Short abstract
Neuropathic pain is a kind of chronic intractable disease. HBO explored the involvement of mitophagy which is associated with energy metabolism by AMPK. CaMKKβ/AMPK signaling pathway may involve in mitophagy process. We randomly divided mice into four groups: C, S, CCI, and CCI + HBO group. Pain-related behaviors were evaluated using mechanical withdrawal threshold and thermal withdrawal latency analysis. Western blot was employed to assess expression of CaMKKβ, pAMPK, AMPK, NIX, BNIP3, and Drp1. Spinal cord was observed under the electron microscope. Immunofluorescence changes in NeuN and CaMKKβ or AMPK were examined. AMPK inhibitor compound C (CC) and CaMKKβ inhibitor STO609 were administrated prior to CCI, respectively. The changes in behaviors and Western blot are examined. HBO upregulated CaMKKβ and pAMPKα expression. NeuN and CaMKKβ were colocalizated in immunofluorescence. HBO can elevate the pain-related behaviors significantly, while it was downregulated by CC or STO609. HBO upregulated NIX, BNIP3, and Drp1 expressive level more significantly than those in CCI group. However, expression was reduced when CC or STO609 were administered. Electron microscopic examination showed that mitophagy was upregulated after HBO treatment. This phenomenon was not observed when CC or STO609 were administered. Our findings suggest that CaMKKβ/AMPK pathway maybe a potential signal pathway on analgesic mechanism of hyperbaric oxygen via mitophagy.
Keywords: AMPK, CaMKK, Drp1, HBO, mitophagy, neuropathic pain
Introduction
Neuropathic pain (NP) is a kind of chronic pain stimulated when the central or peripheral nerve damage. It is also one of the urgent problems in chronic pain which involved many factors.1 Researchers have discovered that approximately 8% to 10% Chinese currently suffered from NP. And millions of related hospitalizations occur annually resulting in more health-care costs.2 Hyperbaric oxygen (HBO), as a non-invasive treatment, has been widely investigated which can effectively relieve NP.3 But molecular mechanism of HBO is unclear. HBO can enhance the antioxidant activity, accelerate elimination of reactive oxygen species (ROS), and repair or protect the damaged nerve tissue.4 These effects are similar to effects of mitophagy. Our previous research discovered that the alleviated effect of HBO on NP is associated with mitophagy.5 But the detailed mechanisms of how mitophagy alleviated pain are not fully understood.
AMP-activated protein kinase (AMPK) plays an important role in the initiation and maintenance of pain.6 It is also a “monitor” of cellular energy, whose activity is monitoring the imbalance of AMP/ATP ratio.7 AMPK is closely related to mitochondrial energy metabolism.8 Altogether, it plays an important role in the process of mitophagy as an essential target for the regulation of cellular metabolism. Generally, AMPK has two activation modes which can be activated by the cell itself or by the AMPK-mediated upstream enzyme.9 Among its upstream proteins, calmodulin-dependent protein kinase kinase β (CaMKKβ) is an important upstream activated enzyme which is associated with cellular metabolism.10 And CaMKKβ/AMPK signaling pathway is one of the classical pathways that regulate cell energy metabolism.
CaMKKβ, an important Ca2 + channel kinase, plays an important role in regulating cellular function, exciting neuron and releasing neurotransmitter.11 It also takes part in regulating genetic transcriptive process, which can adjust the metabolic function of organ.12 And some studies reported that CaMKKβ may participate in cell-mediated neuroinflammatory responses to regulate the occurrence and maintenance of pain.13 Since CaMKKβ mediates the opening of Ca2 + channels in chronic pain and plays the corresponding regulatory role, it may be an important target between NP and calcium signal pathways.
HBO is beneficial to form new blood vessels, establish and accelerate lateral branch circulation, and repair or protect mitochondrial function of nerve tissue.14 All of these results are related to the increasing of ATP production and improving of tissue energy metabolism.15 And ATP production is mainly related to participation of AMPK. Therefore, HBO is likely to influence and regulate AMPK signaling pathways.16 On the other hand, it has been reported that HBO can protect the function of brain cells, improve the ischemia state of brain tissue, and reduce the risk of memory impairment in elderly mice by regulating the concentration of Ca2 + .17 In the preliminary experiment, we found that HBO can enhance pAMPK and CaMKKβ expression. Based on a series of preliminary work, we hypothesized that HBO may regulate mitophagy and relieve pain by mediating CaMKKβ/AMPK signal pathway.
Materials and methods
Materials
The following materials were used in this study including enhanced chemiluminescence (ECL) Western blotting kit (Solarbio, Beijing, China), horseradish peroxidase-conjugated rabbit anti-goat IgG and goat anti-rat IgG (Pierce, USA), rabbit anti-rat CaMKK (ab80066, Abcam, UK), rabbit anti-rat AMPKα (ab3759, Abcam, UK), rabbit anti-rat pAMPKα (ab131357, Abcam, UK), rabbit anti-rat Drp1 (ab56788,Abcam,UK), rabbit anti-rat NIX and BNIP3 IgG (D4R4B, CST, USA), mouse anti-rat NeuN (ab104224, Abcam, UK), rabbit anti-rat STO609 (ab141591, Abcam, UK), rabbit anti-rat Dorsomorphin/Compound C (ab120843, Abcam, UK), a fluorescence microscope X81 (Olympus, Tokyo, Japan), total protein extraction kit (Keygen Biotech, Nanjing, China), and Transmission Electron Microscope H-600 (Hitachi, Japan).
Animals
Male Sprague Dawley (SD) rats (260 ± 20 g) were obtained from the Animal Care Center at Shengjing Hospital of China Medical University. The study protocol was approved by the Institutional Animal Care and Use Committee, Shengjing Hospital of China Medical University and was in accordance with the ethical guidelines for the use of experimental animals. A total of 108 rats were randomly separated into a normal control group without treatment (n = 36), a CaMKKβ inhibitor group treated by intrathecal injection of STO609 (n = 36), and an AMPK inhibitor group treated by intrathecal injection of compound C (CC) (n = 36). Each group included four sub-groups (n = 9 rats per group) as follows: control group (C group) in which both chronic constrictive injury (CCI) and HBO were not performed; a sham operation group (S group) in which the sciatic nerve was exposed, but not ligated and without HBO treatment; sciatic nerve with CCI group (CCI group) in which the sciatic nerve was ligated without HBO treatment; and a CCI plus HBO treatment group (CCI + HBO group) in which HBO was initiated 6 h after CCI once a day for the following five days.
