Abstract
Purpose:
To compare plan quality and delivery efficiency of noncoplanar volumetric modulated arc therapy with coplanar volumetric modulated arc therapy, intensity-modulated radiation therapy, and CyberKnife for multiple brain metastases.
Methods:
For 15 patients with multiple brain metastases, noncoplanar volumetric modulated arc therapy, coplanar volumetric modulated arc therapy, intensity-modulated radiation therapy, and CyberKnife plans with a prescription dose of 30 Gy in 3 fractions were generated. Noncoplanar volumetric modulated arc therapy and coplanar volumetric modulated arc therapy plans consisted of 4 noncoplanar arcs and 2 full coplanar arcs, respectively. Intensity-modulated radiation therapy plans consisted of 7 coplanar fields. CyberKnife plans used skull tracking to ensure accurate position. All plans were generated to cover 95% target volume with prescription dose. Gradient index, conformity index, normal brain tissue volume (V 3Gy − V 24Gy), monitor units, and beam on time were evaluated.
Results:
Gradient index was the lowest for CyberKnife (3.49 ± 0.65), followed by noncoplanar volumetric modulated arc therapy (4.21 ± 1.38), coplanar volumetric modulated arc therapy (4.87 ± 1.35), and intensity-modulated radiation therapy (5.36 ± 1.98). Conformity index was the largest for noncoplanar volumetric modulated arc therapy (0.87 ± 0.03), followed by coplanar volumetric modulated arc therapy (0.86 ± 0.04), CyberKnife (0.86 ± 0.07), and intensity-modulated radiation therapy (0.85 ± 0.05). Normal brain tissue volume at high-to-moderate dose spreads (V 24Gy − V 9Gy) was significantly reduced in noncoplanar volumetric modulated arc therapy over that of intensity-modulated radiation therapy and coplanar volumetric modulated arc therapy. Normal brain tissue volume for noncoplanar volumetric modulated arc therapy was comparable with noncoplanar volumetric modulated arc therapy at high-dose level (V 24Gy − V 15Gy) and larger than CyberKnife at moderate-to-low dose level (V 12Gy − V 3Gy). Monitor units was highest for CyberKnife (28 733.59 ± 7197.85), followed by intensity-modulated radiation therapy (4128.40 ± 1185.38), noncoplanar volumetric modulated arc therapy (3105.20 ± 371.23), and coplanar volumetric modulated arc therapy (2997.27 ± 446.84). Beam on time was longest for CyberKnife (30.25 ± 7.32 minutes), followed by intensity-modulated radiation therapy (2.95 ± 0.85 minutes), noncoplanar volumetric modulated arc therapy (2.61 ± 0.07 minutes), and coplanar volumetric modulated arc therapy (2.30 ± 0.23 minutes).
Conclusion:
For brain metastases far away from organs-at-risk, noncoplanar volumetric modulated arc therapy generated more rapid dose falloff and higher conformity compared to intensity-modulated radiation therapy and coplanar volumetric modulated arc therapy. Noncoplanar volumetric modulated arc therapy provided a comparable dose falloff with CyberKnife at high-dose level and a slower dose falloff than CyberKnife at moderate-to-low dose level. Noncoplanar volumetric modulated arc therapy plans had less monitor units and shorter beam on time than CyberKnife plans.
Keywords: multiple brain metastases, noncoplanar VMAT, coplanar VMAT, IMRT, CyberKnife, dosimetry
Introduction
Brain metastases have been reported in up to 40% of patients with systemic cancer,1,2 and the incidence of brain metastases is increasing due to more sophisticated examination, such as brain magnetic resonance imaging screening and improved outcome of systemic therapy against primary cancers.
