Abstract
Background:
Liposomal irinotecan (nal-IRI) plus 5-fluorouracil and leucovorin (5-FU/LV) was effective and well-tolerated in patients with metastatic pancreatic adenocarcinoma (mPAC) that progressed on gemcitabine-based therapy in the global NAPOLI-1 trial. Real-world data may further clarify the outcomes and safety profile of nal-IRI + 5-FU/LV in clinical practice.
Methods:
This retrospective analysis included patients with mPAC who received nal-IRI + 5-FU/LV following gemcitabine-based therapy under a Managed Access Program in Korea.
Results:
From January 2017 to April 2018, 86 patients across 10 institutions received nal-IRI + 5-FU/LV (median age, 61 years; 60% male; ECOG performance status, 0–1). A total of 35 (41%) and 51 (59%) patients had received less than two and two or more lines of chemotherapy before inclusion, respectively. At a median follow up of 6.4 months, median overall survival (OS) was 9.4 months (95% confidence interval [CI] 7.4–11.4) and median progression-free survival (PFS) was 3.5 months (95% CI 1.3–5.7). Six-month OS and PFS rates were 65.1% and 37.5%, respectively. Objective response and disease control rates were 10% and 55%, respectively. Most common grade 3–4 toxicities were neutropenia (37.2%), nausea (10.5%), vomiting (9.3%), anorexia (8.1%) and diarrhoea (4.7%).
Conclusion:
Real-life data for Korean patients indicate that, consistent with NAPOLI-1, nal-IRI + 5-FU/LV is effective and well-tolerated in patients with mPAC that progressed on gemcitabine-based therapy.
Keywords: chemotherapy, liposomal irinotecan, pancreatic adenocarcinoma, real-world evidence
Introduction
Pancreatic adenocarcinoma (PAC) is an aggressive disease with a dismal prognosis, which is mainly associated with its detection at an advanced stage and frequent recurrence even after curative resection. Fewer than 10% of patients survive at 5 years after diagnosis.1,2
Gemcitabine has been the standard therapy for unresectable or metastatic PAC (mPAC) for the past two decades.3 The development of two combination chemotherapeutic regimens, FOLFIRINOX [oxaliplatin, irinotecan, fluorouracil (5-FU) and leucovorin (LV)] and gemcitabine plus nab-paclitaxel, has significantly improved patient outcomes as front-line chemotherapy.4,5 These regimens delay the deterioration of quality of life for patients with mPAC, improving the chance for salvage chemotherapy after progression on first-line chemotherapy.6–8
Few phase III trials have addressed the role of salvage chemotherapy after failure of first-line chemotherapy. Although the addition of oxaliplatin to 5-FU/LV improved survival outcomes compared with 5-FU/LV in patients showing disease progression on gemcitabine in the previous CONKO-003 trial,9 this finding was not reproduced in the PANCREOX study, in which oxaliplatin was combined with 5-FU/LV infusion.10 The role of oxaliplatin in the management of patients with mPAC that progressed on first-line gemcitabine-based therapy is therefore controversial.
Liposomal irinotecan (nal-IRI) consists of irinotecan sucrosofate salt encapsulated in liposome particles, which increase and prolong the intratumoural levels of both irinotecan and its active metabolite SN-38.11 The promising activity of nal-IRI against PAC in a phase II trial12 led to the design of the pivotal phase III NAPOLI-1 trial, in which nal-IRI + 5-FU/LV showed higher effectiveness than 5-FU/LV alone and manageable toxicities in patients with mPAC following previous gemcitabine-based therapy.13 In the NAPOLI-trial, nal-IRI + 5-FU/LV was used as first-line (13%), second-line (53%) and third-line or later (34%) chemotherapy for the management of metastatic disease.
