Abstract
Background:
Although adjuvant chemotherapy is recommended for patients with stage II colon cancer characterized by poor prognostic features, its pros and cons remain a controversial issue. We aim to evaluate the real effectiveness of chemotherapy on stage II colon cancer as well as select suitable patients.
Methods:
Patients during 1988–2013 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. The competing risk regression model and propensity score matching method were used to evaluate colon-cancer-specific death (CCSD) and non-CCSD. Also, a competing-risk nomogram was constructed to identify risk of patients. Risk score (RS) was calculated according to nomogram.
Results:
A total of 58,133 patients were included, 25.66% received chemotherapy, and 74.34% were without chemotherapy. In total, 19.95% and 25.78% of patients died of CCSD and non-CCSD, respectively. Univariate and multivariate analyses showed that receiving chemotherapy appears to be associated with more CCSD and less non-CCSD (HR 1.23, 95% CI 1.18–1.28; HR 0.45, 95% CI 0.43–0.47, respectively), even after adjustment for covariates and propensity score weighting. A competing-risk nomogram was established; the model was relatively good with a C-index of 0.661. Based on the RS, risk stage could only predict prognosis but failed to predict the benefit from chemotherapy.
Conclusions:
The value of chemotherapy is much less than we thought. It is time to de-escalate chemotherapy for stage II colon cancer. CCSD, rather than overall survival, should be considered as an appropriate primary end point for future trials in stage II colon cancer.
Keywords: chemotherapy, colon cancer, competing, nomogram, SEER, survival
Introduction
Colon cancer is the second leading cause of death from cancer, and it is estimated that 95,270 new cases were diagnosed in the United States in 2016.1 Among patients with colon cancer, approximately one-third of cases are diagnosed with stage II disease. Generally, surgery is the curative treatment for stage I colon cancer, and adjuvant chemotherapy is widely accepted as the standard of care for patients with stage III colon cancer on the basis of improved survival outcomes from large trials and the pivotal IMPACT meta-analysis.2–4 However, the role of adjuvant chemotherapy in patients with stage II colon cancer remains an area of controversy as different studies present inconsistent results. The Quick and Simple and Reliable (QUASAR) trial showed that chemotherapy has small survival benefit in patients with stage II cancer,4 while some clinical studies concluded that adjuvant chemotherapy fail to improve overall survival (OS) or disease-free survival (DFS).5–8 Also, several studies have suggested that a subset of patients with stage II colon cancer with high-risk features might achieve improved survival with adjuvant chemotherapy.9–11 Two recent studies12,13 derived from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER)-Medicare database, both indicated that adjuvant chemotherapy does not improve survival, regardless of the presence of poor prognostic features or tumor location. Guidelines of the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) recommend that chemotherapy might be considered in stage II colon cancer with high-risk prognostic characteristics, including T4 lesion, poorly differentiated tumors, lymphovascular or perineural invasion, obstruction or perforation, positive margins, and inadequate (<12) number of lymph nodes analyzed after surgery.14,15 However, the benefit of adjuvant chemotherapy is still lacking solid evidence in stage II colon cancer. Furthermore, prognostic factors do not always correctly predict the benefit of adjuvant chemotherapy.13 Several microarray-based multigene assays that can predict prognosis also fail to predict the benefit from chemotherapy.16
Previously studies have addressed the significance of covariates in identifying prognostic and predictive markers, but they all failed to consider the rationality of the endpoints such as OS or DFS. The incidence of OS or DFS is generally estimated by the Kaplan–Meier method. It is not possible to test for covariate effects on subdistribution in cause-specific hazard analysis. OS generally means the absolute risk of death, which fails to differentiate the cause of death [colon-cancer-specific death (CCSD) or noncancer specific death (non-CCSD)]. Patients with stage II colon cancer have favorable prognosis, and where long-term survival from cancer is expected, are at a greater risk of non-CCSD. That is, the OS will be diluted by other causes of death and fails to correctly interpret the real effectiveness of chemotherapy. The benefits of disease-free DFS or OS in stage II patients may not translate into real cancer-related survival benefits.
Moreover, in view of the high 5-year survival probability of patients with stage II colon cancer, the short- and long-term toxicities, adverse events, expense, and inconvenience of adjuvant chemotherapy, especially chemotherapy-related mortality, should also be incorporated into the decision-making process.
In 2016, the 21st Century Cures Act was passed by the House of Representatives, which approved using real-world evidence as an expanded evidence for clinical decisions. At the same time, the United States Food and Drug Administration (FDA) published guidance for using real-world evidence.17 Therefore, the aim of the present study was to describe the real impact of chemotherapy on cause-specific death (CCSD or non-CCSD) by using the competing-risk survival model, and to develop a convenient individual assessment model for clinicians to speculate whether these patients, whose prognosis is extremely favorable, merit further adjuvant therapy at all.
Materials and methods
Data collection
Data on colon cancer records were obtained from a total of 18 cancer registries utilizing the National Cancer Institute’s SEER Cancer database released in April 2017, based on the November 2017 submission (http://seer.cancer.gov/). We received permission to access the research data (Account Number: 14075-Nov2015) and treatment data was obtained via further application. SEER*Stat software was utilized to identify patients with Stage II colon cancer. Chemotherapy information was obtained by submitting a special data request to the SEER program. The study was approved by the ethics committee of Zhejiang University Jinhua hospital.
Specific inclusion criteria were as follows: years of diagnosis 1988–2013; patients diagnosed with stage II colon cancer; histological type ICD-O-3 limited to 8140/3, 8480/3, 8481/3 and 8490/3. Exclusion criteria were as follows: patients lacking documentation of age at diagnosis, gender, race, marital status, differentiated grade, and classification T; patients younger than 20 years old or older than 80 years old; patients with multiple primary tumors; patients who survived less than 1 month.
