Abstract
The plasma kynurenine to tryptophan ([Kyn]/[Trp]) ratio is frequently used to express or reflect the activity of the extrahepatic Trp-degrading enzyme indoleamine 2,3-dioxygenase (IDO). This ratio is increasingly used instead of measurement of IDO activity, which is often low or undetectable in immune and other cells under basal conditions, but is greatly enhanced after immune activation. The use of this ratio is valid in in vitro studies, eg, in cell cultures or isolated organs, but its ‘blanket’ use in in vivo situations is not, because of modulating factors, such as supply of nutrients; the presence of multiple cell types; complex structural and functional tissue arrangements; the extracellular matrix; and hormonal, cytokine, and paracrine interactions. Determinants other than IDO may therefore be involved in vivo. These are hepatic tryptophan 2,3-dioxygenase (TDO) activity and the flux of plasma-free Trp down the Kyn pathway. In addition, conditions leading to accumulation of Kyn, eg, inhibition of activities of Kyn monooxygenase and kynureninase, could lead to elevation of the aforementioned ratio. In this review, the origin of use of this ratio will be discussed, variations in extent of its elevation will be described, evidence against its indiscriminate use will be presented, and examining determinants other than IDO activity and their correlates will be proposed for future studies.
Keywords: Albumin; flux of tryptophan down the kynurenine pathway; indoleamine 2,3-dioxygenase; non-esterified fatty acids; plasma-free tryptophan; tryptophan 2,3-dioxygenase
Introduction
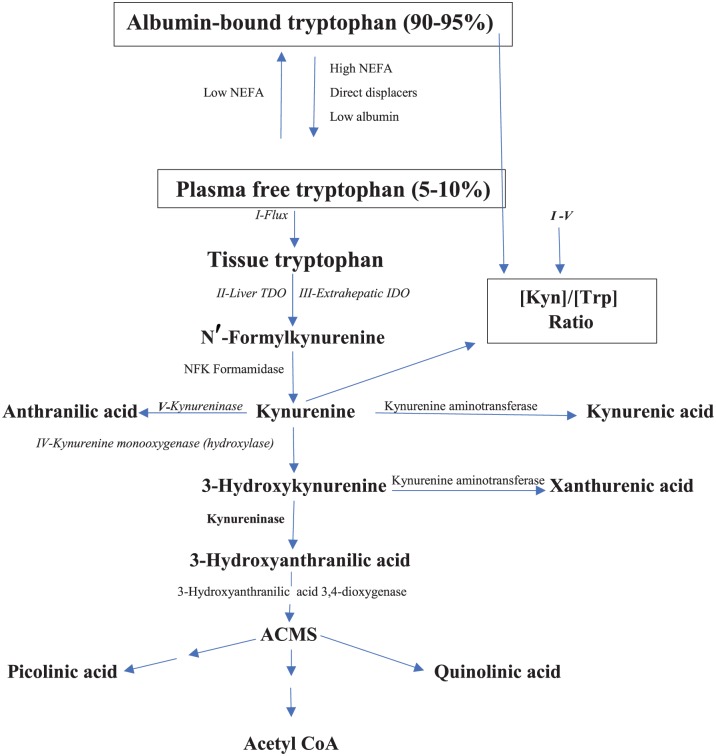
The kynurenine (Kyn) pathway (KP) (Figure 1) is the major route of degradation of the essential amino acid l-tryptophan (Trp), accounting for ~95% of the dietary Trp disposal. Functional and regulatory aspects of this pathway have recently been reviewed.1 The liver is the major site of the KP, being responsible for ~90% of overall Trp degradation under normal physiological conditions. The extrahepatic KP, by contrast, contributes very little (<2%) to Trp degradation under normal conditions, but assumes a greater quantitative significance after immune activation. The KP is controlled by the first enzyme: tryptophan 2,3-dioxygenase (TDO, formerly Trp pyrrolase; EC 1.13.11.11) in liver and indoleamine 2,3-dioxygenase (IDO: EC 1.13.11.17) elsewhere. Liver TDO activity is regulated by a number of mechanisms: induction of synthesis de novo by glucocorticoids, activation and stabilisation by the Trp substrate, activation by the heme cofactor, and end product inhibition by NAD(P)H.1 By contrast, IDO is induced mainly by interferon-γ (IFN-γ) and certain other cytokines and inhibited by NO and excess Trp.1 The discovery of IDO and its induction by IFN-γ2 heralded a new era in immunologic research centred on Trp metabolism and in particular IDO (for an extensive review, see the study by Yeung et al3). The KP now occupies a central position in studies of immune function and disease.
