Abstract
The treatment of severe chronic immune thrombocytopenia (SCITP) in pediatric patients is challenging. We evaluated the clinical efficacy and safety of eltrombopag in children with SCITP in China. This observational study was carried out at the Hematology Oncology Center, Beijing Children’s Hospital between April 2017 and July 2018. Patients with SCITP who had at least 12 weeks of eltrombopag treatment and follow-up data were included. Baseline data, such as age, drug dosage, pre-study platelet count, concomitant medications, and bleeding severity, were collected. Treatment response rates, durable response rates, bleeding events, and adverse events were assessed during eltrombopag therapy for at least 12 weeks. The median duration of eltrombopag therapy was 16 (12–48) weeks. The overall, complete, and partial response rates were 75% (15/20), 35% (7/20), and 40% (8/20), respectively. The durable response rate was 70% (14/20). No serious bleeding events or serious adverse events occurred during the study period. Eltrombopag appears to be effective and safe in children with SCITP, although additional research is needed to confirm this.
Keywords: children, eltrombopag, severe chronic immune thrombocytopenia
Introduction
Immune thrombocytopenia (ITP) is the most common autoimmune cytopenia in children. ITP is defined as an isolated low platelet count (<100 × 109/L) in the absence of an underlying cause, and those with thrombocytopenia lasting >12 months are considered to have chronic immune thrombocytopenia (CITP).1 Severe ITP is reserved for patients who have clinically relevant bleeding, defined as bleeding at presentation of sufficient magnitude to mandate treatment or by the occurrence of new bleeding symptoms requiring additional interventions or an increase in drug dose, which usually occurs when the platelet count is below 10–20 × 109/L. It is important that children with severe chronic immune thrombocytopenia (SCITP) receive appropriate treatment due to the bleeding risk associated with a low platelet count.
Eltrombopag is an oral, nonpeptide thrombopoietin receptor agonist (TPO-RA) that stimulates thrombopoiesis and is considered suitable for use in children.2 In pivotal clinical trials, the reported response rates of ITP to eltrombopag ranged from 59% to 88%, and the treatment was well tolerated.3,4 Continuous long-term treatment with eltrombopag for up to 3 years was reported to be safe, and the drug maintained its efficacy when used at a stable dose.5,6 Two international, randomized, double-blind, placebo-controlled trials of eltrombopag in pediatric patients with CITP demonstrated that the platelet response rate was 36%–44%.7,8 However, the safety of eltrombopag in children of China with severe ITP is largely unknown. To add to the growing body of knowledge regarding the use of eltrombopag in children, we describe our single-center experience of using eltrombopag to treat patients with SCITP. The primary aim of our study was to observe the clinical efficacy and safety of eltrombopag in the treatment of children with SCITP.
Materials and methods
Patients
This observational study of pediatric patients with SCITP who had received previous therapy was undertaken at Beijing Children’s Hospital, Capital Medical University, Beijing, China between April 2017 and July 2018. Patients who had at least 12 weeks of eltrombopag treatment and follow-up data were included in the analyses. The study was approved by the Ethics Committee of Beijing Children’s Hospital, and informed consent was obtained from each enrolled patient or his or her legally authorized guardian.
Treatment with eltrombopag
Before the start of therapy with eltrombopag, all patients were informed about potential adverse events, appropriate administration of the drug, and possible interaction with certain foods. Clinical hematology and liver function tests were evaluated regularly throughout therapy with eltrombopag, and the dosage regimen was adjusted based on the platelet count. The initial dose of eltrombopag was 50 mg once daily for pediatric patients aged 6–17 years and weighing ⩾27 kg and 1.5 mg/kg once daily for pediatric patients aged 1–5 years or weighing <27 kg.7 Platelet count was monitored weekly for 2 weeks and then monthly. Dose adjustments were permitted up to a maximum dosage of 75 mg/day with the aim of obtaining a platelet response that was at least a doubling of the baseline count in the absence of bleeding. If the platelet count was <50 × 109/L after treatment for at least 2 weeks, the daily dose was increased by 12.5 mg for children aged 1–5 years and 25 mg for children aged 6–17 years. We decreased the dose by 12.5 mg once per day at 2-week intervals if the platelet count increased to more than 200 × 109/L. If the platelet count increased to more than 400 × 109/L, we interrupted treatment and it was resumed at the next lower dose based on 12.5 mg increments once the patient’s platelet count had decreased to less than 150 × 109/L.9
Efficacy and safety analysis
Demographic and clinical characteristics were recorded, including age, gender, time since diagnosis (weeks), treatments for ITP prior to starting eltrombopag, platelet counts at diagnosis and before and during eltrombopag therapy, doses of eltrombopag used, duration of therapy, treatment response rates (see below), bleeding events, and adverse events during treatment with eltrombopag.