Statistical analyses
Age was classified into young (⩽60 years old) and old (>60 years old) groups. Race was divided into White, Black, and Other. Marital status was regrouped as married, single (never married or domestic partner), or divorce (separated, single, and divorced). Tumor location was grouped as left colon and right colon. Left colon includes rectosigmoid junction, sigmoid colon, descending colon, and splenic flexure. Right colon includes transverse colon, hepatic flexure, ascending colon, cecum, and appendix. Histological type was grouped as adenocarcinoma, mucinous adenocarcinoma, and ring signet cell cancer. All cases were regrouped according to the 7th American Joint Committee on Cancer (AJCC) TNM staging system. Number of lymph nodes (nLN) sampled was regrouped as 0, 1– 3, 4– 6, 7– 11, and ⩾12 according to X-tile program.18 The variable chemotherapy was classified as chemotherapy ‘yes’ or ‘no/unknown’ according to the SEER program.19 Year of diagnosis was divided into two periods: 1988–2004 and 2005–2013.
The distribution of chemotherapy subgroups was analyzed using Chi-squared tests. The time to CCSD, the primary endpoint, was calculated from the date of diagnosis to the date of death from cancer. The time to non-CCSD, as the second endpoint, was calculated from the date of diagnosis to the date of death of a cause other than cancer. The 5- and 10-year probability of CCSD were calculated by the Gray test.20 CCSD was the failure event, while non-CCSD was the competing event, and vice versa. The subdistribution hazard ratio (SHR) of variables for cause-specific death was estimated by the Fine and Gray proportional hazard model.21 A stacked cumulative incidence function plot was used to describe the actual prognosis of specific causes of death.22 Propensity scores were calculated for each patient using models to estimate each patient’s probability of receiving adjuvant chemotherapy conditional on the clinical characteristics most related to survival outcome.23 Pair-matching used the nearest neighbor method within the calipers of a width of 0.2 of the standard deviation of the logit of the propensity score.24,25
A competing-risk nomogram was constructed based on the results of multivariate analysis for CCSD using the package of rms and cmprsk in R software (http://www.r-project.org/). The nomogram performance was evaluated in terms of the concordance index (C-index) and calibration performance. Patients were further divided into three groups according to quartiles of predicted risk. When the two-sided p value was less than 0.05, the difference was considered statistically significant. All statistical analyses were performed using R software (version 3.5.3).
Results
Demographic and characteristics of patients
A total of 58,133 eligible patients were included in the analysis. The latest follow-up date was November 2017, and the median follow-up time was 82.0 months (range 1–335 months). Of all the patients in the study, 14,917 (25.66%) patients received chemotherapy, and 43,216 (74.34%) patients were without chemotherapy. Patients with chemotherapy were younger, more often male, more often White, to a higher percentage married, had more often left cancer, presented more often with advanced T classification, and had a poorer differentiated grade. The detailed clinicopathological characteristics of the chemotherapy subgroups are presented in Table 1.
Table 1.
The characteristics of patients in chemotherapy and nonchemotherapy subgroups.
| Risk factors | n (%) | Nonchemotherapy n (%) | Chemotherapy n (%) |
p * |
|---|---|---|---|---|
| Total | 58,133 | 43,216 (74.34) | 14,917 (25.66) | |
| Age | <0.001 | |||
| ⩽60 years | 18,885 | 11,437 (26.46) | 7448 (49.93) | |
| >60 years | 39,248 | 31,779 (73.54) | 7469 (50.07) | |
| Gender | <0.001 | |||
| Female | 28,820 | 21,647 (50.09) | 7173 (48.09) | |
| Male | 29,313 | 21,569 (49.91) | 7744 (51.91) | |
| Marital status | <0.001 | |||
| Married | 35,203 | 25,562 (59.15) | 9641 (64.63) | |
| Unmarried | 8056 | 5905 (13.66) | 2151 (14.42) | |
| Divorced | 14,874 | 11,749 (27.19) | 3125 (20.95) | |
| Race | 0.011 | |||
| White | 46,664 | 34,666 (80.22) | 11,998 (80.43) | |
| Black | 6519 | 4928 (11.40) | 1591 (10.67) | |
| Other | 4950 | 3622 (8.38) | 1328 (8.90) | |
| Location$ | <0.001 | |||
| Left colon | 27,564 | 19,255 (44.56) | 8309 (55.70) | |
| Right colon | 30,569 | 23,961 (55.44) | 6608 (44.30) | |
| Histology | <0.001 | |||
| Adenocarcinoma | 50,390 | 37,578 (86.95) | 12,812 (85.89) | |
| Mucinous adenocarcinoma | 7452 | 5444 (12.60) | 2008 (13.46) | |
| Signet ring cell carcinoma | 291 | 194 (0.45) | 97 (0.65) | |
| Differential grade | <0.001 | |||
| Grade I | 4775 | 3693 (8.55) | 1082 (7.25) | |
| Grade II | 44,445 | 33,313 (77.08) | 11,132 (74.63) | |
| Grade III | 8913 | 6210 (14.37) | 2703 (18.12) | |
| T classification‡ | <0.001 | |||
| T3 | 48,883 | 37,689 (87.21) | 11,194 (75.04) | |
| T4 | 9250 | 5527 (12.79) | 3723 (24.96) | |
| nLN | <0.001 | |||
| 0 | 1346 | 905 (2.09) | 441 (2.96) | |
| 0–3 | 1879 | 1373 (3.18) | 506 (3.39) | |
| 3–6 | 5004 | 3716 (8.60) | 1288 (8.63) | |
| 6–12 | 14,479 | 10,770 (24.92) | 3709 (24.86) | |
| ⩾12 | 35,425 | 26,452 (61.21) | 8973 (60.15) | |
| Year of diagnosis | 0.741 | |||
| 1988–2004 | 32,386 | 24,093 (55.75) | 8293 (55.59) | |
| 2005–2013 | 25,747 | 19,123 (44.25) | 6624 (44.41) |
nLN, number of lymph nodes.
p values obtained from the χ2 test. All statistical tests were two-sided.