Figure 1.
The Kyn pathway of Trp degradation. The 5 determinants of the [Kyn]/[Trp] ratio(Flux, TDO, IDO, kynureninase, and KMO) are in italics and numbered in Latin numerals. ACMS indicates 2-amino-3-carboxymuconic acid-6-semialdehyde or acroleyl aminofumarate; IDO, indoleamine 2,3-dioxygenase; KMO, kynurenine monooxygenase; Kyn, kynurenine; NEFA, non-esterified fatty acids; TDO, tryptophan 2,3-dioxygenase; Trp, tryptophan.
We would like to discuss in this review an aspect of IDO research that requires critical appraisal, namely that of the somewhat indiscriminate use of the ratio in plasma or serum of concentration of Kyn to that of Trp, ie the [Kyn]/[Trp] ratio, to express IDO activity. In the following text, the origin of use of this ratio will be discussed, variations in extent of its elevation will be described, evidence against its indiscriminate use will be presented, and examining factors other than IDO activity which determine it will be proposed for future studies.
Origin of the Use of the Plasma [Kyn]/[Trp] Ratio to Express IDO
In demonstrating IDO induction by IFN-γ, Yasui et al2 observed a decrease in [Trp] and an increase in [Kyn] in human lung slices. Similar changes were subsequently observed in culture media after IFN-γ induction of IDO in human peripheral blood monocytes4 and a number of human cell lines.5 In these in vitro studies, the ‘closed’ system allows the monitoring of Kyn and Trp levels in the isolated tissue or culture media and thus justifies the valid use of the [Kyn]/[Trp] ratio to express or at least reflect changes in IDO activity. A wide array of published studies in cell cultures too numerous to be listed here have established beyond doubt the role of IDO in controlling the [Kyn]/[Trp] ratio in these systems. A similar situation is observed after cortisol induction of TDO in the isolated perfused rat liver.6 Here, a ~50% decrease in perfusion medium [Trp] is accompanied by a ~4-fold increase in [Kyn], with a consequent dramatic increase in the [Kyn]/[Trp] ratio. Although monitoring this ratio in culture media and other isolated systems is free from interference from or participation of ‘external’ modulating factors, this is not the case in the in vivo situation, wherein supply of nutrients; the presence of multiple cell types; complex structural and functional tissue arrangements; the extracellular matrix; and hormonal, cytokine, and paracrine interactions are some such modulators.7 Thus, extrapolation from cell culture to the whole organism is not justified without adequate control of the prevailing in vivo conditions.
Extrapolation was adopted by many researchers, because either they did not have the means of direct measurement of IDO activity in immune cells or such activity is generally hardly detectable under basal conditions. Other authors resorted to measuring the IDO gene expression as a second best, but increased mRNA expression is not synonymous with increased enzyme functional activity. A case in point is that of the ability of 1-methyl tryptophan to prevent the increase in the [Kyn]/[Trp] ratio, but not the increased gene expression of IDO in Bacillus Calmette-Guérin (BCG)-treated mice.8 A situation has thus arisen wherein never in the history of biochemistry has so much been concluded about changes in an enzyme activity without having had it measured directly in suitable sources. That IDO activity is hardly detectable under normal conditions has been amply demonstrated in a variety of cells, eg, human monocytic THP-1 cells, a range of mouse cell lines, and rat glial cells.9-12 However, there is no reason why it could not be detectable when measured in immune cells after immune activation in test subjects in comparison with the low or undetectable activity in similar cells from controls.