Most previous treatments include whole brain radiotherapy (WBRT), but more normal brain tissue was irradiated with WBRT resulting in more side effects. Therefore, it is crucial to spare normal brain tissue to decrease damages.3,4 Recent studies have shown that adjuvant WBRT for patients with limited brain metastases increased cognitive decline without improving survival.5,6 Brown et al 5 had compared patients (1-3 brain metastases) randomly treated with stereotactic radiosurgery (SRS) or SRS plus WBRT and found that SRS alone resulted in less cognitive deterioration at 3 months with no difference in overall survival. Yamamoto et al 7 found that SRS alone might be an alternative for multiple5–10 brain metastases patients in a multi-institutional prospective observational study. However, the toxicity of SRS given in a single fraction increases risk of neurological morbidity from radionecrosis for large brain metastases.8–11 Minniti et al 8 found a significant subset of patients who were treated with SRS developing neurological complications and suggested to consider hypofractionated stereotactic radiotherapy (HFSRT) to minimize the risk of symptomatic radionecrosis. It had been reported that HFSRT was an effective and safe way to treat large brain metastases.12–16 Minniti et al 12 had found that HFSRT was effective to treat brain metastases, associated with better local control and reduced risk of radionecrosis as compared to SRS. Ogura et al 13 had found that HFSRT for brain metastases yielded high local control probabilities without increasing severe adverse events.
Currently, radiotherapy devices for brain metastases mainly include Gamma Knife (GK; Elekta AB, Stockholm, Sweden), CyberKnife (CK; Accuray, Sunnyvale, California), TomoTherapy (Accuray), and conventional C-arm linear accelerator. Treatment plan of multiple brain metastases is relatively complex because the targets are often surrounded by many critical and radiation-sensitive structures including brainstem, eyes, and lenses. Brain radionecrosis is also a very severe side reaction. A sharper dose falloff outside the targets is needed to protect organs at risk (OARs) better. The delivery of adequate radiation dose to the targets with lower dose to OARs is challenging for brain metastases, and each technique has its own advantages and disadvantages. The advantages of GK are quick dose falloff and better protection of normal brain tissue. CyberKnife combines a high-resolution image–guided tracking system to adjust the angle of beams during treatment to guarantee the accuracy of the treatment. The shortcoming of GK and CK is that the delivery time is much longer than conventional linear accelerator-based plan.17–19 The dose falloff of intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) is not as quick as GK and CK.20
There are several studies investigating these techniques. But no systematic study was published comparing conventional C-arm linear accelerator and robotic radiosurgery for brain metastases. The aim of this study is to compare plan quality and delivery efficiency of noncoplanar VMAT (NC-VMAT) with coplanar VMAT (C-VMAT), IMRT, and CK plans for multiple brain metastases and to find the strengths and weaknesses of conventional C-arm linear accelerator and robotic radiosurgery.
Materials and Methods
This study was approved by Peking University Third Hospital Ethics Committee (approval no. M2019273), and written-informed consent requirement was waived. All the image data were deidentified by anonymization and analyzed retrospectively. Fifteen patients with 48 brain metastases, who were originally treated at our institution, were selected for this retrospective study.
All structures, including gross tumor volume (GTV) and OARs, were delineated by experienced radiation oncologists on Eclipse system (version 13.6; Varian Medical System, Palo Alto, California). The planning target volume (PTV) was generated by adding an isotropic margin of 2 mm to the GTV. Organs at risk included brainstem, eyes, lenses, optic nerves, optic chiasm, and pituitary.21 The normal brain tissue was defined as healthy brain tissue minus PTV. The image sets including all delineated structures were transferred via Digital Imaging and Communications in Medicine–radiotherapy to the CK Multiplan system (version 4.6; Accuray) for CK planning. The prescription dose (D p) was 30 Gy in 3 fractions.22 For OARs, the tolerance was set according to TG 101.23 The treatment plans were generated to cover 95% volume of the PTV with D p.
Linear accelerator plans were designed based on TrueBeam linear accelerator (Varian Medical System, Palo Alto, California) equipped with the Varian High Definition 120 multileaf collimator (MLC) with flattening filter free beams with 6-MV photon beams energy at a maximum dose rate of 1400 monitor unit (MU) per minute at Eclipse system. The type of MLC motion is sliding window. All doses were calculated by the means of an analytic anisotropic algorithm with the grid size of 1.25 mm. The single isocenter defined for all treatment plans was set at the center of mass of all brain metastases. Noncoplanar VMAT plans consisted of 4 noncoplanar arcs: 1 full arc with couch angle of 0° and 3 half arcs with couch angles of 45°, 315°, and 270°, respectively. Coplanar VMAT plans consisted of 2 coplanar arcs of 358° optimized simultaneously and to be delivered with opposite rotation (clock and counterclockwise). The first arc started at a gantry angle of 181° and stopped at 179°, and the second arc started at a gantry angle of 179° and stopped at 181°. The couch angle was set to 0° for both arcs. Intensity-modulated radiotherapy plans were optimized with 7 coplanar fields with the couch angle of 0°. The collimator angle for each technique was adjusted according to the location and size of the tumors in the Beam’s Eye View (BEV).