Although 30% of patients in the NAPOLI-1 trial were of East Asian ethnicity, including patients from Korea,13 FOLFIRINOX or gemcitabine plus nab-paclitaxel were not widely used at the time of the study. These modern front-line regimens are now commonly used after their approval for reimbursement by the Korean National Healthcare Insurance in 2016. Considering the effect of the changes in front-line chemotherapy on the effectiveness of salvage therapy and the heterogeneity of clinical features and management of mPAC, real-world data are needed for nal-IRI + 5-FU/LV treatment.
The present multicentre, retrospective, observational study was conducted by the Korean Cancer Study Group (KCSG) to evaluate the effectiveness and safety of nal-IRI + 5-FU/LV in patients with mPAC following disease progression on previous gemcitabine-based therapy.
Methods
Patients
This retrospective study was a multicentre, open-label, non-comparative observational study that included patients who entered into the nal-IRI Managed Access Program (MAP) in Korea. This analysis was performed by the hepatobiliary and pancreatic cancer division of the KCSG. Effectiveness and safety data were retrospectively collected and analysed and the study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review board (IRB) at the main study site (Asan Medical Center, approval number 2018-0492), with additional local approvals granted at other study sites as required. At 3 of the 10 participating institutions in this study (Dong-A University Hospital, Bucheon St. Mary’s Hospital and Korea University Hospital), additional approval was waived according to local IRB policy (additional local approval not required in the case of multicentre, retrospective analyses). Approval numbers from the remaining six institutions were as follows: Cha University Hospital (2018-07-008-001); Dongguk University Hospital (110757-201808-HR-02-03); Haeundae Baik Hospital (2018-04-009-005); Seoul National University Hospital (1809-012-969); Samsung Medical Center (2018-03-162); and Yonsei University College of Medicine (Severance Hospital; 4-2017-1011). IRBs waived the need for informed consent for this study owing to the nonrequirement of consent in retrospective analysis covered by regulations in Korea.
Patients with histologically or cytologically confirmed mPAC were eligible for inclusion in this MAP if they had evidence of disease progression on prior gemcitabine-based therapy, including neoadjuvant, adjuvant or palliative chemotherapy. All patients who had previously received conventional irinotecan as part of a FOLFIRINOX regimen had progressed prior to administration of nal-IRI during the MAP.
Patients received nal-IRI + 5-FU/LV as described in the NAPOLI-1 trial (80 mg/m2 irinotecan hydrochloride trihydrate salt equivalent to 70 mg/m2 irinotecan free base over 90 min, followed by 400 mg/m2 LV over 30 min and then 2400 mg/m2 5-FU over 46 h, every 2 weeks). Computed tomography scans were performed every 6–8 weeks.
Adverse events were evaluated during every clinic visit and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.03. Effectiveness was measured using radiological assessments, including computed tomography or magnetic resonance imaging scans, and graded according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1.
Statistical analysis
The objective response rate (ORR) and disease control rate (DCR) were assessed using RECIST v1.1. Progression-free survival (PFS) was defined as the time from the initiation of nal-IRI + 5-FU/LV to the date of disease progression determined by RECIST v1.1 or death, whichever occurred first. Overall survival (OS) was defined as the time between the initiation of nal-IRI + 5-FU/LV and death from any cause. Survival outcomes were estimated using Kaplan–Meier curves and subgroups compared using the log-rank test. Univariate and multivariate analyses of OS and PFS were performed using the Cox proportional hazards model. A two-sided p value <0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM, Armonk, NY, USA) version 22.0.
Results
Baseline characteristics
The MAP enrolled 86 patients with mPAC from ten Korean institutions between January 2017 and April 2018. Baseline patient characteristics are summarised in Table 1. Median age was 61 years (range, 37–79) and 52 patients (60.5%) were male. Most patients had a primary tumour in the pancreatic head (n = 41, 47.7%) followed by the tail (n = 27, 31.4%) and body (n = 17, 19.8%). All patients had metastatic disease and Eastern Cooperative Oncology Group performance status 0–1 at the time of nal-IRI + 5-FU/LV initiation. The most common metastatic sites were the liver (n = 49, 57.0%), peritoneum (n = 30, 34.9%) and lung (n = 27, 31.4%). Serum CA 19-9 levels were elevated in 59 (83.1%) of 71 patients with available data at initiation of nal-IRI + 5-FU/LV treatment.
