined.
2. Methods
2.1. Fabricating synthetic cervices
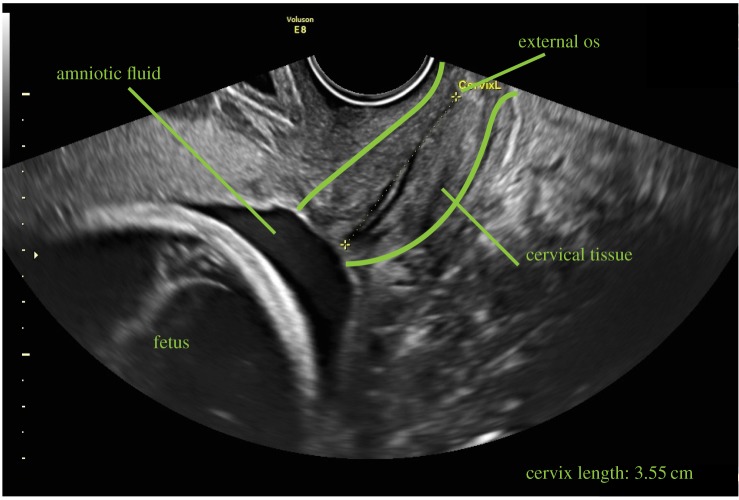
A model for the cervix was generated from ultrasound images (figure 3) by extracting relevant geometric information. From a single patient’s ultrasound images of a 20 week scan of the cervix, we identified the contours of the cervical tissue and cervical canal. Each image provided a different view of the cervix and was imported into Solidworks (Dassault Systèmes SE, Vélizy-Villacoublay, France). We used the select feature to isolate the pieces of the ultrasound image relevant to our model creation. Then we traced the contours to create splines of the tissue outline. The length of the cervix was measured during the scan. Stitching the splines of different images together, a patient-specific, three-dimensional model of the cervix was created.
Figure 3.
This image shows an ultrasound of a patient at 20 weeks with a normal length cervix. Specific anatomical features such as locations of cervical tissue, amniotic fluid and fetus are labelled in the figure. (Online version in colour.)
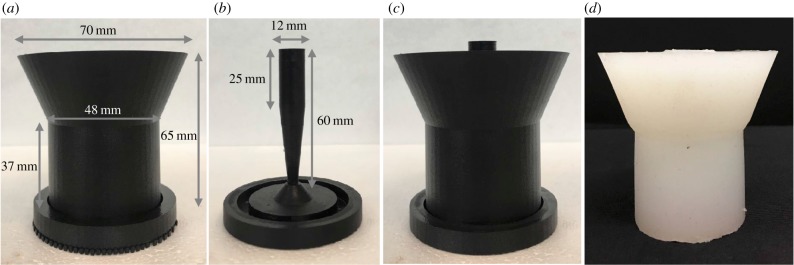
To generalize the model of the cervix, the complex contours, such as those seen in figure 3, were smoothed into a cylinder. Anatomically, the cervix is attached to the pelvic sidewall and the sacrum via the cardinal and uterosacral ligaments. Our model has isolated the cervical tissue away from the surrounding structures and simplified the geometry. The outer diameter of the synthetic cervix is 38 mm and inner diameter varies as described below. The length of the synthetic cervix is 30 mm. This is slightly longer than the physiological length of a short cervix, but will allow investigation into the effects of cervical softness in the current studies while reserving cervical length for future analysis. The cervices are attached to a thicker section of silicone to provide a base for the doctors to perform the cerclage procedure, shown in figure 4a. The inverse of the generalized cervical model was printed with a Markforged Mark Two (MarkForged Inc., Watertown, MA, USA) using onyx material. Onyx is a nylon mixed with chopped carbon fibre, made by MarkForged. This fully assembled three-dimensional printed mould is shown in figure 4c.
Figure 4.
Panel (a) shows the outer piece of the cervical mould; (b) shows the inner section of the cervical mould used in these experiments. Panel (c) shows the outer and inner pieces assembled into one and (d) shows the resulting silicone model removed from the mould after curing. (Online version in colour.)
The inner section of the cervix geometry, corresponding to the cervical canal, can be varied to replicate various stages of cervical softening and dilatation. For these experiments, the middle section has a funnelling diameter where the widest part is 12 mm, shown in figure 4b.