Does the Plasma [Kyn]/[Trp] Ratio Always Reflect IDO Activity?
There is evidence that this is not always the case. In an extensive review of data on Kyn metabolites, Chen and Guillemin13 reported that, of 15 studies reporting the ratio in patients with inflammatory disease and cancer, the ratio was elevated in 11, decreased in 2, and unaltered in 2. An increase in the ratio in alcohol-dependent subjects, 8 weeks after withdrawal, does not correlate with neopterin,14 a marker of IDO induction, as IFN-γ also induces the key enzyme of neopterin synthesis, GTP cyclohydrolase I. In a study15 of patients with haemodialysis from 2 separate centres, the significant increase in the plasma [Kyn]/[Trp] ratio caused by a significant increase in [Kyn] and a significant decrease in [Trp], does not correlate with IDO activity measured directly in peripheral blood mononuclear cells (PBMCs). That the increase in the ratio in this study was independent of IDO activity is further suggested by the failure of these PBMCs to metabolise Trp to Kyn.15 A more likely explanation of the changes in this ratio and its 2 components is enhancement of hepatic TDO activity, which has been demonstrated in rat models of renal failure by 2 independent groups.16,17 By contrast, IDO activity directly measured in 5 tissues16 including lung17 is not altered in these 2 models. Thus, liver TDO activity is another determinant of the ratio. In a recent study of cirrhotic patients with acute-on-chronic liver failure (ACLF) and with kidney failure (ACLF-KF) or without kidney failure,18 in those with infection, in whom IDO induction should be expected, neither plasma [Kyn] nor the [Kyn]/[Trp] ratio is increased. As will be discussed subsequently, liver cirrhosis is associated with TDO inhibition, which may counteract an IDO-induced Trp depletion and thus explain the normal ratio.
Extent of Elevation of the [Kyn]/[Trp] Ratio
The data in Table 1 show the extent of the increase in the ratio and its components in 10 published studies.16,19-27 An increase in the ratio could be due to an increase in [Kyn], a decrease in [Trp], or both. The data in Table 1 give examples of all 3 possibilities. In most of the studies listed, a significant increase in [Kyn] is apparent. However, in many published studies unquoted here, the increase in the ratio is due solely to a decrease in [Trp]. However, the absence of a simultaneous increase in plasma [Kyn] after IDO or TDO induction does not rule out the involvement of these enzymes, because of the nature of Kyn disposition. Thus, Kyn is rapidly cleared by the kidney, where it is transaminated to kynurenic acid (KA) or oxidised to 3-hydroxykynurenine (3-HK) and is mainly excreted in urine as xanthurenic acid (XA).28,29 In line with these features, KA and XA concentrations and kynureninase (KYNU) activity are highest in (rat) kidney,16 thus further reflecting the important role of the kidney in Kyn metabolism and disposition. An increase in plasma [Kyn] can occur when Trp oxidation exceeds renal handling, eg, after robust induction of TDO or IDO or loading with Trp or Kyn. Measurement of plasma Kyn metabolites could be more informative. At plasma levels below 10 µM, Kyn is 100% reabsorbed by kidneys.28 As such levels are reached after IDO induction, it is most likely that the absence of observable Kyn elevation in some studies is due to this renal handling. Another potential explanation of the absence of elevation of plasma [Kyn] in some instances is that of upregulation of kynurenine monooxygenase (KMO), which occurs in inflammatory conditions, which can exert the opposite effect to that of IDO induction (see below).
Table 1.
