Abstract
The only clinically accepted method of fertility preservation in young women facing gonadotoxic chemo- and/or radiotherapy for malignant or autoimmune diseases is cryopreservation of embryos or unfertilized ova, whereas cryopreservation of ovarian tissue for future reimplantation, or in vitro maturation of follicles, and the use of gonadotropin-releasing hormone agonists (GnRHa) are still considered investigational, by several authorities. Whereas previous publications have raised the fear of GnRHa’s possible detrimental effects in patients with hormone receptor-positive breast cancers, recent randomized controlled trials (RCTs) have shown that it either improves or does not affect disease-free survival (DFS) in such patients. This review summarizes the pros and cons of GnRHa co-treatment for fertility preservation, suggesting 5 theoretical mechanisms for GnRHa action: (1) simulating the prepubertal hypogonadotropic milieu, (2) direct effect on GnRH receptors, (3) decreased ovarian perfusion, (4) upregulation of an ovarian-protecting molecule such as sphingosine-1-phosphate, and (5) protecting a possible germinative stem cell. We try to explain the reasons for the discrepancy between most publications that support the use of GnRHa for fertility preservation and the minority of publications that did not support its efficiency.
Keywords: Fertility preservation, GnRH analogues, chemotherapy, premature ovarian insufficiency
The increase in cancer incidence at young ages and the significant increase in long-term survival have brought about interest among reproductive endocrinologists, gynecologists, hematologists, oncologists, pediatricians, rheumatologists, endocrinologists, family physicians, general practitioners, and actually all health care providers in attempts to preserve fertility in young patients exposed to gonadotoxic chemo- and radiotherapy.1-9 Indeed, the request for fertility preservation has dramatically increased, currently representing a standard of care for young patients facing gonadotoxic therapies.1-9 Challenging the remote effects of malignancy is a medical priority in oncology, targeted at preserving survivors’ quality of life, including gonadal function and fertility. The coined specialty “Oncofertility” and preservation of fertility have revolutionized reproductive endocrinology and affected many medical specialties, specifically oncology, assisted reproduction, and infertility treatment.1-10
Whereas other authors elaborate on the other methods, we will specifically elaborate on gonadotropin-releasing hormone agonist (GnRHa) cotreatment for fertility preservation.
Endocrine Suppression Before and Along With Gonadotoxic Chemotherapy
The rationale for endocrine suppression using GnRH analogs
The odds of preserving gonadal function following combined-modality treatment are significantly better for prepubertal girls than for boys.9,11,12 The reduced gonadotoxicity in prepubertal girls is likely due to their higher ovarian reserve, in addition to the hypogonadotropic prepubertal milieu. Whereas ovarian function has been preserved in most long-term female survivors treated prepubertally for lymphoma,12 but only in about half of similarly treated adult reproductive age women,1 it is logical to generate a temporary and reversible prepubertal milieu in women of reproductive age before and during gonadotoxic chemotherapy.1,4-9 Following this hypothesis, GnRHa co-treatment has been used by many groups of clinicians to reduce the gonadotoxic effects of chemotherapy1,4-9,13-15 by simulating a prepubertal hormonal milieu, under the philosophy that preventing premature ovarian failure (POF) in survivors is preferable to treating it, following the dictum: “An ounce of prevention is worth a pound of cure.”
Past and Present Experience Using GnRH Analogs for Fertility Preservation
The possibility of administering a noninvasive adjuvant treatment that may reduce the gonadal damage caused by gonadotoxic chemotherapy is obviously attractive.1,4-9,13-15 Although Glode et al16 suggested, almost 4 decades ago, that GnRHa appeared to protect male mice from the gonadotoxicity of cyclophosphamide, GnRHa co-treatment is not effective in the male,17 as opposed to female.1,4-9,13-15 It has been suggested that primordial follicles (PMFs), which are metabolically inactive, fare better than active growing follicles and cells that are part of an active cell cycle.9 Girls fare better than boys in their odds of preserving gonadal function following gonadotoxic chemotherapy.1,4-9,17 Ovarian function was preserved in over 90% of long-term female survivors who were treated prepubertally for lymphoma, but it was preserved only in a minority of similarly treated adult patients.11 Numerous preclinical and clinical studies have attempted to induce a temporary prepubertal milieu in postpubertal females before and during gonadotoxic chemotherapy.1,4-9,13-15 The only primate study that assessed ovarian histology before and after GnRHa co-treatment along chemotherapy, which obviously cannot be conducted clinically in humans, has been done more than 3 decades ago.18 This prospective randomized study demonstrated that GnRHa might protect the ovary against cyclophosphamide-induced damage.18 Co-treatment with GnRHa from before and along cyclophosphamide has significantly decreased the daily rate of follicular decline, from 0.12% ±0.012% to 0.057% ± 0.019% (P < 0.05), and the total number of PMFs lost during the chemotherapeutic insult from 64.6% ± 2.8% to 28.9% ± 9.1% (P < 0.05), compared with cyclophosphamide without GnRHa co-treatment.18
In the clinical setting, many groups of clinicians have used GnRHa co-treatment along with chemotherapy for fertility preservation and reducing its gonadotoxic effects.1,4-9,13-15 However, a limitation is that not all the studies conducted so far had pregnancy as the primary outcome.19
The philosophy of this approach suggests that preventing POF/Premature Ovarian Insufficiency (POI) in survivors is more advantageous than treating it, by reimplantation of cryopreserved ovarian tissue.1,4-9,13-15,19-37 Therefore, this noninvasive and inexpensive treatment has gained ubiquitous interest and popularity.1,4-9,13-15,19-63 Although 9 studies including 572 patients reported that GnRHa treatment before and along with chemotherapy did not bring about a significant decrease in POF rate, over 24 studies (15 retrospective and 9 randomized controlled trial [RCT]) including over 3100 patients receiving this treatment for breast cancer, hematologic cancers, or autoimmune diseases necessitating chemotherapy reported a significant decrease in POF rate in survivors.1,4-9,13-15,19-63 The GnRHa adjuvant co-treated patients resumed regular menses and normal ovarian function in about 85% to 90% of cases as compared to 40% to 50% in the chemotherapy only group.1,4-9,13-15,19-63 However, return of cyclic ovarian function (COF), normal gonadotropins, and other sex hormonal levels, such as anti-Mullerian hormone (AMH), progesterone, and estradiol, and even antral follicle count (AFC) are only surrogate markers of fertility preservation. Therefore, it is important to compare the natural pregnancy rate (PR) in survivors in the GnRHa-treated group versus controls.1,4-9,13-15,19-63 Indeed, natural PR in survivors who were co-treated with GnRHa adjuvant during gonadotoxic chemotherapy ranged from 23% to 88%, compared to 11% to 35% (P < 0.05) in control patients who did not receive this co-treatment.1,4-9,13-15,19-63
Eighteen meta-analyses of RCTs13 and 4 recent international expert consensus meetings,28,29,54,58 in addition to many smaller studies, have critically summarized the utility of GnRHa adjuvant co-treatment along with chemotherapy, concluding that this co-treatment significantly reduces the risk of POF and increases the PR in survivors.1,4-9,13-15,19-63 Three recent, large prospective RCTs in patients with breast cancer support the use of GnRHa cotreatment for fertility preservation.34,35,46 The US, POEMS–SWOG/S0230 study enrolled only hormone receptors (HR)-negative breast cancer patients,34 whereas most patients in the Italian PROMISE–GIM635,59 study were HR positive. Both RCTs have demonstrated a statistically significant 70% to 72% reduction in ovarian failure rate in the GnRHa arms, (odds ratio [OR]: 0.28-0.30; P < 0.001-0.04; OR: 0.30; P = 0.04). Moreover, the PR was significantly increased by GnRHa (OR: 2.45; P = 0.03).34,35,46 The PROMISE–GIM6 study46 performed a long-term assessment of the survivors, with a median follow-up of 7.3 years (range, 6.3-8.2 years).35 This follow-up shows a 5-year cumulative COF resumption of 72.6% (95% confidence interval [CI], 65.7%-80.3%) in the GnRHa group compared to 64% (95% CI, 56.2%-72.8%) among controls (age-adjusted hazard ratio, 1.48 [95% CI, 1.12-1.95]; P = 0 .006), with no difference in 5-year disease-free survival (DFS).35
In the POEMS–SWOG S0230 study, a National Institutes of Health (NIH)-sponsored prospective RCT in which 257 premenopausal breast cancer patients received chemotherapy with or without GnRHa, the GnRHa-treated patients had an improved COF rate across multiple endpoints and higher fertility (more pregnancies) than the controls.34 Unexpectedly, the GnRHa co-treatment led to more favorable DFS and overall survival (OS) rates compared to the chemotherapy alone controls.34 Two years after chemotherapy, the POF/POI rate was 22% for the standard chemotherapy arm versus only 8% for the GnRHa arm (OR = 0.30, 95% CI [0.09-0.97]; P < 0.04).34 Successful pregnancy was achieved by 12 of the 18 survivors who attempted pregnancy in the chemotherapy alone group compared with 22 of the 25 survivors who successfully conceived in the GnRHa arm (adjusted OR, 2.45; P < 0.03).34 In addition, women in the GnRHa group gave birth to 18 healthy neonates compared to 12 in the standard chemotherapy group.34 Moreover, in a surprising and unexpected finding, the 4-year mortality rate in the GnRHa group was significantly lower than in the chemotherapy without GnRHa group (P = 0.05).34 Most recently, the investigators of the POEMS/SWOG S0230 study published their final analysis after 5 years follow-up.60 Two-sided statistical tests detected more patients in the chemotherapy + GnRHa arm reported at least one pregnancy vs the chemotherapy alone arm (5-year cumulative incidence = 23.1%, 95% CI = 15.3-31.9; and 12.2%, 95% CI = 6.8-19.2, respectively; OR = 2.34; 95% CI = 1.07-5.11; P = 0.03). This long-term follow-up found continued evidence that patients randomly assigned to receive goserelin + chemotherapy were not only more likely to avoid POF/POI but were also more likely to become pregnant without adverse effect on disease-related outcomes.60
The third large, prospective, parallel, Anglo Celtic Group OPTION RCT46 examined the effect of GnRHa administration before and during chemotherapy to 202 stage I-IIIB breast cancer patients. The primary outcome was amenorrhea between 12 and 24 months after randomization, supported by elevated follicle-stimulating hormone (FSH).46 This RCT has found that the GnRHa, goserelin, reduced the prevalence of amenorrhea between 12 and 24 months to 22% vs 38% in the control group (P = 0.015) and the prevalence of POI to 18.5% vs 34.8% in the controls (P = 0.048). Follicle-stimulating hormone concentrations were also lower in all women treated with GnRHa at both 12 and 24 months (P = 0.027, P = 0.001), respectively. The authors concluded that GnRHa had significantly reduced the risk of POI in women treated with chemotherapy for early breast cancer, in women younger than 40 years.46 Furthermore, this study has also found significant changes in bone turnover markers between the GnRHa-treated women and controls, at 36 months (P = 0.0006).46 The authors suggested that GnRHa addition to chemotherapy may offer sufficient ovarian protection to negate long-standing altered bone turnover associated with POI, mainly in patients older than 40.46
Most relevant to this equivocal and highly debatable issue, a study from a previous opponent group to GnRHa use for fertility preservation61 found that the use of GnRHa during chemotherapy has significantly increased the probability of natural conception (OR, 12.87; P = 0.001).37 Furthermore, these investigators37 adjusted the analysis to a high degree and nevertheless “ . . . found surprisingly strong (OR12) > indirect evidence supporting the prophylactic use of GnRHa in women receiving therapy for early unfavorable HL” (HL = Hodgkin Lymphoma). They37 concluded that the multivariate analysis revealed that the use of GnRHa during therapy is a “strong, independent, and highly significant predictor of pregnancies.”