Left colon includes rectosigmoid junction, sigmoid colon, descending colon and splenic flexure; right colon includes transverse colon, hepatic flexure, ascending colon, cecum, and appendix.
T classification according to 7th AJCC staging system.
Univariate and multivariate analysis for CCSD and non-CCSD based on competing-risk survival regression model
In total, 11,600 (19.95%) and 14,987(25.78%) patients died of CCSD and non-CCSD, respectively. Based on Gray method, the 3-, 5-, and 10-year probabilities of CCSD, were 10.20%, 15.27%, and 21.01%, respectively; and the 3-, 5-, and 10-year probabilities of non-CCSD were 6.32%, 10.23%, and 21.42%, respectively. Patients with stage II colon cancer having long-term survival are at a greater risk of non-CCSD (Figure 1). When compared with the Kaplan–Meier method, the Gray test could correct the issue of overestimation of probability of death.
Figure 1.
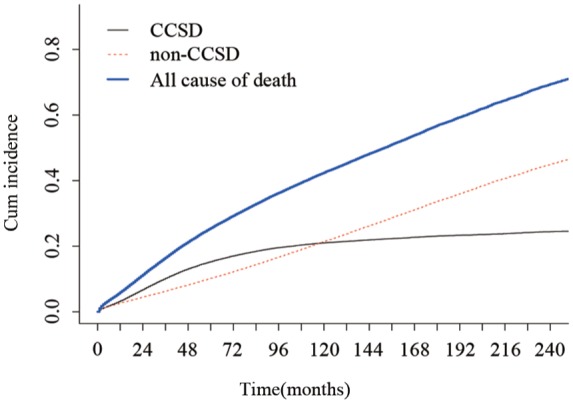
Stacked cumulative incidence plots.
For patients with stage II colon cancer, the non-CCSD and CCSD curves crossed at approximately 120 months. Non-CCSD constituted almost 50% of deaths in the later period.
CCSD, colon-cancer-specific death.
Univariate and multivariate analyses showed that chemotherapy was associated with both CCSD and non-CCSD (Table 2). Unexpectedly, receiving chemotherapy appears to be associated with more CCSD and less non-CCSD (HR = 1.23, 95% CI = 1.18– 1.28, p < 0.001, HR = 0.45, 95% CI = 0.43–0.47, p < 0.001, respectively) (Table 2). Young, Black race, single marital status, poorly differentiated grade, signet ring cell cancer, larger tumor, and less lymph node sampled were other independent factors for greater CCSD (Table 2).
Table 2.
CCSD and non-CCSD in univariate and multivariate analyses.
| Risk factors | CCSD |
Non-CCSD |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analyses |
Multivariate analyses |
Univariate analyses |
Multivariate analyses |
|||||
| SHR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | SHR (95% CI) | p | |
| Age | ||||||||
| ⩽60 | 1 | – | 1 | – | 1 | – | 1 | – |
| >60 | 1.34 (1.28– 1.39) | 0.000 | 1.38 (1.32– 1.44) | 0.000 | 4.73 (4.49– 4.98) | 0.000 | 4.08 (3.87– 4.30) | 0.000 |
| Gender | ||||||||
| Female | 1 | – | 1 | – | 1 | – | 1 | – |
| Male | 1.10 (1.06– 1.14) | 0.000 | 1.16 (1.12– 1.21) | 0.000 | 1.11 (1.08– 1.15) | 0.000 | 1.32 (1.28– 1.37) | 0.000 |
| Marital status | ||||||||
| Married | 1 | – | 1 | – | 1 | – | 1 | – |
| Unmarried | 1.29 (1.23– 1.37) | 0.000 | 1.32 (1.25– 1.39) | 0.000 | 0.98 (0.92– 1.03) | 0.322 | 1.21 (1.15– 1.27) | 0.000 |
| Divorced | 1.27 (1.22– 1.33) | 0.000 | 1.24 (1.18– 1.29) | 0.000 | 1.51 (1.46– 1.57) | 0.000 | 1.38 (1.33– 1.44) | 0.000 |
| Race | ||||||||
| White | 1 | – | 1 | – | 1 | – | 1 | – |
| Black | 1.31 (1.25– 1.39) | 0.000 | 1.35 (1.28– 1.42) | 0.000 | 0.81 (0.77– 0.86) | 0.000 | 0.90 (0.85– 0.95) | 0.000 |
| Other | 0.87 (0.81– 0.93) | 0.000 | 0.90 (0.84– 0.97) | 0.004 | 0.65 (0.61– 0.70) | 0.000 | 0.73 (0.69– 0.78) | 0.000 |
| Location$ | ||||||||
| Left colon | 1 | – | 1 | – | 1 | – | 1 | – |
| Right colon | 0.76 (0.73– 0.79) | 0.000 | 0.81 (0.78– 0.85) | 0.000 | 1.22 (1.18– 1.25) | 0.000 | 1.12 (1.09– 1.16) | 0.000 |
| Histology | ||||||||
| Adenocarcinoma | 1 | – | 1 | – | 1 | – | – | – |
| Mucinous adenocarcinoma | 0.99 (0.94– 1.05) | 0.728 | 0.99 (0.94– 1.05) | 0.849 | 0.99 (0.95– 1.05) | 0.792 | – | – |
| Signet ring cell carcinoma | 1.50 (1.19– 1.89) | 0.001 | 1.37 (1.10– 1.71) | 0.006 | 1.02 (0.80– 1.30) | 0.893 | – | – |
| Differential grade | ||||||||
| Grade I | 1 | – | 1 | – | 1 | – | – | – |