Extent of elevation of the plasma [Kyn]/[Trp] ratio: some published data.
| Reference | % ↓ in [Trp] | % ↑ in [Kyn] | % ↑ in [Kyn]/[Trp] | Condition | |
|---|---|---|---|---|---|
| 19 | 8 | 15 | 24* | Breast cancer | |
| 20 | 9* | 27* | 39* | Renal failure | |
| 21 | 13 | 18 | 38* | Fatigue in lung cancer | |
| 22 | 16* | −5 | 26* | Colorectal cancer | |
| 23 | 11* | 29* | 45* | AIDS: asymptomatic | |
| 23 | 41* | 101* | 243* | AIDS | |
| 23 | 14* | 111* | 128* | AIDS: PN/ARD | |
| 24 | 0-34? | 13-114? | 67-150? | Various cancers | |
| 25 | 51* | 137* | 292* | Sepsis | |
| 26 | 62* | 4 | 55* | Pregnancy | |
| 27 | 59* | 168* | 560* | Chronic kidney disease (stage 5) | |
| 16 | 55* | 75* | 289* | Chronic renal failure (rats CRF3) |
Abbreviations: Kyn, kynurenine; PN/ARD, peripheral neuritis with AIDS-related dementia; Trp, tryptophan.
Differences are expressed as % decreases below or increases above control values. The asterisk (*) denotes a significant difference from control values and the question mark (?) denotes absence of statistical assessment. The references are detailed in the list of references.
Occasionally, as in the study by Lyon et al,19 the significant increase in the ratio is a statistical product of non-significant changes in [Trp] and [Kyn] and is therefore unlikely to have a metabolic basis or clinical relevance.
The greatest increases in the [Kyn]/[Trp] ratio appear to occur in AIDS,23 cancer,24 sepsis,25 and kidney disease.16,27 In general and in the absence of confounders or other determinants, glucocorticoid induction of TDO or IFN-γ induction of IDO in vivo results in decreases in plasma total (free + albumin-bound) [Trp] not exceeding 30%.30 Any greater decrease must therefore involve other factors. As shown in Figure 1, plasma Trp exists largely bound to albumin, with only 5% to 10% being free. The physiological binder of Trp is albumin, whereas the physiological displacers of albumin-bound Trp are nonesterified fatty acids (NEFA). Trp binding can therefore be increased or decreased. It is increased if [NEFA] are decreased by antilipolytic agents such as insulin or nicotinic acid. By contrast, Trp binding is decreased: (a) by direct displacement of albumin-bound Trp by drugs such as salicylate; (b) if [NEFA] are increased, eg, by stimulation of lipolysis by catecholamines, agents releasing catecholamines, such as ethanol, and by phosphodiesterase inhibitors such as the methylxanthines; (c) if [albumin] is decreased, eg, in pregnancy, liver, and kidney diseases and inflammatory conditions. It has been estimated that a decrease in circulating [albumin] of 19% or more can cause a significant release of albumin-bound Trp.31 Plasma-free Trp is therefore a labile parameter that can be influenced by many factors and also by food intake (for details see the study by Badawy32). Equilibration between the free and bound forms is, however, rapid, such that a strong and sustained displacement from albumin-binding sites, coupled with increased tissue uptake, results in depletion of bound or total [Trp]. The extent of this depletion will, however, depend on whether liver TDO activity is simultaneously altered. If TDO activity is enhanced, both the Trp depletion and Kyn elevation will be stronger, whereas a TDO inhibition will exert the opposite effects. Data from the following studies illustrate the aforementioned points. In rats treated 1 h earlier with a 50 mg/kg intraperitoneal dose of sodium salicylate, serum-free [Trp] is increased by 25% and total [Trp] is decreased by 52%.33 The increases in rat plasma [NEFA] of 129% to 300% induced by noradrenaline or 3,4-dihydroxyphenylalanine (l-Dopa) are associated with a 109% to 144% increase in free [Trp] and a 24% to 38% decrease in total [Trp].34 In normal human volunteers, subcutaneous injection of adrenaline induces also a large increase in free [Trp] and a considerable decrease in total [Trp].35 Similarly, the 255% increase in rat serum [NEFA] observed at 2 h after administration of ethanol (acting here by releasing catecholamines) is associated with a 231% increase in free [Trp] and a 45% decrease in total [Trp].36 In late pregnancy, decreases in plasma total [Trp] of 25% to 50% and increases in free [Trp] of up to 132% have been reported (for reference, see the study by Badawy37). These changes are most likely caused by the widely reported decrease in serum [albumin] and increase in [NEFA], which occur in late pregnancy.37 It is noteworthy that these changes in albumin and NEFA do occur in immune diseases and must therefore be considered in studies involving measurement of the [Kyn]/[Trp] ratio.