Table 1.
Patient characteristics.
| Variable | nal-IRI + 5-FU/LV (n = 86) |
|---|---|
| Gender | |
| Male | 52 (60.5%) |
| Female | 34 (39.5%) |
| Age, median (range) | 61 (37–79) |
| <65 | 55 (64.0%) |
| ⩾65 | 31 (36.0%) |
| Primary tumour site | |
| Head | 41 (47.7%) |
| Body | 17 (19.8%) |
| Tail | 27 (31.4%) |
| Multi-centric | 1 (1.2%) |
| Site of metastasis | |
| Liver | 49 (57.0%) |
| Lung | 27 (31.4%) |
| Bone | 4 (4.7%) |
| Peritoneum | 30 (34.9%) |
| Lymph node | 21 (24.4%) |
| Other | 15 (17.4%) |
| Baseline CA 19-9 level (U/ml), median (range) | 844 (1.2–124,073) |
| ⩽1 × UNL | 12 (14.0%) |
| >1 and ⩽2 × UNL | 4 (4.7%) |
| >2 × UNL | 55 (64.0%) |
| N/A | 15 (17.4%) |
| Prior surgical resection | 39 (45.3%) |
| Prior concurrent chemoradiotherapy | 20 (23.3%) |
| Prior lines of palliative chemotherapy, median (range) | 2 (0–4) |
| 0 | 8 (9.3%) |
| 1 | 27 (31.4%) |
| 2 | 36 (41.9%) |
| 3 | 12 (14.0%) |
| 4 | 3 (3.5%) |
| Prior first-line palliative chemotherapy | n = 78 |
| Gemcitabine monotherapy | 12 (14.0%) |
| Gemcitabine plus nab-paclitaxel | 44 (51.2%) |
| FOLFIRINOX | 14 (16.3%) |
| Others | 8 (9.3%) |
| Prior irinotecan-containing chemotherapy (FOLFIRINOX) | 18 (20.9%) |
| Prior gemcitabine plus nab-paclitaxel chemotherapy | 51 (59.3%) |
| Prior 5-FU/LV containing chemotherapy | 59 (68.6%) |
CA 19-9, carbohydrate antigen 19-9; UNL, upper normal limit; N/A, not available.
Curative surgery and chemoradiotherapy were previously performed in 39 (45.3%) and 20 (23.3%) patients, respectively. A median of two lines (range, 1–4) of chemotherapy (including neoadjuvant, adjuvant and palliative therapy) were given prior to nal-IRI + 5-FU/LV treatment. The median number of lines for palliative therapy only for locally advanced or mPAC was also two (range, 0–4). Irinotecan was previously administered as a component of FOLFIRINOX in 18 patients (20.9%). This was received as neoadjuvant therapy in two of these patients, and disease progression was observed in all patients who had previously been treated with irinotecan. Gemcitabine plus nab-paclitaxel and 5-FU/LV were previously administered in 51 (59.3%) and 59 patients (68.6%), respectively.
Effectiveness outcomes
Effectiveness outcomes with nal-IRI + 5-FU/LV are summarised in Table 2. The median follow-up duration was 6.4 months (95% confidence interval [CI] 5.5–7.3 months), median OS was 9.4 months (95% CI 7.4–11.4 months) and median PFS was 3.5 months (95% CI 1.3–5.7 months). Six-month OS and PFS rates were 65.1% (95% CI 53.8–74.3%) and 37.5% (95% CI 27.1–47.8%), respectively (Figure 1). Median OS since the start of first-line therapy for unresectable or metastatic disease was 26.3 months (95% CI 19.3–33.5 months) and the 2-year OS rate was 54.3%.
Table 2.
