Abstract
The primary exchange units in the human placenta are terminal villi, in which fetal capillary networks are surrounded by a thin layer of villous tissue, separating fetal from maternal blood. To understand how the complex spatial structure of villi influences their function, we use an image-based theoretical model to study the effect of tissue metabolism on the transport of solutes from maternal blood into the fetal circulation. For solute that is taken up under first-order kinetics, we show that the transition between flow-limited and diffusion-limited transport depends on two new dimensionless parameters defined in terms of key geometric quantities, with strong solute uptake promoting flow-limited transport conditions. We present a simple algebraic approximation for solute uptake rate as a function of flow conditions, metabolic rate and villous geometry. For oxygen, accounting for nonlinear kinetics using physiological parameter values, our model predicts that villous metabolism does not significantly impact oxygen transfer to fetal blood, although the partitioning of fluxes between the villous tissue and the capillary network depends strongly on the flow regime.
Keywords: microcirculation, human placenta, solute transport, metabolism
1. Introduction
The human placenta is an unusual and often overlooked organ. During pregnancy, it supplies the developing fetus with all its essential nutrients, removes its waste products and has a range of additional endocrine functions [1]. Placental insufficiency compromises fetal growth and can have a life-long impact on the later health of the individual [2]. As an exchange organ, the placenta’s geometric structure plays a crucial role in determining its function. With three-dimensional imaging revealing placental morphological complexity in ever greater detail [3–5], it is important to look at placental structure through the prism of the physical transport processes taking place within it. This allows us to identify the geometrical features that dictate transport capacity, and to characterize in quantitative terms the pathological consequences of structural abnormality.
The present study contributes to a growing literature in which mathematical and computational models have been used to provide insight into placental physiology. These studies have addressed the fetal circulation (involving networks of blood vessels confined within villous trees), the maternal circulation (involving blood flowing outside the branches of the trees, effectively through a porous medium) and solute exchange across the trophoblast barrier between them. Recent reviews are provided by Serov et al. [6], Jensen & Chernyavsky [7] and Plitman Mayo [8]. Here we focus on the primary structural exchange unit associated with the fetal circulation, namely the terminal villus: this is effectively a protruding ‘leaf’ on a villous tree that contains an irregular network of fetal capillaries. The thin-walled villus is bathed in maternal blood, allowing dissolved gases and nutrients to pass between fetal and maternal blood. If blood flow in the capillaries is insufficient to carry available solute we term the transport ‘flow-limited’; if diffusion through villous tissue is the dominant barrier to exchange, we term the transport ‘diffusion-limited’.
Pearce et al. [5] constructed a regression equation describing maternal-to-fetal solute transport in a terminal villus, by taking a harmonic average of limiting approximations for solute fluxes valid under flow-limited and diffusion-limited conditions. As explained by Jensen & Chernyavsky [7], this expression is naturally expressed in terms of suitable dimensionless parameters, namely a Damköhler number Da that measures solute transit time across the villous tissue due to diffusion relative to transit time through the villus due to flow, and a parameter μ that measures the relative diffusive capacities of the villous tissue and the intravillous capillary network. Erlich et al. [9] added a further refinement to the regression equation and then validated it using computational simulations of four villus specimens, each having complex internal structure. The significant physical parameters in their analysis were the solute diffusivities in tissue and plasma (Dt and Dp, respectively), the effective viscosity of blood η (based on an assumption of Newtonian flow), a dimensionless parameter B that captures the advective boost that oxygen acquires from binding to haemoglobin [10], and the imposed pressure drop ΔP driving blood through the vessel network. This analysis also revealed some of the key geometric parameters determining the transport capacity of a villus for most solutes: the flow resistance of the capillary network per unit viscosity (, which has dimensions of inverse volume); the total length of capillary vessels within the villus Lc; and a lengthscale capturing the diffusive capacity of villous tissue (a normalized diffusive flux integrated over an exchange area). A key finding from [9] is that, for the majority of physiologically relevant solutes studied, the diffusive capacity ratio was sufficiently small among all specimens studied for the effects of concentration boundary layers within capillaries to be a secondary factor. Then, assuming the solute is not absorbed by villous tissue, transport was predicted to be flow-limited when Da ≫ 1 and diffusion-limited when Da ≪ 1, where
| 1.1 |
The solute flux N is well approximated [5,9] by
| 1.2 |
where represents the maximum diffusive capacity of the villus under a solute concentration difference Δc between maternal and fetal blood. Setting aside the term involving DaF ≡ μ2Da/166.4 (a correction accounting for concentration boundary layers), (1.2) captures the transition from flow-limited transport () to diffusion-limited transport (N ≈ Nmax) as flow strength (Da−1) increases from low to high values. The simple expressions in (1.1) and (1.2) show how physical processes and villous geometry together influence solute transfer. In particular, they demonstrate how, for given flow conditions, different solutes can have widely varying values of Da (through differing values of Dt/B), implying that flow-limited and diffusion-limited transport may take place simultaneously in the same villus.
This approach can be used to understand the transport of solutes that pass passively through villous tissue. For some solutes, however, the situation is not so simple, either because active transport is required (in the case of amino acids [11]) or because the solute is absorbed by villous tissue. We consider such solutes here, focusing inter alia on oxygen, a proportion of which can be taken up by villous tissue before reaching fetal blood [12,13]. Our primary goal is to refine the estimate of solute transfer N to account for this uptake. We use simulations to compute the transfer rate in terminal villi recovered from imaging using confocal microscopy. To describe uptake of a generic solute under linear (first-order) kinetics, we introduce a kinetic parameter α that describes the uptake rate by villous tissue, and then present a modified version of (1.2) that expresses uptake in terms of Da and α. We identify two new dimensionless parameters
| 1.3 |
where Acap is the area of the capillary interface within the villus and ℓ is a lengthscale (that we compute) relevant to solute uptake under flow-limited conditions. We show how the transition from flow-limited to diffusion-limited transport, which occurs when when , instead occurs when when . We then extend our study to consider nonlinear uptake kinetics associated specifically with oxygen metabolism, exploiting parameters that we determine from ex vivo perfusion measurements, and examine the influence of flow on the partitioning of oxygen fluxes between placental tissue and fetal blood. Our results suggest that oxygen uptake by terminal villous tissue has surprisingly limited impact on oxygen flux to the fetus.
2. Methods
2.1. A mathematical model for feto-placental transport
We summarize the computational model briefly here, providing technical details in appendix A. We model steady-state solute transport in an intravillous feto-placental capillary network as an advection–diffusion–uptake problem, extending existing models [5,9,14] to account for tissue metabolism.
Three-dimensional images of villous microvasculature and the accompanying syncytiotrophoblastic shell (figures 1 and 2, insets) were segmented and meshed from stained confocal microscopy of four specimens taken from two different peripherial lobules of a normal human placenta at term, as reported previously [9,14,15]. For each villous specimen, the images reveal the spatial domain Ωb occupied by the capillary network. This is bounded by an inlet surface Γin, an outlet surface Γout and the capillary endothelium Γcap (figure 5a in appendix A). The network is embedded in villous tissue, with exterior surface, Γvil, representing the interface with maternal blood. A fixed solute concentration cmat is specified at Γvil. Using a Newtonian (Stokes flow) approximation, our model simulates the flow of fetal blood entering through Γin and leaving via Γout, driven by a pressure difference ΔP imposed between the inlet and outlet. In the fetal capillaries, solutes are advected by blood flow and undergo diffusion. In the villous tissue that forms the bulk between the capillary surface Γcap and villous surface Γvil, we assume there is no flow and the solute concentration c is assumed to obey a diffusion–uptake problem , where Dt is the solute diffusivity in tissue and q(c) is the tissue solute metabolic rate. In the present study, we consider first a generic solute (such as a dilute suspension of polystyrene nano-particles or other environmental pollutants [16,17]) characterized by the first-order kinetics
| 2.1 |
where α is the rate of solute uptake by tissue, assumed uniform. We then model oxygen metabolism, using nonlinear Michaelis–Menten kinetics [18,19],
| 2.2 |
where qmax is the maximum rate of oxygen metabolism and c50 is the concentration at which the metabolic rate reaches 50% of its maximum (table 1 summarizes parameter values from the literature). For c ≪ c50, (2.2) approaches (2.1) with α = qmax/c50. In keeping with prior physiological literature [24], (2.2) can also be approximated (more empirically) using α ≈ qmax/cmat. Note that under linear kinetics, using (2.1), transport depends on α and Dt in the combination α/Dt, as reflected in the parameters and in (1.3).