CyberKnife combines a high-resolution image–guided tracking system to adjust the angle of beams during treatment to guarantee the accuracy of the treatment. CyberKnife plans were designed on Multiplan system via skull tracking and RayTracing algorithm with 6-MV photon beams energy at a maximum dose rate of 950 MU per minute and were capable of noncoplanar, nonisocentric delivery. More than one iris collimators were used for each plan to reduce delivered MUs compared to using only one collimator. Collimators were chosen such that one collimator diameter was approximately equal to the central part of the largest lesion and the other was small enough to cover the tumor’s smallest features. Several “auto-shells” were created outside the target volume to constrain the conformity and the extent of the low-dose region.
Gradient index (GI)24 described the steepness of the dose gradient from D p to 50% of D p (GI = V 50%Dp/V p, where V 50%Dp is 50% of the prescription isodose line volume and V p is the prescription volume). Conformity index (CI)24 was calculated to evaluate the degree of conformity of the dose distribution (CI = (V tp)2/(V t × V p), where V tp is the PTV volume within the prescription isodose surface, V t is the PTV volume and V p is the prescription volume). For normal brain tissue, volumes receiving a specific dose in the range of 3 to 24 Gy (V 3Gy − V 24Gy) were evaluated. In addition, delivery parameters were recorded including MUs and beam on time (BT).
To assess the difference among the plans, the Wilcoxon signed rank test was performed using the Statistical Package for Social Science, version 24.0, software (IBM, New York). A P value of <.05 was considered to indicate statistical significance.
Results
There were 15 (10 male and 5 female) patients in this study, and the median age of them was 67 years (range 36-81 years). The number of lesions ranged from 2 to 5 (2 lesions: 7, 3 lesions: 2, 4 lesions: 2, 5 lesions: 4). The median metastases volume was 6.66 cm3 (range 1.49-38.64 cm3). The distances between targets and the nearest OARs for all the patients were larger than 1 cm. All plans generated with 4 techniques were clinically acceptable, but differences were observed in the dosimetric parameters, MUs, and BT. Data were presented as mean values ± standard deviation.
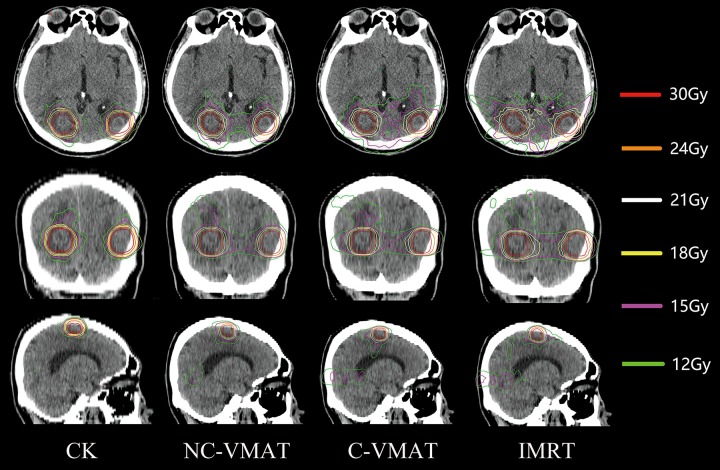
Figure 1 presented the isodose distribution for a representative case for the 4 techniques under investigation. It can be observed that the CK and NC-VMAT plans provided a steeper dose gradient than IMRT and C-VMAT plans (V 12Gy of this example for CK, NC-VMAT, C-VMAT, and IMRT was 164.11, 230.73, 268.53, and 281.53 cm3, respectively).
Figure 1.
Dose distributions of CK, noncoplanar VMAT, coplanar VMAT, and intensity-modulated radiotherapy plans in the axial plane (upper), coronal plane (center), and sagittal plane (lower) for a typical patient. VMAT indicates volumetric modulated arc therapy and CK incicates CyberKnife.