Effectiveness outcomes.
| nal-IRI + 5-FU/LV (n = 86) |
|
|---|---|
| Best response | |
| CR | 2 (2.3%) |
| PR | 7 (8.1%) |
| SD | 38 (44.2%) |
| PD | 32 (37.2%) |
| N/A | 7 (8.1%) |
| 6–month OS, % (95% CI) | 65.1 (53.8–74.3) |
| Median OS, months (95% CI) | 9.4 (7.4–11.4) |
| 6–month PFS, % (95% CI) | 37.5 (27.1–47.8) |
| Median PFS, months (95% CI) | 3.5 (1.3–5.7) |
| Objective response rates (CR + PR) | 9 (10.5%) |
| Disease control rate (CR + PR + SD) | 47 (54.7%) |
CI, confidence interval; CR, complete response; N/A, not available; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Figure 1.

Survival outcomes with nal-IRI + 5-FU/LV.
According to RECIST v1.1, complete response (CR) and partial response (PR) were achieved in two (2.3%) and seven (8.1%) patients, respectively, indicating an ORR of 10.5% (95% CI 3.9–17.1%). Stable disease (SD) and progressive disease (PD) were the best response in 38 (44.2%) and 32 (37.2%) patients, respectively, and response evaluation was not available in seven patients (8.1%). The DCR was 54.7% (95% CI 36.1–58.1%). Among 51 patients with available pre- and post-treatment CA 19-9 levels, 16 (31.4%) achieved a CA 19-9 response (i.e. a ⩾50% decrease in CA 19-9 levels from baseline).13
Survival outcomes by prior chemotherapy
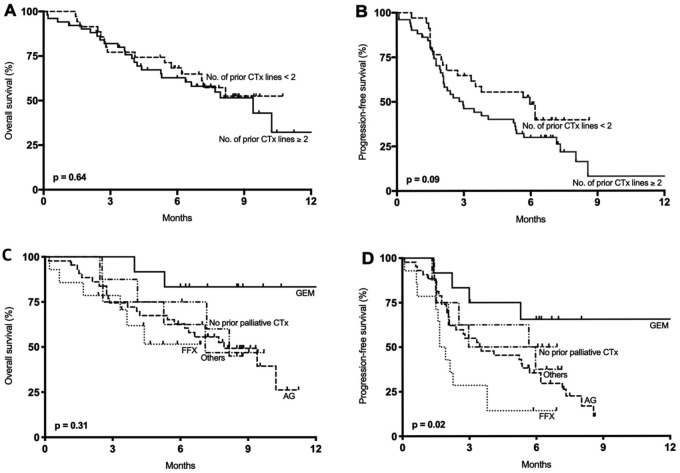
Survival outcomes with nal-IRI + 5-FU/LV were analysed by the number of lines of prior chemotherapy in the palliative setting (Figure 2A and B) and prior first-line chemotherapy regimen (Figure 2C and D). Median OS and PFS did not differ significantly according to the number of lines of previous palliative chemotherapy (<2 versus ⩾2) (p = 0.64 and p = 0.09, respectively). Median OS was 7.9 months (95% CI not available) in patients who received ⩾2 lines (n = 51) of palliative chemotherapy, and was not reached at the time of analysis in patients who received <2 lines (n = 35) of treatment. Median PFS in these groups was 6.0 months (95% CI 3.3–8.6 months) and 3.0 months (95% CI 1.6–4.4 months) in patients who received <2 lines and ⩾2 lines of palliative chemotherapy, respectively. Six-month OS rate was 68.3% (95% CI 50.1–81.0%) in patients who had received <2 lines of palliative chemotherapy, and 62.8% (95% CI 47.5–74.7%) in those who had received ⩾2 lines of palliative chemotherapy. Six-month PFS rate was 48.6% (95% CI 30.8–64.4%) in patients who had received <2 lines of palliative chemotherapy, and 30.0% (95% CI 18.0–42.9%) in those who had received ⩾2 lines of palliative chemotherapy.
Figure 2.