In our own research, we found that GnRHa co-treatment, along with chemotherapy, could significantly increase natural conceptions in survivors (P < 0.006), in addition to preserving COF (OR = 6.87) in a large group of young women followed for more than 2 decades.19 Ninety patients (62%) conceived 178 times in the GnRHa group versus only 32 experiencing 56 pregnancies (42%) in the controls (P < 0.003), generating 131 and 42 newborns (P < 0.01), respectively.19 Natural pregnancies occurred in 58% of the survivors in the GnRHa group versus 38% of the controls (P = 0.009).19 These results are in keeping with the results of the large, recent RCTs and meta-analyses.13-15,34-36,46,60
As the ultimate gold standard of fertility preservation is pregnancy, it is notable to mention that 3 different studies from 3 continents have shown high PRs after GnRHa co-treatment versus controls:
In Wong et al’s39 UK study, 71% (30 of 42 who attempted pregnancy) of survivors treated with GnRHa and chemotherapy achieved pregnancy, in a nonrandomized study.
In the POEMS–SWOG RCT in the United States,34 88% of survivors (22 of 25 who attempted pregnancy) treated with GnRHa and chemotherapy achieved pregnancy, compared with 66.7% (12 of 18 who attempted pregnancy) in the control group (P = 0.03).
In our nonrandomized study,19 in Israel, 68.8% of the survivors (84 of 122 who attempted pregnancy) naturally conceived, compared with 42.4% (28 of 66 who attempted pregnancy) in the control group (P = 0.006).
Furthermore, GnRHa was effective in protecting COF and decreasing the POF/POI rate, not only in conventional chemotherapy, but also in survivors after stem-cell transplantation, who had received aggressive gonadotoxic conditioning chemotherapy.43,44 In the GnRHa group, 38.3% (18/47) of patients resumed COF, compared with 11.1% (4/36) of patients who did not receive GnRHa (P = 0.01). Gonadotropin-releasing hormone agonist had a significant effect on long-term COF in patients with lymphomas (66.7% [14/21] for GnRHa group vs 18.2% [2/11] for control) (P = 0.01).43
Four recent international consensus meetings support the use of GnRHa for fertility preservation: the 2015 St. Gallen International Expert Consensus panel28 and the National Comprehensive Cancer Network guidelines (www.nccn.org).29 The St. Gallen international expert consensus28 stated that GnRHa therapy during chemotherapy “proved effective to protect against POF and preserve fertility” in young women with ER-negative breast cancer undergoing chemotherapy. This consensus states that GnRHa co-treatment also increased the rate of subsequent successful pregnancies and did not compromise disease outcomes.28 The second expert consensus meeting summarized 10 concluding recommendations.29 The recommendations were graded according to the levels of evidence and grades of recommendation (according to the European Society of Medical Oncology [ESMO] Clinical Practice Guidelines for fertility preservation in patients with cancer).29 The only conclusion (out of 10) that received the highest grading was that regarding GnRHa.29 This conclusion states,
Ovarian suppression with the use of luteinizing hormone releasing hormone agonist (LHRHa) during chemotherapy should be considered a reliable strategy to preserve ovarian function and fertility, at least in breast cancer patients, given the availability of new data suggesting both the safety and the efficacy of the procedure have become available (IA).29
A third international consensus meeting, the second international consensus guidelines for breast cancer in young women,54 similarly supported the beneficial protective role of GnRHa in young patients with both HR-positive and HR-negative breast cancer. The fourth international consensus, the European School of Oncology (ESO)-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3), also concluded that GnRHa preserves ovarian function and increases PR.58
The conclusions of the 4 international expert meetings28,29,54,58 are in keeping with most recent summaries and meta-analyses of RCTs, including the most recent Cochrane Collaboration analysis update31 confirming that the use of GnRHa is associated with a reduced risk of POF and significantly increased PR.13-15 Therefore, the Italian association of medical oncology (AIOM) has adopted the routine clinical utility of temporary ovarian suppression with LHRHa during chemotherapy as a strategy to preserve ovarian function and fertility in patients with breast cancer.63 In addition, GnRHa can effectively prevent thrombocytopenia-associated menorrhagia in the treated patients.1,5,9,13-15
A large meta-analysis26 of 12 RCTs including 1231 patients with breast cancer in 2015 concluded the following:
The use of GnRHa was associated with a significant reduced risk of POF/POI (OR = 0.36, 95% CI - 0.23-0.57; P < 0.001), yet with significant heterogeneity (P for heterogeneity = 0.026).
In 8 studies reporting amenorrhea rates 1 year after chemotherapy completion, the addition of GnRHa reduced the risk of POF/POI (OR = 0.55, 95% CI = 0.41-0.73, P < 0.001) without heterogeneity (P for heterogeneity = 0.936).
In 5 studies reporting pregnancies, more patients treated with LHRHa achieved pregnancy (33 vs 19 women; OR = 1.83, 95% CI = 1.02-3.28, P = 0.041; P for heterogeneity = 0.629).
In 3 studies reporting on disease-free survival, no difference was observed (hazard ratio = 1.00, 95% CI = 0.49-2.04, P = 0.939; P for heterogeneity = 0.044).
In several countries, like Italy and Israel, the coverage for the cost of GnRHa is granted to all young patients with breast cancer who are candidates to chemotherapy and are interested in fertility preservation.40
Most recently, Lambertini et al,13 have summarized all the studies on GnRHa for fertility preservation in an extensive overview of the literature. In this recent meta-analysis, out of 7 preclinical studies in female mice evaluating temporary ovarian suppression with GnRHa during chemotherapy, five were in support and two did not conclude this cotreatment to be effective.13 Out of 3 preclinical studies in female primates and human models evaluating GnRHa effect during chemotherapy, two were supportive and the third was not.13 Ten out of 14 randomized trials evaluating temporary ovarian suppression with GnRHa during chemotherapy in patients with breast cancer were in support, whereas four did not conclude this cotreatment to be effective.13 However, another recent meta-analysis14 and systematic review used individual patient-level data of the 5 major RCTs, multivariate logistic regression analysis, and a random effects model for statistical analyses to evaluate the GnRHa strategy.14 This study provided robust evidence for both the efficacy and the safety of GnRHa as a clinical option to reduce POI and improve fertility.14,62 Interestingly, several of the coauthors in this meta-analysis have previously authored publications that did not support the efficacy of GnRHa, including those cited as “negative conclusion” in the recent summary.13 These investigators should be congratulated for their scientific integrity and courage of changing and updating their previous opinion.62
However, whereas most recent RCT of GnRHa cotreatment suggest a beneficial role in reducing POF/POI in patients with breast cancer, a similar conclusion on the benefit of GnRHa for fertility preservation in patients with hematologic malignancies has not been unequivocally accepted.13
The updated results from the study by Demeestere et al38,52 have shown no gonadal protection of GnRHa cotreatment in patients with lymphoma. However, an expert opinion21 claimed that although providing important insights on long-term outcomes, several limitations should be considered in the interpretation of these updated results. The Demeestere et al52 study was underpowered, there was a dropout rate of 50%, and 25% of the study population were lost to follow-up or their data were unavailable.52,62 The authors did not use a composite end point but relied only on one FSH measurement with a possible increased false-positive rate.52 Although the original study design mandated the accrual of 157 patients to ensure a power of 80% and a type error I probability of 5%, enrollment was discontinued after the assignment of 129 patients, but only 63 patients were evaluated for POF/POI, 31 to 32 in each arm, in 15 centers (1-3 patients/arm/center).52 Furthermore, 5 pregnancies were reported in patients with protocol defined POF of “one FSH >40 U/L measurement” challenging the accuracy of POF definition and the resulting conclusions.52 The small number of the evaluated patients (α error) may explain the “negative” results after 1 year, the pendulum swinging to “positive” result at 2 years, and again switching back to negative conclusion at 5 years.38,52,64 At 2 years evaluation, Demeestere et al64 observed a significantly improved ovarian function resumption on their lymphoma patients: “the number of patients who totally restored their ovarian function (FSH ⩽10 IU/L) was higher in the GnRHa group (P = 0.049) confirming results of AMH.” They concluded, at 2 years evaluation, that “Triptorelin . . . has a positive effect on the ovarian reserve . . . ”64 Moreover, in both treatment arms, patients could receive other forms of hormonal treatments (norethisterone acetate and hormonal contraceptives) with potential diminished observed protective effect of GnRHa.38,52
Nevertheless, although reported as a study not supporting GnRHa use, the results in terms of both OR for treatment-related POF and PR observed in this study were claimed to be consistent with the protective effect of GnRHa administration seen in the breast cancer trials.38,40,62 It has been suggested that the results have not achieved statistical significance due to lack of power.40,62 The expert opinion40 claims that patients with lymphoma are younger and treated with either highly gonadotoxic regimens (conditioning before bone marrow transplantation [BMT]) or of low gonadotoxicity (such as Adriamycin. Bleomycin, Vinblastine, Dacarbazine [ABVD]). On the contrary, patients with breast cancer are older and treated with intermediate gonadotoxic chemotherapy.40 These differences may possibly explain the modest but clear benefit of GnRHa cotreatment evident in women with breast cancer but not always evident in patients with lymphoma.40 Therefore, large, well-designed RCTs are still needed to unequivocally determine the protective effect of GnRHa in hematologic and other malignancies.