Experimental process
Intrathecal tube was implemented five days before CCI. Then the rats were observed in the following 48 h to confirm no adverse complication. In addition, 0.9% saline (1 ml) or STO609 (20 mg/kg, 1 ml) or CC(20 mg/kg, 1 ml) were injected in the normal control group or CaMKKβ or AMPK inhibitor group, respectively, three days before CCI intrathecally. We calculated the dosage according to 20 mg/kg and dissolved the drug into 1 ml which were injected into epidural cavity of rats, once a day. After injection of three consecutive days, baseline of mechanical withdrawal thresholds (MWTs) and thermal withdrawal latencies (TWLs) were measured in all rats one day before CCI. Then, CCI or sham operations were performed in the morning. Pain-related behaviors were observed first, third, fifth and seventh days after CCI between 09:00 and 11:00 am each day. Rats in the CCI + HBO group were first exposed to HBO 6 h after CCI once a day for the following five days. Finally, rats were sacrificed with dorsal root ganglion (DRG) and the dorsal part of spinal cord collected on the seventh day after CCI (Figure 1).
Figure 1.
Experimental process. This course is processed in N normal group or in STO609/CC group. 3d: Inject Saline/STO609/CC group for three days, B: baseline values; S: sham operation; CCI: chronic constrictive injury; d: day; D: death sample taken; HBO: hyperbaric oxygenation; T: intrathecal cathetering; C: control group; S: sham group. C+H: CCI plus HBO group; CCI: CCI group; CC: AMPK inhibitor (Compound C) STO609: CaMKKβ inhibitor; −5d: five days before CCI (begin point of intrathecal catheterizing); −3d: three days before CCI (begin point of drug administration); 6h: 6 h after CCI (begin point of HBO); 1d, 3d, 5d, 7d: one, three, five, seven days after CCI (time points of behavior test); light blue: behavior test score pink: surgery dark blue: HBO treatment; green: intrathecal drug administration.
Intrathecal catheterizing
We used inhaled anesthesia. The rats were inhaled 2.5% isoflurane for induction before the surgery and 2% isoflurane for maintenance during the surgery. Intrathecal catheterizing was performed on the special surgical table for rats after anesthesia. The back skin of rats were cleaned and disinfected with iodine gently. We located the L6 by the iliac crest of rats, which made us easy to locate L3-4 interspace. Then, longitudinal skin incision was done to expose muscle and fascia. The inter-spine space was fully exposed by carefully removing redundant lamina and spines. After clearance of L3-4 interspace, we breakthroughed yellow ligament with a fine epidural catheter slowly and gently, then placed it into the cephalic side by 2 cm. The success of subarachnoid cava was confirmed when we saw colorless cerebrospinal fluid flowing out of the catheter. The other end of the catheter was led out of the back neck of the rats subcutaneously. We fixed the tube on the head of rats to prevent it being scratched off by the rats. In addition, 15,000 units of penicillin were injected intraperitoneally to prevent postoperative infection effectively. After 48 h of surgery, the bilateral hind limbs and tail movements of rats were observed to confirm there is no occurrence of spinal nerve injury.
CCI model preparation
We used inhaled anesthesia method, in which 2.5% isoflurane was inhaled for induction and 2% isoflurane for maintenance before and during the surgery. CCI models were prepared using a common method in which we made a posterolateral incision on the right hind limb. The right sciatic nerve trunk was found and loosely ligated it with 4–0 silk thread to produce slight pressure on the epineurium until momentary muscle contraction was observed in the sciatic nerve distribution region. Finally, we closed the incisions representing the completion of CCI model successfully. When regaining consciousness, rats were returned to their cages. In S group, the sciatic nerve was only exposed without ligation.
HBO treatment
All the rats were placed into HBO chamber, while only rats in the CCI + HBO group were treated with HBO five times at a frequency of once per day followed after CCI. On the first day, HBO treatment was given 6 h after CCI. HBO conditions were described as follows. An HBO chamber was first purified with 90% pure oxygen which was allowed to fill the chamber for 10 min. Rats were exposed under high pressure which was increased at a rate of 0.0125 mPa/minute to 0.25 mPa for 60 min and subsequently decreased for 30 min at a constant rate. The rats in other groups simulated the environment of HBO chamber without high oxygen and pressure.
Collect samples
On the seventh day, rats were anesthetized and inhaled 2.5% isoflurane and intubated from the left ventricle to the ascending aorta. Rats were perfused with a 0.9% saline solution until no red perfusate was effused. After 0.1 M phosphate buffer (pH 7.4) was perfused, the L4–L6 spinal cord segments and DRG of L4 and L5 were removed.
Pain-related behaviors
All the rats were habituated 1 h every day for successive two days before the behavioral testing. We carried out the behavioral testing including mechanical and thermal heat tests with 1 h intervals which were observed first, third, fifth, seventh days after CCI between 09:00 and 11:00 am. MWT was tested using von Frey filaments. Rats were placed individually in chamber and allowed to acclimatize for 30 min. Each von Frey filament was applied to the plantar surface of the hind paw for 6 to 8 s to observe the hind paw withdrawal response. TWL was assessed to quantitatively determine thermal sensitivity. Rats were placed on the glass surface of a thermal testing apparatus and allowed to acclimatize for 30 min before testing. A mobile radiant heat source located under the glass was focused on the hind paw of each rat. The paw TWL was the mean of three trials which were recorded by a timer for three times. We use 20 s as cut-off time to prevent potential tissue damage.