The dosimetric parameters and delivery parameters were shown in Table 1. The GI was the lowest for CK, followed by NC-VMAT, C-VMAT, and IMRT. The CI was the largest for NC-VMAT, followed by C-VMAT, CK, and IMRT. The MUs was the highest for CK, followed by IMRT, NC-VMAT, and C-VMAT. The BT was the longest for CK, followed by IMRT, NC-VMAT, and C-VMAT.
Table 1.
Evaluation Parameters of NC-VMAT, C-VMAT, IMRT, and CK Plans (Mean ± SD).a
| Parameter | NC-VMAT | C-VMAT | P | IMRT | P | CK | P |
|---|---|---|---|---|---|---|---|
| GI | 4.21 ± 1.38 | 4.87 ± 1.35 | .001 | 5.36 ± 1.98 | .001 | 3.49 ± 0.65 | .015 |
| CI | 0.87 ± 0.03 | 0.86 ± 0.04 | .008 | 0.85 ± 0.05 | .002 | 0.86 ± 0.07 | .281 |
| MUs | 3 105.20 ± 371.23 | 2 997.27 ± 446.84 | .065 | 4 128.40 ± 1 185.38 | .002 | 28 733.59 ± 7 197.85 | .001 |
| BT/min | 2.61 ± 0.07 | 2.30 ± 0.23 | .001 | 2.95 ± 0.85 | .173 | 30.25 ± 7.32 | .001 |
Abbreviations: BT, beam on time; CI, conformity index; CK, CyberKnife; C-VMAT, coplanar volumetric modulated arc therapy; GI, gradient index; IMRT, intensity-modulated radiation therapy; NC-VMAT, noncoplanar volumetric modulated arc therapy; MUs, monitor units; SD, standard deviation.
a Statistical significance was tested for each technology in comparison with NC-VAMT.
The absolute volume of the brain tissue receiving a specific dose was listed in Table 2 for 4 treatment plans. The mean absolute volume was lower in the NC-VMAT plans than IMRT and C-VMAT plans, and a significant difference was observed at the dose level ranges from 24 to 9 Gy (V 24Gy − V 9Gy). In contrast, a very low-dose volume (V 6Gy − V 3Gy) in NC-VMAT plans resulted in a somewhat larger dose spread than IMRT and C-VMAT plans. It can be observed that NC-VMAT plans provided a comparable dose volume with CK plans at the dose level ranges from 24 to 15 Gy (V 24Gy − V 15Gy). As dose decreased, the mean absolute volume was lower in the CK plans than NC-VMAT plans, and a significant difference was observed at the dose level ranges from 12 to 3 Gy (V 12Gy − V 3Gy).
Table 2.
Dosimetric Results for Normal Brain Tissue of NC-VMAT, C-VMAT, IMRT, and CK Plans (Mean ± SD).a
| OARs | NC-VMAT | C-VMAT | P | IMRT | P | CK | P | |
|---|---|---|---|---|---|---|---|---|
| Normal brain tissue volume, cm3 | V 24Gy | 15.85 ± 7.05 | 17.40 ± 8.64 | .010 | 20.45 ± 8.31 | .001 | 17.98 ± 9.10 | .100 |
| V 21Gy | 25.97 ± 12.44 | 29.81 ± 15.73 | .001 | 34.32 ± 15.55 | .001 | 28.66 ± 14.70 | .156 | |
| V 18Gy | 41.19 ± 21.45 | 49.39 ± 26.96 | .001 | 55.57 ± 26.92 | .001 | 41.63 ± 21.86 | .820 | |
| V 15Gy | 68.19 ± 38.89 | 88.69 ± 53.71 | .001 | 94.49 ± 48.74 | .001 | 61.60 ± 33.02 | .100 | |
| V 12Gy | 124.69 ± 79.48 | 165.98 ± 109.97 | .001 | 168.56 ± 96.88 | .001 | 94.97 ± 52.41 | .015 | |
| V 9Gy | 236.12 ± 157.32 | 285.76 ± 177.29 | .001 | 280.76 ± 172.16 | .008 | 171.50 ± 104.34 | .003 | |
| V 6Gy | 435.47 ± 276.56 | 467.69 ± 250.10 | .088 | 424.23 ± 230.52 | .955 | 324.49±199.67 | .004 | |
| V 3Gy | 793.03 ± 371.40 | 742.99 ± 302.19 | .156 | 635.20 ± 274.93 | .005 | 597.33 ± 293.26 | .001 | |
a Statistical significance was tested for each technology in comparison with NC-VAMT.
