Abstract
Background:
The loss of functional hand skills is a primary characteristic of Rett syndrome. Stereotypies, dyspraxia, and other sensory processing issues severely limit the individual’s ability to reach toward and sustain grasp on objects. This loss of functional reach and grasp severely limits their ability to participate in self-help, play, and school-related activities. We proposed that Ayres Sensory Integration (ASI) treatment would improve sensory processing and motor planning, which would lay the sensory-motor groundwork for improving grasp of objects, an important first step in developing functional hand use.
Objective:
We examined effects of ASI treatment on rate of reaching and grasping for children with Rett syndrome/Rett-related disorders.
Methods:
We used an interrupted time series design to measure changes in outcome variables occurring after intervention initiation and cessation. We analyzed daily video observations during baseline, intervention, and post-intervention periods, over a span of 7 months.
Results:
During baseline, rate of grasping declined moderately. There was a 15% increase in grasping from the end of baseline to end of the post-intervention period. There was no significant change in rate of reaching.
Conclusions:
This study provides preliminary data showing very small improvements in hand grasp of children with Rett syndrome following ASI treatment; larger studies in diverse settings are needed to establish the effectiveness of this approach. This study shows that an interrupted time series research design provides a valid template for evaluating interventions for children with rare disorders.
Keywords: Rett syndrome, occupational therapy, sensory integration, hand function
Introduction
Rett syndrome (RTT)/Rett-related disorder is a rare, neurodevelopmental disorder that primarily affects females and occurs in approximately 1 in every 10 000 live female births. It is associated with a mutation in the x-linked MECP2 gene in approximately 95% of those diagnosed with typical RTT and 50%-70% of those with atypical RTT.1,2 Children who have RTT appear to develop typically until 6 to 18 months of life when they begin to lose previously acquired skills. The 4 main characteristics of RTT are development of hand stereotypies, partial or complete loss of spoken communication, development of dyspraxia of gait or loss of gait, and dyspraxia of hand use with partial or complete loss of functional hand skills.1,3,4 Cognitive impairment ranges from mild to severe, depending on the type of MECP2 mutation.5-10
Children with RTT demonstrate severe dyspraxia, sensory hypersensitivities, and other sensory processing issues.9,11 A praxis deficit, or dyspraxia, is a problem conceptualizing what to do with an object; organizing or recalling previous experiences in relation to a task; planning sequences of movements needed to interact with objects; and/or judging timing, distance, force, and grading of arm and body movements to functionally interact with objects.12,13 Dyspraxia, praxis deficits, and poor motor planning are terms used by occupational therapists and other disciplines to identify this somatosensory-based issue. Praxis has a cognitive component.12,14 A gradual decline in cognition may occur in individuals with RTT9,15 and therefore may affect praxis abilities over time.
An accurate internal sense of postural configuration of the body is integral for planning and executing movement.16 This sense of the body’s shape, spatial properties, and arrangement of body parts in space (body scheme) is derived from current and previous sensory inputs from the skin, muscles, and joints while moving through the environment.17,18 A poorly developed body scheme contributes to praxis deficits.9
Individuals with RTT have difficulty going from intention to action, resulting in delays or absence of motor responses to verbal requests and inability to imitate actions.11 For example, in clinical practice children with RTT may appear to want to reach toward a highly preferred object or food (as seen by sustained visual attention to the object, joint attention, or facial expression), but their arms appear stuck. They seem to lack awareness of how to position and sequence movements of their arms and hands to reach toward and obtain the object, and when the movement is demonstrated, they are unable to imitate it. These praxis deficits severely limit purposeful reach, hand function,9,11 and participation in daily living routines, leading to a marked decrease in quality of life for individuals with RTT and their families.19,20
Mouse models genetically engineered with Mecp2 mutations display symptoms very similar to those observed in humans with RTT/MECP2 mutations.19 These include loss of purposeful and coordinated forepaw use, impaired reaching and grasping, motor apraxia, development of forepaw stereotypies, reduced exploration of their environment, among others. Electroencephalographic (EEG) recordings of these mouse models show abnormalities in transmission of impulses between neurons throughout the cortex, resulting in dysfunction in sensory information processing and motor coordination.21 Neurochemical changes in norepinephrine and serotonin were seen in the cerebrospinal fluid and motor control areas of the brain in Mecp2-null mouse models as well as in individuals with RTT. This functionally manifests as delayed motor initiation and dyspraxia—that is, difficulty with or the inability to plan and initiate purposeful movement.22
Multiple studies of mice with Mecp2 mutations demonstrate that actively moving through enriched environments (EE) promotes the development and maturation of neurons, formation of synapses, plasticity, neuronal activation and signaling between neurons, improved motor coordination, balance, and motor learning.23-27 One EE study of mice with Mecp2 mutations showed significantly improved functional grasping and paw placement compared with a control group in sparse cages.28 Although we cannot directly apply basic animal studies to humans, consistency across animal models suggests that these could be a foundation for the study of improving function in humans with RTT.
There are significant parallels between the enriched environments in EE animal studies and the physical environment and process elements of Ayres Sensory Integration (ASI) therapy.25,29 These similarities include moving through novel, sensory-rich environments; having control over the activity (child-directed) in a playful environment; and simultaneously tapping into more than 1 sensory system.29,30 Ayres Sensory Integration clinicians encourage children to actively play on a variety of swings, therapy balls, scooter boards, ramps, climbing/jumping, and other suspended and movable equipment. They also provide tactile-proprioceptive enrichment opportunities with crash pads, textured objects, brushes, vibrating toys, and/or a ball pit.30,31 Collectively, these stimulate proprioceptors of muscles and joints, motion receptors in the inner ear, visual and auditory receptors, and/or tactile receptors in the skin. Ayres Sensory Integration therapists facilitate just-right challenges that encourage problem-solving and adaptive responses to ever-changing sensory and motor environments. In theory, these activities improve the nervous system’s ability to process, organize, and integrate sensory information, improve functioning,29,32 and provide the building blocks for the development of body scheme and motor planning/praxis.17,18
There are no studies that specifically examine the effects of ASI therapy on motor planning/praxis, functional reaching, or hand use of individuals with RTT. There is preliminary evidence that this type of therapy may benefit children with sensory processing issues who have other diagnoses.94 A randomized controlled trial (RCT) of children with autism showed that children who received ASI intervention scored significantly higher than the control group on individualized Goal Attainment Scale scores and showed a decrease in caregiver assistance needed during self-care.33,34 A study of children with Pervasive Developmental Disorder-not otherwise specified and Sensory Processing Disorder (SPD) who received individual ASI therapy demonstrated a decrease in autistic mannerisms and significant improvement in goals related to sensory processing, self-regulation, and fine motor development.35 In a pilot study of children with high-functioning autism spectrum disorder, the children in the ASI group showed a greater increase in basic motor coordination and complex motor planning compared with control groups with other types of treatments or usual care with no treatment.36 In a study of children with mild intellectual disabilities, the children in the ASI treatment group significantly outperformed those in neurodevelopmental treatment and perceptual-motor treatment groups on the Bruininks-Oseretsky Test of Motor Proficiency subtests of upper-limb coordination, response speed, visual-motor control, and upper limb speed and dexterity.37,38
Standardized assessments of hand function and sensory processing cannot be used with most children who have RTT. Severe praxis deficits affect their ability to physically interact with testing materials and imitate actions.9,11,39 Language processing deficits affect their ability to follow verbal directions.40-44 Also, many test/questionnaire items are not applicable to children with RTT due to their very limited functional abilities and, in some cases, visual processing deficits.45,46 Downs et al47 developed and validated an 8-point measure to describe hand function in people with RTT. Although this scale provides important information about the quality of purposeful hand function, it does not measure the very small yet potentially significant changes necessary to evaluate treatment effects over a relatively short study period.