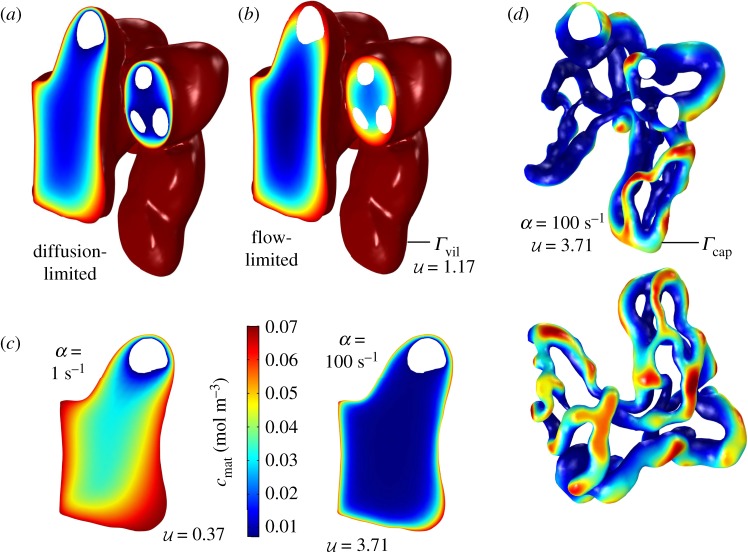
Figure 1.
Features of transport of a generic solute in a terminal villus, assuming first-order kinetics. (a,b) Concentration fields in tissue are shown in a slice through specimen 3 under diffusion-limited and flow-limited conditions at metabolic rate α = 10 s−1. The villous surface is fully oxygenated due to the condition c = cmat on Γvil (shown in dark red). Vessel cross-sections appear as white inclusions. (a) In the extreme diffusion-limited case, c = 0 at the capillary surface, Γcap. (b) In the extreme flow-limited case, at Γcap. (c) Concentration slices (over part of the same surface shown in a, b) for the diffusion-limited case, with uptake rate α ranging over two orders of magnitude. Concentration boundary layers form at the villous surface Γvil as α increases. (d) ‘Hotspots’ emerge with increasing metabolism: only where Γcap and Γvil are in close proximity can solute penetrate to capillaries. The top figure shows the capillary surface Γcap of specimen 3 in the same spatial orientation as panels a, b, with vessel cross-sections shown in white. Colours show the concentration at Γcap for the extreme flow-limited case. The lower panel shows a different projection of the same simulation. (Online version in colour.)
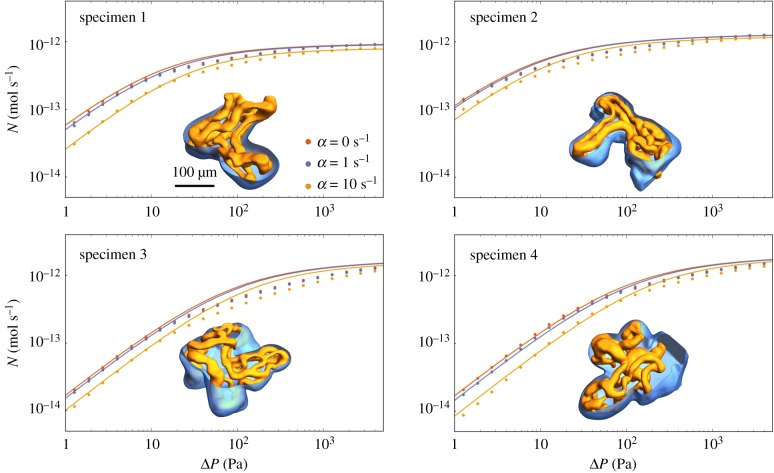
Figure 2.
Symbols show predictions of computational simulations of the full advection–diffusion–uptake problem (appendix A); curves show predictions of the simple regression equation (3.2). The four panels show results for the four vasculatures used in [9]; capillaries and the villous surface are illustrated by insets for each case in orange and blue, respectively. Each panel shows the net uptake N as a function of the inlet–outlet pressure drop ΔP, for no solute uptake (α = 0, identical with fig. 2 in [9]) and increasing uptake (α = 1, 10 s−1; Dt = 1.7 × 10−9 m2 s−1). (Online version in colour.)
Figure 5.
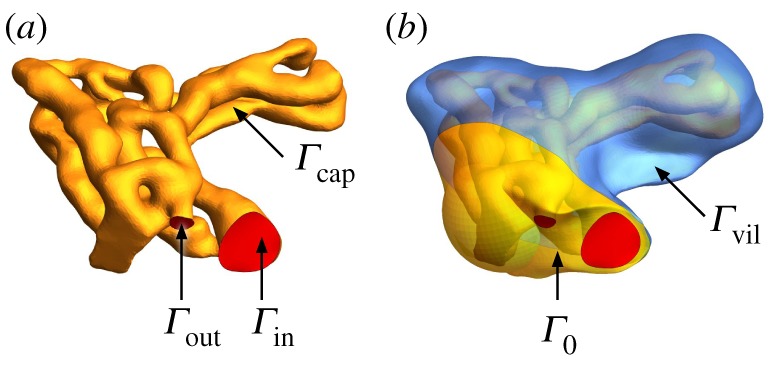
Computational domain of specimen 1, and boundary surfaces Γ. (a) The domain occupied by blood vessels Ωb is bounded by the inlet and outlet surfaces Γin and Γout (red) and the capillary surface Γcap (yellow). (b) The domain occupied by villous tissue Ωt is bounded by the capillary surface Γcap, the no-flux surface Γ0 and the villous surface Γvil (blue). (Online version in colour.)
Table 1.
Reference parameter values used in the model (see table 2 for comparison of O2 kinetics in different tissues and table 3 for villous geometric quantities).
| parameter | units | value | reference |
|---|---|---|---|
| ΔP | Pa | ∼10–102 | [9] |
| η | Pa · s | 2 × 10−3 | [9] |
| 1.4 × 102 | [5] | ||
| mol · (m3 · s)−1 | ∼10−3–10−1 | [13,20,21] | |
| qmax,glucose/fructose | mol · (m3 · s)−1 | ∼10−3–10−2 | [22,23] |
| mol · (m3)−1 | ∼10−3–10−1 | [21,24] | |
| mol · (m3)−1 | 0.7 × 10−1 (≈50 mmHg) | [5] | |
| cmat, glucose | mol · (m3)−1 | ∼1–10 | [22,25] |
| mol · (m3)−1 | ∼10−2–10−1 | [25,26] | |
| m2 s−1 | 1.7 × 10−9 | [5] | |
| m2 s−1 | ∼10−12–10−11 | [9] | |
| m2 s−1 | ∼10−13–10−12 | [9] | |
| ∼0.01–0.1 | |||
| ∼0.01–1 | |||
| ∼1–10 |
Our computational model for three-dimensional flow and transport was implemented in COMSOL Multiphysics® 5.3a, as described in [9]. A specific challenge of the modelling of transport is the emergence of boundary layers within the tissue when the uptake rate is high (corresponding to ), which required a particularly fine mesh resolution near the villous surface. The meshes of the villous domain used in figures 1–3 had approximately 20 million tetrahedral elements. Owing to the weaker uptake in figure 4, a less detailed mesh was required (315 000 tetrahedral elements). In the latter case, a mesh convergence test revealed a change in the solute uptake of at most 2% upon increasing the number of tetrahedral elements from 0.3 to 4 million.
Figure 3.