Abbreviations: CK, CyberKnife; C-VMAT, coplanar VMAT; IMRT, intensity-modulated radiation therapy; NC-VMAT, noncoplanar volumetric modulated arc therapy; OARs, organs at risk; SD, standard deviation.
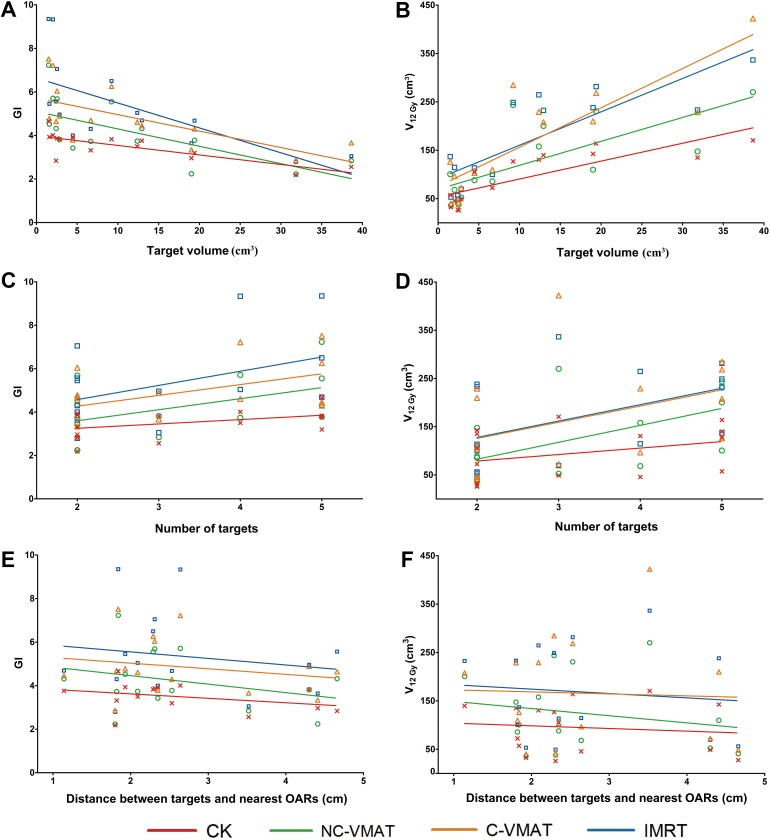
The variations in GI and V 12Gy with different target volume, number of targets, and distance between targets and nearest OARs were plotted in Figure 2. The GI of CK and NC-VMAT were smaller than IMRT and C-VMAT. Gradient index decreased with the increase in target volume and increased with the increase in number of targets. With the distance between targets and nearest OARs increasing, GI changed little. The V 12 Gy of CK and NC-VMAT were smaller than IMRT and C-VMAT. V 12 Gy increased with the increase in target volume and number of targets. With the distance between targets and nearest OARs increasing, V 12 Gy changed little.
Figure 2.
Comparison of gradient index (GI) and V 12Gy variation as a function of target volume, number of targets, and distance between targets and nearest organs at risk for 4 techniques. Dots are the actual value of GI or V 12Gy. Solid lines are fitting lines.