Hand function involves the following 3 essential components: transport (reach), orientation, and manipulation.48 In this study, we chose to quantitatively examine the transport component by studying reach and grasp during classroom activities as children with RTT typically have difficulty at this basic level.
We hypothesized that the ASI intervention would develop underlying foundations of sensory processing, body schema, and motor planning/praxis that would be followed by a visible change in trend and a statistically significant increase in the rate of functional reaches and grasps in the study participants.
We examined the following specific aims—controlling for the 40-day baseline trend in study outcomes among 5 children with RTT:
Is an ASI intervention (36 hours of total treatment, 3 hours per week) associated with an increase in the level and/or slope of the time series of average rates of functional reaching and grasp in 5 children with RTT?
Is there decay in the observed improvements during the 40 days following cessation of the intervention?
Methods
Research design
We used an interrupted time series (ITS) design—one of the most rigorous quasi-experimental designs—to analyze intervention effects on functional reaching and grasp.49 Randomized controlled trials are often administratively and methodologically infeasible for studying populations with rare disorders such as RTT.50,51 Interrupted time series takes advantage of multiple observations over time and allows us to track small changes in trends, even in a small sample size. The ITS research method is recognized by medical and health policy researchers as a strong alternative to weaker designs such as single-observation pre-post designs, cross-sectional designs at one point in time, or single case study that cannot control for basic biases.51-54 Interrupted time series controls for most threats to internal validity, such as history (eg, secular trends) and maturation (eg, aging). Despite the small sample size, it is worth noting that this study may be one of the largest samples of children with these rare disorders in 1 school setting. For statistical purposes, the sample size is the number of observations, not the number of children. Therefore, the ITS design significantly strengthens the study by yielding 104 days of observation after excluding outliers and missing values.
There were 3 observation periods as follows: 8 weeks of baseline (pre-intervention); 12 to 15 weeks of intervention (60-minute sessions of individual, ASI therapy 3 times per week, for a total of 36 hours per child—the intervention period was lengthened when needed due to school absences/illness to ensure that each participant received the full 36 hours of treatment); and 8 weeks following cessation of the intervention (post-intervention). We randomly assigned participants to 1 of 3 staggered start dates to control for history bias as outcomes may vary by time of the school year (Table 1). We defined the index date as the first day of the intervention.
Table 1.
Order of intervention of interrupted time series design.a
| Participant number | October | November | December | January | February | March | April | May | June |
|---|---|---|---|---|---|---|---|---|---|
| 1 and 5 | Baseline | ASI intervention | Follow-up | ||||||
| 3 | Baseline | ASI intervention | Follow-up | ||||||
| 2 and 4 | Baseline | ASI intervention | Follow-up | ||||||
Abbreviation: ASI, Ayres Sensory Integration.
This was a fixed cohort of the same 5 study participants. All 5 children participated in the pre-intervention (baseline), ASI intervention, and post-intervention (follow-up) periods.
Participants
We received approval from the Institutional Review Board to enroll 6 students with diagnoses of RTT. We included the following diagnoses under the umbrella of RTT: classic and atypical RTT and Rett-related disorders involving MECP2 and CDKL5 genes.1 All attended a private, publicly funded school for students with severe disabilities and complex health care needs. We withdrew 1 participant who received the intervention. This is because she consistently walked away from the video-recorded classroom lessons thereby preventing measurement of outcomes. The remaining 5 participants ranged in age and developmental levels (see Table 1). They received their usual therapies within the school setting throughout all phases of the study. No one was enrolled in any other research study prior to or during any of the observation periods. Each participant received the full 36 hours of individualized ASI intervention.
Study intervention
Prior to the intervention period, we assessed participants to identify their sensory and motor strengths and needs to document baseline functioning and to set individual treatment objectives. As standardized assessments could not be used, we created our own Sensory Processing Survey for Children with Multiple Disabilities55 based on applicable portions of commercially available sensory processing assessments/sensory histories and the examiners’ own experience with children who have severe disabilities.56-59 In addition, we used selected items from the Peabody Developmental Motor Scales (PDMS-260), the Assessment, Evaluation and Programming System for Infants and Children (AEPS61,62), and the School Function Assessment (SFA63).
Dosage
There is no precedent for dosage of ASI specific to children who have RTT. In a systematic review of literature on ASI for treatment of a range of conditions, May-Benson and Koomar64 found wide variation in frequency, duration, and amount of intervention. We set the frequency at 3 times weekly over a 3-month period and duration of 60 minutes for a total of 36 hours per participant—roughly within the average range of the most rigorous studies in their review. We estimated that this dosage would be sufficient to demonstrate change.
ASI fidelity
We adhered to the required process and structural elements of the Ayres Sensory Integration Fidelity Measure© (ASIFM30,31) during all intervention sessions. The ASI treatments occurred in a large multipurpose room within a private school for children aged 3 to 21 years with multiple disabilities. The physical space included an area with 3 overhead hooks, a rotational device, and swings (platform, tire, net, and frog) for suspension equipment. The room was equipped with a large ball pit, scooter boards, ramps, mats, cushions, sensory toys, ropes and bungee cords, as well as a quiet space.
Treatments were provided by 2 pediatric occupational therapists with certification in ASI and expertise with children who have severe special needs. The ASI therapy provided child-centered, multisensory input with the goal of eliciting adaptive responses. The sensory input included vestibular, proprioceptive, tactile, visual, and auditory play-based activities that were altered or continued according to the child’s response.30
Due to the significant limitations in communication and motor functioning of this study participants (see Table 2), we used child-specific communication systems to collaborate on activity choice and to support the child’s intrinsic motivation to play—essential aspects of fidelity to ASI. Some children used their own communication books to choose activities. Others made choices by moving themselves to specific activities or materials they were interested in. For children who did not have communication books and were not mobile, we provided assistance to experience activities and then looked for indications that they wanted the activity to continue. These signs included joint attention, repeating a motor action, and signs of pleasure (smiling, playful behavior, reaching, etc.). Conversely, we interpreted the following as indicators that the child was not interested in or finished with the activity and ready for something different: movement away, grimacing, lack of engagement, no observable sign of enjoyment, or adaptive responses.
Table 2.