The primary efficacy endpoints were treatment response rates. A complete response (CR) was defined as a platelet count > 100 × 109/L and the absence of bleeding. A partial response (PR) was defined as a platelet count between 30 × 109/L and 100 × 109/L with at least a doubling of the baseline count and the absence of bleeding. The overall response rate (ORR) was defined as CR rate + PR rate. A durable response (DR) was defined as a PR or CR persisting for ⩾4 weeks with a stable dose of eltrombopag. No response (NR) was defined as a platelet count < 30 × 109/L, less than a doubling of the baseline count or bleeding events when the patient had received an appropriate dose of eltrombopag for 12 weeks. Safety was assessed based on the incidence of serious bleeding events (such as mucosal bleeding) and the occurrence of any adverse events, which were assessed according to the Common Terminology Criteria for Adverse Events.
Statistical analysis
Demographic and baseline clinical data are summarized using descriptive statistics. Descriptive summaries of the data were carried out using Excel (Microsoft, Redmond, WA). Normally distributed continuous variables are presented as the mean and standard deviation (SD), and nonnormally distributed continuous variables are described as the median and interquartile range (IQR). Discrete variables are expressed as percentages. Quantitative and qualitative data were compared using the Mann–Whitney U and Fisher’s exact tests, respectively. Statistical significance was assumed for P-values < 0.05. The statistical analyses were performed using SPSS 21.0 for Windows (IBM Corp., Armonk, NY).
Results
Patient characteristics
A total of 20 patients with SCITP were enrolled (see Table 1). The median age of the patients was 7 (1.4–14.6) years: nine patients (45%) were aged 1 to <6 years, nine patients (45%) were aged 6–12 years, and two patients (10%) were aged >12 years. The male–female ratio was 14:6. The baseline platelet count was 4 (1–12) × 109/L. The median duration of ITP was 1.8 (1.0–13.2) years. Of these patients, 2 (10%) had previously received sirolimus and 18 (90%) had been treated with corticosteroids, high-dose immunoglobulin or rituximab.
Table 1.
Demographic and clinical characteristics of 20 patients with severe chronic immune thrombocytopenia treated with eltrombopag.
| No. | Age (years) | Gender (M/F) | Type of treatment before eltrombopaga | ITP duration before eltrombopag (years) | Platelet count before eltrombopag (×109/L) | Starting dose of eltrombopag (mg/day) | Maximum dose of eltrombopag (mg/day) | Response time (days) | Duration of eltrombopag (months) | Maximum platelet count (×109/L) | Adverse drug reaction | Response after eltrombopag |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 4.2 | M | 1, 2, 3, 5 | 2.2 | 9 | 25 | 25 | 30 | 3 | 89 | N | PR |
| P2 | 10 | F | 1, 2, 3, 6 | 7.1 | 3 | 37.5 | 50 | N | 10 | 7 | N | NR |
| P3 | 1.4 | M | 1, 2, 3, 5 | 1.0 | 2 | 18.5 | 25 | 61 | 11 | 470 | N | CR |
| P4 | 6 | F | 1, 2, 3, 4, 5 | 5.1 | 3 | 37.5 | 37.5 | N | 6 | 15 | N | NR |
| P5 | 14.6 | M | 1, 2, 3, 4, 5, 6 | 13.2 | 3 | 50 | 50 | 6 | 12 | 121 | N | CR |
| P6 | 7 | F | 1, 2, 3, 5 | 5.1 | 2 | 37.5 | 50 | 17 | 3 | 40 | N | PR |