Survival outcomes with nal-IRI + 5-FU/LV according to number of previous lines of palliative chemotherapy and type of first-line therapy: (A) overall survival according to number of previous lines of palliative chemotherapy; (B) progression-free survival according to number of previous lines of palliative chemotherapy; (C) overall survival according to type of first-line palliative chemotherapy; and (D) progression-free survival according to type of first-line palliative chemotherapy.
AG, gemcitabine plus nab-paclitaxel; CTx, chemotherapy; FFX, FOLFIRINOX; GEM, gemcitabine monotherapy.
The median OS with nal-IRI + 5-FU/LV did not differ significantly according to the first-line palliative chemotherapy regimen (p = 0.31, Figure 2C), whereas the difference in PFS was significant (p = 0.02, Figure 2D). The median PFS with nal-IRI + 5-FU/LV was 18.0 months (95% CI not available), 3.5 months (95% CI 0.9–6.2 months), and 1.7 months (95% CI 1.3–2.1 months) in patients who previously received first-line gemcitabine (n = 12), gemcitabine plus nab-paclitaxel (n = 44), and FOLFIRINOX (n = 14), respectively. Median OS was 10.2 months in patients who had not previously received irinotecan; and 4.4 months in patients who had received and progressed on prior neoadjuvant or palliative irinotecan treatment (n = 18; p = 0.011). Median PFS was 4.4 months in patients who had not previously received irinotecan; and 1.7 months in patients who had received and progressed on prior neoadjuvant or palliative irinotecan treatment (p < 0.001).
Multivariate analysis of prognostic factors
In the multivariate analyses of survival outcomes (Table 3: OS and PFS), bone metastases (HR, 8.28; 95% CI 2.06–33.33; p = 0.003) were significantly associated with worse OS outcomes; and age (< versus ⩾65 years: HR, 0.39; 95% CI 0.20–0.78; p = 0.007), liver metastases (HR, 2.96; 95% CI 1.46–6.02; p = 0.003), bone metastases (HR, 7.47; 95% CI 2.12–26.28; p = 0.002), and previous first-line FOLFIRINOX (versus gemcitabine monotherapy: HR, 4.08; 95% CI 1.21–13.73; p = 0.02) were significantly associated with worse PFS outcomes.
Table 3.
Multivariate analyses of survival outcomes.
|
Progression-free survival
|
Overall survival
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Sex (female versus male) | 1.21 | 0.67–2.18 | 0.538 | 0.90 | 0.45–1.81 | 0.772 |
| Age (⩾65 versus <65 years) | 0.39 | 0.20–0.78 | 0.007 | 0.83 | 0.39–1.77 | 0.627 |
| Liver metastases | 2.96 | 1.46–6.02 | 0.003 | – | – | – |
| Bone metastases | 7.47 | 2.12–26.28 | 0.002 | 8.28 | 2.06–33.33 | 0.003 |
| Peritoneum metastases | – | – | – | 1.89 | 0.93–3.87 | 0.080 |
| Prior surgical resection | 1.50 | 0.82–2.73 | 0.184 | – | – | – |
| Prior lines of palliative chemotherapy (⩾2) | 0.62 | 0.27–1.40 | 0.247 | 1.43 | 0.63–3.24 | 0.389 |
| Prior first-line palliative chemotherapy | ||||||
| Gemcitabine monotherapy | Ref | – | 0.100 | Ref | – | 0.458 |
| Gemcitabine plus nab-paclitaxel | 2.55 | 0.89–7.34 | 0.082 | 4.08 | 0.95–17.50 | 0.059 |
| FOLFIRINOX | 4.08 | 1.21–13.73 | 0.023 | 4.10 | 0.78–21.56 | 0.095 |
| Others | 2.69 | 0.65–11.10 | 0.172 | 3.66 | 0.63–21.33 | 0.149 |
| No previous palliative chemotherapy | 0.85 | 0.18–3.92 | 0.836 | 3.92 | 0.60–25.50 | 0.153 |
CI, confidence interval; HR, hazard ratio; Ref, reference.