| Grade II | 1.01 (0.95– 1.08) | 0.712 | 1.08 (1.01– 1.16) | 0.023 | 0.97 (0.92– 1.03) | 0.328 | – | – |
| Grade III | 1.17 (1.09– 1.27) | 0.000 | 1.25 (1.16– 1.36) | 0.000 | 0.97 (0.91– 1.04) | 0.449 | – | – |
| T classification‡ | ||||||||
| T3 | 1 | – | 1 | – | 1 | – | 1 | – |
| T4 | 2.30 (2.21– 2.40) | 0.000 | 2.13 (2.04– 2.22) | 0.000 | 0.79 (0.76– 0.83) | 0.000 | 0.84 (0.80– 0.88) | 0.000 |
| nLN | ||||||||
| 0 | 1 | – | 1 | – | 1 | – | 1 | – |
| 0–3 | 0.60 (0.54– 0.68) | 0.000 | 0.63 (0.56– 0.71) | 0.000 | 1.30 (1.14– 1.48) | 0.000 | 1.13 (0.99– 1.29) | 0.065 |
| 3–6 | 0.54 (0.49– 0.60) | 0.000 | 0.58 (0.53– 0.64) | 0.000 | 1.33 (1.19– 1.50) | 0.000 | 1.15 (1.03– 1.30) | 0.016 |
| 6–12 | 0.41 (0.37– 0.45) | 0.000 | 0.46 (0.42– 0.51) | 0.000 | 1.27 (1.14– 1.42) | 0.000 | 1.14 (1.02– 1.27) | 0.024 |
| ⩾12 | 0.28 (0.25– 0.30) | 0.000 | 0.34 (0.31– 0.37) | 0.000 | 0.99 (0.89– 1.11) | 0.906 | 1.03 (0.92– 1.15) | 0.584 |
| Chemotherapy | ||||||||
| Nonchemotherapy | 1 | – | 1 | – | 1 | – | 1 | – |
| Chemotherapy | 1.23 (1.19– 1.28) | 0.000 | 1.19 (1.15– 1.25) | 0.000 | 0.45 (0.43– 0.47) | 0.000 | 0.59 (0.56– 0.61) | 0.000 |
| Year of diagnosis | ||||||||
| 1988–2004 | 1 | 1 | 1 | 1 | ||||
| 2005–2013 | 0.72 (0.69– 0.74) | 0.000 | 0.85 (0.81– 0.88) | 0.000 | 0.60 (0.57– 0.62) | 0.000 | 0.64 (0.61– 0.67) | 0.000 |
CCSD, colon cancer specific death; nLN, number of lymph nodes; SHR, subdistribution hazard ratio.
Left colon includes rectosigmoid junction, sigmoid colon, descending colon and splenic flexure; Right colon includes transverse colon, hepatic flexure, ascending colon, cecum, and appendix.
T classification according to 7th AJCC staging system.
Analysis of CCSD and non-CCSD after propensity score matching
To further corroborate the findings from univariate and multivariable competing-risk survival regression analyses, a propensity score matching (PSM) method was performed to eliminate the bias caused by differences in characteristics of patients with chemotherapy and without chemotherapy. During the propensity score analysis, 30,521 patients without chemotherapy were excluded from the analysis because no counterpart propensity score was identified. Finally, a total of 13,806 patients with chemotherapy were matched with 13,806 patients without chemotherapy. Supplemental Figure S1 displays the distribution of the propensity scores of the two groups prior to, and after, PSM and weighting. Supplemental Table S1 summarizes patient characteristics after propensity score weighting. The matched data demonstrate an identical distribution of all confounders. When performing a univariable and multivariable competing-risk survival regression analysis after PSM, chemotherapy persisted to be associated with more CCSD and less non-CCSD (HR = 1.16, 95% CI = 1.10– 1.22, p < 0.001 and HR = 0.60, 95% CI = 0.57–0.63, p < 0.001, respectively). The results after PSM and weighting are listed in Supplemental Table S2.
Stratified analyses according to administration of chemotherapy
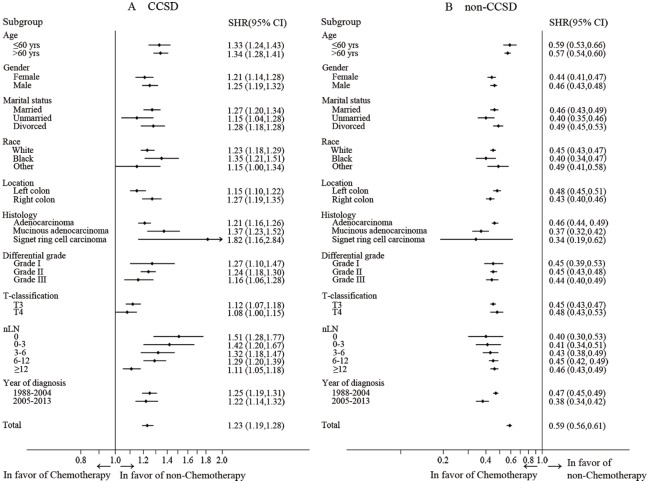
To gain a deeper understanding of the chemotherapeutic impacts on CCSD and non-CCSD, we performed further subgroup analyses based on the competing-risk survival regression model (Figure 2). Surprisingly, the forest plot clearly showed that, for all subgroups, receiving chemotherapy had more risk of CCSD, while receiving chemotherapy appeared to have a protective role on non-CCSD with fewer patients dying of non-CCSD. In different age subgroups, chemotherapy was associated with less non-CCSD.
Figure 2.
Stratified analyses according to administration of chemotherapy.