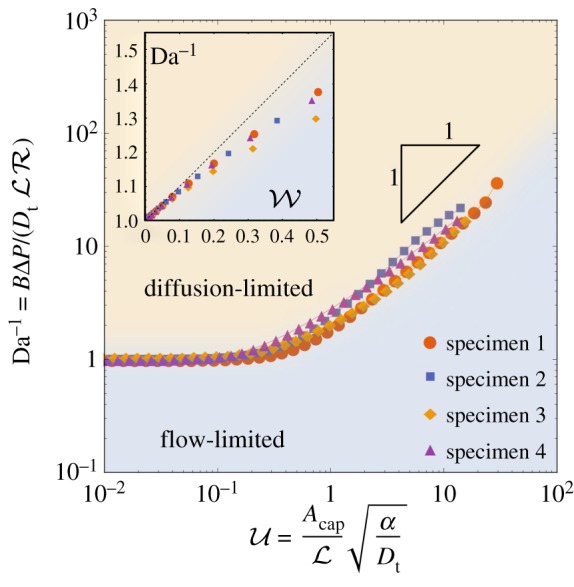
Flow-limited and diffusion-limited regimes in the presence of metabolism. The curves show for all four specimens the location where flow-limited and diffusion-limited regimes balance (which we define by Da−1 = F/G), as a function of the non-dimensional uptake parameter . For weak uptake (), Da−1 ≈ 1; for strong uptake (), . The boundary between flow-limited and diffusion-limited regimes should be understood as a smooth transition, indicating where contributions of both regimes are of comparable strength. The curves for all four specimens collapse appreciably. The inset illustrates how, when uptake is weak, the boundary between flow- and diffusion-limited uptake is approximated more precisely by (dashed line), highlighting as a significant dimensionless measure of uptake in this regime. Geometric parameters are reported in table 3. (Online version in colour.)
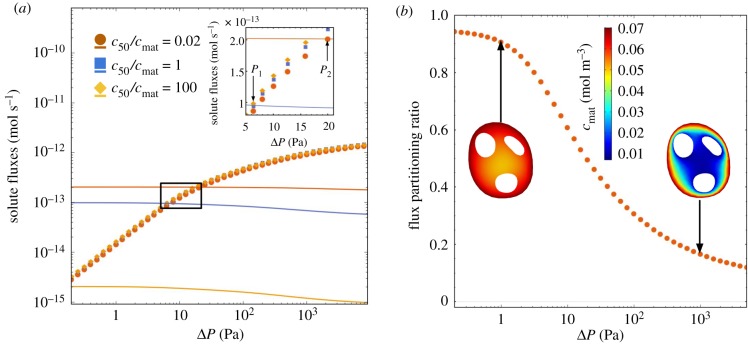
Figure 4.
The effect of nonlinear uptake kinetics on solute transport. (a) The coloured symbols show the solute uptake N which is delivered to the fetus, plotted against the inlet–outlet pressure drop ΔP, for specimen 3. For comparison, the solid lines with matched colours show the solute metabolized by villous tissue for the same specimen. All curves are plotted for the same maximum rate of oxygen metabolism qmax = 0.1 mol/(m3 · s), which we identify as a physiological value in metabolizing tissue (table 1). The different curves show changes in the parameter c50, spanning from predominantly zeroth-order (red symbols and curve) to predominantly first-order (yellow symbols and curve) uptake kinetics. Intersections in the inset, marked P1 and P2, show where the metabolized oxygen flux balances the flux delivered to fetal blood. (b) The flux partitioning ratio Ntissue/Ntotal of the solute flux metabolized by the villous tissue to the total flux of solute entering the terminal villus (for c50/cmat = 0.02; see appendix A). The flux partitioning ratio depends strongly on the flow regime. (Online version in colour.)
In addition to full advection–diffusion–uptake computations, we employed a set of simulations of transport by diffusion and uptake alone, satisfying (2.1) subject to simplified boundary conditions appropriate to flow-limited and diffusion-limited transport (described in appendix B).
2.2. Ex vivo measurement of placental oxygen metabolism
In order to inform models of oxygen transport, we conducted experiments to estimate values of q and c50 for use in (2.2). All tissues were acquired from two full-term human placentas delivered at St Mary’s Hospital, Manchester, UK, with appropriate informed written consent and ethics approval (15/NW/0829). Ex vivo dual perfusion was established in an isolated lobule, as described previously [27,28]. Briefly, each placental lobule was perfused via a peristaltic pump at an inflow rate of 14 ml min−1, oxygen concentration of 21% (volume per cent in air) delivered via a single cannula from the maternal side, and at 6 ml min−1, 0% O2 from the fetal side. Oxygen in tissue was recorded using a needle-type optical oxygen sensor (PyroScience FireStingO2 OXF500PT; Aachen, Germany) with an outer diameter of 500 μm and diameter of the tip of 230 μm. The optical sensors were 2-point calibrated as per the manufacturer’s instructions.
To record the tissue oxygen metabolic rate, the lobule was perfused until the oxygen reading reached a steady value. The oxygen drop-off curve (figure 6a in appendix C) was recorded after cessation of both maternal and fetal inflows, while the needle-type oxygen probe was held at a fixed position approximately 8 mm below the decidual surface. The measured oxygen decay rate was fitted to a nonlinear Michaelis–Menten law to estimate parameter values; details are provided in appendix C.
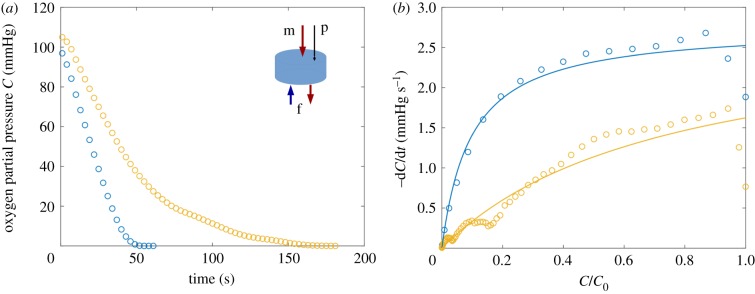
Figure 6.
Oxygen metabolism kinetics in the human placenta ex vivo. (a) Tissue oxygen partial pressure decay after the cessation of flow in two dually perfused placentas; inset shows the dual perfusion set-up, with maternal (m) and fetal (f) cannulas and the optical sensor probe (p). (b) Fitted (equation (C 1), solid) versus measured (circles) oxygen metabolic rates. See Methods and appendix C for more details. (Online version in colour.)
3. Results
3.1. Transport with linear uptake kinetics
We first consider a model of linear uptake kinetics for a generic solute, as described by (2.1). Predictions of the computational model are shown in figure 1. To show the possible range of behaviour, we first show the extreme cases of diffusion-limited transport (with negligible solute concentration in the capillary, a limit addressed in two spatial dimensions in [29]) and flow-limited transport (when the solute concentration in the capillary equilibrates with the surrounding tissue, so that there is negligible flux across the capillary surface); these simplified limits are described in more detail in appendix B. In both cases, the solute concentration falls with distance from the villous surface, but does so more rapidly under diffusion-limited conditions (figure 1a,b). These concentration fields were computed assuming a moderate metabolic rate (α = 10 s−1, for which ). The impact of changing α is demonstrated in figure 1c, which shows how, under diffusion-limited conditions, concentration gradients become steeper as the uptake rate increases. For sufficiently large α (i.e. ), most transport is reduced to a thin boundary layer (of thickness ) near the villous surface, significantly reducing the solute flux reaching more internal capillaries. This is illustrated in figure 1d, which shows the solute concentration at the capillary surface Γcap for specimen 3 in the flow-limited regime (the same scalebar applies as in figure 1c). The solute concentration on Γcap (and therefore the flux across the capillary surface) is highly heterogeneous in this example. As tissue metabolism increases, localized regions of concentration (hotspots) become more pronounced and solute transport becomes increasingly focused at a few regions at which the distance between the capillary and villous surfaces is locally minimal.
The symbols in figure 2 show computational predictions of the solute flux N entering four fetal capillary networks versus the pressure drop ΔP driving flow through the network, obtained using the full advection–diffusion–uptake model for the four specimens investigated. In each case, N rises approximately linearly with small ΔP (under flow-limited conditions) before saturating at large ΔP (under diffusion-limited conditions). In the absence of uptake, we can use equation (1.2) to describe the flux/pressure-drop relationship: N ≈ Nmax/Da ∝ ΔP when Da−1 ≪ 1, and N ≈ Nmax when Da−1 ≫ 1, where Nmax is specific to each villus (see appendix B). The symbols in figure 2 also show that the impact of increasing the uptake parameter α is to reduce N by an amount that diminishes slightly as ΔP increases. Overall, the change in metabolic uptake from α = 0 to α = 1 s−1 causes a relative decrease in solute net uptake N of at most 16%, considering all pressure drops across all four specimens.