The synthetic cervices were fabricated from EcoFlex 00-30 (SmoothOn, Inc., Macungie, PA, USA). Part A and Part B of EcoFlex 00-30 were mixed together in equal amounts. Silicone thinner was added to this mixture to adjust the material softness. Once the thinner was added, the mixture was placed in a vacuum chamber to remove any air bubbles. After degassing, the mixture was poured into the three-dimensional printed model and left to cure for approximately 8 h. The resulting model can be seen in figure 4d. We created 10 different types of synthetic cervical tissue with increasing softness by varying the percentage of silicone thinner from 0% by volume up to 90%. Because there are no conclusive data on the material parameters of the cervix for women with the condition, we relied on the training of physicians at the George Washington University (GWU) Medical Faculty Associates (MFA) Department of Obstetrics and Gynecology (OBGyn) to asses the synthetic cervical tissue based on their clinical experience. Our physicians were able to qualitatively feel and assess each synthetic cervical tissue and determine which most closely related to a hard (healthy) cervix, a medium and a soft cervix. From this assessment, we determined the percentages of thinner added for a hard (10%), medium (50%) and soft (70%) model of the cervix.
2.2. Model cervix properties
A material testing sweep was conducted on the 10 models of synthetic cervical tissue material to determine the elasticity moduli of the samples given to our physicians. This was done by creating solid cylindrical pieces of silicone with a height of 38 mm and a diameter of 32 mm. These samples were subjected to uniaxial compression testing to find their stress–strain relationships using an MTS Criterion Model 43 (MTS Systems Corporation, Eden Prairie, MN, USA). The load cell was model LPS.503 with a force rating of 5 kN (MTS Systems Corporation). The extension rate was 10 mm min−1. Force on the solid pieces was measured as a function of time.
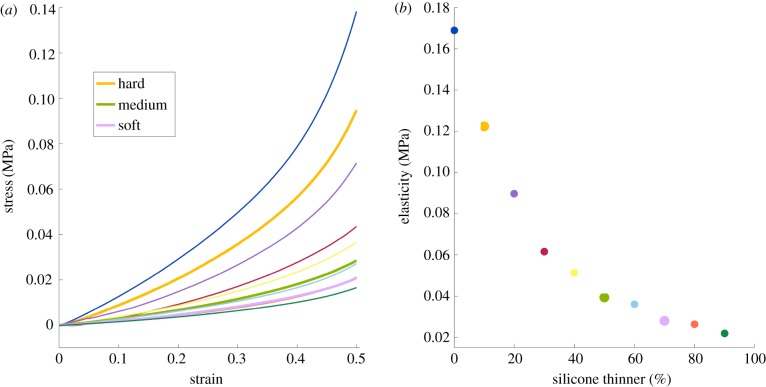
The elastic modulus, E, is a relationship between the stress and strain on the material where E = (σ/ε), with σ as the stress and ε as the strain. From measuring force as a function of time with a known extension rate, we were able to calculate the elastic moduli of the 10 silicone mixture materials by taking the slope of the linear regions on the stress–strain curves (figure 5). These moduli are representative of the silicone behaviour within the operating parameters of the experiment.
Figure 5.
The material testing results of the silicone samples (a) The stress–strain curves for each of the 10 types of silicone tested. (b) The modulus of elasticity of the linear region is reported as a function of silicone thinner added to the sample. (Online version in colour.)
2.3. Cerclage testing
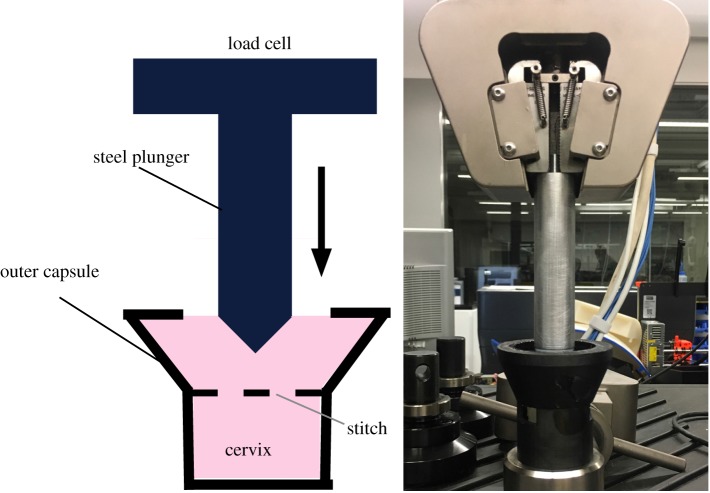
For each of the hard, medium and soft silicone mixtures described above, four complete cervical and base models were created for a total of 12 model cervices. A Maternal Fetal Medicine physician performed a cerclage on each model. The suture was placed at the highest point possible along the cervix, closest to the base. The cerclage technique used was a McDonald procedure with a 5 mm wide Mersilene tape on a taper needle. The needle moved in and out of the cervix at eight points circumferentially around the diameter of the cervix starting at the 12 o’clock position and moving counterclockwise; the two ends of the Mersilene tape were pulled tight and knotted seven to eight times at the 12 o’clock position (figure 2). After the cervices were stitched, they were inserted into a three-dimensional printed plastic capsule to contain the cervix during testing. The cervix is bound by the capsule at the bottom and around the circumference. A custom steel insert with a conical head and a maximum diameter of 25 mm was attached to the compression machine and passed through the silicone base. As the steel insert moves downward through the cervix, it applies force directly to the cerclage stitch until failure (figure 6).