Construction and validation of a competing risk nomogram
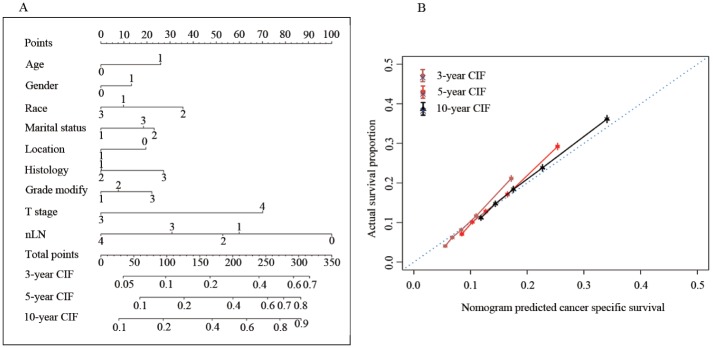
Prognostic factors (except for chemotherapy) associated with CCSD were included in the competing-risk survival regression model used to construct a nomogram (Figure 3A). Age, race, gender, marital status, tumor location, histological type, differential grade, T classification, and nLN were incorporated into the model. Beta-coefficients from the model were used for allocation of scale (Table 3). By adding up the total scales and locating them on the total points, we could easily draw a straight line to give estimates of 3-, 5-, or 10-year predicted CCSD. In the model, differentiated grade and nLN were the largest contributors to prognosis. The model demonstrated good accuracy for predicting CCSD, with a C-index of 0.661 (95% CI, 0.650–0.671). The calibration plots presented excellent agreement between the nomogram prediction and actual observations for 3-, 5-, and 10-year CCSD (Figure 3B).
Figure 3.
Nomogram model for patients with stage II colon cancer. (A) Individual patient’s values are located on each variable axis, and a line is drawn upward to determine the number of points received for each variable value. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the likelihood of 3-, 5-, or 10-year CCSD. (B) Calibration curve for predicting patient survival at 3-, 5-, and 10-years.
CCSD, colon-cancer-specific death; CIF, cumulative incidence function, nLN number of lymph nodes.
Table 3.
Point assignment and prognostic score in nomogram.
| Variables | Score | Estimated 5-year CIF (%) |
|---|---|---|
| Age | ||
| ⩽60 | 0 | |
| >60 | 26 | |
| Gender | ||
| Female | 0 | |
| Male | 13 | |
| Race | ||
| White | 10 | |
| Black | 36 | |
| Other | 0 | |
| Marital status | ||
| Married | 0 | |
| Unmarried | 23 | |
| Divorced | 18 | |
| Location$ | ||
| Left colon | 20 | |
| Right colon | 0 | |
| Histology | ||
| Adenocarcinoma | 0 | |
| Mucinous adenocarcinoma | 0 | |
| Signet ring cell carcinoma | 27 | |
| Differential grade | ||
| Grade I | 0 | |
| Grade II | 8 | |
| Grade III | 22 | |
| T classification‡ | ||
| T3 | 0 | |
| T4 | 70 | |
| nLN | ||
| 0 | 100 | |
| 0–3 | 60 | |
| 3–6 | 53 | |
| 6–12 | 31 | |
| ⩾12 | 0 | |
| Total prognostic score (5-year CIF) | ||
| 59 | 0.1 | |
| 126 | 0.2 | |
| 200 | 0.4 | |
| 253 | 0.6 | |
| 277 | 0.7 | |
| 303 | 0.8 |
CIF, cumulative incidence function; nLN, number of lymph nodes.
Left colon includes rectosigmoid junction, sigmoid colon, descending colon and splenic flexure; Right colon includes transverse colon, hepatic flexure, ascending colon, cecum, and appendix.
T classification according to 7th AJCC staging system.
CCSD–risk score to predict efficacy of chemotherapy and prognosis
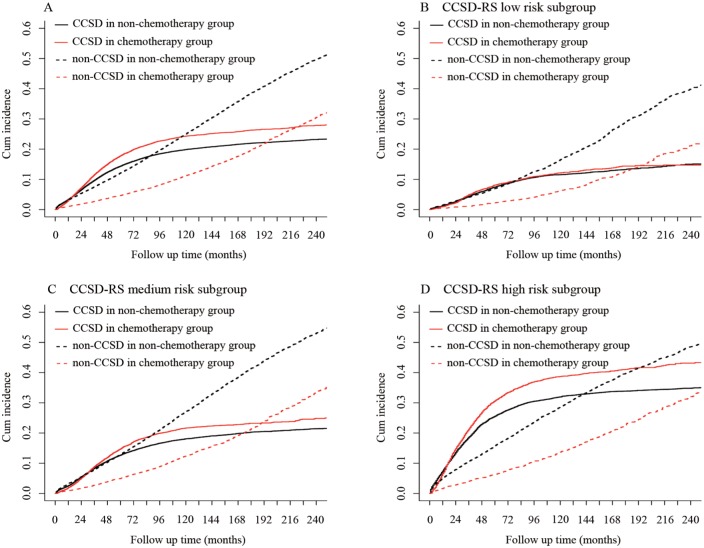
The risk score (RS) of CCSD for each case was calculated by adding the scale of all variables incorporated into nomogram. Using two cut-off values of 25% and 75% of RS, the cohort was classified into three subgroups: low CCSD–RS: 0–58, medium CCSD–RS: 59–115, and high CCSD–RS: >116. CCSD–RS acted as a strong prognostic factor to discriminate the whole cohort; the 5- and 10-year probabilities of CCSD were 7.61% and 11.71% in the low subgroup, 13.18% and 18.92% in the medium subgroup, and 27.10% and 33.97% in the high subgroup, with statistical significance (p < 0.001) (Figure 4).
Figure 4.
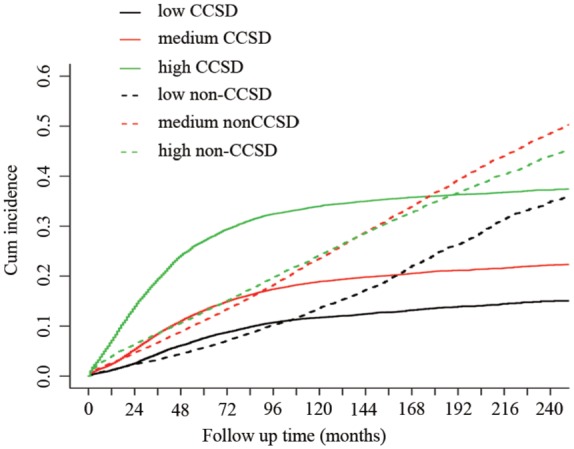
Non-CCSD and CCSD of different risk subgroups according to the Gray method.
CCSD, colon-cancer-specific death.