To extend these predictions beyond the specific cases studied, it is helpful to approximate the relationship between N and ΔP using a regression equation that incorporates relevant geometric parameters as well as the effect of metabolism. (Our regression strategy is to identify simple algebraic expressions that capture key relationships with reasonable accuracy, rather than unwieldy but more precise formulae.) To generalize equation (1.2), we first focus on how metabolism affects the extreme flow-limited and diffusion-limited transport fluxes NFL and NDL. Computing these cases independently for each specimen, we determine the metabolic dimensionless scale functions G(α) and F(α) that vary between 0 and 1 (appendix D, figure 7) for which
| 3.1 |
A simple algebraic approximation for generic solute uptake under linear kinetics across both flow-limited and diffusion-limited transport regimes is then provided by constructing the harmonic mean of NFL and NDL as
| 3.2 |
This result provides an approximation for the net flux through any villus, requiring only a small set of computations of equation (2.1) under different boundary conditions, from which (and hence Nmax), F and G can be determined. Figure 2 shows that equation (3.2) provides a reasonable approximation of numerical solutions of the full advection–diffusion–uptake problem. (The boundary layer correction in (3.2) is not essential to this argument but it improves the accuracy of the approximation at intermediate Da.) The largest relative error between computational results and equation (3.2) across all specimens and all pressure drops is 33% and occurs in specimen 3.
Figure 7.
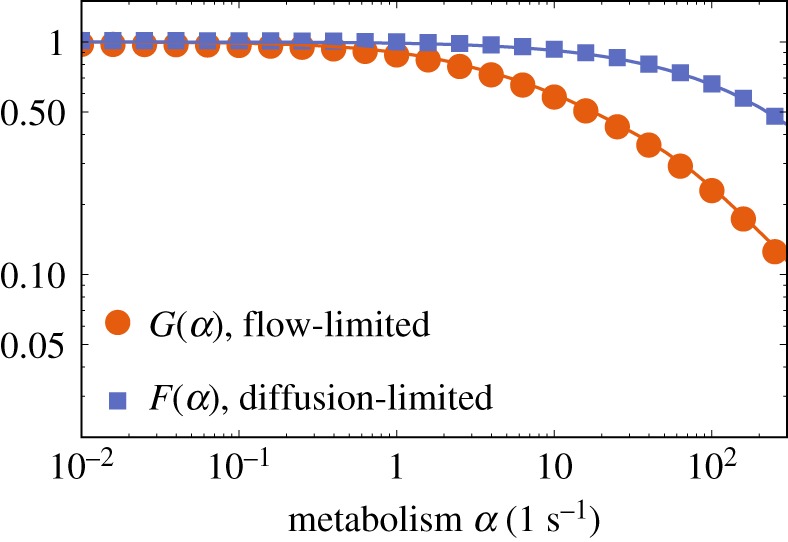
Metabolic scale functions computed for specimen 3. By definition, F = 1 and G = 1 for the case of no metabolism, α = 0. The points result from finite-element calculations and the lines connecting the points are meant as visual guides. (Online version in colour.)
The regression equation (3.2) reveals the factors defining the transition between flow- and diffusion-limited transport. Specifically, this arises when the two primary terms in the denominator are of comparable magnitude, i.e. when Da−1 ≈ F(α)/G(α). This relationship is plotted for all four specimens in figure 3. The thresholds between the diffusion-limited and flow-limited regimes collapse onto a near-universal curve when F/G is plotted not against α but instead against the non-dimensional parameter given in equation (1.3). This parameter emerges from an analysis of the large-α limit, described in appendices D and E.
For weak metabolism (), the solute distribution resembles that in the no-uptake limit discussed in [9], for which F and G are both close to unity. Uptake in this case takes place across the whole volume of the villous tissue; this limit is examined further in appendix F and is relevant to oxygen transport, as explained below. For solutes that are taken up strongly by villous tissues, delivery to fetal blood takes place via hotspots, i.e. local minima in the distance between Γcap and Γvil that are sufficiently small to penetrate the solute boundary layer adjacent to Γvil (as illustrated in figure 1d). We show in appendices D and E how common features between flow- and diffusion-limited transport explain the scaling relationship when . Both functions decay exponentially fast as increases (with metabolism becoming the dominant barrier to delivery to the fetus), but G falls off faster than F with increasing uptake (appendix D, figure 7), lowering the N versus ΔP curve more at low flow rates than at high flow rates, and hence promoting flow-limited transport relative to diffusion-limited transport when uptake is sufficiently strong.
In addition to the lengthscale (identified in our previous study [9]), the capillary surface area Acap becomes an important geometric determinant when solute uptake is strong (appendix E). By contrast, when solute uptake is weak (appendix F) we identify an independent geometric quantity (the lengthscale ℓ, appearing in the parameter in (1.3)) which captures the weak-uptake approximation (see the inset to figure 3). ℓ2 is determined by solving Poisson’s equation over the villous volume, and is a normalized measure of the solute reduction by uptake through the tissue, communicated to the internal capillaries by diffusion.
In summary, we have shown how uptake of a generic solute in villous tissue under first-order kinetics reduces the rate of delivery of solute to the fetus (figure 2). We have quantified this reduction under flow-limited and diffusion-limited conditions, showing a greater impact in the former case and implying that stronger fetal flows are needed to achieve maximal delivery in the presence of uptake (figure 3). By using appropriate dimensionless parameters (Da, and ), we have identified relationships that are independent of the details of individual villous geometries. We now specialize our study to consider the important case of oxygen transport and uptake.
3.2. Nonlinear oxygen metabolism
To establish the effect of metabolism on oxygen transfer in a realistic physiological context, we consider a Michaelis–Menten reaction-kinetics model for solute uptake in villous tissue, equation (2.2). The model parameters are given in table 1. In particular, the Michaelis–Menten parameters qmax and c50 are informed by a novel ex vivo oxygen measurement study discussed in detail in appendix C. The parameter values emerging from this experimental study are compared with the literature values in other metabolizing tissues in table 2. The ex vivo measured maximal rate of placental tissue metabolism qmax agrees with the lower end of metabolic activity reported in other tissues (table 2, with the brain and cardiac tissue being more metabolically active). However ex vivo estimates suggest high variability in the ‘half-maximal metabolic rate’ concentration c50, which appears larger in the human placenta than in other tissues (table 2), indicating that a first-order kinetics approximation could be appropriate in less oxygenated regions of the intervillous space.
Table 2.
Characteristic kinetic parameters for oxygen metabolism in different tissues. Oxygen solubility is taken to be 1.35 × 10−3 mol/(m3 · mmHg) [30], tissue density is ≈103 kg m−3, and the molar volumetric content of oxygen (at 37°C) is taken equal to ≈40 μmol/(ml O2).
| tissue | qmax, mol/(m3 · s) (ml/(kg · min)) | c50, mol · m−3 (mmHg) | reference |
|---|---|---|---|
| brain (mouse, in vivo) | ∼10−2 | [31] | |
| brain (rat, ex vivo) | ∼10−1 (≈140) | ∼10−3 (≈0.8) | [24] |
| brain | ∼10−1 (140) | ∼10−3 (1) | [18,21] |
| liver (rat, ex vivo) | ∼10−1 (≈88) | ∼10−3 (≈2.2) | [24] |
| tumour | ∼10−2 (15) | ∼10−3 (1) | [18,21] |
| cardiac parenchyma | ∼10−2 –10−1 | ∼10−4 (≈0.05) | [30] |
| placenta (human, ex vivo) | ∼10−3 –10−2 (2–11) | [13,20] | |
| placenta (ex vivo, this study) | ∼10−2 (∼10) | ∼10−2 –10−1 (∼10–102) |
Figure 4a shows the predicted solute uptake N of the fetal capillary network versus the inlet–outlet pressure drop ΔP using the specimen 3 geometry (coloured symbols). We consider three cases: c50 ≪ cmat (red symbols), when we expect to recover zeroth-order kinetics; c50 = cmat (blue symbols), when the nonlinearity in the Michaelis–Menten approximation should be most apparent; and c50 ≫ cmat (yellow symbols), when we expect to recover the first-order kinetics. Although there is uncertainty in the value of c50 (table 1), the case c50/cmat = 0.02 (red symbols in figure 4a) lies within the range of physiological values of metabolizing tissues (table 1). As the inset shows, the variation in c50 across four orders of magnitude (spanning a transition from predominantly zeroth-order to predominantly first-order kinetics) reveals only modest variation in net oxygen delivery to fetal blood, affecting no more than 12% change at an intermediate drop of ΔP = 10 Pa. This is in contrast to the solute flux metabolized by the villous tissue (solid lines, matching colours), for which the increase of c50/cmat from 0.02 to 100 leads to a decrease in the metabolized flux by two orders of magnitude. We thus conclude that oxygen delivery to fetal blood in physiological conditions is not strongly affected by variation in c50. Correspondingly, for a physiologically relevant oxygen metabolism rate α ∼ 10−1 s−1 [24], we estimate the non-dimensional transport parameters (1.3) and (tables 1 and 3), placing oxygen transport in the weak metabolism regime.