Possible mechanisms
Five possible mechanisms have been put forward to explain the effect of GnRHa in minimizing the gonadotoxic effect of chemotherapy (Table 1).
Table 1.
Possible mechanisms whereby GnRHa may minimize POF.
| # | Mechanism | Support/Pro | Limitations/Con |
|---|---|---|---|
| 1 | Simulating the prepubertal hypogonadotropic state | Prepubertal girls are more resistant to the gonadotoxic effect of chemotherapy than adult women are. Figure 1 suggests a possible pathophysiologic mechanism, similar to the “burn-out” theory. | The relative resistance of prepubertal girls may be due to their significantly larger pool of primordial follicles and not due to the low gonadotropin level. |
| 2 | Possible direct ovarian effect | It has been demonstrated that Buserelin, a GnRHa, may minimize the gonadotoxic effect of doxorubicin, in vitro, regardless of the hypogonadotropic milieu. Ovarian GnRH receptors have been demonstrated in several species, including human. | It is unknown what is the exact mechanism whereby GnRH receptors may minimize gonadotoxicity and which ovarian cells are responsible for such a direct effect. |
| 3 | Hypoestrogenic decreased ovarian perfusion | The decreased utero-ovarian perfusion in the hypoestrogenic milieu generated by GnRHa-induced pituitary desensitization may result in lower total cumulative exposure of the ovaries to the chemotherapeutic agents, secondarily resulting in a decreased gonadotoxic effect. | Theoretically, in cases of ovarian metastases, limited ovarian exposure to chemotherapy may increase the risk of persistent ovarian disease. However, such an effect has not been reported. |
| 4 | Up-regulation of an ovarian-protecting molecule such as sphingosine-1-phosphate | Monkeys administered S1P or its analogue by direct intraovarian cannulation for a week before ovarian irradiation resumed menstrual cycles and maintained the ovarian follicles. Not only ovarian follicles were preserved but also fertility and spontaneous conception. | There is no proof that GnRHa up-regulates the intraovarian S1P concentration. |
| 5 | Protecting a possible germinative stem cell (GSCs) | Menopausal FSH and undetectable AMH were observed in one-third of patients for up to 1 year after chemotherapy + GnRHa. Afterward, FSH decreased to normal and AMH increased in most patients, and over 60% conceived. Possibly, the protected GSCs started growing and producing inhibin, AMH, and sex hormones about a year after chemotherapy, decreasing FSH levels. | The hypothesis is a theoretical speculation, without supporting evidence. |
Abbreviations: AMH, anti-Mullerian hormone; FSH, follicle-stimulating hormone; GnRHa, gonadotropin-releasing hormone agonists; POF, premature ovarian failure.
Five theoretical mechanisms to explain how the GnRHa may preserve fertility: (1) simulating the prepubertal hypogonadotropic state; (2) direct ovarian effect; (3) hypoestrogenic decreased ovarian perfusion; (4) upregulation of an ovarian-protecting molecule such as sphingosine-1-phosphate; and (5) protecting a possible germinative stem cell.
Simulating a prepubertal hypogonadotropic milieu
The hypogonadotropic state, induced by GnRHa, simulates the prepubertal hormonal milieu. The gonadotoxic chemotherapy induces follicular loss resulting in decreased secretion of sex hormones and inhibins.4-9 The resultant low systemic concentrations of steroid sex hormones and inhibin feedback on the pituitary and hypothalamus to increase gonadotropins secretion, mainly FSH. The increased FSH concentrations may enhance and augment the rate of resting preantral follicle recruitment and maturation and the ability to enter the unidirectional process of folliculogenesis. Due to the active metabolism of dividing cells during folliculogenesis, these growing follicles are subjected to the gonadotoxic effects of chemotherapy, ending in an accelerated rate of follicular demise4-9 (Figure 1). Similarly, Meirow’s group55,56 coined the “burnout theory” to describe the enhanced follicular demise due to accelerated folliculogenesis in the ovary after gonadotoxic chemotherapy. They postulated that alkylating agents increase phosphorylation of proteins through the phosphatidylinositol 3-kinase signaling pathway that stimulates accelerated PMF activation of both oocytes and granulosa cells, resulting in the “burnout” effect and follicle loss.55,56 The decreased AMH further enables the enhanced recruitment of the PMFs (Figure 1).
Figure 1.
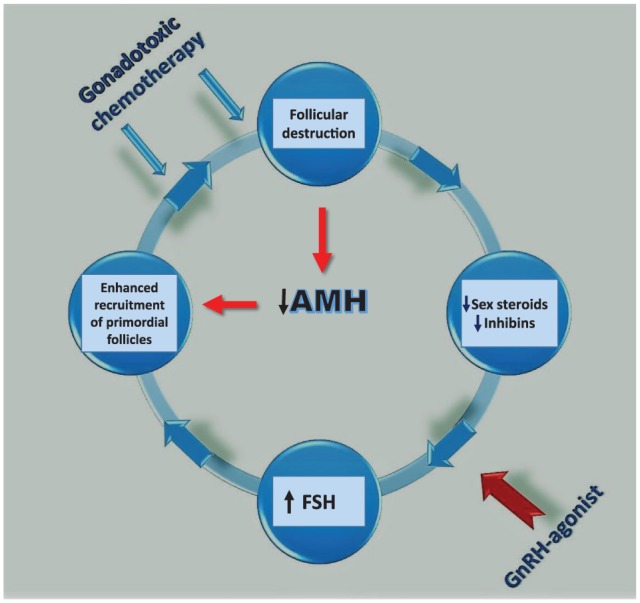
A suggested pathophysiologic mechanism whereby gonadotoxic chemotherapy may destroy the growing follicles, decrease estrogen, AMH, and inhibin levels, and increase FSH concentration, which may augment the recruitment of primordial and primary follicles entering the differentiation process and be further subjected to the detrimental effect of chemotherapy, leading to POF. The suggested rescuing effect of GnRHa is through prevention of high gonadotropins levels. AMH indicates anti-Mullerian hormone; FSH, follicle-stimulating hormone; GnRHa, gonadotropin-releasing hormone agonists; POF, premature ovarian failure.
Morgan et al65 summarized the mechanisms of gonadotoxicity induced by chemotherapy, concluding that POF results from the loss of PMFs not only through a direct effect but also due to an increased rate of folliculogenesis to replace the damaged developing follicles. Gonadotoxicity not only directly damages oocytes but also indirectly causes damage to somatic granulosa cells. The rate at which PMFs leave the non-active resting pool is influenced by the presence or absence of the more advanced gonadotropin-dependent growing follicles.65 Thus, the administration of GnRHa may interrupt this destructive vicious cycle by causing desensitization of GnRH receptors in the pituitary, preventing the increase in FSH concentration despite low estrogen and inhibin concentrations (Figure 1).4-9,65,66
Others67 have suggested a possible detrimental effect of high gonadotropin concentrations on the resting PMF pool. Transgenic mice for β-LH, with high levels of LH, have at birth a similar number of follicles as wild-type controls.67 However, after several weeks of exposure to increased LH concentrations, they suffer a significant premature loss of their primordial and primary follicle pool, in keeping with the suggested pathophysiologic “vicious cycle”1,4-9,20-24 (Figure 1).