Safety profile
Adverse events that occurred in >10% of patients are listed in Table 4. Any-grade adverse events were observed in the majority of patients (n = 78, 90.7%), and severe grade 3–4 toxicities were observed in 49 patients (57.0%). There was no treatment-related mortality. The most common adverse events were neutropenia (n = 45, 52.3%), anaemia (n = 44, 51.2%), nausea (n = 40, 46.5%), anorexia (n = 32, 37.2%), and diarrhoea (n = 26, 30.2%). Grade 3–4 neutropenia (n = 32, 37.2%), nausea (n = 9, 10.5%), vomiting (n = 8, 9.3%) and diarrhoea (n = 4, 4.7%) were the most frequent severe toxicities reported. Febrile neutropenia occurred in seven patients (8%).
Table 4.
Adverse events occurring in >10% of patients for any grade.
| Preferred terms (PT) | Any grade | Grade 3–4 |
|---|---|---|
| All, n (%) | 78 (90.7%) | 49 (57.0%) |
| Neutropenia, n (%) | 45 (52.3%) | 32 (37.2%) |
| Anaemia, n (%) | 44 (51.2%) | 7 (8.1%) |
| Alopecia, n (%) | 16 (18.6%) | NA |
| Fatigue, n (%) | 25 (29.1%) | 2 (2.3%) |
| Anorexia, n (%) | 32 (37.2%) | 7 (8.1%) |
| Nausea, n (%) | 40 (46.5%) | 9 (10.5%) |
| Vomiting, n (%) | 28 (32.6%) | 8 (9.3%) |
| Diarrhoea, n (%) | 26 (30.2%) | 4 (4.7%) |
n = 86; NA, not applicable.
Treatment exposure and dose modification
The median treatment duration of nal-IRI + 5-FU/LV was 3.1 months (range, 0.5–16.7). At the time of this analysis, nal-IRI + 5-FU/LV was ongoing in 18 patients (20.9%). Treatment was discontinued because of disease progression in 45 patients (66.2%) and adverse events in 8 (11.8%). The adverse events were grade 3 fatigue (n = 2), grade 3 diarrhoea (n = 2), grade 3 anorexia (n = 1), grade 3 febrile neutropenia (n = 1), grade 3 pneumonia (n = 1) and grade 4 neutropenia (n = 1). nal-IRI + 5-FU/LV doses were reduced or delayed in 43 patients (50.0%). The most frequent reasons for dose modification of nal-IRI + 5-FU/LV were neutropenia (n = 20, 23.3%), fatigue (n = 10, 11.6%) and nausea/vomiting (n = 5, 5.8%). Diarrhoea was a reason for dose modification in only 2 (4.7%) patients. Subsequent chemotherapy was administered to 15 patients (22.1%) following failure of nal-IRI + 5-FU/LV.
Discussion
The present multicentre retrospective study is one of the largest real-world analyses of nal-IRI + 5-FU/LV since modern front-line chemotherapy regimens such as FOLFIRINOX and gemcitabine plus nab-paclitaxel became available.
In the current study, the 6-month OS rate was 65.1%, and the median OS was 9.4 months. nal-IRI + 5-FU/LV resulted in a median PFS of 3.5 months and an ORR of 10.5%. These findings were consistent with the results of the NAPOLI-1 study and previous retrospective analyses,13,14 particularly with the Asian subgroup analysis.15 In the NAPOLI-1 study, the median OS and PFS were 6.1 and 3.1 months, respectively, and the ORR was 16% in the nal-IRI + 5-FU/LV group.13 In a recent retrospective analysis of nal-IRI + 5-FU/LV, the median OS and PFS were 5.3 and 2.9 months, respectively, and the ORR was 5%.14 The consistent clinical outcomes of these studies, despite variation in baseline patient characteristics, support the clinical relevance of nal-IRI + 5-FU/LV in patients with mPAC that progressed following prior gemcitabine-based therapy.