Participant characteristics and functional status at baseline.
| Participant number | Age (years) | Diagnosis and health issues | Sitting/mobility | Hand use (non-food) |
|---|---|---|---|---|
| 1 | 9.1 | Atypical RTT CDKL5 Erratic sleep Upper respiratory infections |
Sits: assumes sitting and sits unsupported Mobility: does not roll, scoot, crawl, or walk |
Reach: reaches toward highly motivating objects Grasp: grasps only food-related Hold: holds only food-related Use: uses only food-related |
| 2 | 8.3 | Atypical RTT Seizure disorder controlled by medication |
Sits: assumes sitting and sits unsupported Mobility: rolls and crawls. Walks with >75% contact assistance without a device |
Reach: reaches for highly motivating objects Grasp: grasps Hold: sustains grasp >2 seconds for highly motivating objects Use: emerging |
| 3 | 9.10 | Classic RTT G-tube Frequent GI distress requiring venting |
Sits: does not assume sitting. Sits with 50%-75% support Mobility: does not roll, crawl or walk |
Reach: occasionally reaches in direction of objects Grasp: does not grasp Hold: does not hold Use: does not use objects functionally |
| 4 | 3.10 | Atypical RTT CDKL5 Seizure disorder not well-controlled by medication |
Sits: unable to assume sitting or sit without full support Mobility: rolls. Does not crawl or walk |
Reach: occasionally swipes toward objects Grasp: does not grasp Hold: does not hold Use: does not use objects functionally |
| 5 | 7.9 | Classic RTT G-tube Frequent GI distress Upper respiratory infections |
Sits: assumes sitting and sits unsupported Mobility: does not roll, scoot, or crawl. Walks with dyspraxia, halting gait, with <25% contact assistance or prompting |
Reach: reaches for highly motivating objects Grasp: grasps highly preferred sensory materials Hold: does not sustain grasp Use: does not use objects functionally |
Abbreviations: GI, gastrointestinal; RTT, Rett syndrome.
We observed for additional adaptive responses that demonstrated progress toward their individual treatment goals and objectives, some of which included making postural adjustments during balance challenges, shifting weight and rotating trunk during transitions, visually guided reaching, sustaining hand grasp on objects, motor planning functional activities with arms and hands, and so on.
Staff from The Koomar Center/Spiral Foundation evaluated the fidelity of our ASI intervention using the ASIFM.30 They inspected the physical space, equipment, and materials to measure the structural elements and observed video recordings to assess the process elements. We video recorded 1, uninterrupted, session per study participant during regularly scheduled ASI treatments. The date of the recording was determined by the availability of volunteer video recorders. The fidelity raters used the Ayres Sensory Integration Fidelity Measure Scoring Summary to score each of the hour-long video-recorded sessions. All treatment videos demonstrated high fidelity to ASI, with a mean of 95 of a possible 100 points (a passing score is 80 or above).
Data collection
To study the effects of the intervention, we video recorded65 each participant during 30-minute, structured classroom observations, Monday through Friday, between 10 o’clock and noon, throughout all 3 phases of the 7-month study period for a total of 554 videos—1 to 2 hours after receiving the ASI intervention (see Table 3). During these video-recorded observations, we counted the number of times the participant reached for and/or grasped materials, according to specific criteria described below. In our experience, children with RTT demonstrate vastly different behaviors when reaching for classroom materials versus food-related items. Therefore, we deliberately excluded food-related activities from our structured observations.
Table 3.
Number of video-recorded observations per participant.
| Participant number | Total number of 30-minute videos | Total time recorded (hours) |
|---|---|---|
| 1 | 105 | 52.5 |
| 2 | 123 | 61.5 |
| 3 | 92 | 46 |
| 4 | 112 | 56 |
| 5 | 125 | 62.5 |
| Total | 554 | 277 |
Observations occurred during structured lessons featuring best practices for teaching students with moderate and severe disabilities.66 We designed the lessons using set elements established within a structured routine (greeting, initial review of activity, sample product exploration, choice of materials, clean up, final review of activity, and goodbye). During each lesson, the teacher followed a standard prompting hierarchy with specific wait time between prompts (30 or 60 seconds based on each child’s Individualized Education Plan).
Study variables
Functional reach
We defined functional reach as forward or lateral motion of the arm/hand away from the body or support surface to bring the hand in contact with a target. Targets included objects related to the lesson or the teacher’s hand if holding a lesson item. We eliminated reaches involving hand stereotypies. We defined hand stereotypies as involuntary rhythmic, patterned, coordinated, repetitive, and seemingly purposeless hand movements that included hand mouthing.11,67,68 A reach was determined to be intentional if 1 or more of the following criteria was met11:
Participant looked at target.
Participant’s head was oriented toward the target.
Participant used the targeted object.
Coder believed the participant intentionally reached for the target, based on contextual evidence. The following are examples of implied intentionality: child opened hand to explore sensory attributes of the object; smiled after coming into contact with target; and oriented to the object using peripheral vision or a fleeting glance.69-71 The following is an example of a reach that implied that the reach was unintentional: child’s contact with a target appeared to be caused by repositioning her body.
Grasp
We coded whether the intentional reach resulted in a grasp of the target.11,72 We did not include grasps that involved bringing objects onto/into the mouth as hand/object-mouthing stereotypies are very common among children with RTT67 and occurred often among our study participants. We targeted grasp of objects that support classroom learning.
Outcome measures
To observe changes in outcome measures over time, we calculated the number of reaches and grasps per child during daily (5 days per week), 30-minute video-recorded observations (hereafter referred to as rate of reaching or rate of grasping) before, during, and after the study intervention period over a span of 7 months.
Data coding
Graduate assistants coded the videos using Datavyu software—a video coding/data visualization tool designed for collecting behavioral data from videos.73,74 Datavyu links videos with an extensible coding spreadsheet that appears on the computer screen beside the video. Custom-programmed scripts enabled precise coding of targeted behaviors during each 30-minute video, including exactly when each reach and grasp occurred, how many seconds/minutes it lasted, and other specific qualities of the action. We eliminated videos that included 15 minutes or more of missing data, such as when a child fell asleep or had a medical incident that interrupted the observation for more than 15 minutes. Coded data columns were hidden to allow blinded coding by multiple research assistants.
Inter-rater reliability
We selected graduate students from a talented pool of applicants enrolled in the field of special education or a related field at a college in the greater Boston area. They were blinded from the study objectives, the actual dates of the videos, and the intervention. We established reliability between coders using the following procedure. We trained coders to identify reaches, grasps, and hand stereotypies that were specific to each participant using the Manual for Coding Reach and Stereotypies.75 All coders were trained on the same sample 30-minute training videos using Datavyu coding software for each of the 5 participants. The investigators evaluated the accuracy of each coder’s work and calculated percentages of agreement between coders, using customized inter-rater scripts. We repeated the training process until the coders achieved inter-rater reliability (IRR) correlation coefficient of 90% or better.
Once IRR was established, we continued to sample 25% of each video for inter-rater agreement throughout the entire coding process. The coders achieved 96% or better agreement for the coding of all 554 videos. Kappa statistics for IRR were 0.99 for grasp (95% confidence interval [CI]: 0.996-1) and 0.91 for reach (95% CI: 0.905-0.931) indicating excellent IRR.76
Statistical analysis
We used ITS analysis/segmented regression models to determine the size and significance of changes in levels and trends of outcome variables occurring after initiation and cessation of the intervention. These models include controls for baseline trends and most internal threats to validity and indicators for level and slope changes coincident with the change points.49,77 We also included quadratic terms to account for the non-linear pattern we observed from the plots.78 We kept the terms with P values less than .05 in the models.