Although the resting follicular pool of primordial and primary follicles is believed to be gonadotropin independent, several investigations have suggested that these follicles may express messenger RNA (mRNA) for FSH and LH receptors.66-75 These findings support the concept that even immature follicles such as the primordial and primary follicles may be gonadotropin dependent.68-72,76
More recently, Patel et al71 have reported that FSH can modulate ovarian germinative stem cells (OGSCs) such as pluripotent, very small embryonic-like stem cells (VSELs) and their “progenitors,” located in adult mammalian ovarian surface epithelium (OSE). Four isoforms of FSH receptors (FSHR) have been reported, but only FSH-R1 and FSH-R3 isoforms have biological activity.71 These investigators examined the effect of FSH on FSH-R1 and FSH-R3 isoforms and stem cell-specific markers for VSELs (such as Oct-4A and Sox-2) and OGSCs (Oct-4) in ovine ovaries.71 They found an increase in FSH-R3 mRNA transcripts, but no increase in FSH-R1 after FSH incubation.71 FSH-R1 is a 75-kDa protein, a member of the GPR superfamily of receptors, expressed on granulosa cells of growing follicles, and its activation by ligand stimulation leads to steroidogenesis via cAMP signal transduction.71-73 FSH-R3, a 39-kDa protein expressed by OSE and granulosa cells, is a growth factor receptor and promotes DNA synthesis leading to proliferation via a MAPK pathway, specifically the extracellular-regulated kinase (ERK).71-73 FSH-R1 and FSH-R3 transcripts differ from each other in exons 9 to 11. Whereas FSH-R1 possesses exons 9 and 10 but lacks exon 11, FSH-R3 lacks exons 9 and 10 but has exon 11.71-73 If indeed FSH-R3 (lacking exon 10) is the key actor to mediate FSH action on ovarian stem cells resulting in neo-oogenesis during postnatal life, one could easily explain why the extensive studies undertaken to search for mutations in exon 10 of the FSH receptor have failed to yield any results and concluded that PFs lack FSH receptors.70-73,76 Therefore, studies that failed to detect these receptors on PFs used rtPCR primers selected from exon 10 of the FSH-R1 receptor, whereas the study that did find active FSH receptors on ovarian germinative cells demonstrated that FSH-R3 lacks exon 10 on PFs and germinative stem cells (GSCs).70-74,76 It was concluded that FSH modulates ovarian stem cells via FSH-R3 to undergo self-renewal, clonal expansion, and differentiation into follicles and oocytes.71
For those who remain unconvinced and persistently hold the view that PMFs are gonadotropin independent, an alternative explanation may be put forward.4-9 The PMF and primary follicles are dependent on many growth factors (GFs) such as bone morphogenic proteins (BMP)-4, -7, and -9, activins, and others.70 These GFs and possibly others that are secreted by the more advanced and gonadotropin-dependent follicles may induce the exit of PMFs from the dormant inactive pool.70 FSH stimulates the secretion of these GFs by the more advanced ovarian follicles.70 The GnRHa co-treatment, after the initial flare-up effect, decreases FSH through pituitary desensitization, preventing the secretion of GFs by the more advanced FSH-dependent follicles, and secondarily preserves more PMFs in the uncommitted, “dormant” stage, minimizing their ultimate destruction by alkylating agents.4-9,70 Thus, although the exit of PMFs from the dormant state and the early stages of folliculogenesis may be gonadotropin independent, FSH may affect the rate of preantral follicle growth.74,75 Therefore, the belief that primordial and primary follicles are completely gonadotropin independent may need updating and reevaluation.9
A possible direct effect on GnRH receptors
Human gonads also contain GnRH receptors, similar to the ovaries of rodents, although at lower concentration.9,74,75,77-79 Activation of the GnRH receptor by its ligand may decrease apoptosis.77 Imai et al80 demonstrated that GnRHa may decrease the in vitro gonadotoxic effect of doxorubicin, regardless of the hypogonadotropic milieu. These investigators have shown a direct in vitro protective effect from chemotherapy-induced GC damage by GnRHa.80
Decreased utero-ovarian perfusion
One of the biologic effects of estrogens is to increase utero-ovarian perfusion.81 Another suggested possible explanation for the beneficial effect of GnRHa co-treatment in reducing the chemotherapy-associated gonadotoxicity is the decreased utero-ovarian perfusion resulting from the hypoestrogenic milieu generated by pituitary–gonadal desensitization.9,81,82 High estrogen levels increased ovarian perfusion in a rat model of ovarian stimulation, and administration of GnRHa significantly inhibited this effect in a dose-dependent manner.81 The decreased utero-ovarian perfusion in the hypoestrogenic milieu generated by GnRHa-induced pituitary desensitization may result in lower total cumulative exposure of the ovaries to the chemotherapeutic agents, secondarily resulting in a decreased gonadotoxic effect. Such a possibility inevitably raises the question whether this decreased exposure of the internal genital organs may not increase the risk of metastases to the internal genitalia in patients treated with GnRHa in addition to chemotherapy. Until now, the OS and DFS of GnRHa co-treated women did not differ from controls9,13-15,19-32,34-36,40-42 and in one RCT, the DFS was even significantly better.34
Sphingosine-1-phosphate
It has been speculated that sphingosine-1-phosphate (S1P) and its agonistic analogs such as FTY720 may possibly affect chemotherapy-induced oocyte apoptosis.9,83-86 The S1P molecule has ubiquitous and different activities and is a pleiotropic lipid mediator involved in cell viability and cancer progression in addition to cell growth, survival, invasion, inflammation, angiogenesis and vascular maturation, allergy, and asthma.87-89 The balance between sphingosine kinases that synthesize the molecule and S1P lyases and phosphatases which degrade it determines its intracellular levels.90 It has been speculated that GnRHa may possibly upregulate ovarian S1P and, thus, reduce follicular loss.9 In mice, disruption of the Bax gene, Bcl-2, or targeted expression of the Bax antagonist to the female mouse germ line can prevent the gonadotoxic effect of doxorubicin and protect oocytes from destruction in vivo85,91 or in vitro.84,85 Oocytes that are deficient of acid sphingomyelinase, which degrades S1P and generates ceramide,92 are resistant to the apoptosis induced in vitro by doxorubicin.85 Administration of S1P into the murine periovarian bursa prevented irradiation-induced ovarian follicle demise.83,85,93 More recently, Zelinsky et al94 demonstrated that female macaque monkeys administered S1P or FTY720 by direct intraovarian cannulation for a week before ovarian irradiation resumed menstrual cycles and maintained the ovarian follicles.94 FTY720 was more potent than S1P in its fertility preservation effect.94 Even more convincing, not only ovarian follicles were preserved but also fertility and spontaneous conceptions.94 Offspring conceived and delivered by the radio-protected females developed normally and showed no evidence of genomic instability.94 Adult human ovarian cortical slices pre-treated with S1P before being xenografted into nude mice lost significantly fewer PMFs than controls.94 Furthermore, S1P protected the female germ line from radiation without a discernible propagation of genomic damage.93 Obviously, such a long-term ovarian cannulation for S1P administration along the whole chemo- or radiotherapy period is not practical in human patients. Future endeavors should challenge the methodology of gonadal administration of S1P without systemic absorption, to reduce follicular loss without jeopardizing the ability of chemo- and radiotherapy to efficiently destroy malignant cells. Nevertheless, it is tempting to speculate that the GnRHa adjuvant co-treatment’s beneficial effect may possibly be associated with an intraovarian increase in S1P or similar anti-apoptotic molecule(s).9 This speculation of GnRHa possibly upregulating the intraovarian S1P effect is hypothetical and awaits validation.
Protection of GSCs
Fifteen years ago, Johnson et al95,96 challenged the concept of a terminal follicular pool in mammals, suggesting that rodent ovaries may possess mitotically active germ cells that continuously replicate themselves. They95,96 concluded that these GSCs might rejuvenate the ovarian PMF pool. Their publications revolutionized the doctrine of reproductive medicine, whereby mammalian females are born with a fixed, determined, and non-increasing reserve of follicles and lose the capacity for germ-cell renewal during fetal life.95-97 The idea that mammalian oogenesis does not occur after birth was established and upheld for more than half a century. This revolutionary concept ignited an ongoing debate about whether the ovaries of adult mammals can generate follicles de novo or not.97-101 The ongoing controversy is about the existence of GSCs and whether there is postnatal mitosis in mammal female germ cells, and generation of ovarian follicles, de novo, or not. More recent studies suggest that oocyte-producing GSCs may indeed exist and can be isolated from ovaries of adult rodents and even humans.99,100 Following publication of this revolutionary work, we may theoretically hypothesize that the GnRHa may possibly protect the undifferentiated GSCs, which ultimately generate de novo PMF.8,9 Indeed, we have measured high menopausal FSH levels and undetectable AMH levels in about one-third of our patients who were treated with GnRHa before and along with chemotherapy for up to 1 year after chemotherapy.1,4-9 Surprisingly and unexpectedly, about a year after the chemotherapeutic ovarian insult, FSH concentrations decreased to normal levels and AMH increased in 90% of these patients, and more than 60% of those who were interested conceived naturally.1,4-9,19-24 We hypothesized that most, if not all, growing follicles were destroyed by the gonadotoxic chemotherapy and the GSCs, preceding the PMFs, were protected by the GnRHa.1,4-9,19-24 Since folliculogenesis, from the stage of GSC to mature Graafian follicle may last somewhere between 6 and 12 months,101 it is conceivable why it took almost a year to rejuvenate the ovaries. The protected GSC started folliculogenesis, maturation, and secretion of AMH, inhibin, and estrogens; and the latter two fed back to decrease FSH levels to normal.99 This hypothesis also needs further investigation to validate if this effect can be attributed to GnRHa.