Western blot analysis
To achieve enough proteins, the ipsilateral DRGs from three rats were put together. The tissues were homogenized and ultrasonicated in chilled lysis buffer (10 mM Tris, 1 mM phenylmethylsulfonyl fluoride, 5 mM MgCl2, 5 mM EGTA, 1 mM EDTA, 1 mM DTT, 40 μM leupeptin, 250 mM sucrose). The total protein was separated using a total protein extraction kit according to the instructions provided. Protein content was determined by diluting the protein with PBS to the same concentration. A total of 20 pg protein was loaded for electrophoresis. After electrophoresis, gels were transferred to a cellulose membrane and immersed in confining liquid for 60 min. The membranes were incubated with anti-NIX, anti-BNIP3, anti-Drp1, anti-pAMPK, anti-AMPK, anti-CaMKKβ primary antibody (1:200), and anti-β-actin primary antibody (1:200) overnight. β-actin served as an internal reference. Membranes were washed with PBS and incubated with secondary antibody (1:1) for 30 min, followed by rinsing. Expression of NIX, BNIP3, Drp1, pAMPK, AMPK, and CaMKKβ were detected with ECL Western blotting kit using Quantity One software on a DocTM XR gel imaging system (Bio-Rad, USA).
Electron microscopic observation
The rats were anesthetized by inhaling 2.5% isoflurane. And then, the right heart was perfused with physiological saline. When there were no bloody fluids flowing out, we removed the lumbar spinal cord from L4 to L6 quickly. The spinal cord was placed on the ice for 1 min. We cut the tissue into blocks (smaller than 1 mm×1 mm ×1 mm) which were fixed with 2.5% glutaraldehyde in 4°C environment for 3 h. In the following, the specimen was treated timely to avoid the cell autolysis which was caused by various internal enzymes. In addition, the specimen was dehydrated with 70%, 80%, 90%, and 100% acetone in turn. Finally, they were treated with osmic acid and embedded in porous template. The most important process is making specimen pieces on the copper which was assisted by the teacher in the electron microscope room. The morphology, structure, and number of mitochondria were observed under transmission electron microscope. We also could see the structure of the lysosomes. After the right field of vision was selected, we adjusted the multiples to focus on the photograph and record the mitochondria.
Immunofluorescence
L4 and L5 DRG on the ipsilateral side was dissected on the seventh day after CCI. After separating, the DRG was fixed with 2.5% glutaraldehyde. Before observing under the electron microscope, samples were placed in the refrigerator for 12 h at 4°C. Then, samples were washed with PBS buffer, fixed with osmic acid, dehydrated with acetone, and embedded with resin. In the following, samples were cut into thin sheets and observed under fluorescence microscopy. To determine whether mitophagy was involved in neurons, the samples were subjected to immunofluorescence using mouse monoclonal anti-neuron antibody, rabbit anti-AMPK and CaMKKβ antibody which were observed on an inverted fluorescence microscope X81 (Olympus, Tokyo, Japan).
Statistical analysis
Data analysis was performed using SPSS version 21.0 (IBM Corporation, USA). Data are expressed as the mean ± SD. Two-way ANOVA were used to compare the indexes between groups. Differences were considered statistically significant at P < 0.05.
Results
AMPK, pAMPK, and CaMKKβ levels
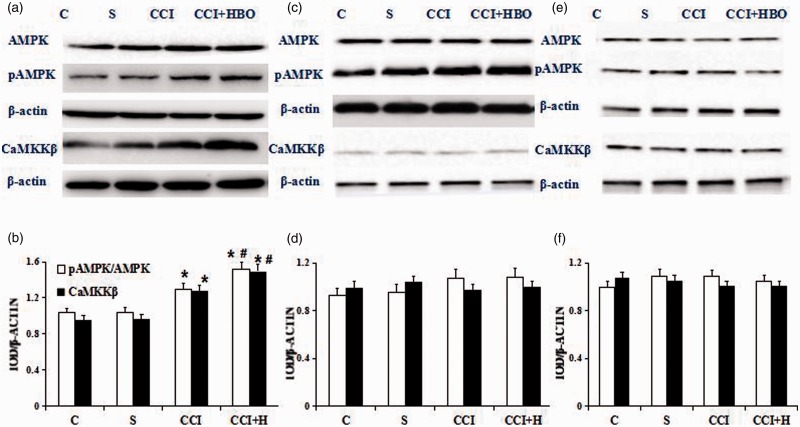
In our previous study, we had verified HBO can upregulate mitophagy.5 But we don’t know whether the mechanisms were associated with AMPK, pAMPK, and CaMKKβ. We evaluated the expressive levels of them by Western blot in rats. Expression of these protein were analyzed using L4, L5 ipsilateral side of DRG on the seventh days after CCI. Compared with C and S groups, pAMPK and CaMKKβ expression was higher in the CCI and CCI + HBO groups. Samples in CCI + HBO groups showed higher pAMPK and CaMKKβ levels than that in CCI group (n = 9 rats per group for three repeats, P < 0.05). However, expression of AMPK did not change significantly among the groups (Figure 2(a) and (b), P > 0.05). There were no significant differences in pAMPK/AMPK ratio between all groups when the inhibitor STO609 or CC was administered (n = 9 rats per group for three repeats, P > 0.05) (Figure 2(c) to (f)).
Figure 2.
Qualitative (a) and quantitative (b) Western blot of AMPK, pAMPK, and CaMKKβ after treatment with C, S, CCI+ HBO, and CCI groups. Western blot was present in the S group and blots became gradually stronger after CCI and especially CCI+HBO therapy (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). And Western blot in the CCI+HBO group is stronger than that in CCI group (# presented compared with CCI group, P < 0.05). Qualitative (c) and quantitative (d) Western blot of AMPK, pAMPK, and CaMKKβ after treatment with C, S, CCI+ HBO, and CCI groups represented the corresponding groups after CaMKKβ inhibitor STO609 administration. The blots presented in the CCI and CCI+HBO group became similar with C and S group after CaMKKβ inhibitor STO609 administration (n = 9 rats per group for three repeats, P > 0.05). Qualitative (e) and quantitative (f) Western blot of AMPK, pAMPK, and CaMKKβ after treatment with C, S, CCI+ HBO, and CCI groups represented the corresponding groups after AMPK inhibitor Compound C(CC) administration. The blots presented in the CCI and CCI+HBO group became similar with C and S group after AMPK inhibitor CC administration(n = 9 rats per group for three repeats, P > 0.05). C: control; S: sham operation; CCI: chronic constriction injury; HBO: hyperbaric oxygenation.