Missing data
We applied statistical methods to adjust for missingness of the data and to smooth the data. To adjust for missing observations at the beginning and end of the observation periods, we removed observations during the first 10 days of baseline observation and the last 10 days after cessation of the intervention. We removed 4 to 5 outlier data points of a total of approximately 91 data points for each study participant that were above the 95th percentile of the distribution.79 Outliers represent potentially unreliable data and they distort the best-fitting trend lines creating autocorrelation (pulling the line above or below the data points, thereby no longer fitting most data points). Because of significant variation in the daily observations, we constructed moving averages80,81 of 10 observations for each study participant to more accurately observe underlying trends. To preserve scientific rigor, we made those decisions before the data analysis.
Results
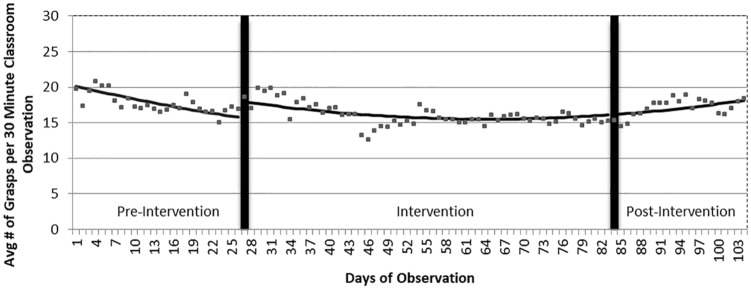
During the baseline period, the average number of grasps per day was already declining from 20.2 to 15.8 (a relative decline of 22%; see Figure 1). Controlling for this declining trend, there was a significant increase of 2.3 grasps per day (95% CI: 0.7-3.9, P = .006) immediately following the start of the intervention. During the 3-month intervention period, the best-fitting line followed a curvilinear (or u-shaped) trend during which the number of grasps per day declined from 17.9 to a low level of 15.5 (−2.4). This trend of grasps per day began to climb after 2 months of the 3-month intervention period from 15.5 to 16.1 (+0.6). By the end of the post-intervention period, this number rose to 18.2, moderately above the number of grasps per day at the end of the baseline period—an increase of 2.4 grasps or a relative increase of 15%.
Figure 1.
Changes in trends in rates of grasping before, during, and after the ASI intervention. ASI indicates Ayres Sensory Integration.
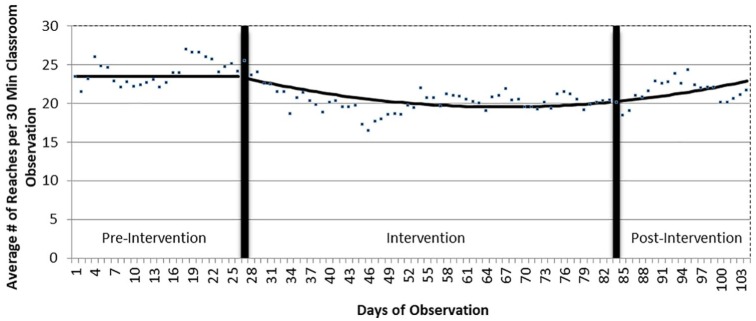
There was no change in the baseline trend of the average number of reaches (23.5 per day; see Figure 2). However, at the start of the intervention there was a modest decline in the slope of reaches (−0.19 reaches per day; 95% CI: −0.29 to −0.098; P = .000127). Although the trend of the raw data declined somewhat during the first 2 months of the intervention period, there was also a modest quadratic (curvilinear) increase in trend of 0.0024 reaches per day at the beginning of the intervention (95% CI: 0.0011-0.0037, P = .006; see Figure 2). In other words, the quadratic term, which best fits the data, declined slowly, stabilized, and rose modestly from the end of the intervention to the end of the post-intervention period. Thus, the estimated number of reaches was approximately the same at the start of the intervention (23.3) and at the end of the post-intervention periods (22.9).
Figure 2.
Changes in trends in rates of reaching before, during, and after the ASI intervention. ASI indicates Ayres Sensory Integration.
Discussion
We conducted an ITS study to investigate the effects of an ASI intervention on functional reaching/grasping in 5 children with RTT/Rett-related disorders. During the baseline period the average rate of grasps declined moderately to an average of 15.8 per day (see Figure 1). This decline in the rate of grasping during the baseline period was not expected.
Although regression is a feature of RTT in the early stages, it likely does not explain the decline in grasp during baseline among our participants who ranged in age from just under 4 to 10 years of age at the start of the study. Studies of hand function in children who have RTT report relatively stable hand function during these years.2,47,82,83 An important distinction from those studies, however, is that we deliberately excluded food, drink, and related utensils/objects, as our objective was to demonstrate improved hand usage to facilitate participation in classroom learning. In our experience, children with RTT may have skill reaching for food/drinks but lack the ability to reach for non-food related objects. Lotan et al82 discussed the importance of motivation and routine practice for maintaining and developing skills. As grasp and use of feeding/drinking tools—inherently motivating and repetitive—occur multiple times per day, it is probable that those skills are more stable than those involving grasp of classroom tools.
Immediately after the start of the intervention period, there was a significant increase in the level of grasping, above the predicted rate from the baseline trend (see Figure 1). That was followed by a modest decline that continued throughout the first 2 months of this 3-month intervention period. The novelty of beginning the intervention process may have caused this temporary increase in level of grasp. One explanation for the decline after that initial increase is that the added physical exertion associated with the intervention resulted in fatigue. We observed frequent periods of fatigue during the intervention sessions as well as during the classroom video recordings.
For children with RTT, the set dosage of 3, 1-hour ASI treatments per week may have been too ambitious. Sleep disorders are common,84-86 occurring in 80% of the individuals with RTT between 2 and 35 years of age.86 These include disorders of initiating sleep, frequent and long duration awakenings during the night, sleep breathing disorders including central apneas during sleep, and daytime somnolence. These issues, common among our study participants, combined with the significant increase in physical activity each week during the intervention period, appeared to affect participation and performance during treatment sessions as well as classroom observations.
The rate of grasping began to climb at the beginning of the third month of the intervention, and this gradual improvement continued throughout the post-observation period (see Figure 1). From the end of the baseline period to the end of the post-observation period, there was a 15% increase in grasping, suggesting that the participants may have needed time to acclimate to the rigors of the intervention. Contemporaneous treatment notes and video recordings during the third month of the intervention document less somnolence and fatigue, improved physical conditioning and motor skills, and increases in strength and active participation. The continuing increase in grasp throughout the post-intervention period may reflect improvements in underlying sensory processing that were manifest as improvements in function after fatigue was no longer a factor.
During the baseline period, the rate of reaching remained the same. The total number of reaches declined modestly (see Figure 2) at the start of the intervention. This decline could be related to the fatigue factor discussed earlier. Also, as the distinction between intentional and random reaching is extremely difficult, actual grasps of objects are likely to be more valid measures of functioning among individuals with RTT. Reaches that do not result in grasping or using an object do not necessarily reflect improvements in function. Despite our careful attempts to differentiate intentional from random reaches, there is a likelihood of some false positives or negatives. Our observation of the more stable and better fitting trend line for grasps versus reaches further supports this hypothesis. It is interesting to note that the rate of reaching began to increase slightly after 2 months of the intervention—roughly the same time as the rate of grasping began to climb.