Discussion
There are many treatment techniques for multiple brain metastases, each with its own characteristics. This is the first study to compare plan quality and delivery efficiency of NC-VMAT, C-VMAT, IMRT, and CK for multiple brain metastases, with the aim to find the strengths and weaknesses of conventional C-arm linear accelerator and robotic radiosurgery. As the results indicated, dose falloff of NC-VMAT and CK plans was sharper than C-VMAT and IMRT plans. It was observed in GI and the normal brain tissue volume receiving a specific dose from 3 to 24 Gy, although some differences were not statistically significant. For cranial SRS/HFSRT, the V 12Gy was an important factor for the risk of radionecrosis.25,10 In this study, the V 12Gy of CK was reduced by 31.3%, 74.8%, and 77.5% compared to NC-VMAT, C-VMAT, and IMRT plans, respectively. The V 12Gy of NC-VMAT was reduced by 33.1% and 35.2% compared to C-VMAT and IMRT, respectively. CyberKnife and NC-VMAT plans were superior to C-VMAT and IMRT plans in terms of sparing of the normal brain tissue. Alongi et al 26 reported the clinical results of HyperArc (Varian Medical System, Palo Alto, California) and proved that the utilization of NC-VMAT treatment was safe and effective for brain metastases. Thomas et al 19 compared GK and multiarc VMAT plans. Multiarc VMAT plans were designed as 1-arc, 2-arc(noncoplanar), 4-arc(noncoplanar) with single isocenter. Compared to GK, multiarc VMAT had similar dose falloff. Ma et al 27 compared the plans of GK, CK, and Novalis. They found that dose of PTV and OARs was similar, but the dose of normal brain tissue was strongly apparatus-dependent. Compared to Novalis, GK and CK decreased the dose of normal brain tissue.
As shown in Figure 2, GI and V 12Gy varied with the target volume and number of targets. The volume of normal brain tissue that received high-dose irradiation around targets increased with the increase in target volume and number of targets. Although GI and V 12Gy varied with target volume and number of targets, the GI and V 12Gy of CK and NC-VMAT were both smaller than IMRT and C-VMAT. It demonstrated that noncoplanar irradiation could spare normal brain tissue better for the studied cases. In this study, the distance between targets and nearest OARs was larger than 1 cm. In this condition, the GI and V 12Gy of NC-VMAT and CK was smaller than C-VMAT and IMRT, which demonstrated the dose falloff advantage of noncoplanar irradiation. As for targets close to critical OARs, the dose falloff of noncoplanar technique was also sharper than coplanar technique.28 Cao et al 28 compared the dosimetric characterization of different techniques for a patients with brain metastasis close to brainstem. The results showed that the GI of noncoplanar technique (GK, CK, and NC-VMAT) was smaller than coplanar technique (C-VMAT), which demonstrated the dose falloff advantage of noncoplanar irradiation. In this study, we found that normal brain tissue volume receiving a specific dose from 3 to 24 Gy was not always smaller for NC-VMAT than IMRT plans. The mean absolute volume was lower in the NC-VMAT plans than IMRT plans, and a significant difference was observed at the dose level ranges from 24-9 Gy (V 24Gy − V 9Gy). In contrast, a very low-dose volume (V 6Gy − V 3Gy) in NC-VMAT plans resulted in a somewhat larger dose spread than IMRT plans. This was because that the distance of opposite collimators was large enough to contain all targets that changing position with gantry rotating, and 2 or more targets share the same MLC leaf pair with gantry rotating, and the moving MLC would not block radiation to the normal tissue around targets in VMAT plans.29 For IMRT plans, the distance of opposite collimators was set smallest according to the location and size of the tumors in the BEV, and MLC was arranged to block as much radiation as possible. Therefore, normal brain tissue volume was smaller for IMRT than NC-VMAT at low-dose level. Wu et al 30 reported the effect of using collimator optimization algorithm, which led to significant improvement in reducing the low dose to normal brain tissue, while retaining similar dose coverage to PTV.
The BT of CK was longer than linear accelerator plans. Slosarek et al 31 compared CK and VMAT and found that the delivery time of CK was longer than VMAT. This was because that the dose rate and size of collimator for CK was different from linear accelerator, and CK system consisted of a high-resolution image-guided tracking system that collected images during treatment and registered with previously generated projection images derived from the planning CT volume data set to reposition the linear accelerator automatically. The BT of VMAT was shorter than IMRT that was consistent with Yang’s32 and Zhao’s33 studies.