| P7 | 3.7 | M | 1, 2, 3, 5 | 1.8 | 7 | 25 | 25 | N | 3 | 7 | N | NR |
| P8 | 11 | M | 1, 2, 3, 4, 5, 6 | 10.3 | 1 | 50 | 50 | 6 | 9 | 100 | Rash | PR |
| P9 | 3 | M | 1, 5, 6 | 2.1 | 4 | 25 | 37.5 | 7 | 5 | 229 | N | CR |
| P10 | 10 | M | 1, 2, 3, 4, 5 | 6.0 | 2 | 50 | 50 | N | 6 | 52 | N | NR |
| P11 | 2.4 | M | 1, 3, 4 | 1.5 | 10 | 25 | 25 | 44 | 3 | 32 | N | PR |
| P12 | 2.6 | M | 1, 5 | 1.8 | 12 | 25 | 25 | 14 | 3 | 167 | N | CR |
| P13 | 13 | M | 1, 2, 3, 4, 5, 6 | 11.0 | 6 | 25 | 75 | 36 | 3 | 61 | N | PR |
| P14 | 7 | M | 1, 3, 5 | 1.8 | 11 | 37.5 | 37.5 | 6 | 4 | 81 | N | PR |
| P15 | 1.5 | M | 1, 2, 5 | 1.2 | 7 | 25 | 25 | 13 | 3 | 51 | N | PR |
| P16 | 1.4 | M | 1, 2, 3, 5 | 1.1 | 2 | 12.5 | 25 | N | 4 | 15 | N | NR |
| P17 | 1.9 | M | 1, 2, 5 | 1.4 | 10 | 25 | 25 | 7 | 3 | 51 | N | PR |
| P18 | 11 | F | 1, 2, 3, 4, 5 | 2.2 | 6 | 50 | 50 | 14 | 4 | 138 | N | CR |
| P19 | 10 | F | 1, 2, 3, 4, 5 | 1.8 | 5 | 50 | 50 | 26 | 4 | 122 | N | PR |
| P20 | 9.8 | F | 1, 2, 3, 4, 5 | 3.5 | 12 | 50 | 50 | 12 | 3.5 | 156 | N | CR |
ITP: immune thrombocytopenia; N: No; PR: partial response; NR: no response; CR: complete response; R: response; M: male; F: female.
1: prednisone; 2: intravenous immunoglobulin; 3: dexamethasone; 4: rituximab; 5: thrombopoietin; 6: sirolimus.
Eltrombopag dosage and efficacy
The median duration of eltrombopag therapy and follow-up was 16 (12–48) weeks. Median time to response was 14 (5–40) days (Figure 1). The median platelet count at the initial response was 32 × 109/L (12–167 × 109/L). Median peak platelet count achieved during eltrombopag therapy was 100 × 109/L (40–470 × 109/L).
Figure 1.
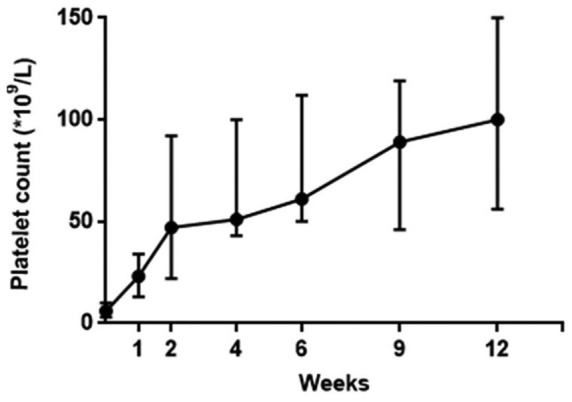
Platelet counts in children with severe chronic immune thrombocytopenia during 12 weeks of treatment with eltrombopag.
An escalating dose of eltrombopag after the 2-week observation period was used in 5/20 patients (25%), while the initial dose was maintained in 15/20 patients (75%). The ORR was 15/20 (75%), the CR rate was 7/20 (35%), and the PR rate was 8/20 (40%). A DR was observed in 14/20 patients (70%) and in 14/15 patients (93.3%) showing CR or PR. The median maintenance dose of eltrombopag was 1.67 mg/kg (1.2–2.3 mg/kg) in children aged 1–5 years, and the drug was effective in eight patients (mean dose: 1.60 mg/kg) and ineffective in three patients (mean dose: 1.76 mg/kg). The median maintenance dose was 50 mg (37.5–75 mg) in children aged 6–17 years, and the drug was effective in two patients (mean dose: 50 mg) and ineffective in two patients (mean dose: 50 mg). Statistical analysis showed that the efficacy of eltrombopag in each age group was independent of the drug dose (P = 0.87 and 0.73, respectively). Among the patients (15 cases) in whom eltrombopag therapy was effective, 10 patients received the reducing dose according to the rules and 5 effective patients received the initial dose. In 10 “reducing dose” patients, an effective level was maintained in 9 patients and one patient’s platelet count decreased after a reduction in eltrombopag dose but recovered after subsequent dose escalation (initial dose). Besides, five patients (received the initial dose) still kept an effective level.