For another explanation of a delayed treatment effect on grasping, we consulted with neuroscientists working with genetically engineered RTT mouse models. Their research suggests that there may be an underlying process caused by dysregulation of the Mecp2 protein that affects neural plasticity. Neural plasticity is the nervous system’s ability to respond to environmental experiences by developing new neurons, reorganizing neuronal circuits, and adapting functionally.87,88 Krishnan et al., 89,90 hypothesize that this dysregulation causes transient, abnormal plasticity involving inhibitory neurons during sensitive time periods of learning. Inhibitory neurons release neurochemicals that make it harder for the electrical signals to travel across the synapses to neighboring neurons.89,90 Further research is needed to determine whether this inhibitory process and abnormal plasticity also occur in children with RTT and/or Rett-related disorders. If so, it may be another explanation for the temporary decline in reach and grasp prior to the upswing during the intervention period.
Increased body tension and emotional dysregulation can occur in individuals with dyspraxia when efforts to perform challenging motor actions are attempted or are not successful.91 The significant increase in physical exertion may also have triggered this response in our participants, which in turn may have muted the effect and progress during the intervention period. The modest, delayed increase in grasp that began at the beginning of the third month of the intervention may accurately reflect the benefit of the intervention without this burden of fatigue and resulting tension/dysregulation.
All our study participants exhibited neurological signs involving altered muscle tone, weakness, and balance that contributed to deficits in postural control, mobility, and hand function. Although we observed steady improvements in postural stability/control, balance, and mobility in all participants from the beginning of the intervention period to the end of the post-intervention period, tracking these outcomes was not an aim of this study. We did, however, document these promising observations and we will examine them in a future qualitative study.
Maciaszek et al92 found that engaging children with poor balance in a variety of whole body gross motor activities rich in vestibular, tactile, and proprioceptive input played a critical role in improving postural stability and balance.92 As motor development typically occurs from proximal (core) to distal,93 our observations of the earliest improvements occurring in core physical abilities during ASI intervention suggest that a longer intervention period may have a more pronounced effect on distal changes necessary to improve hand function, particularly in participants with severe motor involvement.
Limitations
This study has several limitations. First, there is significant variation in participants’ conditions within the umbrella of RTT/Rett-related disorders (Table 2). For example, some have active seizure disorders and significant gastrointestinal issues that temporarily affected their ability to participate, whereas others are more medically stable. We need to study if and how the level of severity of RTT impacts responses to ASI.
Another limitation involves sleep disorders that are common in individuals with RTT.84-86 We frequently observed low levels of energy and alertness among study participants during intervention sessions as well as during the video-recorded classroom observations. We anticipated that there would be a sizable amount of missing data given the participants’ conditions that included sleep disturbances and childhood illnesses. However, the amount of missing data at both the beginning and ending of the 7-month study periods was higher than anticipated, effectively shortening the pre- and post-intervention periods. This limited our ability to measure long-term post-intervention trends.
To standardize the study conditions, treatments were scheduled each morning at either 9 or 10 o’clock and video observations occurred before the noon lunch hour to accommodate the participants’ 6-hour school day. Undoubtedly, this additional amount of activity—all occurring between 9 o’clock and noon each school day—contributed to fatigue and possibly anxiety and may have affected performance. We need to learn more about optimal dosing and timing of treatments to maximize gains, given the special considerations of fatigue and neurological limitations associated with the condition.
Finally, the results of this study may not demonstrate a direct effect on hand function because ASI treatment does not specifically provide training of hand skills. Our intention was to use ASI more broadly to improve praxis by addressing underlying sensory processing abilities.
Implications for practice
Rett syndrome is a neurodevelopmental disorder that primarily occurs in females. Poor motor planning/praxis, severely limited hand use, and persistent hand stereotypies significantly impact their independence, participation in daily activities, and quality of life. There are currently no known effective treatments for developing hand function of children in this population. The potential to achieve even small improvements in function would be significant for these children whose ability to move and use their hands with purpose is so severely compromised. This study provides preliminary data suggesting that ASI may have small positive effects on the rate of grasping in children with RTT and warrants further study before recommending it in routine practice.
Our observations of fatigue and decline in functioning during the first 2 months of intervention warrant further study to examine the possible effects of ASI dosage on performance for children with RTT.
This study may be the first to use ASI as an intervention for children with RTT—a severely disabling condition. This form of therapy has traditionally been used with individuals who have milder motor and functional issues. Further study is needed to determine whether ASI might benefit children who have this and other severe conditions.
When studying and/or working with children who have RTT and other severe conditions, it is important to look at the whole body—recording both proximal and distal functions over time. That is, in addition to tracking functional hand use, therapists should also track changes in proximal trunk stability, balance, and mobility, as these are important foundation skills for developing more distal control and function of the hand.
Suggestions for further research
Future research is needed to determine the effectiveness of ASI to improve hand function in children with RTT. These should include a larger sample size and longer post-intervention period to study whether changes in hand grasp following an ASI intervention persist and for how long.
Further research is needed to establish whether the inhibitory process and abnormal plasticity observed in Mecp2 mouse models also occur in children with RTT and/or Rett-related disorders and whether this could account for a delayed treatment effect—in other words, when the stimulus (intervention) is removed, plasticity resumes and learning is observed. This may help us determine the optimal timing and dosing of ASI treatment.
The gold standard research design—RCTs—is often infeasible for evaluating the effectiveness of promising therapies for children with rare conditions such as RTT. Interrupted times series offers a strong and rigorous alternative. Interrupted time series can detect relatively small changes in stable outcome measures over time and controls many of the threats to validity better than alternative research designs. It measures changes in multiple observations occurring both before and during the intervention; this is an added benefit over simple single-point before-and-after designs that cannot observe changes in outcomes during each observation period—for example, a trend that initially declines and then increases within the intervention period as we found in this study.52 For statistical purposes, the sample size is the number of observations, not the number of children, thereby increasing statistical power even when studying small numbers of children. This under-used research design is growing rapidly in health policy and medical research. We believe that this study demonstrates that ITS provides an effective and valid template for researching the effectiveness of ASI and other therapeutic interventions for children with rare disorders. These children deserve the same high standards of evidence-based interventions that we provide the general population.