Figure 6.
At left is a schematic of the experimental set-up and at right is a photo of the experiment running. The experimental set-up consists of a custom steel insert with conical end and a maximum diameter of 25 mm attached to a 5 kN load cell. The stitched cervix is bound inside a plastic capsule designed to restrict its lateral movement. The steel insert moves through the cervix, applying force to the stitch until rupture. (Online version in colour.)
The machine used was the MTS Criterion Model 43. The load cell was model LPS.503 with a force rating of 5 kN. The extension rate was 10 mm min−1. As the cerclage suture was compressed by the machine, force was measured and outputted as a function of time. The force trace and the maximum force before the suture ruptures were recorded.
3. Results
3.1. Material testing of synthetic cervices
The material testing for the 10 silicone mixtures shows a clear linear region for all of the mixtures from which a modulus of elasticity can be determined. Figure 5a shows the stress–strain curves for all 10 materials tested. In each case, there is a significant linear region that corresponds to the range of extension experienced during the model cerclage testing. The silicone mixtures physicians identified as being the most similar to hard (10%), medium (50%) and soft (70%) cervical tissue are shown in bold. Figure 5b gives the modulus of elasticity for each mixture, taken from the linear region of each curve. We see that modulus of elasticity is not linear with the percentage of thinner added. Additionally, the values for the hard, medium and soft cervices are E = 0.12, 0.04 and 0.03 MPa, respectively.
3.2. Cerclage procedure
For each cervix, force as a function of time and extension was recorded, shown in figure 7. The averaged force traces as a function of extension over all trials are shown in figure 8. In each case, the amount of extension is limited by the physical set-up, but the amount of force required for rupture is taken to be the maximum force recorded in each test. It is noted that failure occurs because of failure of the cervical model, not the suture; this is a method of clinical cerclage failure.
Figure 7.
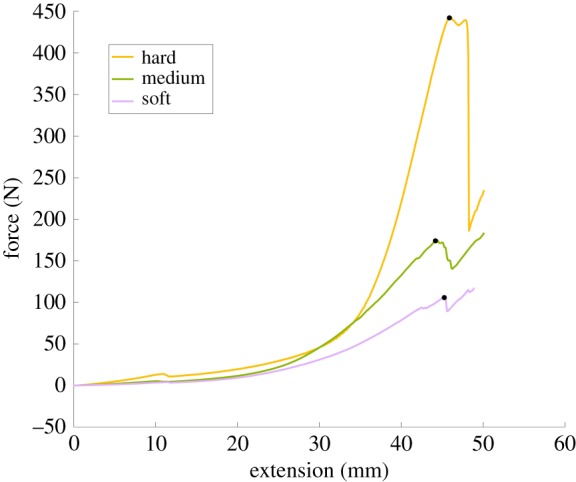
The three curves in this plot are single, non-averaged trials for a hard (yellow line), a medium (green line) and a soft (purple line) cervix. The black markers define the maximum force prior to rupture. (Online version in colour.)
Figure 8.
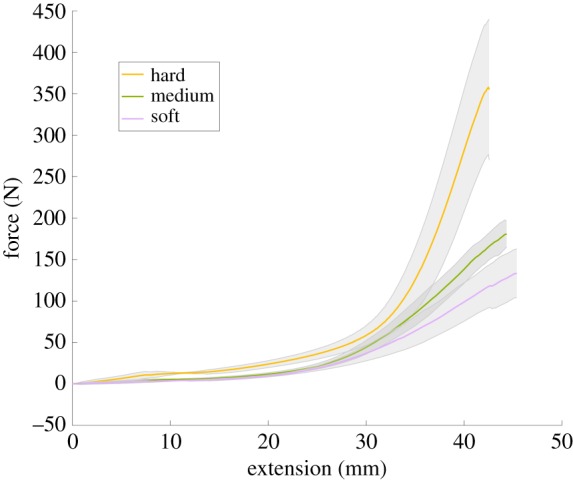
The three curves in this plot represent the averaged force traces for the hard (yellow line), medium (green line) and soft (purple line) cervices. The shaded regions indicate the standard deviation of these results. (Online version in colour.)