Improved effectiveness of first-line chemotherapy has led to an increase in the number of patients treated with salvage therapy for unresectable or metastatic disease. In the current study population, the median OS from the start of first-line palliative chemotherapy for unresectable or metastatic disease was 26.3 months. Although this analysis included patients with good performance status and organ function who were eligible for subsequent chemotherapy following disease progression on prior therapies, the findings support the notion that long-term survival may be achieved using appropriate sequential chemotherapy in the subset of patients with mPAC. This underscores the importance of selecting optimal chemotherapy regimens after progression on first-line chemotherapy. A reduced OS effect with nal-IRI + 5-FU/LV in patients who previously received and progressed on conventional irinotecan has already been reported.13,14 In the current study, survival outcomes were poorer in patients who had previously received conventional irinotecan. Discrepancy in the survival outcomes with nal-IRI + 5-FU/LV according to the prior exposure and progression to conventional irinotecan in the studies might be caused by the development of resistance to irinotecan or SN-38 (active metabolite of irinotecan) during prior treatment using conventional irinotecan. Although nal-IRI modifies the pharmacological properties of irinotecan, resulting in greater exposure of irinotecan and SN-38, this might be insufficient to overcome the resistance to this molecule. However, the number of patients in this group in the studies including ours was small, and thus further evaluation is needed.
The safety profile of nal-IRI + 5-FU/LV reported in this real-world study was consistent with the results of the NAPOLI-1 trial and its associated Asian subgroup analysis.13,15 The most common grade 3–4 toxicities were neutropenia, nausea, vomiting, anaemia and diarrhoea. Febrile neutropenia occurred in seven patients. The incidence of non-haematological toxicities such as diarrhoea was lower than reported in the intention-to treat population of the NAPOLI-1 trial. This might be explained by potential ethnic differences in pharmacokinetics and pharmacogenomics governing the metabolism of nal-IRI, or toxicities may have been underestimated owing to the retrospective nature of this analysis.16
The effectiveness of nal-IRI + 5-FU/LV demonstrated in this study and the NAPOLI-1 Asian subgroup analysis has important clinical implications for the future investigation of nal-IRI-containing regimens. These include neoadjuvant and adjuvant chemotherapy for potentially curative surgery or front-line chemotherapy for unresectable or metastatic disease. Future trials of nal-IRI with oral 5-FU to reduce infusion time may also be clinically relevant in terms of patient convenience.
The present study had several limitations. It was retrospective, which may result in potential selection or recall bias. Moreover, nal-IRI + 5-FU/LV was administered as several lines of chemotherapy following progression on gemcitabine-containing regimens. Some caution is therefore warranted in the interpretation of these results. This program did not include any patients with ECOG performance status >2, although this is likely due to physician concern over the potential for toxicities in patients with poorer performance status, reflecting clinical practice. Moreover, our findings were based on an ethnically homogeneous population as all patients were of East Asian origin and were from South Korea. The advantages of this study include its use of extensive real-world data gathered from multiple centres, and that patient characteristics were similar to those observed in the NAPOLI-1 trial, allowing balanced comparisons to be made between the two studies.
In conclusion, this multicentre, retrospective, observational study demonstrated that the effectiveness and safety of nal-IRI + 5-FU/LV in clinical practice was similar to that observed in NAPOLI-1, particularly to the results observed in Asian patients who were enrolled in that study.13,15 The results presented here show that nal-IRI + 5-FU/LV was an effective and feasible therapy in patients with mPAC after failure of gemcitabine-based therapy in a real-world clinical setting. nal-IRI + 5-FU/LV is a clinically relevant and valuable addition to the arsenal of treatments for mPAC, characterised by a high unmet need, exemplified by limited survival and lack of treatment options. Future investigation of nal-IRI and optimal sequence of chemotherapy is warranted to improve clinical outcomes of patients with PAC across different clinical settings.