In this study, IMRT and VMAT plans were designed with single isocenter. Certainly, conventional C-arm linear accelerator can also be used to design multi-isocenters’ plans. Clark et al 34 found that single-isocenter VMAT plans could be used to deliver conformity equivalent to that of multi-isocenters VMAT plans. Single-isocenter VMAT radiosurgery for multiple targets could be delivered extremely efficiently compared to multi-isocenters VMAT. Huang et al 35 had compared single-isocenter dynamic conformal arcs (SIDCA) with multi-isocenters dynamic conformal arcs (MIDCA) in radiosurgery treatment of multiple brain metastases. The plan quality of SIDCA was similar with MIDCA, and the delivery time was shorter than MIDCA. Patients only need to be placed once for single-isocenter plans and technicians do not have to re-enter treatment room during treatment process. It saves delivery time and improves clinical efficiency.
Conclusion
For brain metastases far away from OARs, NC-VMAT generated more rapid dose falloff and higher conformity compared to IMRT and C-VMAT. Noncoplanar VMAT provided a comparable dose falloff with CK at high dose level and a slower dose falloff than CK at moderate-to-low dose level. Noncoplanar VMAT plans had less MUs and shorter BT than CK plans.
Acknowledgments
The authors would like to thank Yuxi Pan, Xile Zhang, Jun Li, and Hongguo Sheng (Department of Radiation Oncology, Peking University Third Hospital, Beijing, China) for their suggestions and guidance in planning.
Abbreviations
- CI
conformity index
- CK
CyberKnife
- C-VMAT
coplanar volumetric modulated arc therapy
- GI
gradient index
- GK
Gamma Knife
- GTV
gross tumor volume
- HFSRT
hypofractionated stereotactic radiotherapy
- NC-VMAT
noncoplanar volumetric modulated arc therapy
- BEV
Beam’s Eye View
- BT
beam on time
- IMRT
intensity-modulated radiation therapy
- MIDCA
multi-isocenters dynamic conformal arcs
- MLC
multileaf collimator
- OARs
organs at risk
- PTV
planning target volume
- SIDCA
single-isocenter dynamic conformal arcs
- SRS
stereotactic radiosurgery
- VMAT
volumetric modulated arc therapy
- WBRT
whole brain radiotherapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China81071237 and the Interdisciplinary Medicine Seed Fund of Peking University (BMU20160585).
ORCID iD: Ruijie Yang, PhD  https://orcid.org/0000-0002-3459-0075
https://orcid.org/0000-0002-3459-0075
References
- 1. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. [DOI] [PubMed] [Google Scholar]
- 2. Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249(10):1357–1369. [DOI] [PubMed] [Google Scholar]
- 3. Aiyama H, Yamamoto M, Kawabe T, et al. Clinical significance of conformity index and gradient index in patients undergoing stereotactic radiosurgery for a single metastatic tumor. J Neurosurg. 2018;129(suppl 1):103–110. [DOI] [PubMed] [Google Scholar]
- 4. Zhao B, Wen N, Chetty IJ, et al. A prediction model of radiation-induced necrosis for intracranial radiosurgery based on target volume. Med Phys. 2017;44(8):4360–4367. [DOI] [PubMed] [Google Scholar]
- 5. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 8. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases. analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. [DOI] [PubMed] [Google Scholar]
- 10. Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 11. Zhong J, Press RH, Olson JJ, Oyesiku NM, Shu HG, Eaton BR. The use of hypofractionated radiosurgery for the treatment of intracranial lesions unsuitable for single-fraction radiosurgery. Neurosurgery. 2018;83(5):850–857. [DOI] [PubMed] [Google Scholar]
- 12. Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3×9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142–1148. [DOI] [PubMed] [Google Scholar]
- 13. Ogura K, Mizowaki T, Ogura M, et al. Outcomes of hypofractionated stereotactic radiotherapy for metastatic brain tumors with high risk factors. J Neurooncol. 2012;109(2):425–432. [DOI] [PubMed] [Google Scholar]
- 14. Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115(4):890–898. [DOI] [PubMed] [Google Scholar]