GnRHa: Pros and Cons
Up to date, over 50 publications (14 RCTs, 25 non-RCTs, and 20 meta-analyses) have reported on over 3100 patients treated with GnRHa in parallel to chemotherapy.1,4-9,19-24,19-44,102-109 Most, but not all, have demonstrated a significant decrease in the POF/POI rate in survivors of malignant diseases, or for autoimmune diseases such as lupus erythematosus nephritis who received cyclophosphamide pulses.13-15,110-113 However, there are also over 10 publications not supporting GnRHa as an effective modality of fertility preservation.45,48-53,114-120 Over the past 3 decades, the pendulum had swung from positive to negative conclusions and positive again regarding the efficiency of GnRHa in reducing gonadotoxicity and preserving fertility.13 One may carefully suggest that in the last few years, the pendulum is now swinging back toward a positive conclusion, possibly advocating the end of the debate, as recently suggested by a few editorials and international expert consensus committees, concluding that GnRHa adjuvant co-treatment may indeed preserve ovarian function and fertility without adverse effect on DFS.13-15,28,29,54,58,121-124 Indeed, several investigators who have previously authored papers claiming that GnRHa was not effective, changed their conclusion, later authoring publications which concluded this cotreatment is beneficial and minimizes POI.14,15,37,46,53,61,125
Possible Explanations for the Diverse Results of GnRHa Co-Treatment
The published dispute and controversy between the supporters of GnRHa co-treatment to preserve fertility and its opponents has occasionally been volatile.23,117-120,126 Elgindy et al49,50 concluded that ovarian suppression by GnRHa did not significantly increase COF versus controls (P = 0.07). However, their meta-analysis has been criticized for assigning low and inappropriate weight to 2 recent large RCTs34,35 and excluding RCTs in support of GnRHa, with a possible bias and consequent underestimation of the beneficial effect of the GnRHa adjuvant co-treatment.127,128 As previously mentioned, Demeestere et al38,52 did not initially find a difference in the POF rate after 1 year between the GnRHa and control arms. However, subsequently presented 2 years of follow-up of the same patients in their previous publication64 at the third World Congress of the International Society for Fertility Preservation in Valencia, Spain in November 2013. They declared that “ . . . the number of patients who totally restored their ovarian function was significantly higher in the GnRHa group (P = 0.049), confirming results of significantly higher AMH” levels in the GnRHa arm (1.4 ng/mL) versus the control arm; AMH = 0.5 ng/mL; P = 0.04.64 Indeed, a short follow-up period may be responsible for the discrepancy between these 2 studies leading to premature conclusions.38,64 More recently, the same group has published a follow-up of patients treated with GnRHa + norethisterone + chemotherapy vs norethisterone + chemotherapy, concluding there was no difference between the groups.52 However, the authors themselves52 mention their study limitations: “dropout rate of 50%, and 25% loss of follow-up or data unavailability.” Although the original study “mandated the accrual of 157 patients to ensure a power of 80% and a type I error probability of 5%, enrollment was discontinued after the assignment of 129 patients, . . . but only 63 patients were evaluated for POF,” 31 to 32 in each arm in the 15 participating centers (1-3 patients/arm/center). Furthermore, 5 pregnancies occurred in patients with protocol-defined POF of “one FSH > 40U/L measurement,” challenging the accuracy of the POF definition and the reliability of the resulting conclusions. The accepted definition of POF necessitates at least 2 FSH measurements in the menopausal range, 1 month apart between assessments. The occurrence of 5 spontaneous pregnancies in the so-called “POF patients,” combined with the small number of patients, significantly higher AMH in the GnRHa group, and the opposing conclusions at 1, 2, and 5 years of follow-up suggests that the conclusions of this study may not be robust.38,52,64,123,124
Several other studies were also underpowered or prematurely ended, before reaching the power-calculated number of patients necessary to detect a significant difference between the 2 arms.125 Similarly, Munster et al’s 53 study aimed at including 124 patients with a planned 5-year follow-up was “stopped for futility after 49 patients were enrolled,” and only 47 were evaluable—obviously not enough to draw solid conclusions.1,9 For protocols in which 88% to 90% of patients resume regular menstruation within 2 years after chemotherapy, such as in the ABVD or AC (adriamycin, cyclophosphamide) chemotherapy combinations, for HL and breast cancer in young patients, respectively, there is apparently little need for any intervention toward preservation of ovarian function.1,9,123,124 For such low gonadotoxic protocols, the number of patients needed to enroll in each arm for detecting significant differences is hundreds of patients, much higher than 21 to 26 in each arm, vs the planned power calculation required inclusion of 124 patients.53 Only in protocols resulting in a POF/POI risk higher than 30% could GnRHa co-treatment have a significant effect on fertility preservation, as suggested by Del Mastro et al and others.27-30,35,36,46,47,78,127-129
Possible causes for the different and contradictory reported results may be the different ages, different timing of ovarian function assessment, different chemotherapeutic protocols, and differences in genetic and environmental constitution (variable gonadotoxicity). One has to take into consideration that resumption of ovarian function may occur as late as 24 months after concluding chemotherapy; therefore, an early assessment of the outcome at 6 months, as in the ZORO study48 or in Munster et al’s53 study, may underestimate the true effect of GnRHa treatment.123-125 Indeed, in the ZORO study,48 the number of patients resuming cyclic menstruation at 2 years after chemotherapy was significantly higher than those with “two menses at 6 months,” as chosen to define resuming ovarian function.
Another problem with the ZORO study48 is that contradictory to the authors conclusion of “no difference” between the study (GnRHa) and control arms, the gonadotropin levels in the control group were significantly higher (P = 0.015), in the menopausal range, compared with the GnRHa arm, suggesting a beneficial effect of GnRHa on the ovarian reserve. This difference was persistently detected at 6, 12, 18, and 24 months after concluding chemotherapy.48
Another possible diverting parameter is the upper age of inclusion. Whereas most studies included patients younger than 40, Munster et al53 included patients up to 45 years of age. The ovarian reserve after age 40 is so low that the efficiency of fertility preservation is minimal or negligible, and many hundreds of patients may be needed to detect a significant difference.1,9
Studies where chemotherapeutic protocol has either very low28,53,115 or very high gonadotoxicity, such as the escalated BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, prednisone) protocol118 are problematic. The power needed to detect a difference in such studies is hundreds of patients, many more than the 17 to 60 patients included in most studies concluding that GnRHa has no protective effect.48,53,61,115
Good evidence-based medicine requires RCTs and/or meta-analyses.130 Although no randomized trials assessed the role of ovarian or oocyte cryopreservation strategies for fertility preservation, many investigators, but not recent ASCO recommendations,130 refer to cryopreservation of ovarian tissue as an established method of fertility preservation. More recently, an argument was raised119,120,131 whereby none of the studies favoring GnRHa effect for fertility preservation was blinded or placebo controlled; therefore, they are unacceptable. On the other hand, these authors119,120,131 claim that the successful reports on ovarian cryopreservation should lead to accepting this method as an unequivocally established method for fertility preservation. However, none of the reports on cryopreservation of embryos, ova, or ovarian tissue were “blinded or placebo controlled” nor RCT. Why apply a double standard when judging and comparing different methods for fertility preservation?124 The quality of evidence for recommending a strategy of only cryopreservation may be considered low, according to Fleisher et al,130 because it is based on non-randomized, case-control, or observational studies. On the contrary, the role of GnRHa therapy in preserving ovarian function has been assessed both in RCTs and in non-randomized, case-control studies, meta-analyses, and 4 recent international experts’ committees’ conclusions.1,9,13-15,22-32,121-124
Another argument raised by the opponents to GnRHa use claims that: “A clinical example for why gonadal suppression may not protect ovaries is the fact that prepubertal children receiving high-dose chemotherapy given before hematopoietic stem-cell transplantation still suffer from ovarian failure.”45 Remérand et al132 described 4 spontaneous pregnancies and successful deliveries in a patient after prepubertal high-dose busulphan and cyclophosphamide (Bu-Cy, the most toxic gonadotoxic chemotherapy combination) conditioning and BMT. Similarly, repeated spontaneous pregnancies and 2 successful deliveries after 2 autologous BMTs, 10 years apart, and GnRHa co-treatment has been described in a postpubertal lymphoma patient, suggesting that the prepubertal milieu induced by the GnRHa co-treatment might have contributed to the preserved fertility despite repeated BMTs.44 After this publication,44 the reported patient experienced a fifth natural conception and a third successful delivery. According to an extensive European survey of stem cell transplantation (SCT) involving 37,362 female patients in the European group for blood and marrow transplantation, only 0.6% of patients conceived after one autologous or allogeneic BMT.133 Thus, the calculated odds for pregnancy after 2 BMTs are negligible (theoretically: 0.006 × 0.006 = 0.000036).1,9,44 Others134 have found a 3% PR after BMT. Thus, theoretically, according to the latter, the estimated odds for conceiving after 2 SCTs are 0.03 × 0.03, ∼1/1000.134 The GnRHa adjuvant co-treatment along the gonadotoxic conditioning chemotherapy simulated the prepubertal hormonal milieu and might have reduced the gonadotoxic effect, enabling ovulation, 5 spontaneous conceptions, and 3 successful deliveries of healthy children.44