Table 3.
Geometric parameters used in defining and , computed assuming the first-order kinetics (with α = qmax/cmat and f(C) = C). was reported previously in [9]. See appendix F for more details.
| specimen | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 8.2 | 11.4 | 15.4 | 17.9 | |
| Acap, mm2 | 0.125 | 0.0830 | 0.124 | 0.122 |
| 0.0016 | 0.0014 | 0.0021 | 0.0021 | |
| ℓDL, mm | 0.0056 | 0.0042 | 0.0050 | 0.0040 |
| ℓ, mm | 0.0185 | 0.0128 | 0.0146 | 0.0181 |
| 0.57 | 0.75 | 0.64 | 0.36 |
Figure 4b shows how the partitioning of fluxes between villous tissue and fetal blood depends on the flow regime. The ratio of fluxes Ntissue/Ntotal is defined as the amount of solute metabolized by the villous tissue divided by the total flux that enters into the terminal villous from the maternal circulation through the villous surface (see appendix A, equation (A 16)). In the flow-limited regime (with very low values of ΔP), almost all of the solute entering the terminal villus is metabolized by the villous tissue. Conversely, in the diffusion-limited regime (with high values of ΔP), a small fraction of total flux is metabolized, and a larger fraction of the solute enters the fetal capillary. This highlights how the flux is partitioned differently depending on the flow regime. For a physiological range of terminal pressure drops of ∼10–100 Pa, the model predicts relative oxygen consumption by terminal villous tissue of approximately 30–60% of the total oxygen supply, which is comparable to the upper range of 22–54% reported as the relative oxygen consumption rate by the human placenta ex vivo and in vivo [12,13].
In summary, parameter estimates from measurements of dynamic oxygen uptake rate in placental tissue and our computational model together suggest that the rate at which oxygen is metabolized by a terminal villus is substantially smaller than the maximum (diffusion-limited) transfer rate of the villus, and also sufficiently small for oxygen to penetrate throughout the villous tissue. However under strongly flow-limited conditions, our model predicts that villous tissue can absorb a substantial proportion of the oxygen supplied from the maternal circulation.
4. Discussion
Computational models of physiological function are important both in developing fundamental scientific understanding and in advancing medical therapies. Like many organs, the placenta has a complex multi-scale organization that challenges current methodologies. We present here a set of results for terminal villi, the primary functional exchange units of the fetal circulation, in which we use three-dimensional simulations to derive simplified expressions of solute transport that can be readily integrated within larger scale models of solute transport. Despite high variability among the four samples of a terminal villus investigated, we have shown that a handful of geometric statistics are sufficient to characterize transport of solutes that are taken up by the villous tissue itself. It is hoped that these results will guide future studies of microvascular anatomy so that function can be assessed more readily from three-dimensional structural datasets.
Our strategy in the present study has been to explore a broad range of parameters, to illustrate possible outcomes for a variety of solutes (table 1), before focusing attention on oxygen. One benefit of this approach is that the simplifications emerging for extreme parameter values shed light on underlying physical mechanisms, which then help us understand the more complex interactions that emerge under physiological conditions.
In formulating approximations of solute exchange, we have sought to use dimensionless quantities that naturally characterize dominant physical balances. In the absence of solute uptake in tissue, we showed previously [5,7,9] that the Damköhler number (1.1) is useful in distinguishing flow-limited from diffusion-limited transport (with the transition between the two cases occurring when Da is of order unity). Additional parameters emerge when solute metabolism is accounted for. When solute uptake (assuming first-order kinetics) is sufficiently strong to induce solute boundary layers within villous tissue, the relevant uptake parameter is (see (1.3)), as illustrated in figure 3. For more moderate uptake (a limit of relevance to oxygen), however, a further parameter emerges, as shown in appendix F. Distinct geometric quantities appear in each parameter, reflecting the differing physical balances: in Da measures a mean exchange area over exchange distance, as is appropriate to diffusion-limited transport; the total area of the capillary endothelium Acap in is relevant to transport under flow-limited conditions when fetal blood is exposed to a varying concentration field over this surface; and the lengthscale ℓ appears in , reflecting uptake of solute throughout the bulk of the tissue. Our study demonstrates how solutions of simple canonical partial differential equations (here, the three-dimensional Laplace and Poisson equations) in complex spatial domains can be used to extract these functionally significant geometric measures from imaging data.
Our linear and nonlinear uptake models allow insight into how the transport of different solutes is affected by metabolism. A key finding of the linear uptake model for a generic solute is that sufficiently strong uptake can drive solute exchange towards the flow-limited regime (figure 3). This may be relevant for certain sugars: our estimate of for fructose (in excess of unity, table 1) suggests that metabolism can have a strong impact on its exchange. To test the effect of metabolism on oxygen transport, we implemented a nonlinear Michaelis–Menten uptake model and parametrized it with physiological literature values from different metabolizing tissues, including our own experiments on placental tissue using an oxygen probe (appendix C, figure 6). Our simulations predict that oxygen transport to fetal blood is only modestly affected by metabolism (figure 4a). This implies that, for physiological values, zero-uptake predictions (such as [9]) provide viable leading-order estimates of oxygen delivery to the fetus, allowing us to determine the impact of oxygen uptake by tissue as linear corrections (see (F 3) and (F 5)). Nevertheless, the metabolic flux is much larger than the flux delivered to fetal blood at low fetal flow rates (more precisely, when ; see appendix F), and vice versa at high flow rates (figure 4b).
The present model rests on numerous assumptions. We demonstrated previously [9] that non-Newtonian effects of fetal blood flow can be neglected in a first approximation, although numerous features of oxygen transport by red blood cells and dynamic haematocrit distribution in complex networks require further assessment [32]. Clearly, there will be value in performing additional studies in a wider sample of villous networks, in order to test the robustness of the present approximations and to consider the impact of structural and metabolic abnormalities that may arise in disease. Future studies should also address the maternal flow exterior to the surface of the villus, to test the assumption that the source of solute is uniformly distributed and to identify appropriate lengthscales that determine transport (see also [33,34]). Given that there is spatial heterogeneity across the whole placenta, the kinetics may switch from zeroth order to first order in different locations within the same organ. Active transport of some solutes by the syncytiotrophoblast is a further refinement that will be required to make robust predictions of placental function. Prior studies of transport in other physiological systems [35–38] suggest that there is value in using mixed (Robin) boundary conditions on Γcap to explore states between the extremes considered in appendices B and D. Finally, our study considers uptake only in the terminal branches of a villus, and does not account for solute metabolism by other placental tissues, which will influence overall delivery to the fetus.
In summary, this study offers an integrated approach to characterize the transport of solutes, such as oxygen, that are metabolized by tissue with complex embedded microvasculature. A robust algebraic relationship (3.2) provides a computationally efficient tool to upscale micro-structural features to the organ-scale function of the human placenta; this approach should be adaptable to other physiological systems with complex vasculature. Although our realistic image-based model offers a general insight into relative contributions of villous tissue metabolism, diffusive capacity and feto-capillary flow, more data are needed to further quantify the identified transport determinants in healthy and abnormal placentas.