MWT and TWL
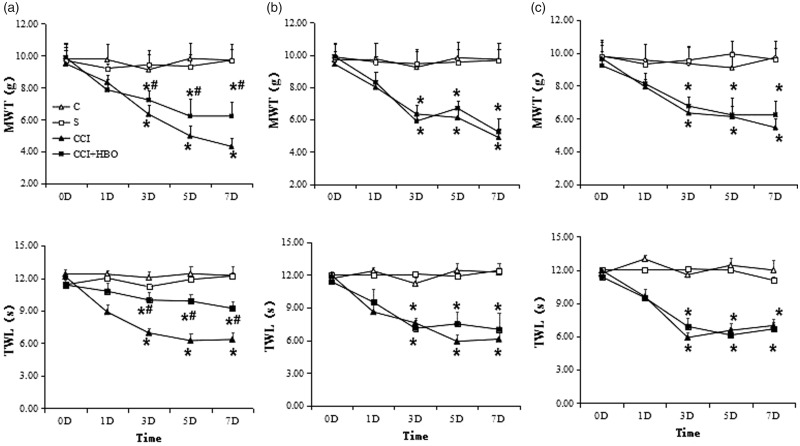
Chronic constriction of the spinal nerve via ligation induced severe mechanical allodynia. Ligation of the spinal nerve produced early onset and long-lasting mechanical hypersensitivity.18 Tactile hypersensitivity determined by Von Frey filaments and heat stimulation developed on the first, third, fifth, seventh days after CCI. Scores of MWT and TWL increased dramatically in the CCI + HBO group compared with the CCI group, which indicates that HBO can alleviate this hypersensitivity (Figure 3(a)) (n = 9 rats per group for three repeats, P < 0.05). After CC or STO609 administration, the value of MWT and TWL still decreased in CCI and CCI + HBO group (n = 9 rats per group for three repeats, P < 0.05). However, there was no significant difference between CCI and CCI + HBO group which indicated HBO lost its effect when CC or STO609 was added (Figure 3(b) and (c)) (n = 9 rats per group for three repeats, P > 0.05).
Figure 3.
MWT and thermal withdrawal latency (TWL) in each group (n = 9 rats per group). In (a), we could see the value of MWT and TWL decreased on third, fifth, and seventh day gradually after CCI in normal group which decreased most in CCI group than those in CCI+HBO group (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). And the value of MWT and TWL in the CCI+HBO group was higher than that in CCI group (# presented compared with CCI group, P < 0.05). After CC administration, the value of MWT and TWL also decreased on third, fifth, and seventh day after CCI in (b) (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). However, the difference of MWT and TWL in CCI group and in CCI+HBO group is smaller than before (n = 9 rats per group for three repeats, P > 0.05). The similar phenomenon happened after STO609 administration. The value of MWT and TWL also decreased on third, fifth, and seventh day after CCI in (c) (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). However, the difference of MWT and TWL in CCI group and in CCI+HBO group was not significant (n = 9 rats per group for three repeats, P > 0.05). MWT: mechanical withdrawal threshold; TWL: thermal withdrawal latency.
NIX, BNIP3, and Drp1 levels
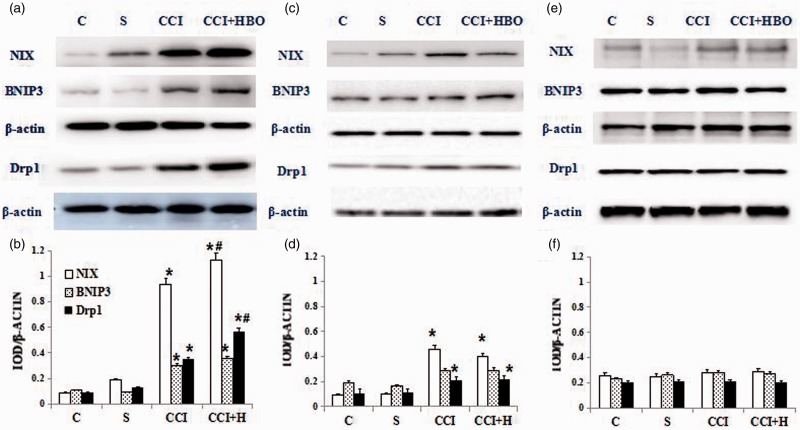
In our previous study, we had verified HBO can upregulate autophagy and mitophagy of nervous cells by affecting the expression of LC3, P62, BNIP3, and NIX.5 Now we try to evaluate the change of Drp1, BNIP3 and NIX expressive levels by Western blot in rats which underwent CCI or sham surgeries. Compared with the C and S groups, NIX, BNIP3 and Drp1 expression was higher in the CCI and CCI + HBO groups (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). Samples from rats which underwent HBO showed higher NIX, BNIP3, and Drp1 levels than that in CCI group (Figure 4(a), n = 9 rats per group for three repeats, # presented compared with CCI group, P < 0.05). However, HBO did not have the same effect after CC or STO609 was administered (Figure 4(b) and (c), n = 9 rats per group for three repeats, P > 0.05).
Figure 4.