Another argument brought up by opponents of GnRHa use is that since 8% of prepubertal children exposed to gonadotoxic chemotherapy may suffer POF/POI by age 40, there is no logic for creating a prepubertal milieu in young women in parallel to chemotherapy.118-140 Several studies in the last decade136-139 found that survivors of childhood cancer have about 8% risk of suffering POF/POI before age 40. This hazard risk is in keeping with the published results whereby women of reproductive age treated with adjuvant GnRHa along chemotherapy experienced POF in the range of 8% to 13% (simulating prepubertal exposure), whereas control survivors who received chemotherapy without the agonist had a 30% to 60% risk of POF.1,9 It may not contradict the rationale of GnRHa administration; on the contrary, it supports simulating a prepubertal milieu.1,4-9,44,132 Patients resuming menses after chemotherapy may indeed experience early menopause.139 However, there are no published data on whether those who received the GnRHa adjuvant along chemotherapy would also suffer early or premature menopause. It has not been robustly established whether these survivors resume menses for a limited period and then suffer menopause, or if this resumption of menses is long-standing.1,9 In our experience of over 25 years with almost 300 patients who received the GnRHa adjuvant with chemotherapy for different indications, those who resumed COF continued experiencing it for at least 5 to 15 years before entering menopause.1,4-9,19 Whereas natural pregnancies in the control survivors occurred in patients younger than 30 at the time of chemotherapy, in the GnRHa group several patients were 30 to 38 years old at treatment, and one spontaneously conceived at 41 years of age (8 years after chemotherapy plus GnRHa), and a few patients conceived and delivered 4 to 6 times.1,4-9,19
Another raised theoretical concern speculates that the agonist may reduce the efficiency of chemotherapy. However, a meta-analysis published in The Lancet,25 based on 11,906 young breast cancer patients randomized in 16 trials, concluded that the addition of GnRHa to tamoxifen reduced recurrence by 12.7% (P < 0.02), and death after recurrence by 15% (P < 0.03), contradicting the raised hypothetical speculation. Furthermore, the publications of Moore et al,34 and those by Recchia et al’s41,42 and Del-Mastro’s groups13-25,26-30 demonstrate similar or improved 5-year survival rates with GnRHa compared to survival without the agonist in both ER+ and ER– patients. The survival rate of almost 300 patients treated in our medical center with GnRHa/chemotherapy for different indications was similar to the survival of those who did not receive the agonist.1,4-9
Similarly, the International Breast Cancer Study Group, SOFT study140 concluded that for women who were at sufficient risk for recurrence to warrant adjuvant chemotherapy and who remained premenopausal, the addition of ovarian suppression such as GnRHa improved disease outcomes. More recently, ASCO published a clinical practice guideline update on ovarian suppression adjuvant endocrine therapy for women with HR+ breast cancer.141 It stated that the addition of ovarian suppression to standard adjuvant therapy with tamoxifen or with an aromatase inhibitor improved DFS and improved freedom from breast cancer and distant recurrence, compared with tamoxifen alone, among the subset of patients who were at sufficient risk for recurrence.141 The panel recommended that high-risk patients receive ovarian suppression with GnRHa, in addition to adjuvant endocrine therapy.141 The results of all these publications imply that GnRHa might either improve or not affect the survival of patients receiving chemotherapy.13-15,28,29,34
Ethical Issues: Combination of Methods
Many unknown and equivocal matters remain to be addressed in fertility preservation. Therefore, the data appear to suggest that clinical medicine is still far from having a ubiquitous solution for all survivors interested in fertility and raising children. None of the suggested methods for fertility preservation guarantees unequivocal success in achieving pregnancy and delivering healthy infants. Therefore, several modalities need to be offered and practiced for maximizing patients’ odds for future fertility. ASCO has updated the practice guideline for fertility preservation, recommending that health care providers discuss with patients and consider all fertility preservation options, including GnRHa cotreatment, as early as possible before beginning gonadotoxic therapy, to allow for the widest array of options.135
Whereas not all methods are 100% successful, young patients deserve to be informed of all the possible options to reduce gonadotoxicity and preserve ovarian function.1,5-9,19-32 In our opinion, GnRHa co-treatment should be offered in addition to IVF and cryopreservation of embryos, ova, and ovarian tissue for fertility preservation. There is no contraindication to ovarian biopsy for cryopreservation combined with GnRHa adjuvant co-treatment and follicular aspiration, as done in the FertiPROTEKT consortium.113,142 In cases where chemotherapy has induced POF/POI, as is frequently the case after total body irradiation and BMT, the patient has cryopreserved ova, embryos, or PMFs to fall back upon. However, in conventional chemotherapy regimens such as those commonly practiced for young breast cancer and lymphoma patients, GnRHa co-treatment may preserve ovarian function and prevent POF without necessitating the use of cryopreserved ova, embryos, or ovarian tissue.1,5-9,13-15,28,29,34
Conclusions
In our opinion, all young women (even patients with high-risk leukemia) facing gonadotoxic chemotherapy should be offered the 3 avenues for fertility preservation: cryopreservation of embryos, ova, and ovarian tissue, along with GnRHa. The rationale leading to this policy is the hope that in a few years, the “artificial ovary”–IVM technology of PMFs to mature Graafian follicles containing M-II fertilizable oocytes may become clinically possible, bypassing the ovarian autotransplantation need in those patients who become prematurely menopausal. Although this technology is not yet available in humans, the previous success in rodents and the 3D follicle culture in alginate gel may hopefully become feasible in the next several years. Therefore, we should offer and discuss with these young patients, and their parents, in the case of children, all the possibilities to preserve fertility.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Zeev Blumenfeld  https://orcid.org/0000-0003-3929-7940
https://orcid.org/0000-0003-3929-7940
References
- 1. Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention is worth a pound of cure’: the case for and against GnRH-agonist for fertility preservation. Ann Oncol. 2014;25:1719-1728. [DOI] [PubMed] [Google Scholar]
- 2. De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384:1302-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace WHB, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209-218. [DOI] [PubMed] [Google Scholar]
- 4. Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012;26:379-390. [DOI] [PubMed] [Google Scholar]
- 5. Blumenfeld Z, Evron A. Endocrine prevention of chemotherapy-induced ovarian failure. Curr Opin Obstet Gynecol. 2016;28:223-229. [DOI] [PubMed] [Google Scholar]
- 6. Blumenfeld Z, Dann E, Avivi I, Epelbaum R, Rowe JM. Fertility after treatment for Hodgkin’s disease. Ann Oncol. 2002;13(Suppl. 1):138-147. [DOI] [PubMed] [Google Scholar]
- 7. Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6:229-239. [DOI] [PubMed] [Google Scholar]
- 8. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? the role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12:1044-1054. [DOI] [PubMed] [Google Scholar]
- 9. Blumenfeld Z, von Wolff M. GnRH-analogues and oral contraceptives for fertility preservation in women during chemotherapy. Hum Reprod Update. 2008;14:543-552. [DOI] [PubMed] [Google Scholar]
- 10. Linkeviciute A, Boniolo G, Chiavari L, Peccatori FA. Fertility preservation in cancer patients: the global framework. Cancer Treat Rev. 2014;40:1019-1027. [DOI] [PubMed] [Google Scholar]
- 11. Ortin TT, Shostak CA, Donaldson SS. Gonadal status and reproductive function following treatment for Hodgkin’s disease in childhood: the Stanford experience. Int J Radiat Oncol Biol Phys. 1990;19:873-880. [DOI] [PubMed] [Google Scholar]
- 12. Fidler MM, Reulen RC, Winter DLet al. Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: population based cohort study. BMJ. 2016;354:i4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lambertini M, Horicks F, Del Mastro L, Partridge AH, Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65-77. [DOI] [PubMed] [Google Scholar]
- 14. Lambertini M, Moore HCF, Leonard RCFet al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. 2018;36:1981-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Mastro L, Lambertini M. Gonadotropin-releasing hormone analogs for ovarian function protection during chemotherapy in young early breast cancer patients: the last piece of the puzzle? Ann Oncol. 2017;28:1683-1685. [DOI] [PubMed] [Google Scholar]
- 16. Glode LM, Robinson J, Gould SF. Protection from cyclophosphamide induced testicular damage with an analogue of gonadotropin-releasing hormone. Lancet. 1981;1:1132-1134. [DOI] [PubMed] [Google Scholar]
- 17. Johnson DH, Linde R, Hainsworth JDet al. Effect of a luteinizing hormone releasing hormone agonist given during combination chemotherapy on posttherapy fertility in male patients with lymphoma: preliminary observations. Blood. 1985;65:832-836. [PubMed] [Google Scholar]
- 18. Ataya K, Rao LV, Laurence E, Kimmel R. Luteinizing hormone-releasing agonist inhibits cyclophosphamide induced ovarian follicular depletion in Rhesus monkeys. Biol Reprod. 1995;52:365-372. [DOI] [PubMed] [Google Scholar]
- 19. Blumenfeld Z, Zur H, Dann EJ. Gonadotropin-releasing hormone agonist cotreatment during chemotherapy may increase pregnancy rate in survivors. Oncologist. 2015;20:1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenfeld Z, Avivi I, Eckman R, Epelbaum R, Rowe JM, Dann EJ. Gonadotropin-releasing hormone agonist decreases chemotherapy-induced gonadotoxicity and premature ovarian failure in young female patients with Hodgkin lymphoma. Fertil Steril. 2008;89:166-173. [DOI] [PubMed] [Google Scholar]
- 21. Blumenfeld Z. GnRH-agonists in fertility preservation. Curr Opin Endocrinol Diabet Obes. 2008;15:523-528. [DOI] [PubMed] [Google Scholar]