Acknowledgments
We acknowledge the many contributions of volunteers, research assistants, teachers, and study participants.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from the International Rett Syndrome Foundation (grant number: 3102) and the Michael and Susan Argyelan Education Research Fund.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: WD, KR, SD, SB, and SS conceptualized and designed the study. FZ performed calculations and data analysis. All authors discussed the results, drafted the manuscript, and revised the article.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
ORCID iD: Wendy Drobnyk  https://orcid.org/0000-0002-0019-180X
https://orcid.org/0000-0002-0019-180X
References
- 1. Neul JL, Kaufmann WE, Glaze DGet al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944-950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smeets EEJ, Chenault M, Curfs LMG, Schrander-Stumpel C, Frijns JP. Rett syndrome and long-term disorder profile. Am J Med Genet Part A. 2009;149:199-205. doi: 10.1002/ajmg.a.32491. [DOI] [PubMed] [Google Scholar]
- 3. Humphreys P. Measuring gross motor activities in Rett syndrome. Develop Med Child Neurol. 2015;57:1086-1087. doi: 10.1111/dmcn.12848. [DOI] [PubMed] [Google Scholar]
- 4. Umansky R, Watson JS, Colvin Let al. Hand preference, extent of laterality, and functional hand use in Rett syndrome. J Child Neurol. 2003;18:481-487. doi: 10.1177/08830738030180070201. [DOI] [PubMed] [Google Scholar]
- 5. Ahonniska-Assa J, Polack O, Saraf Eet al. Assessing cognitive functioning in females with Rett syndrome by eye-tracking methodology. Eur J Paediatr Neurol. 2018;22:39-45. doi: 10.1016/j.ejpn.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 6. Berger-Sweeney J. Cognitive deficits in Rett syndrome: what we know and what we need to know to treat them. Neurobiol Learn Memory. 2011;96:637-646. doi: 10.1016/j.nlm.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7. Djukic A, Valicenti McDermott M. Social preferences in Rett syndrome. Pediatr Neurol. 2012;46:240-242. doi: 10.1016/j.pediatrneurol.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 8. Fabio RA, Billeci L, Crifaci G, Troise E, Tortorella G, Pioggia G. Cognitive training modifies frequency EEG bands and neuropsychological measures in Rett syndrome. Research in Develop Disabil. 2016;53-54:73-85. doi: 10.1016/j.ridd.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 9. Lotan M, Ben-Zeev B. Rett syndrome. A review with emphasis on clinical characteristics and intervention. Sci World J. 2006;6:1517-1541. doi: 10.1100/tsw.2006.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rose SA, Djukic A, Jankowski JJ, Feldman JF, Fishman I, Valicenti-Mcdermott M. Rett syndrome: an eye-tracking study of attention and recognition memory. Develop Med Child Neurol. 2013;55:364-371. doi: 10.1111/dmcn.12085. [DOI] [PubMed] [Google Scholar]
- 11. Downs J, Parkinson S, Ranelli S, Leonard H, Diener P, Lotan M. Perspectives on hand function in girls and women with Rett syndrome. Develop Neurorehabil. 2014;17:210-217. doi: 10.3109/17518423.2012.758183. [DOI] [PubMed] [Google Scholar]
- 12. May-Benson T, Cermak SA. Development of an assessment for ideational praxis. Am J Occup Ther. 2007;61:148-153. doi: 10.5014/ajot.61.2.148. [DOI] [PubMed] [Google Scholar]
- 13. Miller LJ, Anzalone ME, Lane SJ, Cermak SA, Osten ET. Concept evolution in sensory integration: a proposed nosology for diagnosis. Am J Occup Ther. 2007;61:135-140. doi: 10.5014/ajot.61.2.135. [DOI] [PubMed] [Google Scholar]
- 14. Ayres AJ. Developmental Dyspraxia and Adult-Onset Apraxia: A Lecture. Torrance, CA: Sensory Integration International; 1985. [Google Scholar]
- 15. Pidcock FS, Salorio C, Bibat Get al. Functional outcomes in Rett syndrome. Brain Develop. 2016;38:76-81. doi: 10.1016/j.braindev.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parkinson A, Condon L, Jackson SR. Parietal cortex coding of limb posture: in search of the body-schema. Neuropsychologia. 2010;48:3228-3234. doi: 10.1016/j.neuropsychologia.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 17. Morasso P, Casadio M, Mohan V, Rea F, Zenzeri J. Revisiting the body-schema concept in the context of whole-body postural-focal dynamics. Front Hum Neurosci. 2015;9:83. doi:org/10.3389/fnhum.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roschin VY, Frolov AA, Burnod Y, Maier MA. A neural network model for the acquisition of a spatial body scheme through sensorimotor interaction. Neur Comput. 2011;23:1821-1834. doi: 10.1162/NECO_a_00138. [DOI] [PubMed] [Google Scholar]
- 19. De Filippis B, Musto M, Altabella L, Romano E, Canese R, Laviola G. Deficient purposeful use of forepaws in female mice modelling Rett syndrome. Neur Plast. 2015;2015:326184. doi: 10.1155/2015/326184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Retzlaff R. Families of children with Rett syndrome: stories of coherence and resilience. Fam Syst Health. 2007;25:246-262. doi: 10.1037/1091-7527.25.3.246. [DOI] [Google Scholar]
- 21. Goffin D, Zhou Z. The neural circuit basis of Rett syndrome. Front Biol. 2012;7:428-435. doi: 10.1007/s11515-012-1248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Santos M, Summavielle T, Teixeira-Castro Aet al. Monoamine deficits in the brain of methyl-CpG binding protein 2 null mice suggest the involvement of the cerebral cortex in early stages of Rett syndrome. Neuroscience. 2010;170:453-467. doi: 10.1016/j.neuroscience.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 23. Kerr B, Silva PA, Walz K, Young JI. Unconventional transcriptional response to environmental enrichment in a mouse model of Rett syndrome. PLoS ONE. 2010;5:e11534. doi: 10.1371/journal.pone.0011534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondo M, Gray LJ, Pelka GJ, Christodoulou J, Tam PPL, Hannan AJ. Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome Mecp2 gene dosage effects and BDNF expression. Eur J Neurosci. 2008;27:3342-3350. doi: 10.1111/j.1460-9568.2008.06305.x. [DOI] [PubMed] [Google Scholar]
- 25. Lane SJ, Schaaf RC. Examining the neuroscience evidence for sensory-driven neuroplasticity: implications for sensory-based occupational therapy for children and adolescents. Am J Occup Ther. 2010;64:375-390. doi: 10.5014/ajot.2010.09069. [DOI] [PubMed] [Google Scholar]
- 26. Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T. Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry. 2010;67:657-665. doi: 10.1016/j.biopsych.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 27. Nag N, Moriuchi JM, Peitzman CGK, Ward BC, Kolodny NH, Berger-Sweeney JE. Environmental enrichment alters locomotor behaviour and ventricular volume in Mecp21lox mice. Behav Brain Res. 2009;196:44-48. doi: 10.1016/j.bbr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28. Megra M, Diener PS. Testing efficacy of sensorimotor enrichment in ameliorating symptoms of Rett syndrome in a mouse model (Yale review of undergraduate research in psychology), 2013. Website. https://cpb-us-w2.wpmucdn.com/campuspress.yale.edu/dist/a/1215/files/2015/11/2013_YRURP-FOURTH-ISSUE-2hxmmxf.pdf.