From this information, we are able to track where and when the cerclage suture fails. The average maximum force on the suture before rupture is 133.2 (N) for the soft model, 180.4 (N) for the medium model and 357.7 (N) for the hard model. This is shown in figure 9, with the rupture force being plotted against the modulus of elasticity for the model cervix material. Errors bars indicate the standard deviation of all the tests for each cervix model. Investigating the impact of tissue stiffness and elasticity on force to rupture, we can see that increasing tissue stiffness also increases the force to rupture. While only three model cervical stiffnesses were tested in this study, figure 9 indicates a linear relationship between the expected failure force and the modulus of elasticity of the model cervix material.
Figure 9.
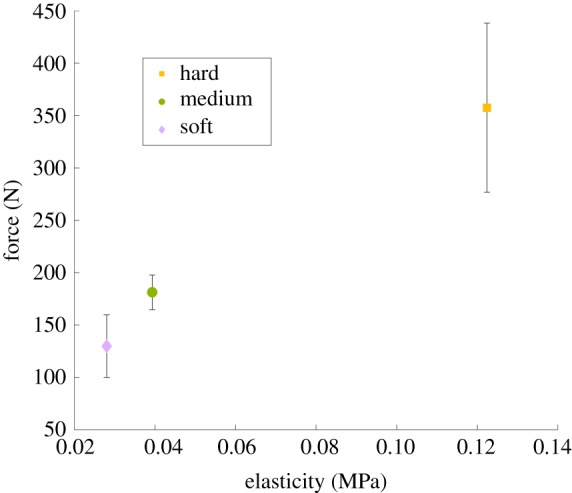
The average rupture force for the tests is reported as a function of material elasticity. The vertical lines are the standard deviations of these results. (Online version in colour.)
4. Discussion
Obtaining data about the material properties of human cervical tissue is a particularly challenging problem. It becomes even more difficult when those properties are changing dynamically throughout gestation. While efforts are underway to develop tools that can measure cervical softness externally, cervical insufficiency is typically diagnosed based on ultrasound measurement and patient history of preterm birth. We have used physician expertise to quantify this very qualitative diagnostic. Our model simplifies the tissue and the complex anatomical structures surrounding the cervix. Cervical tissue is dynamic and complex, unlike the homogeneous silicone mixtures used in this model; and by isolating our cervix model away from the surrounding ligaments and tissues, we lose information on how force is distributed and transferred. However, this is a first step towards providing a material property that can be used in both physical and computational models of cervical stretching and remodelling, as well as further investigations into successful modes of treating cervical insufficiency and other pregnancy and post-pregnancy-related conditions.
Figure 5b shows that the relationship between the percentage of silicone thinner used and E is not linear over the range that we tested. This is important when choosing a silicone mixture to make a model. While it is true that more thinner decreases the modulus of elasticity, the results presented here, or direct testing of the mixture to be used in the model would be needed to determine the resulting model properties.
It is noted that the difference in properties between the ‘soft’ and ‘medium’ consistency cervices is much less than the difference in properties between the ‘hard’ and ‘medium’ consistency cervices. Because the strength of the cerclage procedure appears to be linear (figure 9), it indicates that the procedure is more likely to be successful when the cervix is ‘harder’. As the tissue properties can continue to change as the cervix remodels, this can be very hard to assess. However, these results do indicate that cervical softness should be a strong indication of the necessity and likelihood of success of the cerclage procedure. It should be noted that this experiment examines only one mode of mechanical failure of the cerclage procedure. There are multiple ways the intervention can fail including premature rupture of the membranes and preterm labour. In the future, the method and model developed in this work can be used to explore in more depth when the procedure may be successful. For example, the length of the cervix is often used as a primary diagnostic for the necessity of a cervical cerclage. Model cervices can be created with various cervical lengths, and the process can be repeated as above. We can also modify the cervical canal geometry or cerclage method.
5. Conclusion
Premature cervical remodelling is a distressing condition where no standard preferred treatment option has been identified. Deciding on a treatment option is often left to the physician who relies on prior experience and preference. Studying this topic has been limited to clinical investigations, which provide valuable insight but have no interest in understanding the underlying mechanism of failure. By modelling the cervix in a biomechanics framework, we can gain insight into why the cerclage stitch fails in specific situations and succeeds in others. Building on this preliminary work, we will investigate the impact of geometry differences and suture material in the future.
Data accessibility
This article has no additional data.
Competing interests
We declare we have no competing interests.