- 22. Blumenfeld Z, Evron A. Preserving fertility when choosing chemotherapy regimens: the role of gonadotropin-releasing hormone agonists. Expert Opin Pharmacother. 2015;16:1009-1020. [DOI] [PubMed] [Google Scholar]
- 23. Blumenfeld Z. Pregnancy rate and preservation of cyclic ovarian function with gonadotropin-releasing hormone agonist cotreatment during chemotherapy. JAMA Oncol. 2016;2:545-546. [DOI] [PubMed] [Google Scholar]
- 24. Blumenfeld Z. Preservation of ovarian function and fertility despite gonadotoxic chemotherapy. Expert Rev Endocrinol Metab. 2012;7:567-576. [DOI] [PubMed] [Google Scholar]
- 25. LHRH-Agonists in Early Breast Cancer Overview Group, Cuzick J, Ambroisine Let al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711-1723. [DOI] [PubMed] [Google Scholar]
- 26. Lambertini M, Ceppi M, Poggio Fet al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26:2408-2419. [DOI] [PubMed] [Google Scholar]
- 27. Lambertini M, Ginsburg ES, Partridge AH. Update on fertility preservation in young women undergoing breast cancer and ovarian cancer therapy. Curr Opin Obstet Gynecol. 2015;27:98-107. [DOI] [PubMed] [Google Scholar]
- 28. Coates AS, Winer EP, Goldhirsch A. Tailoring therapies: improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2015;26:1533-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambertini M, Del Mastro L, Pescio MCet al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Del Mastro L, Ceppi M, Poggio Fet al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40:675-683. [DOI] [PubMed] [Google Scholar]
- 31. Chen H, Xiao L, Li J, Cui L, Huang W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2019;11:CD3008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Del Mastro L, Giraudi S, Levaggi A, Pronzato P. Medical approaches to preservation of fertility in female cancer patients. Expert Opin Pharmacother. 2011;12:387-396. [DOI] [PubMed] [Google Scholar]
- 33. Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15: 1124-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moore HCF, Unger JM, Phillips KAet al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lambertini M, Boni L, Michelotti Aet al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314:2632-2640. [DOI] [PubMed] [Google Scholar]
- 36. Munhoz RR, Pereira AA, Sasse ADet al. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Behringer K, Thielen I, Mueller Het al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin Lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann Oncol. 2012;23:1818-1825. [DOI] [PubMed] [Google Scholar]
- 38. Demeestere I, Brice P, Peccatori FAet al. Gonadotropin-releasing hormone agonist for the prevention of chemotherapy-induced ovarian failure in patients with lymphoma: 1-year follow-up of a prospective randomized trial. J Clin Oncol. 2013;31:903-909. [DOI] [PubMed] [Google Scholar]
- 39. Wong M, O’Neill S, Walsh G, Smith IE. Goserelin with chemotherapy to preserve ovarian function in pre-menopausal women with early breast cancer: menstruation and pregnancy outcomes. Ann Oncol. 2013;24:133-138. [DOI] [PubMed] [Google Scholar]
- 40. Lambertini M, Dellepiane C, Viglietti G, Del Mastro L. Pharmacotherapy to protect ovarian function and fertility during cancer treatment. Expert Opin Pharmacother. 2017;18:739-742. [DOI] [PubMed] [Google Scholar]
- 41. Recchia F, Candeloro G, Rosselli Met al. Adjuvant ovarian suppression, high-dose chemotherapy and immunotherapy for premenopausal patients with high-risk breast cancer. Anticancer Res. 2015;35:6847-6853. [PubMed] [Google Scholar]
- 42. Recchia F, Necozione S, Bratta M, Rosselli M, Guerriero G, Rea S. LH-RH analogues in the treatment of young women with early breast cancer: long-term follow-up of a phase II study. Int J Oncol. 2015;46:1354-1360. [DOI] [PubMed] [Google Scholar]
- 43. Blumenfeld Z, Patel B, Leiba R, Zuckerman T. Gonadotropin-releasing hormone agonist may minimize premature ovarian failure in young women undergoing autologous stem cell transplantation. Fertil Steril. 2012;98:1266-1270.e1. [DOI] [PubMed] [Google Scholar]
- 44. Blumenfeld Z, Zuckerman T. Repeated spontaneous pregnancies and successful deliveries after repeated autologous stem cell transplantation and GnRH-agonist. Oncologist. 2010;15:59-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nitzschke M, Raddatz J, Bohlmann MK, Stute P, Strowitzki T, von Wolff M. GnRH analogs do not protect ovaries from chemotherapy-induced ultrastructural injury in Hodgkin’s lymphoma patients. Arch Gynecol Obstet. 2010;282: 83-88. [DOI] [PubMed] [Google Scholar]
- 46. Leonard R, Adamson D, Bertelli Get al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017;28:1811-1816. [DOI] [PubMed] [Google Scholar]
- 47. Del Mastro L, Lambertini M. Temporary ovarian suppression with gonadotropin-releasing hormone agonist during chemotherapy for fertility preservation: toward the end of the debate? Oncologist. 2015;20:1233-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gerber B, von Minckwitz G, Stehle Het al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334-2341. [DOI] [PubMed] [Google Scholar]
- 49. Elgindy EA, El-Haieg DO, Khorshid OMet al. Gonadotrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78-86. [DOI] [PubMed] [Google Scholar]
- 50. Elgindy EA, Sibai H, Abdelghani A, Mostafa M. Protecting ovaries during chemotherapy through gonad suppression: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:187-195. [DOI] [PubMed] [Google Scholar]
- 51. Yang B, Shi W, Yang Jet al. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2013;22:150-157. [DOI] [PubMed] [Google Scholar]
- 52. Demeestere I, Brice P, Peccatori FAet al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016;34:2568-2574. [DOI] [PubMed] [Google Scholar]
- 53. Munster PN, Moore AP, Ismail-Khan Ret al. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paluch-Shimon S, Pagani O, Partridge AHet al. Second international consensus guidelines for breast cancer in young women (BCY2). Breast. 2016;26:87-99. [DOI] [PubMed] [Google Scholar]
- 55. Kalich-Philosoph L, Roness H, Carmely Aet al. Cyclophosphamide triggers follicle activation causing ovarian reserve ‘burn out’: AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;15:185. [DOI] [PubMed] [Google Scholar]
- 56. Gavish Z, Peer G, Roness H, Cohen Y, Meirow D. Follicle activation and “burn-out” contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod. 2014;29:989-996. [DOI] [PubMed] [Google Scholar]
- 57. Sinha N, Letourneau JM, Wald Ket al. Antral follicle count recovery in women with menses after treatment with and without gonadotropin-releasing hormone agonist use during chemotherapy for breast cancer. J Assist Reprod Genet. 2018;35: 1861-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paluch-Shimon S, Pagani O, Partridge AHet al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203-217. [DOI] [PubMed] [Google Scholar]
- 59. Del Mastro L, Boni L, Michelotti Aet al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269-276. [DOI] [PubMed] [Google Scholar]
- 60. Moore HCF, JM Unger, Phillips KAet al. Final analysis of the prevention of early menopause study (POEMS)/SWOG intergroup S0230. J Natl Cancer Inst. 2019;111:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Behringer K, Wildt L, Mueller Het al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21:2052-2060. [DOI] [PubMed] [Google Scholar]
- 62. Blumenfeld Z. Fertility preservation by endocrine suppression of ovarian function using gonadotropin-releasing hormone agonists: the end of the controversy? J Clin Oncol. 2018;36:1895-1897. [DOI] [PubMed] [Google Scholar]
- 63. Lambertini M, Cinquini M, Moschetti Iet al. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: a GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. Eur J Cancer. 2017;71:25-33. [DOI] [PubMed] [Google Scholar]
- 64. Demeestere I, Brice P, Peccatori FAet al. Triptorelin to prevent chemotherapy-induced ovarian failure in lymphoma patients: a prospective randomized study. Paper presented at the Third World Congress of the International Society for Fertility Preservation; November 7-9, 2013; Valencia, Spain https://difusion.ulb.ac.be/vufind/Record/ULB-DIPOT:oai:dipot.ulb.ac.be:2013/151560/Details. [Google Scholar]
- 65. Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525-535. [DOI] [PubMed] [Google Scholar]
- 66. Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64-73. [DOI] [PubMed] [Google Scholar]
- 67. Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod. 1997;57:1233-1237. [DOI] [PubMed] [Google Scholar]
- 68. Patsoula E, Loutradis D, Drakakis P, Michalas L, Bletsa R, Michalas S. Messenger RNA expression for the follicle-stimulating hormone receptor and luteinizing hormone receptor in human oocytes and preimplantation-stage embryos. Fertil Steril. 2003;79:1187-1193. [DOI] [PubMed] [Google Scholar]
- 69. Zheng W, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148:47-53. [PMC free article] [PubMed] [Google Scholar]
- 70. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191-206. [DOI] [PubMed] [Google Scholar]
- 71. Patel H, Bhartiya D, Parte S, Gunjal P, Yedurkar S, Bhatt M. Follicle stimulating hormone modulates ovarian stem cells through alternately spliced receptor variant FSH-R3. J Ovarian Res. 2013;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72. Babu PS, Danilovich N, Sairam MR. Hormone-induced receptor gene splicing: enhanced expression of the growth factor type I follicle-stimulating hormone receptor motif in the developing mouse ovary as a new paradigm in growth regulation. Endocrinology. 2001;142:381-389. [DOI] [PubMed] [Google Scholar]
- 73. Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398-408. [DOI] [PubMed] [Google Scholar]
- 74. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Webb R, Garnsworthy PC, Gong JG, Armstrong DG. Control of follicular growth: local interactions and nutritional influences. J Anim Sci. 2004;82(Suppl. E):E63-E74. [DOI] [PubMed] [Google Scholar]
- 76. Oktay K, Briggs D, Gosden RG. Ontogeny of the follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748-3751. [DOI] [PubMed] [Google Scholar]
- 77. Grundker C, Emons G. Role of gonadotropin-releasing hormone (GnRH) in ovarian cancer. Reprod Biol Endocrinol. 2003:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11:725-748. [DOI] [PubMed] [Google Scholar]
- 79. Leung PC, Cheng CK, Zhu XM. Multi-factorial role of GnRH-I and GnRH-II in the human ovary. Mol Cell Endocrinol. 2003;202:145-153. [DOI] [PubMed] [Google Scholar]