Further, the association between CCSD-RS and chemotherapy was also analyzed; 4488 patients (30.93%), 6531 patients (22.98%), and 3898 patients (25.64%) received chemotherapy in the high, medium, and low CCSD–RS subgroups, respectively, with significant difference (p < 0.001). To our surprise, chemotherapy increased the risk of CCSD in high CCSD–RS and medium CCSD–RS subgroups (high: HR 1.24, 95% CI 1.16–1.31, p < 0.001; medium: HR 1.18, 95% CI 1.10–1.25, p < 0.001), while, in the low RS subgroup, chemotherapy was not associate with higher CCSD (p = 0.454). (Figure 5, Supplemental Figure S4)
Figure 5.
Effects of chemotherapy on CCSD and non-CCSD in different risk subgroups.
CCSD, colon-cancer-specific death.
Discussion
In most clinical trials, OS is widely accepted as the primary end point. OS is based on absolute risk of death, which does not take into account any competing cause of death (cancer-specific death or noncancer-specific death).26 As patients with stage II colon cancer have a favorable prognosis, they are expected to survive long after their diagnosis of cancer, and, inevitably, are at a greater risk of noncancer-specific death. Based on our results, for patients with stage II colon cancer, the risk of noncancer specific death is comparable with, or even exceeds, the risk of cancer-specific death. When a competing risk model was applied, chemotherapy proved to have less value in stage II colon cancer, even in the high-risk subset.
Casadaban and colleagues analyzed data from the National Cancer Data Base,6 and further revealed that improved OS was associated with adjuvant chemotherapy, regardless of treatment regimen, patient age, or high-risk pathologic risk features. Several previous studies have also suggested that a subset of patients (T4 classification) with stage II colon cancer with high-risk features might achieve improved survival with adjuvant chemotherapy.9–11 Surprisingly, our results indicated that chemotherapy failed to have any benefits, and might even be associated with poorer cancer-specific survival outcome. Furthermore, to corroborate our findings from univariate and multivariable competing-risk survival regression analyses, PSM was performed to minimize the bias caused by differences in characteristics of patients with chemotherapy and without chemotherapy. The results before and after PSM were almost the same. In addition, we developed an individual assessment model to evaluate the real benefit of chemotherapy for high-risk patients; no survival benefit was shown in ‘high-risk’ patients. The main difference between previous studies and our research lies in the end point. As is shown visually in Figure 1, the risk of CCSD was exceeded by non-CCSD at the very beginning, making non-CCSD the main contributor to OS. If OS is chosen as the endpoint of interest, an incorrect interpretation will inevitably be made. The ‘benefit’ of chemotherapy might be a false impression. Furthermore, to further revalidate the difference, a sensitivity analysis using our data showed that chemotherapy indeed provided a modest benefit for OS (results not shown), but when cause-specific death accounting for non-CCSD was evaluated, we found that chemotherapy significantly decreased the incidence of non-CCSD but not CCSD. Therefore, we recommend choosing CCSD as the first endpoint instead of OS in further clinical trials on stage II colon cancer.
The definition of N1c (no regional lymph nodes are positive, but there are tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic, or perirectal/mesorectal tissues) was put forward in 2010 and our main data were collected prior to 2010. It is possible that Stage II patients identified in our data might be mixed up with some stage III (TXN1cM0) patients. However, this selection bias does not affect our results. Until now, the important milestones of chemotherapy benefit in a stage II setting were prospective data demonstrating OS improvement derived from the QUASAR trial in which the median number of resected lymph nodes was 6, far less than the desired 12.27 This suggested that the stage II population was likely contaminated by stage III patients. For stage III disease, significant survival benefit of chemotherapy is evident.4,28,29 Also, analysis of another stage III cohort also revealed a consistent survival benefit of CCSD (data not shown). A stage II population, if mixed with several unrecognized stage III patients, is more likely to register a positive outcome of chemotherapy.
Several previous studies of stage II disease have actually indicated that chemotherapy was ineffective for patients with high-frequency microsatellite instability,30,31 and patients without poor prognosis.13 O’Connor’s study, using the SEER-Medicare dataset, indicated that adjuvant chemotherapy did not substantially improve OS for patients older than 65 years.13 Their study did not account for the effect of non-CCSD on CCSD, and benefits of treatment may thus be overestimated, as we have illustrated above. Once CCSD and non-CCSD are taken into consideration, the real effect of adjuvant chemotherapy will be seen to be less. Furthermore, at the most recent 2019 ASCO Gastrointestinal (GI) Meeting, Galon and colleagues revealed the surprising result that patients older than 70 years old with high immunoscore who did not receive chemotherapy exhibited better survival than those who received chemotherapy (for patients with low immunoscore, there is no obvious benefit of chemotherapy either).32 That is to say, chemotherapy is detrimental in some stage II patients. To some extent, their research support the main points of our findings. Furthermore, among patients with high-risk features, there is a group of patients with low immunoscore whose prognosis is quite poor.32 Though the value of chemotherapy is quite limited in our results, we propose that chemotherapy may still benefit a certain group of patients (e.g. those with low immunoscore). Despite the presence of high-risk clinic-pathological features that usually trigger adjuvant chemotherapy, maybe it is really time to reconsider the latter’s clinical utility!