Four patients (20%) received concomitant ITP medication (low doses of prednisone, 0.3–0.5 mg/kg, separately, Qod-Biw) during treatment with eltrombopag. It is not yet possible to evaluate the synergistic effect of glucocorticoid combined with eltrombopag. A clinical response to eltrombopag was also shown by a reduction in bleeding events from 75% at baseline to 10% at week 16.
Safety
No serious adverse events were reported. Only one case (5%) of rash occurred. Headache (usually the most frequently reported adverse event) was not observed. No adverse events caused treatment withdrawal. Two patients (10%) had minor bleeding episodes during treatment with eltrombopag. Liver transaminases were not elevated during eltrombopag therapy. No patients developed new cataracts or thromboembolic events during the study, and no malignancies or thromboses were reported. In all of the 20 cases, only 1 patient’s platelet count rose to 470 × 109/L, which was reduced to the normal range by timely dosage adjustment, and no adverse reaction was found.
Discussion
Treatment approaches for children with SCITP are largely based on evidence from studies in adults, and few randomized trials have assessed the treatment response in children. This is the first study to systematically evaluate the clinical efficacy and safety of eltrombopag in the treatment of pediatric patients with SCITP in China. This report describes our experience with eltrombopag in real-world practice.
Efficacy
The ORR of our study was 75%, which is higher than that reported previously for children with CITP but lower than that reported for adults with ITP. We speculate that dietary restrictions and swallowing difficulties may make it difficult for the youngest children to comply with treatment. Due to fewer research data in real-world and application experience, especially for children, we pay more attention to its safety. For careful consideration, we initially used it as a third-line treatment. These may also contribute to a lower response rate to that observed in adults with CITP.
Previous studies10 have found a correlation between the efficacy of eltrombopag and its dosage, but this was not detected in our study. This discrepancy may be related to certain factors relevant to the children in our study, such as the underlying pathophysiology or the use of concomitant medication. In our study, the mean effective and ineffective doses of eltrombopag in children aged 1–5 years were 1.60 and 1.76 mg/kg, respectively. For children aged 6–17 years, the effective and ineffective doses were the same. Younger children generally are prescribed higher doses of drugs relative to body size than older patients probably because they have higher clearance7 and tolerance levels. Whether pediatric patients who do not respond to 75 mg eltrombopag would respond to higher doses is currently being investigated and will be reported in a future study.
Clinical evidence obtained in patients with ITP and other thrombocytopenias has demonstrated the feasibility of combining eltrombopag with immunosuppressants. It is possible that simultaneous treatment of insufficient platelet production with eltrombopag and increased platelet destruction with other ITP medications may elicit a synergistic response in patients refractory to monotherapy-based approaches. However, we were unable to test this possibility in this study due to the very small sample size (only four cases of concomitant therapy).
Interestingly, the number of patients showing bleeding symptoms in our study was reduced following treatment with eltrombopag irrespective of whether or not they were responders, although the median platelet count remained between 12 × 109/L and 18 × 109/L in those who did not respond during the course of treatment. It is possible that enhanced platelet adhesion may have contributed to this finding: eltrombopag upregulates the platelet collagen receptor (glycoprotein VI (GPVI)),11 and this action may have reduced the risk of hemorrhage in both treatment responders and nonresponders.
Safety
The adverse event profile was similar to that seen in adults with CITP, except that we observed no thrombotic events (this may be related to the physical characteristics of children) or malignancies (perhaps because children tolerate eltrombopag better than adults). Our findings are consistent with a previous randomized, placebo-controlled, phase 3 study.7,8 However, other studies12 reporting the effects of TPO-RAs on the bone marrow in adults have shown a small risk of progressive fibrosis after more than 2–5 years of treatment. Although the results of adult studies are reassuring, the impact of long-term eltrombopag therapy on the bone marrow of children has not been studied and remains unknown. Longer follow-up and serial bone marrow examinations will be needed to establish whether long-term administration of eltrombopag has detrimental effects on the bone marrow. Although hepatotoxicity and thrombotic events are important side effects of eltrombopag, they are infrequent. In such a small number of patients, it is very possible that they would not encounter any significant adverse events due to the rare nature of these events. Additional studies with larger sample sizes are needed to assess the long-term efficacy and safety of eltrombopag in the treatment of pediatric patients with SCITP. Eltrombopag was effective and safe as a second- or third-line treatment option in patients who were intolerant or refractory to first- and/or second-line therapy. Treatment with eltrombopag resulted in a stable increase in platelet count and a reduction in the need for concomitant therapy for ITP. Our findings suggest that the use of eltrombopag might be a reasonable approach to supporting the platelet count while waiting for a spontaneous improvement in children. The efficacy of eltrombopag in the treatment of children with SCITP is encouraging. Thus, eltrombopag could be a valuable addition to the limited treatment options available for the management of SCITP in children who show an inadequate response to first-line therapies. However, our study is limited in its retrospective design, small sample, and relatively short follow-up. We are unable to comment on the impact of eltrombopag on health-related quality of life (HRQoL) or specifics regarding bleeding symptoms that may guide treatment decisions. Because a retrospective assessment of bleeding severity is difficult, we only describe whether bleeding events occurred. Since improved HRQoL was endorsed as a reason for initiating a TPO-RA, the application of HRQoL measures both a priori and throughout the treatment may provide useful information. Prospective trials collecting information on decision making, HRQoL, and bleeding assessment are critically needed.