Qualitative (a) and quantitative (b) Western blot of NIX, BNIP3 and Drp1 in C, S, CCI+ HBO, and CCI group. Weakest blot was present in the C and S group and blots became gradually stronger after CCI and especially CCI+HBO therapy (n = 9 rats per group for three repeats, *presented compared with S group, # presented compared with CCI group, P < 0.05).Qualitative (c) and quantitative (d) Western blot of NIX, BNIP3 and Drp1 represented the corresponding groups after AMPK inhibitor CC administration. The blots of BNIP3 presented in the CCI and CCI+HBO group became similar with C and S group (n = 9 rats per group for three repeats, P > 0.05). While the blots of NIX and Drp1 are stronger in CCI and CCI+HBO group than the C and S groups (n = 9 rats per group for three repeats, *presented compared with S group, P < 0.05). There is no significant difference between CCI and CCI+HBO group (n = 9 rats per group for three repeats, P > 0.05). Qualitative (e) and quantitative (f) Western blot of NIX, BNIP3, and Drp1 represented the corresponding groups after CaMMKβ inhibitor STO609 administration. The blots presented in the CCI and CCI+HBO group became similar with C and S group (n = 9 rats per group for three repeats, P > 0.05). CCI: chronic constriction injury; HBO: hyperbaric oxygenation.
Electron microscopic results
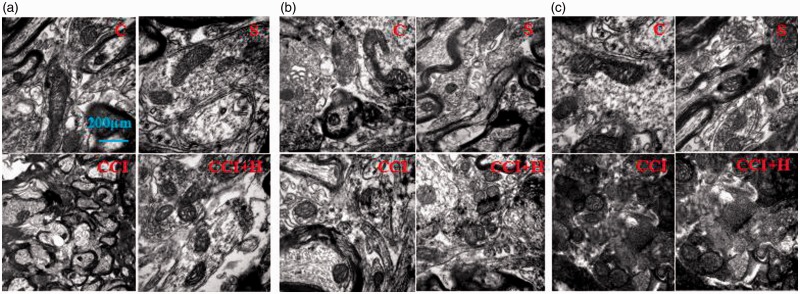
We could see only few mitochondria appeared in C and S with normal round or oval morphology. There were no autophagosomes or less lysosomes in it. While in CCI group, the morphology changed with increasing the number of mitochondria and lysosomes. In addition, some cells showed obvious necrosis and apoptosis. And the mitochondria were significantly increased in CCI + HBO group with a double membrane structure and a large number of lysosome or autophagosome. We also can see some fusion of mitophagy-lysosome with monolayer membrane structure or the residue after digestion. In addition, the mitochondrial crest structure disappeared without obvious necrosis and apoptosis (Figure 5(a)). The structure of double membrane, lysosome, and autophagosome disappeared when the inhibitor CC or STO609 was administered. There were no differences between them (Figure 5(b) and (c)).
Figure 5.
There are only few mitochondria appeared in picture C and S in normal morphology, round or oval without autophagosomes or lysosomes in it. After CCI, the morphology of nervous cells changed, such as increase in number of mitochondria and appearance of lysosomes and other structures. The mitochondria of nervous cells were significantly increased in CCI+HBO group with a double membrane structure and a large number of lysosome or autophagosome. At the same time, the mitochondrial crest structure disappeared without obvious necrosis and apoptosis in (a). There were no differences between all groups when the inhibitor CC or STO609 was administered ((b) and (c)).
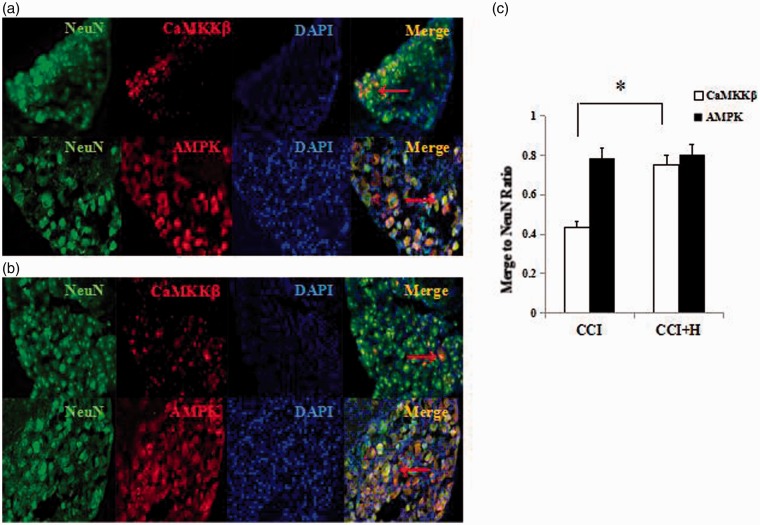
Pathological changes
To show the process of mitophagy which was associated with CaMKKβ and AMPK, we observed the neuron marker (NeuN), CaMKKβ, and AMPK by fluorescence microscopy. It has been shown to be an effective method for detecting colocalization. We immunostained CCI and CCI + HBO group with NeuN, AMPK, CaMKKβ, and DAPI. Compared with the CCI group (Figure 6(a)), there were a large number of red and green fluorescence signals merging into an orange signal in CCI + HBO groups (Figure 6(b)) on expression of CaMKKβ (Figure 6(c), n = 9 rats per group for three repeats, P < 0.05). The number of red and green fluorescence signals merging into an orange signal were similar in CCI and CCI + HBO groups in the expression of AMPK (Figure 6(c), n = 9 rats per group for three repeats, P > 0.05). This indicates that CaMKKβ can colocalize with NeuN, and HBO may upregulate this process.
Figure 6.
Immunostaining of CCI and CCI+H group with NeuN, AMPK, CaMKKβ, and DAPI. Compared with the CCI group, there were a large number of red and green fluorescence signals merging into an orange signal in CCI+H groups on expression of CaMKKβ. The number of red and green fluorescence signals merging into an orange signal were similar in CCI and CCI+H groups on expression of AMPK. CCI: chronic constrictive injury.