Decreased [albumin] is also an important feature of diseases of the liver, where synthesis of this major plasma protein takes place.38 In patients with fulminant hepatic failure, free [Trp] is increased by 425%.39 A similar increase (409%) occurs in patients with liver cirrhosis with oesophageal varices.40 Because the TDO activity is inhibited in liver diseases,41 plasma total [Trp] was not decreased in these 2 studies,39,40 but underwent gradual increases with the severity of the liver dysfunction.40 Patients with advanced renal disease also have a decreased [albumin] and/or an increased [NEFA] and exhibit a 43% decrease in total [Trp] and a 38% increase in free [Trp].42 In another study of patients with renal failure, plasma total [Trp] is decreased by 25% to 40% in those with normal [albumin] and by 60% in those with hypoalbuminemia.43 Stronger changes in renal failure in humans and rats have been reported in studies16,27 listed in Table 1. As stated earlier, renal failure is associated with liver TDO enhancement,16,17 whereas IDO activity is not impaired.16,17 In addition to TDO enhancement, kidney failure is associated with changes in other Kyn pathway enzymes, notably enhancement of kynurenine aminotransferase (KAT) and inhibition of KYNU and ACMSD.16,17 These changes explain the recently reported18 greater accumulation of Kyn, KA, and quinolinic acid in plasma of patients with ACLF-KF than in those without kidney failure (ACLF), which the authors attributed to further stimulation of Trp oxidation in kidney failure.
Large increases in plasma-free [Trp] and in the [Kyn]/[Trp] ratio are also observed in patients with cancer (for reference, see the study by Badawy44). [NEFA] is also elevated in patients with cancer and most also exhibit a low [albumin].45 In AIDS, low [albumin] predicts survival45,46 and is associated with HIV progression,45 which may explain the progressive increases in [Kyn] and the [Kyn]/[Trp] ratio reported in the study by Huengsberg et al23 in Table 1. [NEFA] is also increased in patients with HIV,47,48 especially if malnutrition is also present.49,50
There are several mechanisms of the decreases in circulating albumin in the aforementioned conditions. In pregnancy, haemodilution (oedema) is responsible, whereas in liver diseases impaired hepatic albumin synthesis is the cause. In AIDS, cancer, and other inflammatory conditions, decreased albumin may result from elevation of the cytokines interleukin (IL)-6, tumour necrosis factor alpha (TNF-α), and IL-1β.50,51 As IDO is likely to be induced under these conditions, the extent of elevation of the [Kyn]/[Trp] ratio is likely to be the combined influence of IDO induction and albumin depletion as a function of level of release of proinflammatory cytokines.
Determinants of the Plasma [Kyn]/[Trp] Ratio
From the above discussion, IDO and TDO activities can determine the plasma [Kyn]/[Trp] ratio by influencing either or both components of the ratio. A potential group of third determinants are KP enzymes that directly influence Kyn levels. These are KMO (also known as kynurenine hydroxylase), KYNU, and to a partial extent KAT. Upregulation of KMO and KYNU can lead to a decrease in circulating and tissue [Kyn] and thus lower the [Kyn]/[Trp] ratio, whereas their downregulation or inhibition can have the opposite effect. With KAT, the situation is less certain, because its catalytic activity on upregulation is determined by an increase in Kyn availability through other mechanisms, eg, TDO/IDO induction or downregulation of KMO or KYNU. Downregulation of KAT is unlikely to influence the [Kyn]/[Trp] ratio. However, little information is currently available to ascertain the role of KAT in the aforementioned ratio changes.