Funding
A.B. is grateful to the Provost’s Diversity Fellowship Program for financial support of her research and to Melody Weigel for her assistance in the data collection. The authors also thank the George Washington University Institute of Biomedical Engineering and the National Science Foundation grant no. CBET-1437611 for financial support of this project.
Reference
- 1.Badir S, Mazza E, Zimmermann R, Bajka M. 2013. Cervical softening occurs early in pregnancy: characterization of cervical stiffness in 100 healthy women using the aspiration technique. Prenat. Diagn. 33, 737–741. ( 10.1002/pd.4116) [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. 2014. Cerclage for the management of cervical insufficiency. ACOG practice bulletin no. 142 Obstet. Gynecol. 123, 372–379. [DOI] [PubMed] [Google Scholar]
- 3.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. 2010. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 88, 31–38. ( 10.2471/BLT.08.062554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blencowe H. et al. 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. ( 10.1016/S0140-6736(12)60820-4) [DOI] [PubMed] [Google Scholar]
- 5.Ward RM, Beachy JC. 2003. Neonatal complications following preterm birth. BJOG 110, 8–16. ( 10.1046/j.1471-0528.2003.00012.x) [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371, 75–84. ( 10.1016/S0140-6736(08)60074-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shennan A, Jones B. 2004. The cervix and prematurity: aetiology, prediction and prevention. Semin. Fetal Neonatal Med. 9, 471–479. ( 10.1016/j.siny.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 8.Ciavattini A, Carpini GD, Boscarato V, Febi T, Giuseppe JD, Landi B. 2016. Effectiveness of emergency cerclage in cervical insufficiency. J. Maternal-Fetal Neonatal Med. 29, 2088–2092. ( 10.3109/14767058.2015.1075202) [DOI] [PubMed] [Google Scholar]
- 9.Aoki S, Ohnuma E, Kurasawa K, Okuda M, Takahashi T, Hirahara F. 2014. Emergency cerclage versus expectant management for prolapsed fetal membranes: a retrospective, comparative study. J. Obstet. Gynaecol. Res. 40, 381–386. ( 10.1111/jog.12207) [DOI] [PubMed] [Google Scholar]
- 10.Namouz S, Porat S, Okun N, Windrim R, Farine D. 2013. Emergency cerclage: literature review. Obstet. Gynecol. Surv. 68, 379–388. ( 10.1097/OGX.0b013e31828737c7) [DOI] [PubMed] [Google Scholar]
- 11.Çavuş Y, Uysal A, Balsak D, Acar Z, İnce Z, Uysal F. 2014. Emergency cervical cerclage: effect on pregnancy outcome and mode of delivery. J. Maternal-Fetal Neonatal Med. 27, 80–83. ( 10.3109/14767058.2013.805196) [DOI] [PubMed] [Google Scholar]
- 12.Ciancimino L, Laganà AS, Imbesi G, Chiofalo B, Mancuso A, Triolo O. 2015. Evaluation of maternal-fetal outcomes after emergency vaginal cerclage performed with Shirodkar-McDonald combined modified technique. J. Clin. Med. Res. 7, 319–323. ( 10.14740/jocmr2108w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güdücü N, İşçi H, Aydinli K. 2013. Results of midtrimester emergency cerclage. J. Reprod. Med. 58, 143–148. [PubMed] [Google Scholar]
- 14.Hume H, Rebarber A, Saltzman DH, Roman AS, Fox NS. 2012. Ultrasound-indicated cerclage: Shirodkar vs. McDonald. J. Maternal-Fetal Neonatal Med. 25, 2690–2692. ( 10.3109/14767058.2012.716465) [DOI] [PubMed] [Google Scholar]
- 15.Naqvi M, Barth WH. 2016. Emergency cerclage: outcomes, patient selection, and operative considerations. Clin. Obstet. Gynecol. 59, 286–294. ( 10.1097/GRF.0000000000000197) [DOI] [PubMed] [Google Scholar]
- 16.Myers K, Paskaleva A, House M, Socrate S. 2008. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 4, 104–116. ( 10.1016/j.actbio.2007.04.009) [DOI] [PubMed] [Google Scholar]
- 17.Myers KM, Feltovich H, Mazza E, Vink J, Bajka M, Wapner RJ, Hall TJ, House M. 2015. The mechanical role of the cervix in pregnancy. J. Biomech. 48, 1511–1523. ( 10.1016/j.jbiomech.2015.02.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hee L, Liao D, Sandager P, Gregersen H, Uldbjerg N. 2014. Cervical stiffness evaluated in vivo by endoflip in pregnant women. PLoS ONE 9, e91121 ( 10.1371/journal.pone.0091121) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.