- 15. Zindler JD, Schiffelers J, Lambin P, Hoffmann AL. Improved effectiveness of stereotactic radiosurgery in large brain metastases by individualized isotoxic dose prescription: an in silico study. Strahlenther Onkol. 2018;194(6):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregucci F, Fiorentino A, Corradini S, et al. LINAC-based radiosurgery or fractionated stereotactic radiotherapy with flattening filter-free volumetric modulated arc therapy in elderly patients: a mono-institutional experience on 110 brain metastases. Strahlenther Onkol. 2019;195(3):218–225. [DOI] [PubMed] [Google Scholar]
- 17. Treuer H, Hoevels M, Luyken K, et al. Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery: comparison of dosimetric treatment plan quality. Strahlenther Onkol. 2015;191(6):470–476. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Andrews DW, Evans JJ, et al. Plan quality and treatment efficiency for radiosurgery to multiple brain metastases: non-coplanar RapidArc vs. Gamma Knife. Front Oncol. 2016;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas EM, Popple RA, Wu X, et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75(4):409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong P, Pérez-Andújar A, Pinnaduwage D, et al. Dosimetric characterization of hypofractionated Gamma Knife radiosurgery of large or complex brain tumors versus linear accelerator-based treatments. J Neurosurg. 2016;125(suppl 1):97–103. [DOI] [PubMed] [Google Scholar]
- 21. Brouwer CL, Steenbakkers RJ, Bourhis J, et al. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. [DOI] [PubMed] [Google Scholar]
- 22. Murai T, Ogino H, Manabe Y, et al. Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol. 2014;26(3):151–158. [DOI] [PubMed] [Google Scholar]
- 23. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. [DOI] [PubMed] [Google Scholar]
- 24. Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105(suppl):194–201. [DOI] [PubMed] [Google Scholar]
- 25. Korytko T, Radivoyevitch T, Colussi V, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64(2):419–424. [DOI] [PubMed] [Google Scholar]
- 26. Alongi F, Fiorentino A, Gregucci F, et al. First experience and clinical results using a new non-coplanar mono-isocenter technique (HyperArc™) for LINAC-based VMAT radiosurgery in brain metastases. J Cancer Res Clin Oncol. 2019;145(1):193–200. [DOI] [PubMed] [Google Scholar]
- 27. Ma L, Petti P, Wang B, et al. Apparatus dependence of normal brain tissue dose in stereotactic radiosurgery for multiple brain metastases. J Neurosurg. 2011;114(6):1580–1584. [DOI] [PubMed] [Google Scholar]
- 28. Cao H, Xiao Z, Zhang Y, et al. Dosimetric comparisons of different hypofractionated stereotactic radiotherapy techniques in treating intracranial tumors > 3 cm in longest diameter. J Neurosurg. 2019;22:1–9. [DOI] [PubMed] [Google Scholar]
- 29. Kang J, Ford EC, Smith K, Wong J, McNutt TR. A method for optimizing LINAC treatment geometry for volumetric modulated arc therapy of multiple brain metastases. Med Phys. 2010;37(8):4146–4154. [DOI] [PubMed] [Google Scholar]
- 30. Wu Q, Snyder KC, Liu C, et al. Optimization of treatment geometry to reduce normal brain dose in radiosurgery of multiple brain metastases with single-isocenter volumetric modulated arc therapy. Sci Rep. 2016;6:34511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Slosarek K, Bekman B, Wendykier J, Grządziel A, Fogliata A, Cozzi L. In silico assessment of the dosimetric quality of novel, automated radiation treatment planning strategy for LINAC-based radiosurgery of multiple brain metastases and a comparison with robotic methods. Radiat Oncol. 2018;13(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang R, Wang J, Xu S, Li H. SmartArc-based volumetric modulated arc therapy for endometrial cancer: a dosimetric comparison with helical tomotherapy and intensity-modulated radiation therapy. BMC Cancer. 2013;13:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao N, Yang R, Wang J, Zhang X, Li J. An IMRT/VMAT technique for nonsmall cell lung cancer. Biomed Res Int. 2015;2015:613060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clark GM, Popple RA, Young PE, Fiveash JB. Feasibility of single-isocenter volumetric modulated arc radiosurgery for treatment of multiple brain metastases. Int J Radiat Oncol Biol Phys. 2010;76(1):296–302. [DOI] [PubMed] [Google Scholar]
- 35. Huang Y, Chin K, Robbins JR, et al. Radiosurgery of multiple brain metastases with single-isocenter dynamic conformal arcs (SIDCA). Radiother Oncol. 2014;112(1):128–132. [DOI] [PubMed] [Google Scholar]