The content of Table 2 is an outline of the changes in plasma or tissue Trp and Kyn metabolites expected with up- or downregulation (or inhibition) of TDO, IDO, and the aforementioned 3 KP enzymes. Measurements of Kyn metabolites in conjunction with the [Kyn]/[Trp] ratio could provide confirmatory evidence of potential changes in the status of KP enzymes. In almost all but 2 of the following study examples, the [Kyn]/[Trp] ratio was not assessed, but can be deduced indirectly from available information.
Table 2.
Expected changes in plasma tryptophan and kynurenine metabolites after up- or downregulation (inhibition) of kynurenine pathway enzymes.
| Enzyme | Trp | Kyn | [Kyn]/[Trp] | KA | AA | 3-HK | XA | 3-HAA | QA |
|---|---|---|---|---|---|---|---|---|---|
| TDO↑ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| IDO↑ | ↓? | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| ↓ | − | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| KMO↑ | − | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ |
| ↓ | − | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ | ↓ | ↓ |
| KAT↑ | − | (↑) | ↑ | ↑ | ↑ | − | ↑ | − | − |
| ↓ | − | ? | ? | ↓ | ? | ? | ↓ | ? | ? |
| KYNU↑ | − | − | − | − | ↑ | − | − | ↑ | ↑ |
| ↓ | − | ↑ | ↑ | ↑? | ↓ | ↑ | ↑ | ↓ | ↓ |
Abbreviations: AA, anthranilic acid; 3-HAA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; KA, kynurenic acid; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; KMO, kynurenine monooxygenase; KYNU, kynureninase; QA, quinolinic acid; TDO, tryptophan 2,3-dioxygenase; Trp, tryptophan; XA, xanthurenic acid.
Targeted deletion of the KMO gene in mice increases [Kyn] by 4.4-fold in liver and 15.6-fold in plasma and elevates the [Kyn]/[Trp] ratio in liver by 5.4-fold.52 KMO inhibition by Ro-61-8048 increases mouse brain [KA] by 7.8-fold,53 suggesting Kyn elevation as the cause, and KMO inhibition by m-nitrobenzoylalanine elevates both [Kyn] and [KA] in brain, liver, blood, and kidney.54 The downregulation of KMO in schizophrenia55 may explain the increased production of KA from Kyn, although upregulation of TDO may also contribute to the Kyn elevation. The increase in plasma [Kyn] in schizophrenia is reflected in increases in the [Kyn]/[Trp] ratio and [KA], whereas the concomitant decrease in [3-HK] implies KMO downregulation.56 Confirmation of downregulation or inhibition of KMO requires demonstration of no decrease in plasma [Trp], a decrease in [3-HK] and subsequent metabolites, and elevation of [Kyn], [KA], and [AA]. Kynurenine monooxygenase is upregulated in many inflammatory conditions,57 mainly by the major proinflammatory cytokine IFN-γ.58 Although under these conditions, [Kyn] is expected to fall, IDO and possibly also TDO induction may compensate, thus resulting in a potentially normal [Kyn].
The less studied KYNU has been shown to be upregulated in chronic inflammatory skin disease and many human autoimmune and auto inflammatory diseases are associated with increased KYNU expression.59 This is likely to result in elevation of AA, 3-HAA, and QA levels. Downregulation or inhibition of KYNU, by contrast, should decrease levels of these 3 metabolites and elevate those of Kyn, 3-HK, and possibly also their transamination products KA and XA. Christensen et al60 reported a massive increase in urinary Kyn, 3-HK, and XA in a young Somali boy and his brother due to a mutation of a gene encoding KYNU.
With KAT, upregulation can enhance its activity only if adequate amounts of the Kyn substrate are available (due to its high Km). Here, an elevation of the [Kyn]/[Trp] ratio cannot be attributed to changes in KAT. A potential effect of downregulation of KAT on the ratio is unlikely (see below).