Why, then, does chemotherapy have a harmful effect on patients with stage II colon cancer. Firstly, the antitumor immune response characterized by the lymphocytic infiltrate characteristic of tumors might be abrogated by the immunosuppressive effects of chemotherapy.33 On the other hand, lymphopenia is a common side effect of many anticancer drugs, and has also been assumed to be detrimental to any potential immune response.34 Secondly, clinically, patients with chemotherapy are more prone to exhibit high-risk features that will cause a higher incidence of CCSD. Furthermore, patients not offered chemotherapy usually had some degree of frailty, postoperative complication, or pre therapy comorbidity (14.96% and 29.51% of patients died of noncancer specific death in the chemotherapy and nonchemotherapy subgroups, respectively). If one is more likely to die of non-CCSD due to frailty, then one is less likely to die from tumor-related consequences, or, for that matter, to have been followed up as rigorously to identify lower CCSD as opposed to higher non-CCSD. This phenomenon would be more obvious in a high-risk group (in the high-risk group, 17.62% and 37.05% of patients died of noncancer-specific death in the chemotherapy and nonchemotherapy subgroups, respectively). To evaluate the value of chemotherapy on high-risk patients more accurately, we developed two models to evaluate the risk of CCSD and non-CCSD, respectively. We screened out patients with high CCSD risk features and low non-CCSD risk features based on the two models. In the high CCSD/low non-CCSD risk subcohort, patients often were high risk, but without the influence of noncancer-specific death, which the analysis of chemotherapy made more accurate. The results showed that, in the high CCSD/low non-CCSD risk subgroup, the SHR of chemotherapy is 1.11 (95% CI = 0.97–1.28), without statistical significance (p = 0.129) (Supplemental Figure S5). That is, in the high-risk patients (with low risk of non-CCCSD), the value of chemotherapy is still quite limited.
Another issue is that the impact of chemotherapy on non-CCSD is still unclear. Most previous studies focused mainly on the benefit of chemotherapy, and relatively few were designed to discuss the real risks and evaluate the side-effects of chemotherapy balanced against the potential minimal improvements in OS. The short- and long-term toxicities, adverse events, expense, and inconvenience caused by adjuvant chemotherapy, especially oxaliplatin, can result in significant patient morbidity. Our non-CCSD results revealed that chemotherapy would not result in an increase in drug-related death. Conversely, before and after PSM, patients treated with chemotherapy consistently lowered the rate of non-CCSD by about 50%. This can be explained by the following biases. Patients without chemotherapy were always weaker. The effect of this frailty on the incidence of non-CCSD is higher, while suggesting that chemotherapy is in some way protective against non-CCSD. Therefore, we hypothesized that chemotherapy was spuriously associated with non-CCSD through some additional unmeasured confounder related with the patient’s state of health. Based on these vital findings, we further performed a sensitive analysis of non-CCSD in a stage III setting; the outcome showed a similar trend that chemotherapy led to less non-CCSD (data not shown).
As mentioned above, patients with stage II colon cancer might exhibit weaker signs of overall benefits than we thought, and this is an inevitable bias in a non-RCT study. However, as is well known, clinical trials always enrol patients with quite strict inclusion and exclusion criteria (in relatively well condition). How can results from patients who are well be applied to more complex patients in reality. In this sense, our analysis based on a large cohort might reflect the ‘real effect’ of controversial chemotherapy of stage II colon cancer. The results indicated that the incidence of CCSD in patients with stage II colon cancer remained higher by 20% in the higher CCSD–RS subgroup than in the low CCSD–RS subgroup. Andre and colleagues35 reported that the adverse effects (peripheral sensory neuropathy) of oxaliplatin can last as long as 4 years. As few as 1% of patients, or even less, enjoy any survival benefit of chemotherapy; it is definitely not advisable to subject 99% patients to higher non-CCSD levels or long-term side-effects of chemotherapy. Thus, for most patients, chemotherapy should be applied conservatively.
Our study is not devoid of limitations. We analyzed the effect of chemotherapy only by comparing a chemotherapy group with one without chemotherapy, without estimating the detailed role of different chemotherapy. Also, the information on chemotherapy from the SEER dataset will inevitably cause a confounding bias, with a sensitivity of 72.1%.19 Because the inclusion of patients dated from 1988 to 2013, there remains the possibility of error related to miscoding and selection biases. Important information related to stage II colon cancer, such as lymphovascular invasion, perineural invasion, surgical margin, and presence of obstruction or perforation, is not available in the SEER dataset. Information on microsatellite instability (MSI) was also not available in most institutions from 1988 to 2013.
To the best of our knowledge, this is the first study to describe real-world numbers of tumor- and nontumor-related death in stage II colon cancer as well as to evaluate the side-effects of chemotherapy. The value of chemotherapy is much lower than we thought. Coincidently, The Adjuvant Therapy (IDEA) demonstrated that a lower duration of chemotherapy for stage III colon cancer might be optional.36 From our point of view, a decreased necessity for chemotherapy for stage II colon cancer should be accepted. Maybe it is time to de-escalate chemotherapy as standard care for stage II colon cancer, applying chemotherapy more individually based on further biomarkers. Also, CCSD, rather than OS, should be considered as an appropriate primary end point for future adjuvant trials in stage II colon cancer. More prospective validation is warranted.
Supplemental Material
Supplemental material, Supplemental_figure1 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_figure2 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_figure3 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_figure5 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_Figure_4 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_Table1-PSM for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental Material
Supplemental material, Supplemental_Table2-PSM for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Acknowledgments
Authors Jianfei Fu and Lunpo Wu contributed equally.
Footnotes
Authors’ note: This work was presented as a poster discussion at the 2018 European Society for Medical Oncology (ESMO) Congress in Munich, Germany. The abstract will also be published in the Journal of Oncology.
Author contributions: Conception and design: Jinin Du, Liangjing Wang, Jianfei Fu and Lunpo Wu
Data collection analysis and interpretation: Jianfei Fu and Lunpo Wu
Methodology and Statistical review: Jianfei Fu, Lunpo Wu and Wei Fu
Manuscript writing and editing: All authors
Final approval of manuscript: All authors
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The research was supported by the National Natural Science Foundation of China (81472214, 81702308), the Natural Science Foundation of Zhejiang Province (LQ17H160010), National Key R&D Program of China (2016YFC0107003 and 2016YFC1303200), Public Welfare Technology Research Program of Zhejiang Province (LGF18H160029), Natural Science Foundation of Zhejiang province (Y19H160126) and Jinhua Science and Technology Project (2016-3-005).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Liangjing Wang  https://orcid.org/0000-0001-8227-8855
https://orcid.org/0000-0001-8227-8855
Jinlin Du  https://orcid.org/0000-0001-6117-3835
https://orcid.org/0000-0001-6117-3835
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jianfei Fu, Department of Medical Oncology, Jinhua Hospital, Zhejiang University School of Medicine, China.