- 29. Reynolds S, Lane SJ, Richards L. Using animal models of enriched environments to inform research on sensory integration intervention for the rehabilitation of neurodevelopmental disorders. J Neurodevelop Disord. 2010;2:120-132. doi: 10.1007/s11689-010-9053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parham LD, Roley SS, May-Benson TAet al. Development of a fidelity measure for research on the effectiveness of the Ayres Sensory Integration® intervention. Am J Occup Ther. 2011;65:133-142. doi: 10.5014/ajot.2011.000745. [DOI] [PubMed] [Google Scholar]
- 31. Parham LD, Cohn ES, Spitzer Set al. Fidelity in sensory integration intervention research. Am J Occup Ther (Bethesda). 2007;61:216-227. doi: 10.5014/ajot.61.2.216. [DOI] [PubMed] [Google Scholar]
- 32. Ayres AJ. Sensory Integration and the Child. Los Angeles, CA: Western Psychological Services; 1972. [Google Scholar]
- 33. Schaaf RC, Benevides T, Mailloux Zet al. An intervention for sensory difficulties in children with autism: a randomized trial. J Autism Develop Disord. 2014;44:1493-1506. doi: 10.1007/s10803-013-1983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mailloux Z, May-Benson TA, Summers CAet al. The issue is—goal attainment scaling as a measure of meaningful outcomes for children with sensory integration disorders. Am J Occup Ther. 2007;61:254-259. doi: 10.5014/ajot.61.2.254. [DOI] [PubMed] [Google Scholar]
- 35. Pfeiffer BA, Koenig K, Kinnealey M, Sheppard M, Henderson L. Effectiveness of sensory integration interventions in children with autism spectrum disorders: a pilot study. Am J Occup Ther. 2011;65:76-85. doi: 10.5014/ajot.2011.09205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwanaga R, Honda S, Nakane H, Tanaka K, Toeda H, Tanaka G. Pilot study: efficacy of sensory integration therapy for Japanese children with high-functioning autism spectrum disorder: sensory integration therapy for ASD. Occup Ther Int. 2014;21:4-11. doi: 10.1002/oti.1357. [DOI] [PubMed] [Google Scholar]
- 37. Wuang YP, Wang CC, Huang MH, Su CY. Prospective study of the effect of sensory integration, neurodevelopmental treatment, and perceptual-motor therapy on the sensorimotor performance in children with mild mental retardation. Am J Occup Ther. 2009;63:441-452. doi: 10.5014/ajot.63.4.441. [DOI] [PubMed] [Google Scholar]
- 38. Wuang YP, Lin YH, Su CY. Rasch analysis of the Bruininks-Oseretsky test of motor proficiency—second edition in intellectual disabilities. Res Develop Disabil. 2009;30:1132-1144. doi: 10.1016/j.ridd.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 39. Lotan M, Isakov E, Merrick J. Improving functional skills and physical fitness in children with Rett syndrome. J Intell Disabil Res. 2004;48:730-735. doi: 10.1111/j.1365-2788.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 40. Bartolotta TE, Remshifski P. Coaching communication partners: a preliminary investigation of communication intervention during mealtime in Rett syndrome. Commun Disord Quart. 2013;34:162-171. doi: 10.1177/1525740112453165. [DOI] [Google Scholar]
- 41. Bartolotta TE, Zipp GP, Simpkins SD, Glazewski B. Communication skills in girls with Rett syndrome. Focus Autis Other Develop Disabil. 2011;26:15-24. doi: 10.1177/1088357610380042. [DOI] [Google Scholar]
- 42. Urbanowicz A, Downs J, Girdler S, Ciccone N, Leonard H. An exploration of the use of eye gaze and gestures in females with Rett syndrome. J Speech Lang Hear Res. 2016;59:1373-1383. doi: 10.1044/2015_JSLHR-L-14-0185. [DOI] [PubMed] [Google Scholar]
- 43. Didden R, Korzilius H, Smeets Eet al. Communication in individuals with Rett syndrome: an assessment of forms and functions. J Develop Phys Disabil. 2010;22:105-118. doi: 10.1007/s10882-009-9168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marschik PB, Kaufmann WE, Sigafoos Jet al. Changing the perspective on early development of Rett syndrome. Res Develop Disabil. 2013;34:1236-1239. doi: 10.1016/j.ridd.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stauder JEA, Smeets EEJ, van Mil SGM, Curfs LGM. The development of visual- and auditory processing in Rett syndrome: an ERP study. Brain Develop. 2006;28:487-494. doi: 10.1016/j.braindev.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 46. Jain D, Singh K, Chirumamilla Set al. Ocular MECP2 protein expression in patients with and without Rett syndrome. Pediatr Neurol. 2010;43:35-40. doi: 10.1016/j.pediatrneurol.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Downs J, Bebbington A, Jacoby Pet al. Level of purposeful hand function as a marker of clinical severity in Rett syndrome. Develop Med Child Neurol. 2010;52:817-823. doi: 10.1111/j.1469-8749.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marotta JJ, Medendorp WP, Crawford JD. Kinematic rules for upper and lower arm contributions to grasp orientation. J Neurophysiol. 2003;90:3816-3827. doi: 10.1152/jn.00418.2003. [DOI] [PubMed] [Google Scholar]
- 49. Wagner AK, Soumerai SB, Zhang F, Ross Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Therapeut. 2002;27:299-309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 50. Anaby D, Lal S, Huszczynski J, Maich J, Rogers J, Law M. Interrupted time series design: a useful approach for studying interventions targeting participation. Phys Occup Ther Pediatr. 2014;34:457-470. doi: 10.3109/01942638.2013.866612. [DOI] [PubMed] [Google Scholar]
- 51. Soumerai SB, Ceccarelli R, Koppel R. False dichotomies and health policy research designs: randomized trials are not always the answer. J Gen Intern Med. 2017;32:204-209. doi: 10.1007/s11606-016-3841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fretheim A, Soumerai SB, Zhang F, Oxman AD, Ross-Degnan D. Interrupted time-series analysis yielded an effect estimate concordant with the cluster-randomized controlled trial result. J Clin Epidemiol. 2013;66:883-887. doi: 10.1016/j.jclinepi.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 53. Majumdar SR, Soumerai SB. The unhealthy state of health policy research. Health Affair. 2009;28:900. doi: 10.1377/hlthaff.28.5.w900. [DOI] [PubMed] [Google Scholar]
- 54. Shadish WR, Cook TD, Campbell DT. Experimental and Quasi Experimental Designs for Generalized Causal Inference. Boston, MA: Houghton Mifflin Company; 2002. [Google Scholar]
- 55. Rocco K, Drobnyk W. Sensory processing survey for children with multiple disabilities [in press]. 2010. [Google Scholar]
- 56. Dunn W, Daniels DB. Initial development of the infant/toddler sensory profile. J Early Interv. 2002;25:27-41. doi: 10.1177/105381510202500104. [DOI] [Google Scholar]
- 57. Ermer J, Dunn W. The sensory profile: a discriminant analysis of children with and without disabilities. Am J Occup Ther. 1998;52:283-290. doi: 10.5014/ajot.52.4.283. [DOI] [PubMed] [Google Scholar]
- 58. Occupational Therapy Associates-Watertown (OTA-Watertown). Developmental Sensory History 0-3 and 4-12. Watertown, SD: OTA-Watertown; 1996. [Google Scholar]