In reality, modulation of more than one of the aforementioned enzymes occurs simultaneously in disease states and even with the use of inhibitors in experimental settings, which makes it difficult to attribute the change in the ratio to any particular determinant. For example, in schizophrenia, upregulation is not limited to KAT, but extends to TDO, whereas KMO is downregulated.55,56 An example of involvement of multiple determinants is provided by the results of experiments in rats with Trp, benserazide (BSZ), and carbidopa (CBD).61 The mean [Kyn]/[Trp] ratios in Figure 2 have been calculated at 0 and 3 h after intraperitoneal administration of Trp (50 mg/kg), BSZ (100 mg/kg), and CBD (50 mg/kg). The 3-h time point was chosen because it was the time at which liver Kyn elevation was maximal. The aforementioned dose of Trp does not activate TDO in rats,62 but its disposition reflects increased flux of Trp down the KP. Apart from their inhibition of aromatic l-amino acid decarboxylase, BSZ and CBD are both inhibitors of KYNU and KAT activities. In vitro, their KYNU inhibition is comparable at concentrations of up to 100 µM, above which CBD becomes a stronger inhibitor.63 With KAT, CBD is the stronger inhibitor at all concentrations (10-500 µM).61 Both BSZ and CBD appear to enhance TDO activity, as suggested by the significant decreases in liver [Trp] and concomitant increases in liver [Kyn], with the decreases in [Trp] being comparable.61 The ratio changes in Figure 2 therefore demonstrate the effects of increased Trp flux by Trp and the combined effects of TDO enhancement and inhibition of KAT and KYNU by BSZ and CBD. Trp administration elevates the [Kyn]/[Trp] ratio maximally at 3 h by 3.03-fold, compared with the zero-time control value. The effects of flux in humans will be considered below. The increase in the ratio by CBD (2.97-fold) was comparable with that by Trp, whereas that by BSZ (8.77-fold) was greater. Although it is difficult to assign any particular mechanism to the ratio elevation, only the following conclusions can be made: (a) the flux of Trp down the KP is as important as modulation of [Kyn] by means of enzymatic changes; (b) the greater inhibition of KAT by CBD does not lead to a greater increase in the ratio, thus supporting the notion that KAT inhibition does not exert a significant effect on [Kyn].
Figure 2.
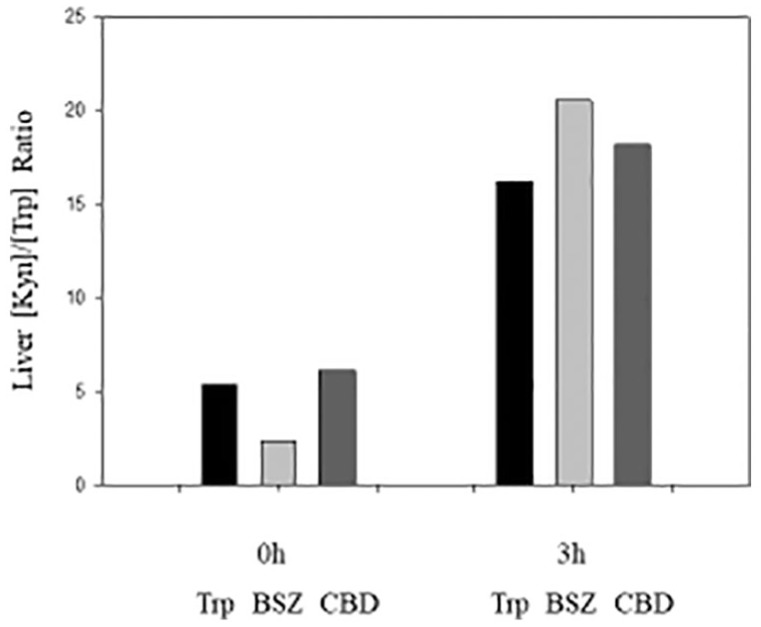
The rat liver [Kyn]/[Trp] ratio after acute administration of Trp, benserazide (BSZ), and carbidopa (CBD). Ratios were determined at zero-time and at 3 h after intraperitoneal administration to male Wistar rats of Trp (50 mg/kg body wt), BSZ (100 mg/kg), or CBD (50 mg/kg). Ratios were calculated from the Kyn and Trp values reported previously in the study by Badawy and Bano.61 Kyn indicates kynurenine; Trp, tryptophan.