Acknowledgements
The authors are grateful to Romina Plitman Mayo (Tel Aviv University, Israel) for sharing the villous geometries, and we thank Edward D. Johnstone (University of Manchester, UK) and Rohan M. Lewis (University of Southampton, UK) for helpful discussions.
Appendix A. The computational model
We model fetal blood flow within a fetal capillary network using the Stokes equations, which are solved over the domain Ωb occupied by blood vessels:
| A 1 |
Here x is a spatial coordinate, u(x) is the fluid velocity field, p(x) the fluid pressure and η the dynamic viscosity of fetal blood, which is treated as Newtonian in our three-dimensional simulations. We take (table 1), which is appropriate for blood with 48% haematocrit in a 20 μm vessel [9]. The capillary domain Ωb is bounded by the inlet surface Γin, the outlet surface Γout and the capillary surface Γcap (figure 5a). The tissue domain Ωt is bounded internally by the capillary surface Γcap and externally by the no-flux surface Γ0 and the villous surface Γvil (figure 5b). Blood enters through the inlet surface Γin and leaves via Γout, driven by a pressure difference ΔP imposed between inlet and outlet. A no-slip condition is imposed on the interior of Γcap. The boundary conditions on the flow are therefore
| A 2 |
| A 3 |
| A 4 |
The volume flux is defined as
| A 5 |
and the resistance of the network is
| A 6 |
Within Ωb, the solute concentration c(x) is assumed to obey the linear advection–diffusion equation
| A 7 |
where Dp is the solute diffusion coefficient in plasma. The parameter B = 1 for most solutes, but B > 1 for species that bind to haemoglobin, modelling facilitated transport by red blood cells. For oxygen in fetal blood, B ≈ 141 [5,33]. Fetal blood is assumed to enter solute-free at the inlet Γin and zero diffusive solute flux is imposed at the outlet Γout,
| A 8 |
| A 9 |
The capillaries are surrounded by villous tissue, which occupies the domain Ωt. Here the solute concentration is assumed to obey a diffusion–uptake equation
| A 10 |
where Dt is the solute diffusion coefficient in tissue and q(c) is the solute uptake rate, which is taken either to satisfy first-order kinetics for a generic solute, q(c) = αc (§3.1 of the Results), or to obey the nonlinear Michaelis–Menten relationship (2.2) for oxygen (§3.2). Here α is a rate of metabolic uptake; α = 0 reproduces the case discussed in [9].
The maternal solute concentration c = cmat is imposed on Γvil and no solute flux is imposed at the intermediate region Γ0 on the villous surface to avoid artificial sharp gradients (we assume that the geometric domain was sliced across Γ0 from the larger network of a villous tree). Thus
| A 11 |
and
| A 12 |
We couple the problems in Ωb and Ωt by imposing continuity of the concentration across Γcap as well as matching the diffusive fluxes
| A 13 |
Once a solution c to (A 1)–(A 13) has been obtained, we compute the net solute flux delivered to the fetus, defined as the integral of the advective flux over the outlet boundary
| A 14 |
Given (A 8) and (A 11), N is equal to the sum of diffusive fluxes across the inlet and capillary surface,
| A 15 |
The proportion of the solute flux metabolized by the villous tissue relative to the total flux supplied by the maternal blood to the villous surface is given by
| A 16 |
Appendix B. Asymptotic transport regimes under first-order kinetics
The computation of N requires the numerical solution of a complex boundary value problem (A 1)–(A 13) over multiple domains, Ωb and Ωt. In order to simplify the problem and gain physical understanding, we consider the simpler asymptotic regimes of extreme diffusion- and flow-limited transport, in which the computation can be restricted to Ωt. Concentration profiles in each regime are shown in figure 1a, and are determined as follows.
In extreme diffusion-limited transport, the flow is sufficiently rapid to impose a fixed concentration difference Δc = cmat between the villous boundary Γvil and the capillary boundary Γcap. Assuming linear kinetics for a generic solute, we define the diffusion-limited boundary value problem as
| B 1 |
The net solute flux to fetal blood can be evaluated as
| B 2 |
This is a function of the uptake rate α as well as the domain shape. In the case α = 0, we retrieve the maximum exchange capacity Nmax ≡ NDL|α=0 from [9]. The lengthscale associated with a villus is defined as .
Extreme flow-limited transport arises when ΔP is sufficiently weak for solute to be fully saturated in fetal blood before it leaves the vessel network. In this case, N is proportional to the (weak) flow rate through the outlet. The flow-limited problem differs from (B 1) by the boundary condition on the capillary surface, which we approximate by assuming that the flux to the vessel is negligible to leading order
| B 3 |
Within the capillary, the concentration profile is almost equilibrated, and swept away by weak flow at the outlet. We model solute mixing by advection and diffusion within the capillary by assuming that the advective flux at the outlet (A 14) is determined by a surface average of the concentration obtained as a solution to (B 3)
| B 4 |
where 〈c〉 is the mean concentration over the capillary surface Γcap and Q is the volume flux (A 5).
Appendix C. Ex vivo oxygen metabolism in the human placenta
We estimate local oxygen metabolism parameters, assuming Michaelis–Menten kinetics [18,19] for the concentration measured by the optical sensor, volume-averaged over a region of size approximately 50 μm in the intervillous space. Provided the diffusion time scale across the measured volume is smaller than the tissue metabolic time scale (tables 1 and 2), the local response to a cessation of flow is assumed to satisfy
| C 1 |
where and are volume-averaged effective kinetic parameters and ϕt is the villous volume fraction.
We use nonlinear least-squares fitting via the Levenberg–Marquardt algorithm implemented in the nlinfit function of MathWorks MATLAB® R2018a to estimate the parameter values of (C 1) from experimental data (figure 6).The fitted values of the volume-averaged parameter (mean ± s.e.) are 2.77 ± 0.11 mmHg s−1 (subject 1) and 2.8 ± 0.3 mmHg s−1 (subject 2), and the values for vary from 9.3 ± 1.6 mmHg to 78 ± 16 mmHg. Assuming ϕt ≈ 0.5 [34] and oxygen solubility of ≈1.35 × 10−3 mol · m−3 · mmHg−1 [30], we obtain qmax ∼ 10−2 mol · s−1 · m−3 and c50 ∼ 10–102 mmHg (or, ∼10−2–10−1 mol m−3); see table 2 for a comparison of placental oxygen metabolism with other tissues.
We emphasize that the reported values are from just two placentas and further studies are necessary to assess inter- and intra-placental variability.
Appendix D. Metabolic scale functions under first-order kinetics
Extending the framework of appendix B, we can recast the diffusion-limited flux (B 2) and the flow-limited flux (B 4) into a more convenient form by introducing the dimensionless metabolic scale functions 0 < F(α) ≤ 1 and 0 < G(α) ≤ 1, satisfying
| D 1 |
and
| D 2 |
These functions satisfy F(0) = 1 and G(0) = 1, retrieving the no-metabolism forms of flow-limited and diffusion-limited flux from [9]. The diffusion-limited metabolic scale function F(α) is defined in terms of an integral over the concentration c, which is the solution to the Helmholtz problem (B 1)
| D 3 |
The flow-limited metabolic scale function G(α) is derived from the solution to the Helmholtz problem (B 3) as
| D 4 |
These functions are illustrated in figure 7, for one villus (specimen 3). Each function becomes exponentially small as the uptake parameter increases, because Γcap falls outside the concentration boundary layer adjacent to Γvil. The dependence of F and G on villous geometry is explored further below.
To explain why G falls off more rapidly than F for large α, consider that when uptake is strong the c field is confined to a boundary layer of thickness adjacent to Γvil, so that F and G will be dominated by localized regions (hotspots) in which Γcap is closest to Γvil. Suppose that in such a region the distance between the two surfaces can be represented by a paraboloid with mean radius of curvature R; then the region over which any uptake takes place is confined to distances of the point of closest approach of the two surfaces, i.e. an area of size R/θ. F, being an area integral (D 3) of where c varies over a lengthscale 1/θ, will be proportional to ; G, being an area integral (D 4) of c, will be proportional to (R/θ)/Acap. As explained in further detail in appendix E, their ratio F/G therefore scales like (see (1.3)) for , implying G ≪ F in this limit.