Discussion
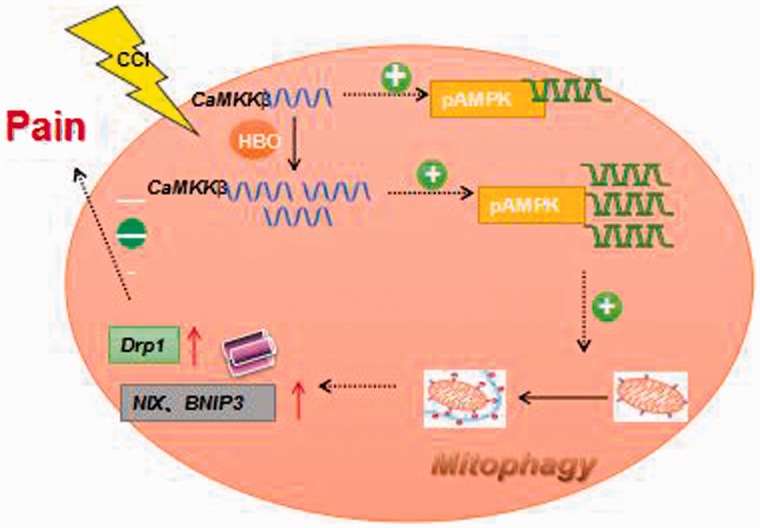
Our previous study observed the effect of HBO on NP through mitophagy in rats. In order to specify its possible mechanism, we did the further study on the molecular mechanism of HBO. But why do we investigate the mechanism of CaMKKβ/AMPK signaling pathways on this duration? First, mitophagy is associated with energy metabolism and ATP utilization.19 And AMPK can play a role in improving the overall expressive level of proteins and changing the transcription and translation process of proteins.20 It can also reduce ATP utilization and improve ATP level by promoting glucose catabolism and inhibiting protein metabolism.21 Second, NP is a persistent injured signal which can be transmitted through ion channels or signaling pathways.8 Ca2 + channels are closely related to pain and excitability of neuron. Many studies have shown that mitophagy also has relationship with Ca2 + channels.22 It can be seen that Ca2 + channels are associated with both NP and mitophagy. Studies have found that CaMKKβ is involved in axon growth, dendritic branches synapse formation, and remodeling the skeletal structure of memory-related nerve cells.23 Finally, CaMKKβ/AMPK signal pathway is a hot research topic at present. But there are few studies on its mechanism of NP. Therefore, it is necessary to further explore its related mechanisms. Therefore, we explored a hypothesis based on our previous study (Figure 7).
Figure 7.
The hypothesis of the mechanism how HBO act on mitophagy is described in picture.
AMPK protein consists of three subunits, which can change the conformation into activated state through phosphorylation of different subunits and then activate the regulating function of cell.24 AMPKα, the main subunit, which is associated with phosphorylation, is mainly investigated in our study. Phosphorylation of AMPK can adjust the expression of downstream protein directly or indirectly to prevent excessive consumption of ATP and activate mitochondrial enzyme activity to generate more ATP to maintain the steady state at the same time.25 Therefore, we used pAMPK and AMPK to evaluate both the energy metabolism and mitochondrial function. In our study, the level of AMPK is stable in all groups, whereas the level of pAMPK increased after CCI, especially in CCI + HBO group. The result indicated that the mitophagy induced by HBO was related to pAMPK, not AMPK. In addition, many studies show that AMPKα contains 548 amino acids, which can be divided into N terminal, middle automatic inhibition zone, and C terminal of binding zone.26 There are eight phosphorylation sites (e.g., serine 485, threonine 172, and threonine 258, etc.) in which the site of threonine 172 plays an important role in the regulation of AMPK activity.27 Due to the time and expense of the experiment, this study did not conduct further research on the phosphorylation site. In the future, we will explore the phosphorylation site of AMPK when HBO acts through CaMKKβ/AMPK signaling pathway. At the same time, the promoting effect between Ca2 + and AMPK indicated that they can be regulated in both directions. They have attracted more and more attention in terms of cellular energy metabolism. Altogether, the role of CaMKKβ/AMPK pathway is a highly concerned but unclear issue for the study of the mechanism of NP at present. In our study, the level of CaMKKβ increased after CCI, especially in CCI + HBO group. The result indicated that the mitophagy induced by HBO was related to CaMKKβ.
Mitochondrial split power-related protein 1 (dynamin related protein 1, Drp1) can regulate the division of the mitochondria in mammals, which is surrounded the split point of mitochondrial membrane and shuttle from inside to outside of the mitochondria.28 Drp1 can induce and regulate mitochondrial division. In addition, Drp1 protein is very important to maintain the normal morphology and distribution of mitochondria.29 Its N terminal plays the role of enzyme and its C terminal plays the role of intermolecular integration and mitochondria localization.30 It can also participate in mitochondrial shearing, mitochondrial outer membrane lipid hydrolysis, gene expressive regulation, and other functions of protein coupling.31 Drp1 is also associated with excess of ROS generation, energy metabolic disorders, and increasing oxidative stress, which are similar to the NP.32 Therefore, the importance of Drp1 is essential for further study of mitophagy. The research on Drp1’s relationship with CaMKKβ and AMPK is helpful to further understand the relationship between NP, mitophagy, and HBO and explore its action mechanism.
HBO has the ability to enhance antioxidant enzyme activity, reduce or eliminate edema in nerve tissue, accelerate the scavenging of free radicals, increase the content of blood oxygen, generate more ATP, promote capillary regeneration, and improve microcirculation.33 Meanwhile, HBO therapy specifically inhibits apoptosis after injury by improving the metabolism of nerve cells and increasing the energy necessary for protein synthesis.34 It is very likely that mitophagy occurs in cells through mitochondrial energy changes. In this study, the pressure and duration of HBO were in normal range. And no adverse reactions or HBO poisoning occurred in all experimental rats.