Acknowledgments
Changhoon Yoo and Hyeon-Su Im contributed equally and should be considered as co-first authors.
nal-IRI (Onivyde®) was generously provided without charge by Shire. Rights for nal-IRI now reside with Ipsen in the USA (April 2017); PharmaEngine, Inc. hold the rights in Taiwan; Servier holds rights in the rest of the world through a licensing agreement with Ipsen. The authors acknowledge Floris A. de Jong (former employee of Shire, Zug, Switzerland and Servier Global Medical Affairs, Zurich, Switzerland) for his valuable contribution to the development of this manuscript. Editorial support for the preparation of this manuscript was provided by Carol McNair of Physicians World Europe GmbH, Mannheim, Germany, and funded by Global Medical Affairs Servier (Suresnes, France).
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant number HI14C2640) and by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (grant number 1720150).
Conflict of interest statement: CY has received research funding from Shire and Servier, and has acted as an advisor for Shire.
DYO and JOP have acted as advisors for Shire. All other authors declare that they have no conflicts of interest.
ORCID iD: Changhoon Yoo  https://orcid.org/0000-0002-1451-8455
https://orcid.org/0000-0002-1451-8455
Contributor Information
Changhoon Yoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Hyeon-Su Im, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Kyu-pyo Kim, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Do-Youn Oh, Division of Medical Oncology, Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Kyung-Hun Lee, Division of Medical Oncology, Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Hong Jae Chon, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
Joo Hoon Kim, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
Myoungjoo Kang, Division of Oncology, Department of Internal Medicine, Inje University College of Medicine, Haeundae Paik Hospital, Busan, Korea.
Ilhwan Kim, Division of Oncology, Department of Internal Medicine, Inje University College of Medicine, Haeundae Paik Hospital, Busan, Korea.
Guk Jin Lee, Division of Medical Oncology, Department of Internal Medicine, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea.
Sung Yong Oh, Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
Younak Choi, Division of Hemato-Oncology, Department of Internal Medicine, Dongguk University Gyeongju Hospital, Gyeongsangbuk-do, Korea.
Hye Jin Choi, Yonsei University College of Medicine, Seoul, Korea.
Seung Tae Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Joon Oh Park, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul, 06351, Korea.
Baek-Yeol Ryoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Pungnap 2(i)-dong, Seoul, 05505, Korea.
References
- 1. Jung KW, Won YJ, Oh CMet al. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat 2017; 49: 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Burris HA, III, Moore MJ, Andersen Jet al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Desseigne F, Ychou Met al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Ervin T, Arena FPet al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiorean EG, Von Hoff DD, Tabernero Jet al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer 2016; 115: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne Fet al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013; 31: 23–29. [DOI] [PubMed] [Google Scholar]
- 8. Kang J, Hwang I, Yoo Cet al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 9. Oettle H, Riess H, Stieler JMet al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014; 32: 2423–2429. [DOI] [PubMed] [Google Scholar]
- 10. Gill S, Ko YJ, Cripps Cet al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016; 34: 3914–3920. [DOI] [PubMed] [Google Scholar]
- 11. Kalra AV, Kim J, Klinz SGet al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res 2014; 74: 7003–7013. [DOI] [PubMed] [Google Scholar]
- 12. Ko AH, Tempero MA, Shan YSet al. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer 2013; 109: 920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang-Gillam A, Li CP, Bodoky Get al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 14. Glassman DC, Palmaira RL, Covington CMet al. Nanoliposomal irinotecan with fluorouracil for the treatment of advanced pancreatic cancer, a single institution experience. BMC Cancer 2018; 18: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen LT, Li CP, Chiu CFet al. Efficacy and safety of nanoliposomal irinotecan (nal-IRI, MM-398, PEP02, BAX-2398) in patients with metastatic pancreatic cancer in Asia: a subgroup analysis of the phase 3 NAPOLI-1 study. Ann Oncol 2016; 27(Suppl. 9): ix68–ix85. [Google Scholar]
- 16. Adiwijaya BS, Kim J, Lang Iet al. Population pharmacokinetics of liposomal irinotecan in patients with cancer. Clin Pharmacol Ther 2017; 102: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]