- 59. Roley SS, Blanche EI, Schaaf RC. Understanding the Nature of Sensory Integration With Diverse Populations. Austin, TX: Pro-Ed, Inc.; 2001. [Google Scholar]
- 60. Wang HH, Liao HF, Hsieh CL. Reliability, sensitivity to change, and responsiveness of the peabody developmental motor scales—second edition for children with cerebral palsy. Phys Ther. 2006;86:1351-1359. doi: 10.2522/ptj.20050259. [DOI] [PubMed] [Google Scholar]
- 61. Bricker D. Assessment, evaluation, and programming system (AEPS) for infants and children. Volume 1: AEPS measurement for birth to three years. Pediatr Phys Ther. 1994;6:43. [Google Scholar]
- 62. Bricker D, Capt B, Pretti-Frontczak K. Assessment, Evaluation, and Programming System for Infants and Children (AEPS), Test: Birth to Three Years and Three to Six Years. 2nd ed. Baltimore, MD: Brookes Publishing Company; 2002. [Google Scholar]
- 63. Hwang JL, Davies PL. Rasch analysis of the school function assessment provides additional evidence for the internal validity of the activity performance scales. Am J Occup Ther. 2009;63:369-373. doi: 10.5014/ajot.63.3.369. [DOI] [PubMed] [Google Scholar]
- 64. May-Benson TA, Koomar JA. Systematic review of the research evidence examining the effectiveness of interventions using a sensory approach for children. Am J Occup Ther. 2010;64:403-414. doi: 10.5014/ajot.2010.09071. [DOI] [PubMed] [Google Scholar]
- 65. Fyfe S, Downs J, McIlroy Oet al. Development of a video-based evaluation tool in Rett syndrome. J Autis Develop Disord. 2007;37:1636-1646. doi: 10.1007/s10803-006-0293-9. [DOI] [PubMed] [Google Scholar]
- 66. Collins BC. Systematic Instruction for Students With Moderate and Severe Disabilities. Baltimore, MD: Brookes Publishing Company; 2012. [Google Scholar]
- 67. Carter P, Downs J, Bebbington Aet al. Stereotypical hand movements in 144 subjects with Rett syndrome from the population-based Australian database. Movement Disord. 2009;25:282-288. doi: 10.1002/mds.22851. [DOI] [PubMed] [Google Scholar]
- 68. Temudo T, Oliveira P, Santos Met al. Stereotypies in Rett syndrome: analysis of 83 patients with and without detected MECP2 mutations. Neurology. 2007;68:1183-1187. doi: 10.1212/01.wnl.0000259086.34769.78. [DOI] [PubMed] [Google Scholar]
- 69. Bosch DG, Boonstra FN, Willemsen MA, Cremers FP, Vries BB. Low vision due to cerebral visual impairment: differentiating between acquired and genetic causes. BMC Ophthalmol. 2014;14:59. doi: 10.1186/1471-2415-14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Glaze DG. Neurophysiology of Rett syndrome. J Child Neurol. 2005;20:740-746. doi: 10.1177/08830738050200082301. [DOI] [PubMed] [Google Scholar]
- 71. Swaminathan M. Cortical visual impairment in children—a new challenge for the future? Oman J Ophthalmol. 2011;4:1-2. doi: 10.4103/0974-620x.77654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sangole AP, Levin MF. Palmar arch dynamics during reach-to-grasp tasks. Exp Brain Res. 2008;190:443-452. doi: 10.1007/s00221-008-1486-6. [DOI] [PubMed] [Google Scholar]
- 73. Datavyu Team. Datavyu: A Video Coding Tool (Databrary project). New York: New York University; 2014. Website. http://datavyu.org. [Google Scholar]
- 74. Adolph K, Gilmore R, Kennedy J. Video data and documentation will improve psychological science. Website. https://www.apa.org/science/about/psa/2017/10/video-data.aspx American Psychological Association Psychological Science Agenda; Up-dated 2017. Accessed December 9, 2018. [Google Scholar]
- 75. Davidson S, Rocco K, Drobnyk W. Manual for Coding Reach and Stereotypies in Children with Rett Syndrome/Rett Related Disorders [in press]; 2014.
- 76. Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley; 1981. [Google Scholar]
- 77. Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol. 2011;64:1252-1261. doi: 10.1016/j.jclinepi.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 78. Lu CY, Zhang F, Lakoma MDet al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ (Clin Res Ed.). 2014;348:g3596. doi: 10.1136/bmj.g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Barnett V, Lewis T. Outliers in Statistical Data. 3rd ed. Hoboken, NJ: Wiley; [1978] 1994. [Google Scholar]
- 80. Brockwell PJ, Davis RA. Time Series: Theory and Methods. New York, NY: Springer-Verlag; 1991. [Google Scholar]
- 81. Hamilton JD. Time Series Analysis. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- 82. Lotan M, Schenker R, Wine J, Downs J. The conductive environment enhances gross motor function of girls with Rett syndrome. A pilot study. Develop Neurorehabil. 2012;15:19-25. doi: 10.3109/17518423.2011.629374 [DOI] [PubMed] [Google Scholar]
- 83. Neul JL, Lane JB, Lee HSet al. Developmental delay in Rett syndrome: data from the natural history study. J Neurodevelop Disord. 2014;6:20. doi: 10.1186/1866-1955-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Boban S, Wong K, Epstein Aet al. Determinants of sleep disturbances in Rett syndrome: novel findings in relation to genotype. Am J Med Gen Part A. 2016;170:2292-2300. doi: 10.1002/ajmg.a.37784. [DOI] [PubMed] [Google Scholar]
- 85. Hagebeuk EEO, Van Den Bossche RAS, De Weerd AW. Respiratory and sleep disorders in female children with atypical Rett syndrome caused by mutations in the CDKL5 gene: case report. Develop Med Child Neurol. 2013;55:480-484. doi: 10.1111/j.1469-8749.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 86. Wong K, Leonard H, Jacoby P, Ellaway C, Downs J. The trajectories of sleep disturbances in Rett syndrome. J Sleep Res. 2015;24:223-233. doi: 10.1111/jsr.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baroncelli L, Braschi C, Spolidoro M, Begenisic T, Maffei L, Sale A. Brain plasticity and disease: a matter of inhibition. Neur Plast. 2011;2011:1-11. doi: 10.1155/2011/286073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697-709. [DOI] [PubMed] [Google Scholar]
- 89. Krishnan K, Wang BS, Lu Jet al. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc Natl Acad Sci. 2015;112:E4782-E4791. doi: 10.1073/pnas.1506499112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Krishnan K, Lau BYB, Ewall G, Shea SD. MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nat Commun. 2017;8:14077. doi: 10.1038/ncomms14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Arntzen C, Elstad I. The bodily experience of apraxia in everyday activities: a phenomenological study. Disabil Rehabil. 2013;35:63-72. doi: 10.3109/09638288.2012.687032. [DOI] [PubMed] [Google Scholar]
- 92. Maciaszek J, Kilan N, Bronikowski M. Reaction to the sensory integration therapy in children with postural stability deficits. Minerv Pediatr. 2016;2016:27706121. [DOI] [PubMed] [Google Scholar]
- 93. Payne VG, Isaacs LD. Human Motor Development: A Lifespan Approach. Abingdon, England: Routledge; 2017. [Google Scholar]
- 94. Schaaf RC, Dumont RL, Arbesman M, May-Benson TA. Efficacy of occupational therapy using Ayres Sensory Integration®: a systematic review. Am J Occup Ther. 2018;72:028431. doi: 10.5014/ajot.2018.028431. [DOI] [PubMed] [Google Scholar]