To clarify the mechanism on how HBO regulates mitophagy, our study used AMPK and CaMKKβ synergistic inhibitors to firstly observe the changes of pain threshold and specific proteins of mitophagy. Then, the correlation between specific proteins of mitophagy and pain was detected. Dorsomorphin (Compound C) is an effective, reversible, and selective AMPK inhibitor. It has strong specificity and stable expression which has no significant inhibitory effect on ZAPK, SYK, PKC, PKA, JAK3, and other kinases with similar structures.35 Therefore, Compound C was selected as AMPK inhibitor in our study. In addition, STO609 is a competitive inhibitor of CaMKKβ. It is easy to penetrate cell membranes and block the biological effect of CaMKKβ at the catalytic competitive site with ATP.36 In our study, it was found that MWT and TWL in HBO group reversed the original upward trend after the administration of Compound C and STO609, especially on the seventh day with the strongest sensitivity of pain. It indicates that the protective effect of HBO on NP disappeared after giving inhibitors. Ethologically, it is suggested that HBO is most likely to relieve NP through CaMKKβ/AMPK signal pathway. Mitochondria play an important role in cell survival and apoptosis. We detect the changes of mitophagy which occur in mitochondria-related protein NIX, BNIP3, and Drp1. The expression of mitophagy-related protein in CCI and CCI + HBO group increased significantly compared with C group and S group. The increase in CCI + HBO group was more obvious. But after given Compound C and STO609, there are no statistical difference in all the groups on the expression of NIX, BNIP3, and Drp1. The increased trend of mitophagy-specific protein induced by HBO was reversed. These phenomenon also accurred under transmission electron microscope. All these changes hint HBO may be related to CaMKKβ/AMPK pathway on NP mechanism.
Intrathecal catheter is a very important means for continuous administration of drugs in subarachnoid space of rats. However, it is difficult to ensure the accurate position of catheter without affecting the complete nerve function of rats. Therefore, successful intrathecal catheter is the key to ensure the whole experiment. According to the physiological characteristics of spinal cord which were terminated at the position of lumber three (L3) where only cauda equina nerve floating in cerebrospinal fluid, we indwelled catheter at L3 or lower where it is not easy to damage the spinal cord. It is also suitable for safe fixation and continuous multiple injection of drugs into the subarachnoid cavity of the lumbar segment. In this study, the surgical method of inserting catheter from lumbosacral segment of rats was adopted, and the soft and thin sterile epidural catheter was selected to minimize the injury on nerve function and effect on the behavior test of pain. In this study, percutaneous catheterization was used to fix the head. We did not select direct fixation because rats could scratch their waist with their paws and remove the catheter. Therefore, the fixation required the catheter to go through a longer neck segment and chest segment to reach the waist segment. The operation was more difficult and may cause greater damage, which may affect behavioral observation. While our results showed that the behavior changes of rats after the intrathecal catheter were stable and consistent. And the development of neuropathic sensitive pain indicated that the technique was successful and had no adverse reactions.
In summary, our data demonstrate that CaMKKβ/AMPK may participate in pain processes. There are several limitations to this study. First, the time points after CCI were optimal for mitophagy, which were discovered through initial experiments. While the formation of mitophagy is a dynamic process, we only selected a single time point, which may have certain limitations. To further clarify the dynamic changes in the mitophagy process, we should observe multiple time points. Second, if using autophagosome or lysosome markers to co-localize and monitor this dynamic process may provide more persuasive results. Finally, the mitochondrial membrane potential, ATP levels, and intracellular calcium ion levels should be monitored in future experiments which can explore the important neuroprotective mechanisms of HBO on mitophagy in depth.
Acknowledgment
The authors thank the Department of Anesthesiology of Shengjing Hospital of China Medical University for supporting the submitted work. We thank the staff who kindly helped in facilitating the experiment.
Authors’ contributions
LK performed immunofluorescence observation and participated in data analysis; LYD performed the behavioral study; LL performed the electron microscopic observation; LXY performed the Western blot experiments; HG designed and supervised experiments and wrote, interpreted, revised the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the National Natural Scientific Foundation of China (NSFC, 81600971).
References
- 1.Stompór M, Grodzicki T, Stompór T, Wordliczek J, Dubiel M, Kurowska I. Prevalence of chronic pain, particularly with neuropathic component, and its effect on overall functioning of elderly patients. Med Sci Monit 2019; 25: 2695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet 2019; 393: 1537–1546. [DOI] [PubMed] [Google Scholar]
- 3.Minami A, Tanaka T, Otoshi T, Kuratsukuri K, Nakatani T. Hyperbaric oxygen significantly improves frequent urination, hyperalgesia, and tissue damage in a mouse long-lasting cystitis model induced by an intravesical instillation of hydrogen peroxide. Neurourol Urodyn 2019; 38: 97–106. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Hei MY, Dai JJ, Hu N, Xiang XY. Effect of hyperbaric oxygenation on mitochondrial function of neuronal cells in the cortex of neonatal rats after hypoxic-ischemic brain damage. Braz J Med Biol Res 2016; 49: e5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han G, Liu K, Li L, Li X, Zhao P. The effects of hyperbaric oxygen therapy on neuropathic pain via mitophagy in microglia. Mol Pain 2017; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan K, Jiang S, Du X, Zeng X, Zhang J, Song L, Zhou J, Kan H, Sun Q, Xie Y, Zhao J. AMPK activation attenuates inflammatory response to reduce ambient PM2.5-induced metabolic disorders in healthy and diabetic mice. Ecotoxicol Environ Saf 2019; 179: 290–300. [DOI] [PubMed] [Google Scholar]