- 80. Imai A, Sugiyama M, Furui T, Tamaya T, Ohno T. Direct protection by a gonadotropin-releasing hormone analog from doxorubicin-induced granulosa cell damage. Gynecol Obstet Invest. 2007;63:102-106. [DOI] [PubMed] [Google Scholar]
- 81. Kitajima Y, Endo T, Nagasawa Ket al. Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology. 2006;147:694-699. [DOI] [PubMed] [Google Scholar]
- 82. Saitta A, Altavilla D, Cucinotta Det al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21: 1512-1519. [DOI] [PubMed] [Google Scholar]
- 83. Paris F, Perez GI, Fuks Zet al. Sphingosine-1 phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8:901-902. [DOI] [PubMed] [Google Scholar]
- 84. Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med. 1997;3:1228-1232. [DOI] [PubMed] [Google Scholar]
- 85. Morita Y, Perez GI, Paris Fet al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109-1114. [DOI] [PubMed] [Google Scholar]
- 86. Tilly JA. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838-848. [DOI] [PubMed] [Google Scholar]
- 87. Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nat Rev Mol Cell Biol. 2003;4:397-407. [DOI] [PubMed] [Google Scholar]
- 88. Spiegel S, Cuvillier O, Edsall LCet al. Sphingosine-1-phosphate in cell growth and cell death. Ann NY Acad Sci. 1998;845:11-18. [DOI] [PubMed] [Google Scholar]
- 89. Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300-307. [DOI] [PubMed] [Google Scholar]
- 91. Reynolds T. Cell death genes may hold clues to preserving fertility after chemotherapy. J Natl Cancer Inst. 1999;91:664-666. [DOI] [PubMed] [Google Scholar]
- 92. Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643-665. [DOI] [PubMed] [Google Scholar]
- 93. Paris F, Perez GI, Kolesnick RN, Tilly JL. Suppression of oocyte apoptosis by sphingosine-1 phosphate therapy in vivo preserves fertility following radiotherapy. Paper presented at 83rd Annual Meeting of the Endocrine Society; June 20-23, 2001; Denver, CO. [Google Scholar]
- 94. Zelinski MB, Murphy MK, Lawson MSet al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95:1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145-150. [DOI] [PubMed] [Google Scholar]
- 96. Johnson J, Bagley J, Skaznik-Wikiel Met al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gargett CE. Review article: stem cells in human reproduction. Reprod Sci. 2007;14:405-424. [DOI] [PubMed] [Google Scholar]
- 98. Telfer EE, Gosden RG, Byskov AGet al. On regenerating the ovary and generating controversy. Cell. 2005;122:821-822. [DOI] [PubMed] [Google Scholar]
- 99. Woods DC, Telfer EE, Tilly JL. Oocyte family trees: old branches or new stems? PLoS Genet. 2012;8:e1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Eggs forever? Differentiation. 2005;73:438-446. [DOI] [PubMed] [Google Scholar]
- 102. Pereyra Pacheco B, Méndez Ribas JM, Milone Get al. Use of GnRH analogs for functional protection of the ovary and preservation of fertility during cancer treatment in adolescents: a preliminary report. Gynecol Oncol. 2001;81: 391-397. [DOI] [PubMed] [Google Scholar]
- 103. Castelo-Branco C, Nomdedeu B, Camus A, Mercadal S, Martínez de, Osaba MJ, Balasch J. Use of gonadotropin-releasing hormone agonists in patients with Hodgkin’s disease for preservation of ovarian function and reduction of gonadotoxicity related to chemotherapy. Fertil Steril. 2007;87:702-705. [DOI] [PubMed] [Google Scholar]
- 104. Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD). Hematology. 2007;12:141-147. [DOI] [PubMed] [Google Scholar]
- 105. Falorio S, Angrilli F, Fioritoni G. Gonadotropin-releasing hormone analog treatment for the prevention of treatment-related ovarian failure and infertility in women of reproductive age with Hodgkin lymphoma. Leuk Lymphoma. 2008;49:1087-1093. [DOI] [PubMed] [Google Scholar]
- 106. Huser M, Crha I, Ventruba Pet al. Prevention of ovarian function damage by a GnRH analogue during chemotherapy in Hodgkin lymphoma patients. Hum Reprod. 2008;23:863-868. [DOI] [PubMed] [Google Scholar]
- 107. Urruticoechea A, Arnedos M, Walsh G, Dowsett M, Smith IE. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC). Breast Cancer Res Treat. 2008;110:411-416. [DOI] [PubMed] [Google Scholar]
- 108. Fox KR, Scialla J, Moore H. Preventing chemotherapy-related amenorrhea using leuprolide during adjuvant chemotherapy for early-stage breast cancer. Proc Am Soc Clin Oncol. 2003;22:13. [Google Scholar]
- 109. Maisano R, Caristi N, Mare Met al. Protective effect of leuprolide on ovarian function in young women treated with adjuvant chemotherapy for early breast cancer: a multicenter phase II study. J Chemother. 2008;20:740-743. [DOI] [PubMed] [Google Scholar]
- 110. Somers EC, Marder W, Christman GM, Ognenovski V, McCune WJ. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52:2761-2767. [DOI] [PubMed] [Google Scholar]
- 111. Blumenfeld Z, Mischari O, Schultz N, Boulman N, Balbir-Gurman A. Gonadotropin releasing hormone agonists may minimize cyclophosphamide associated gonadotoxicity in SLE and autoimmune diseases. Semin Arthritis Rheum. 2011;41:346-352. [DOI] [PubMed] [Google Scholar]
- 112. Liang LQ, Qiu Q, Yang XYet al. Role of gonadotropin releasing hormone analogues for ovarian protection in systemic lupus erythematosus patients treated with cyclophosphamide. Zhonghua Yi Xue Za Zhi. 2008;88:1009-1011. [PubMed] [Google Scholar]
- 113. Henes M, Henes JC, Neunhoeffer Eet al. Fertility preservation methods in young women with systemic lupus erythematosus prior to cytotoxic therapy: experiences from the FertiPROTEKT network. Lupus. 2012;21:953-958. [DOI] [PubMed] [Google Scholar]
- 114. Waxman JH, Ahmed R, Smith Det al. Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol. 1987;19:159-162. [DOI] [PubMed] [Google Scholar]
- 115. Azem F, Samara N, Cohen Tet al. Assessment of ovarian reserve following ovarian tissue banking and/or GnRH-a co-treatment prior to chemotherapy in patients with Hodgkin’s disease. J Assist Reprod Genet. 2008;25:535-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Behringer K, Wildt L, Mueller Het al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010;21:2052-2060. [DOI] [PubMed] [Google Scholar]
- 117. Turner NH, Partridge A, Sanna G, Di Leo A, Biganzoli L. Utility of gonadotropin-releasing hormone agonists for fertility preservation in young breast cancer patients: the benefit remains uncertain. Ann Oncol. 2013;24:2224-2235. [DOI] [PubMed] [Google Scholar]
- 118. Demeestere I, Turan V, Oktay K. Pregnancy rate and preservation of cyclic ovarian function with gonadotropin-releasing hormone agonist cotreatment during chemotherapy–reply. JAMA Oncol. 2016;2:546-547. [DOI] [PubMed] [Google Scholar]
- 119. Oktay K, Bedoschi G. Appraising the biological evidence for and against the utility of GnRHa for preservation of fertility in patients with cancer. J Clin Oncol. 2016;34:2563-2565. [DOI] [PubMed] [Google Scholar]
- 120. Rodriguez-Wallberg K, Turan V, Munster P, Oktay K. Can ovarian suppression with gonadotropin-releasing hormone analogs (GnRHa) preserve fertility in cancer patients? Ann Oncol. 2016;27:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Blumenfeld Z. Ovarian tissue transplantation in leukemia. Fertil Steril. 2018;109:69-70. [DOI] [PubMed] [Google Scholar]
- 122. Blumenfeld Z. Fertility preservation in leukemia. Oncologist. 2018;23:645-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Blumenfeld Z. Fertility preservation by endocrine suppression of ovarian function using gonadotropin-releasing hormone agonists: the end of the controversy? invited editorial. J Clin Oncol. 2018;36:1895-1897. [DOI] [PubMed] [Google Scholar]
- 124. Blumenfeld Z. Reply to V. Turan et al. J Clin Oncol. 2019;37:88-89. [DOI] [PubMed] [Google Scholar]
- 125. Blumenfeld Z. ZORO study: discrepancy between the conclusion and the results. J Clin Oncol. 2011;29:3340. [DOI] [PubMed] [Google Scholar]
- 126. Oktay K, Turan V. Failure of ovarian suppression with gonadotropin-releasing hormone analogs to preserve fertility: an assessment based on the quality of evidence. JAMA Oncol. 2016;2:74-75. [DOI] [PubMed] [Google Scholar]
- 127. Falcone T, Farrell RM, Moore HC. Protecting ovaries during chemotherapy through gonad suppression: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:899-900. [DOI] [PubMed] [Google Scholar]
- 128. Lambertini M, Poggio F, Levaggi A, Del Mastro L. Protecting ovaries during chemotherapy through gonad suppression: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:901. [DOI] [PubMed] [Google Scholar]
- 129. Blumenfeld Z, Evron A. Preserving fertility when choosing chemotherapy regimens: the role of gonadotropin-releasing hormone agonists. Expert Opin Pharmacother. 2015;16:1009-1020. [DOI] [PubMed] [Google Scholar]
- 130. Fleisher LA, Bass EB, McKeown P. Methodological approach: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:17S-23S. [DOI] [PubMed] [Google Scholar]
- 131. Turan V, Bedoschi G, Rodriguez-Wallberg Ket al. Utility of gonadotropin-releasing hormone agonists for fertility preservation: lack of biologic basis and the need to prioritize proven methods. J Clin Oncol. 2019;37:84-86. [DOI] [PubMed] [Google Scholar]
- 132. Remérand G, Merlin E, Froissart Ret al. Four successful pregnancies in a patient with mucopolysaccharidosis type I treated by allogeneic bone marrow transplantation. J Inherit Metab Dis. 2009;32(Suppl. 1):S111-S113. [DOI] [PubMed] [Google Scholar]
- 133. Salooja N, Szydlo RM, Socie Get al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358: 271-276. [DOI] [PubMed] [Google Scholar]
- 134. Carter A, Robison LL, Francisco Let al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37:1023-1029. [DOI] [PubMed] [Google Scholar]
- 135. Oktay K, Harvey BE, Partridge AHet al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:1994-2001. [DOI] [PubMed] [Google Scholar]
- 136. Sklar CA, Mertens AC, Mitby Pet al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890-896. [DOI] [PubMed] [Google Scholar]
- 137. Green DM, Sklar CA, Boice JD, Jret al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Edgar AB, Wallace WH. Pregnancy in women who had cancer in childhood. Eur J Cancer. 2007;43:1890-1894. [DOI] [PubMed] [Google Scholar]
- 139. Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E. Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646-1653. [DOI] [PubMed] [Google Scholar]
- 140. Francis PA, Regan MM, Fleming GFet al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Burstein HJ, Lacchetti C, Anderson Het al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34:1689-1701. [DOI] [PubMed] [Google Scholar]
- 142. von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women: a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]