Lunpo Wu, Department of Gastroenterology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China; Institute of Gastroenterology, Zhejiang University, Hangzhou, China.
Chenyang Ge, Department of Colorectal Surgery, Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, China.
Tiantian Xu, Institute of Translational Medicine, School of Medicine, Zhejiang University, Hangzhou, China.
Dan Li, Department of Medical Oncology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China; Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, Chinese National Ministry of Education; Key Laboratory of Molecular Biology in Medical Sciences, Zhejiang Province, China), Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China.
Wei Fu, Division of Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Liangjing Wang, Department of Gastroenterology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China; Institute of Gastroenterology, Zhejiang University, Hangzhou, China.
Jinlin Du, Department of Colorectal Surgery, Zhejiang University Jinhua Hospital, 351 Mingyue Road, Zhejiang Province, 321000, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Moertel CG, Fleming TR, Macdonald JSet al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990; 322: 352–358. [DOI] [PubMed] [Google Scholar]
- 3. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International multicentre pooled analysis of colon cancer trials (IMPACT) investigators. Lancet 1995; 345: 939–944. [PubMed] [Google Scholar]
- 4. Quasar Collaborative Group, Gray R, Barnwell Jet al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007; 370: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 5. Tournigand C, Andre T, Bonnetain Fet al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol 2012; 30: 3353–3360. [DOI] [PubMed] [Google Scholar]
- 6. Casadaban L, Rauscher G, Aklilu Met al. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer 2016; 122: 3277–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Booth CM, Nanji S, Wei Xet al. Adjuvant chemotherapy for stage II colon cancer: practice patterns and effectiveness in the general population. Clin Oncol (R Coll Radiol) 2017; 29: e29–e38. [DOI] [PubMed] [Google Scholar]
- 8. Yothers G, O’Connell MJ, Allegra CJet al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29: 3768–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verhoeff SR, van Erning FN, Lemmens VEet al. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer 2016; 139: 187–193. [DOI] [PubMed] [Google Scholar]
- 10. Kumar A, Kennecke HF, Renouf DJet al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer 2015; 121: 527–534. [DOI] [PubMed] [Google Scholar]
- 11. Babcock BD, Aljehani MA, Jabo Bet al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol 2018; 25: 1980–1985. [DOI] [PubMed] [Google Scholar]
- 12. Schrag D, Rifas-Shiman S, Saltz Let al. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J Clin Oncol 2002; 20: 3999–4005. [DOI] [PubMed] [Google Scholar]
- 13. O’Connor ES, Greenblatt DY, LoConte NKet al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011; 29: 3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benson AB, III, Schrag D, Somerfield MRet al. American society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004; 22: 3408–3419. [DOI] [PubMed] [Google Scholar]
- 15. Labianca R, Nordlinger B, Beretta GDet al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24(Suppl. 6): vi64–vi72. [DOI] [PubMed] [Google Scholar]
- 16. Hutchins G, Southward K, Handley Ket al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol 2011; 29: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 17. Sherman RE, Anderson SA, Dal Pan GJet al. Real-world evidence – what is it and what can it tell us? N Engl J Med 2016; 375: 2293–2297. [DOI] [PubMed] [Google Scholar]
- 18. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004; 10: 7252–7259. [DOI] [PubMed] [Google Scholar]
- 19. Noone AM, Lund JL, Mariotto Aet al. Comparison of SEER treatment data with medicare claims. Med Care 2016; 54: e55–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howlader N, Mariotto AB, Woloshin Set al. Providing clinicians and patients with actual prognosis: cancer in the context of competing causes of death. J Natl Cancer Inst Monogr 2014; 2014: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 22. Hinchliffe SR, Lambert PC. Flexible parametric modelling of cause-specific hazards to estimate cumulative incidence functions. BMC Med Res Methodol 2013; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brookhart MA, Schneeweiss S, Rothman KJet al. Variable selection for propensity score models. Am J Epidemiol 2006; 163: 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making 2009; 29: 661–677. [DOI] [PubMed] [Google Scholar]
- 25. Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J 2009; 51: 171–184. [DOI] [PubMed] [Google Scholar]
- 26. Fu J, Wu L, Jiang Met al. Real-world impact of non-breast cancer-specific death on overall survival in resectable breast cancer. Cancer 2017; 123: 2432–2443. [DOI] [PubMed] [Google Scholar]
- 27. Le Voyer TE, Sigurdson ER, Hanlon ALet al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003; 21: 2912–2919. [DOI] [PubMed] [Google Scholar]
- 28. Andre T, de Gramont A, Vernerey Det al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015; 33: 4176–4187. [DOI] [PubMed] [Google Scholar]
- 29. Shah MA, Renfro LA, Allegra CJet al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol 2016; 34: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ribic CM, Sargent DJ, Moore MJet al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sargent DJ, Marsoni S, Monges Get al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010; 28: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pages F, Mlecnik B, Marliot Fet al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018; 391: 2128–2139. [DOI] [PubMed] [Google Scholar]
- 33. Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res 2008; 14: 1413–1417. [DOI] [PubMed] [Google Scholar]
- 34. Ogino S, Galon J, Fuchs CSet al. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol 2011; 8: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andre T, Boni C, Navarro Met al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–3116. [DOI] [PubMed] [Google Scholar]
- 36. Grothey A, Sobrero AF, Shields AFet al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018; 378: 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_figure1 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_figure2 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_figure3 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_figure5 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_Figure_4 for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_Table1-PSM for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology
Supplemental material, Supplemental_Table2-PSM for De-escalating chemotherapy for stage II colon cancer? by Jianfei Fu, Lunpo Wu, Chenyang Ge, Tiantian Xu, Dan Li, Wei Fu, Liangjing Wang and Jinlin Du in Therapeutic Advances in Gastroenterology