- 7.Rana S, Blowers EC, Natarajan A. Small molecule adenosine 5′-monophosphate activated protein kinase (AMPK) modulators and human diseases. J Med Chem 2015; 58: 2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng J, Zhang T, Ji H, Tao K, Guo J, Wei W. Functional characterization of AMP-activated protein kinase signaling in tumorigenesis. Biochim Biophys Acta 2016; 1866: 232–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J, Wang H, Hu Y, Zhang YS, Wen L, Yin F, Wang Z, Zhang Y, Li S, Miao Y, Lin B, Zuo D, Wang G, Mao M, Zhang T, Ding J, Hua Y, Cai Z. Inhibition of CaMKIIα activity enhances antitumor effect of fullerene C60 nanocrystals by suppression of autophagic degradation. Adv Sci (Weinh) 2019; 6: 1801233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakanishi A, Hatano N, Fujiwara Y, Sha'ri A, Takabatake S, Akano H, Kanayama N, Magari M, Nozaki N, Tokumitsu H. AMP-activated protein kinase-mediated feedback phosphorylation controls the Ca2 + /calmodulin (CaM) dependence of Ca2 + /CaM-dependent protein kinase kinase β. J Biol Chem 2017; 292: 19804–19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gocher AM, Azabdaftari G, Euscher LM, Dai S, Karacosta LG, Franke TF, Edelman AM. Akt activation by Ca2 + /calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J Biol Chem 2017; 292: 14188–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Wen D, Wang X, Yang L, Liu T, Liu J, Zhu J, Fang X. Growth inhibition of human gastric adenocarcinoma cells in vitro by STO-609 is independent of calcium/calmodulin-dependent protein kinase kinase-beta and adenosine monophosphate-activated protein kinase. Am J Transl Res 2016; 8: 1164–1171. [PMC free article] [PubMed] [Google Scholar]
- 13.Ye C, Zhang D, Zhao L, Li Y, Yao X, Wang H, Zhang S, Liu W, Cao H, Yu S, Wang Y, Jiang J, Wang H, Li X, Ying H. CaMKK2 suppresses muscle regeneration through the inhibition of myoblast proliferation and differentiation. Int J Mol Sci 2016; 17: 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shams Z, Khalatbary AR, Ahmadvand H, Zare Z, Kian K. Neuroprotective effects of hyperbaric oxygen (HBO) therapy on neuronal death induced by sciatic nerve transection in rat. BMC Neurol 2017; 17: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paprocki J, Sutkowy P, Piechocki J, Woźniak A. Markers of oxidant-antioxidant equilibrium in patients with sudden sensorineural hearing loss treated with hyperbaric oxygen therapy. Oxid Med Cell Longev 2019; 2019: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Q, Manaenko A, Matei N, Guo Z, Xu T, Tang J, Zhang JH. Hyperbaric oxygen preconditioning: a reliable option for neuroprotection. Med Gas Res 2016; 6: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Q, Manaenko A, Bian H, Guo Z, Huang JL, Guo ZN, Yang P, Tang J, Zhang JH. Hyperbaric oxygen reduces infarction volume and hemorrhagic transformation through ATP/NAD + /Sirt1 pathway in hyperglycemic middle cerebral artery occlusion rats. Stroke 2017; 48: 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei H, Viisanen H, You HJ, Pertovaara A. Spinal histamine in attenuation of mechanical hypersensitivity in the spinal nerve ligation-induced model of experimental neuropathy. Eur J Pharmacol 2016; 772: 1–10. [DOI] [PubMed] [Google Scholar]
- 19.Glosse P, Föller M. AMP-activated protein kinase (AMPK)-dependent regulation of renal transport. Int J Mol Sci 2018; 19: 3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara Y, Kawaguchi Y, Fujimoto T, Kanayama N, Magari M, Tokumitsu H. Differential AMP-activated protein kinase (AMPK) recognition mechanism of Ca2 + /calmodulin-dependent protein kinase kinase isoforms. J Biol Chem 2016; 291: 13802–13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willows R, Sanders MJ, Xiao B, Patel BR, Martin SR, Read J, Wilson JR, Hubbard J, Gamblin SJ, Carling D. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem J 2017; 474: 3059–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janzen NR, Whitfield J, Hoffman NJ. Interactive roles or AMPK and glycogen from cellular energy sensing to exercise metabolism. Int J Mol Sci 2018; 19: 3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross FA, Hawley SA, Auciello FR, Gowans GJ, Atrih A, Lamont DJ, Hardie DG. Mechanisms of paradoxical activation of AMPK by the kinase inhibitors SU6656 and sorafenib. Cell Chem Biol 2017; 24: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moral‐Sanz J, Mahmoud AD, Ross FA, Eldstrom J, Fedida D, Hardie DG, Evans AM. AMP-activated protein kinase inhibits Kv1.5 channel currents of pulmonary arterial myocytes in response to hypoxia and inhibition of mitochondrial oxidative phosphorylation. J Physiol 2016; 594: 4901–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakur S, Viswanadhapalli S, Kopp JB, Shi Q, Barnes JL, Block K, Gorin Y, Abboud HE. Activation of AMP-activated protein kinase prevents TGF-β1–induced epithelial-mesenchymal transition and myofibroblast activation. Am J Pathol 2015; 185: 2168–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DW, Jiang S, Liu Y, Siegal GP, Inoki K, Abraham E, Zmijewski JW. GSK3β-dependent inhibition of AMPK potentiates activation of neutrophils and macrophages and enhances severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2014; 307: L735–L745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol 2016; 26: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell 2015; 26: 4466–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker DJ, Iyer A, Shah S, Moran A, Hjelmeland AB, Basu MK, Liu R, Mitra K. A new mitochondrial pool of cyclin E, regulated by Drp1, is linked to cell-density-dependent cell proliferation. J Cell Sci 2015; 128: 4171–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald PJ, Stepanyants N, Mehrotra N, Mears JA, Qi X, Sesaki H, Ramachandran R. A dimeric equilibrium intermediate nucleates Drp1 reassembly on mitochondrial membranes for fission. Mol Biol Cell 2014; 25: 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manczak M, Kandimalla R, Yin X, Reddy PH. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet 2019; 28: 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinton RW, Francy CA, Ramachandran R, Qi X, Mears JA. Dynamin-related protein 1 oligomerization in solution impairs functional interactions with membrane-anchored mitochondrial fission factor. J Biol Chem 2016; 291: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobanoglu HB, Vuralkan E, Arslan A, Mirasoglu B, Toklu AS. Is hyperbaric oxygen therapy effective in cisplatin-induced ototoxicity in rats? Clin Exp Otorhinolaryngol 2019; 12: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thiele RH. Subcellular energetics and metabolism: potential therapeutic applications. Anesth Analg 2017; 124: 1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Chhipa RR, Nakano I, Dasgupta B. AMPK inhibitor compound C is a potent AMPK-independent anti-glioma agent. Mol Cancer Ther 2014; 13: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.York B, Li F, Lin F, Marcelo KL, Mao J, Dean A, Gonzales N, Gooden D, Maity S, Coarfa C, Putluri N, Means AR. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci Rep 2017; 7: 11793. [DOI] [PMC free article] [PubMed] [Google Scholar]