Our results represent patients on treatment for variable periods of time, making conclusions about long-term therapy difficult. Hence, we focus on the 12-week treatment therapy, which is similar to the initial time period of many clinical trials. Only 20 patients were included, but this is the first single-center report of eltrombopag for pediatric ITP patients in China. Future trials need to identify the population of Chinese pediatric patients who will benefit the most from the agent, proper dosing strategies to expand their use, and comparison of patient-related outcomes with other therapeutic options.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: English language editing was performed by MedSci and funded by Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the Construction of Clinical Evaluation Technology Platform for Children Demonstration New Drugs (No. 2017ZX09304029) and Beijing Natural Science Foundation of China (No. 7192046).
ORCID iD: Xiaoling Cheng  https://orcid.org/0000-0002-0048-2658
https://orcid.org/0000-0002-0048-2658
References
- 1. Kim TO, Despotovic J, Lambert MP. (2018) Eltrombopag for use in children with immune thrombocytopenia. Blood Advances 2(4): 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merli P, Strocchio L, Vinti Let al. (2015) Eltrombopag for treatment of thrombocytopenia-associated disorders. Expert Opinion on Pharmacotherapy 16(14): 2243–2256. [DOI] [PubMed] [Google Scholar]
- 3. Bussel JB, Cheng G, Saleh MNet al. (2007) Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. The New England Journal of Medicine 357(22): 2237–2247. [DOI] [PubMed] [Google Scholar]
- 4. Cheng G, Saleh MN, Marcher Cet al. (2011) Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. The Lancet 377(9763): 393–402. [DOI] [PubMed] [Google Scholar]
- 5. Noronha V, Philip SD, Joshi Aet al. (2012) Prolonged remission from eltrombopag in chronic refractory idiopathic thrombocytopenic purpura. International Journal of Hematology 96(3): 380–382. [DOI] [PubMed] [Google Scholar]
- 6. Wong RSM, Saleh MN, Khelif Aet al. (2017) Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: Final results of the EXTEND study. Blood 130(23): 2527–2536. [DOI] [PubMed] [Google Scholar]
- 7. Bussel JBD, deMiguel PG, Despotovic JMet al. (2015) Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): A randomised, multicentre, placebo-controlled study. The Lancet. Haematology 2(8): e315–e325. [DOI] [PubMed] [Google Scholar]
- 8. Grainger JD, Locatelli F, Chotsampancharoen Tet al. (2015) Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): A randomised, multicentre, placebo-controlled trial. The Lancet 386(10004): 1649–1658. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Liu X, Wang Let al. (2019) Successful discontinuation of eltrombopag in one child with refractory primary immune thrombocytopenia and literature review. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis 30(2): 71–74. [DOI] [PubMed] [Google Scholar]
- 10. Wire MB, Li X, Zhang Jet al. (2018) Modeling and simulation support eltrombopag dosing in pediatric patients with immune thrombocytopenia. Clinical Pharmacology and Therapeutics 104(6): 1199–1207. [DOI] [PubMed] [Google Scholar]
- 11. Ghadaki B, Nazi I, Kelton JGet al. (2013) Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion 53(11): 2807–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brynes RK, Wong RS, Thein MMet al. (2017) A 2-year, longitudinal, prospective study of the effects of eltrombopag on bone marrow in patients with chronic immune thrombocytopenia. Acta Haematologica 137(2): 66–72. [DOI] [PubMed] [Google Scholar]