Appendix E. WKB approximation for strong metabolism
We seek approximations of (B 1) and (B 3) under strong uptake, assuming first-order kinetics. Let C = c/cmat and θ2 = α/Dt. We consider the limit in which the boundary-layer thickness 1/θ becomes smaller than the thickness of the villous tissue. We pose a WKB expansion, . Then, at successive orders, the diffusion–uptake equation gives
| E 1 |
We assume Γvil is parametrized by surface coordinates s ≡ (s1, s2) and a local normal coordinate n, oriented into the villous tissue. Let be the local unit normal and the curvature, where .
The eikonal equation (E 1a) is solved by S0 = ±n, where n measures distance along . The transport equation (E 1b) becomes , implying that for some constant A. Thus, the leading-order expression for C can be written
| E 2 |
The boundary layer thickness 1/θ must be significantly smaller than the wall radius of curvature 1/κ for this approximation to be valid. Imposing C = 1 on Γvil implies A+ + A− = 1. Ignoring complications arising from caustics, we assume Γcap lies at n(s) = N(s). The boundary condition on this surface determines A±. Let denote the unit normal to Γcap, pointing into the capillary.
Imposing C = 0 on Γcap in the diffusion-limited case implies
| E 3 |
and so
| E 4 |
Imposing Cn = 0 on Γcap in the flow-limited case implies
| E 5 |
and so
| E 6 |
We can estimate the integrals in (E 4) and (E 6) by noting that the exponential will be dominated by the local minima of N. Let such a point be at s = s0, at which . The present analysis addresses the case in which N(s0) ≫ 1/θ. Let λ1 > 0, λ2 > 0 be the eigenvalues of the Jacobian . The local area element may be written as , so that near s0 we have dA ≈ ds1 ds2. We can rotate the coordinates locally so that the Jacobian is diagonal, and then …. Introducing scaled coordinates ui = (θλi)1/2 (s − s0)i for i = 1, 2, we obtain as a contribution to G
| E 7 |
The factor is a lengthscale associated with the mean radius of the ‘hotspot’ at s0. The prefactor in (E 7) is a dimensionless ratio of this length multiplied by the boundary-layer thickness 1/θ to Acap, and is associated with the assumption that the flow absorbs solute from Γcap by (effectively) averaging over the capillary interface. The exponential sensitivity to N(s0)θ in (E 7) shows that proximity of Γvil to Γcap is the predominant factor in determining overall exchange under flow-limited conditions with strong uptake. We can expect G to be dominated by contributions from a small number of such hotspots within a villus, each with its own value of N(s0) and R(s0).
The corresponding contribution to F will have , giving
| E 8 |
In this case, the net exchange capacity under diffusion-limited conditions, proportional to , is determined instead by the hotspot radius R(s0), becoming independent of the global measure .
In general, F and G will be determined by a sum of such contributions from the dominant ‘hotspots’ in each case, but with (from (E 7) and (E 8))
| E 9 |
for sufficiently large α. Thus we see the emergence of the dimensionless parameter in (1.3). The relationship (E 9) is validated in figure 3.
Appendix F. Weak metabolism limit
The case of weak tissue metabolism arises when the total rate of solute uptake by tissue, which we can estimate by where is the volume of villous tissue in Ωt, is smaller than the maximum possible flux reaching fetal blood . Equivalently we require qmax/(Dt cmat) to be smaller than a quantity with dimensions of inverse area, which we estimate provisionally as but which we now determine more precisely. We can decouple the diffusive and uptake fluxes in (A 10) by expanding the solution of
| F 1 |
formally in powers of ɛ, subject to appropriate boundary conditions, as C ≈ C(0) + ɛ C(1) + … . Here f(C) is a non-negative dimensionless metabolic function (equal to 1 for zeroth-order, C for first-order, or C/(C + c50/cmat) for Michaelis–Menten kinetics). While C(0) is dimensionless, C(1) has dimensions of length squared in this formulation and is expected to be negative, reflecting the reduction of the solute field by delivery to tissue.
In diffusion-limited conditions with and , we have, at leading order, , and , and thus the flux to fetal blood is . The following order gives , . The reduction to the flux to fetal blood due to solute uptake in tissue reveals the effective area, which we write as the square of a lengthscale ℓDL,
| F 2 |
which can be computed numerically for a given villous geometry (table 3). In dimensional variables, the approximate total flux becomes
| F 3 |
or equivalently (using (D 1)) for .
In the flow-limited case, and . Thus, at successive orders, (F 1) gives C(0) ≡ 1, and , . In this case, the flux delivered to fetal blood yields the effective area
| F 4 |
The total uptake flux (in dimensional variables) is approximated by
| F 5 |
or equivalently (using (D 2)) G ≈ 1 − ɛℓ2. The extreme flow-limited assumption requires that NFL is substantially smaller than the flux entering villous tissue, which balances the overall rate of uptake .
The limiting cases (F 3) and (F 5) provide an approximate ratio
| F 6 |
As table 3 illustrates, for specimens 1–4 in all cases is less than 12%, indicating that uptake has a stronger relative effect on the flow-limited compared with the diffusion-limited state. Thus, to a good approximation, we can describe the boundary between flow-limited and diffusion-limited uptake using when uptake is weak (see inset to figure 3), highlighting as a significant dimensionless measure of uptake.
The condition underpinning the flow-limited approximation can be expressed for first-order kinetics, using (F 5), as . The geometric index is an order unity parameter for all specimens (table 3). The condition therefore provides an estimate of the conditions at which the flux partition ratio in figure 4b first falls appreciably below unity.
Ethics
The ex vivo data were obtained at the Maternal and Fetal Health Research Centre, St Mary's Hospital, Manchester, UK, with informed written consent and appropriate approval by the North West Research Ethics Committee (ref. no. 15/NW/0829).
Data accessibility
All data needed to evaluate the results and conclusions are present in the paper. The associated datasets and codes can be accessed via the Figshare repository (http://doi.org/10.6084/m9.figshare.7718462). Additional data related to this study may be available from the authors upon request.
Authors' contributions
A.E., O.E.J. and I.L.C. took part in mathematical model design, performed asymptotic and data analysis, interpreted the results and prepared the manuscript; G.A.N., P.B. and I.L.C. conceived the experimental model; A.E. performed numerical simulations; G.A.N. conducted ex vivo experiments. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by MRC research grant no. MR/N011538/1.
References
- 1.Burton GJ, Fowden AL. 2015. The placenta: a multifaceted, transient organ. Phil. Trans. R. Soc. B 370, 20140066 ( 10.1098/rstb.2014.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horikoshi M. et al. 2016. Genome-wide associations for birth weight and correlations with adult disease. Nature 538, 248–252. ( 10.1038/nature19806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth CJ, Haeussner E, Ruebelmann T, Koch FV, Schmitz C, Frank H-G, Wall WA. 2017. Dynamic modeling of uteroplacental blood flow in IUGR indicates vortices and elevated pressure in the intervillous space – a pilot study. Sci. Rep. 7, 40771 ( 10.1038/srep40771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perazzolo S, Lewis R, Sengers B. 2017. Modelling the effect of intervillous flow on solute transfer based on 3D imaging of the human placental microstructure. Placenta 60, 21–27. ( 10.1016/j.placenta.2017.10.003) [DOI] [PubMed] [Google Scholar]
- 5.Pearce P, Brownbill P, Janácek J, Jirkovská M, Kubínová L, Chernyavsky IL, Jensen OE. 2016. Image-based modeling of blood flow and oxygen transfer in feto-placental capillaries. PLoS ONE 11, e0165369 ( 10.1371/journal.pone.0165369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serov AS, Salafia C, Grebenkov DS, Filoche M. 2016. The role of morphology in mathematical models of placental gas exchange. J. Appl. Physiol. 120, 17–28. ( 10.1152/japplphysiol.00543.2015) [DOI] [PubMed] [Google Scholar]
- 7.Jensen OE, Chernyavsky IL. 2019. Blood flow and transport in the human placenta. Annu. Rev. Fluid Mech. 51, 25–47. ( 10.1146/annurev-fluid-010518-040219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plitman-Mayo R. 2018. Advances in human placental biomechanics. Comp. Struct. Biotech. J. 16, 298–306. ( 10.1016/j.csbj.2018.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erlich A, Pearce P, Mayo RP, Jensen OE, Chernyavsky IL. 2019 doi: 10.1126/sciadv.aav6326. Physical and geometric determinants of transport in feto-placental microvascular networks. Sci. Adv.5, eaav6326. (http://arxiv.org/abs/1809.00749. ) [DOI] [PMC free article] [PubMed]
- 10.Käsinger W, Huch R, Huch A. 1981. In vivo oxygen dissociation curve for whole fetal blood: fitting the Adair equation and blood gas nomogram. Scand. J. Clin. Lab Inv. 41, 701–707. ( 10.3109/00365518109090518) [DOI] [PubMed] [Google Scholar]
- 11.Sibley CP, Brownbill P, Glazier JD, Greenwood SL. 2018. Knowledge needed about the exchange physiology of the placenta. Placenta 64, S9–S15. ( 10.1016/j.placenta.2018.01.006) [DOI] [PubMed] [Google Scholar]
- 12.Carter A. 2000. Placental oxygen consumption. Part I: in vivo studies – a review. Placenta 21, S31–S37. ( 10.1053/plac.1999.0513) [DOI] [PubMed] [Google Scholar]
- 13.Schneider H. 2000. Placental oxygen consumption. Part II: in vitro studies – a review. Placenta 21, S38–S44. ( 10.1053/plac.1999.0512) [DOI] [PubMed] [Google Scholar]
- 14.Plitman-Mayo R, Olsthoorn J, Charnock-Jones DS, Burton GJ, Oyen ML. 2016. Computational modeling of the structure-function relationship in human placental terminal villi. J. Biomech. 49, 3780–3787. ( 10.1016/j.jbiomech.2016.10.001) [DOI] [PubMed] [Google Scholar]
- 15.Plitman Mayo R, Charnock-Jones DS, Burton GJ, Oyen ML. 2016. Three-dimensional modeling of human placental terminal villi. Placenta 43, 54–60. ( 10.1016/j.placenta.2016.05.001) [DOI] [PubMed] [Google Scholar]
- 16.Grafmäller S, Manser P, Krug HF, Wick P, von Mandach U. 2013. Determination of the transport rate of xenobiotics and nanomaterials across the placenta using the ex vivo human placental perfusion model. J. Vis. Exp. 76, e50401 ( 10.3791/50401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanie G. et al. 2015. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ. Health Perspect. 123, 1280–1286. ( 10.1289/ehp.1409271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Secomb TW, Hsu R, Park EYH, Dewhirst MW. 2004. Green’s function methods for analysis of oxygen delivery to tissue by microvascular networks. Ann. Biomed. Eng. 32, 1519–1529. ( 10.1114/B:ABME.0000049036.08817.44) [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Yipintsoi T, Bassingthwaighte JB. 1997. Nonlinear model for capillary-tissue oxygen transport and metabolism. Ann. Biomed. Eng. 25, 604–619. ( 10.1007/BF02684839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider H. 2015. Energy metabolism of human placental tissue studied by ex vivo perfusion of an isolated cotyledon. Placenta 36, S29–S34. ( 10.1016/j.placenta.2014.11.022) [DOI] [PubMed] [Google Scholar]
- 21.Fry BC, Roy TK, Secomb TW. 2013. Capillary recruitment in a theoretical model for blood flow regulation in heterogeneous microvessel networks. Physiol. Rep. 1, 1–13. ( 10.1002/phy2.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J-l, Qi M, Chinookoswong N, Scully S, Shi Z-Q. 1999. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology 140, 2117–2124. ( 10.1210/endo.140.5.6681) [DOI] [PubMed] [Google Scholar]
- 23.Nilsson LH, Hultman E. 1974. Liver and muscle glycogen in man after glucose and fructose infusion. Scand. J. Clin. Lab Invest. 33, 5–10. ( 10.3109/00365517409114190) [DOI] [PubMed] [Google Scholar]
- 24.Buerk DG, Saidel GM. 1978. Local kinetics of oxygen metabolism in brain and liver tissues. Microvasc. Res. 16, 391–405. ( 10.1016/0026-2862(78)90072-9) [DOI] [PubMed] [Google Scholar]
- 25.Hwang JJ, Johnson A, Cline G, Belfort-DeAguiar R, Snegovskikh D, Khokhar B, Han CS, Sherwin RS. 2015. Fructose levels are markedly elevated in cerebrospinal fluid compared to plasma in pregnant women. PLoS ONE 10, e0128582 ( 10.1371/journal.pone.0128582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yap C, Vickers MH, Clayton ZE, Sloboda DM. 2011. Maternal fructose intake during pregnancy and lactation alters placental growth and leads to sex-specific changes in fetal and neonatal endocrine function. Endocrinology 152, 1378–1387. ( 10.1210/en.2010-1093) [DOI] [PubMed] [Google Scholar]
- 27.Soydemir F. et al. 2011. Adapting in vitro dual perfusion of the human placenta to soluble oxygen tensions associated with normal and pre-eclamptic pregnancy. Lab Invest. 91, 181–189. ( 10.1038/labinvest.2010.171) [DOI] [PubMed] [Google Scholar]
- 28.Nye GA, Ingram E, Johnstone ED, Jensen OE, Schneider H, Lewis RM, Chernyavsky IL, Brownbill P. 2018. Human placental oxygenation in late gestation: experimental and theoretical approaches. J. Physiol. 596, 5523–5534. ( 10.1113/tjp.2018.596.issue-23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill JS, Salafia CM, Grebenkov D, Vvedensky DD. 2011. Modeling oxygen transport in human placental terminal villi. J. Theor. Biol. 291, 33–41. ( 10.1016/j.jtbi.2011.09.008) [DOI] [PubMed] [Google Scholar]
- 30.Beard D, Bassingthwaighte J. 2001. Modeling advection and diffusion of oxygen in complex vascular networks. Ann. Biomed. Eng. 29, 298–310. ( 10.1114/1.1359450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakadžić S. et al. 2016. Two-photon microscopy measurement of cerebral metabolic rate of oxygen using periarteriolar oxygen concentration gradients. Neurophotonics 3, 045005 ( 10.1117/1.NPh.3.4.045005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellums JD, Nair PK, Huang NS, Ohshima N. 1995. Simulation of intraluminal gas transport processes in the microcirculation. Ann. Biomed. Eng. 24, 1–24. ( 10.1007/BF02770991) [DOI] [PubMed] [Google Scholar]
- 33.Serov AS, Salafia CM, Filoche M, Grebenkov DS. 2015. Analytical theory of oxygen transport in the human placenta. J. Theor. Biol. 368, 133–144. ( 10.1016/j.jtbi.2014.12.016) [DOI] [PubMed] [Google Scholar]
- 34.Serov AS, Salafia CM, Brownbill P, Grebenkov DS, Filoche M. 2015. Optimal villi density for maximal oxygen uptake in the human placenta. J. Theor. Biol. 364, 383–396. ( 10.1016/j.jtbi.2014.09.022) [DOI] [PubMed] [Google Scholar]
- 35.Sapoval B. 1994. General formulation of Laplacian transfer across irregular surfaces. Phys. Rev. Lett. 73, 3314–3316. ( 10.1103/PhysRevLett.73.3314) [DOI] [PubMed] [Google Scholar]
- 36.Sapoval B, Filoche M, Weibel ER. 2002. Smaller is better—but not too small: a physical scale for the design of the mammalian pulmonary acinus. Proc. Natl Acad. Sci. USA 99, 10 411–10 416. ( 10.1073/pnas.122352499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grebenkov DS, Filoche M, Sapoval B, Felici M. 2005. Diffusion-reaction in branched structures: theory and application to the lung acinus. Phys. Rev. Lett. 94, 050602 ( 10.1103/PhysRevLett.94.050602) [DOI] [PubMed] [Google Scholar]
- 38.Grebenkov DS, Filoche M, Sapoval B. 2006. Mathematical basis for a general theory of Laplacian transport towards irregular interfaces. Phys. Rev. E 73, 021103 ( 10.1103/PhysRevE.73.021103) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the results and conclusions are present in the paper. The associated datasets and codes can be accessed via the Figshare repository (http://doi.org/10.6084/m9.figshare.7718462). Additional data related to this study may be available from the authors upon request.