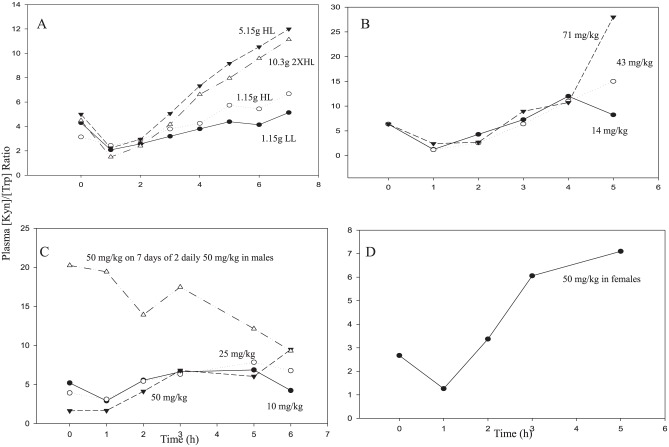
A potential fourth determinant is the flux of Trp through TDO and down the KP, which is enhanced by albumin depletion and/or NEFA elevation. Studies in rat hepatocytes suggest that the flux of Trp down the KP primarily involves plasma-free Trp.64 The increase in [Kyn]/[Trp] ratio following administration of a 50 mg/kg dose of Trp to rats illustrated in Figure 2 the importance of flux in determining this ratio. Flux has not so far been assessed in humans, but can be studied under conditions of acute tryptophan loading (ATL). Several ATL studies have been published65-69 in which plasma [Kyn] and [Trp] were measured over several hours after loading, from which the [Kyn]/[Trp] ratio can be calculated. It should be emphasised that ATL in these studies induced elevations of plasma [Trp] greater than 100 µM, ranging between 106 µM with a 1.15 g dose of Trp (~16 mg/kg) and 410 µM (oral) or 1310 µM (intravenous) with a 5 g dose (~71 mg/kg). As IDO activity is inhibited by [Trp] of >50 µM,1 it is almost certain that the Kyn formed after ATL (or conditions leading to high plasma Trp levels) is produced by the flux of Trp through TDO. Except with small doses of Trp, the increases in [Kyn] after larger doses varied between 10- and 20-fold, in contrast to the relatively modest increases shown in Table 1. The question of whether the [Kyn]/[Trp] ratio is elevated by the simple flux of Trp or requires net activation of TDO can be explored from the data in Figure 3. In the 5 studies examined,65-69 Trp was given intravenously in 167 and orally in the other 4. In 3 studies,67-69 Trp was given alone, whereas in the other 2 studies,65,66 it was given in an amino acid mixture. At the concentrations used, the only amino acid in this mixture that exerts effects on TDO is Leu. It enhances the flux of Trp through TDO (see the study by Badawy et al65 for a discussion). As shown in Figure 3A, Leu was present at low (LL) or high (HL) concentrations in the mixture containing the small (1.15 g) dose of Trp, at HL in the mixtures containing 5.15 g of Trp and at double the HL with the 10.3 g dose of Trp.
Figure 3.
The kynurenine to tryptophan ([Kyn]/[Trp]) ratio after acute tryptophan loading: previous studies. (A) Trp was administered orally in an amino acid mixture at 3 dose levels. The leucine content was either low (LL) or high (HL). Details are in the studies by Badawy and colleagues.65,66 (B) Trp was administered intravenously in doses of 1, 3, or 5 g (doses were approximated as mg/kg for a 70 kg adult). Details are in the study by Heuther et al.67 (C) Trp was administered orally to normal male subjects in doses of 10, 25, and 50 mg/kg and in a 50 mg/kg dose to subjects previously treated with twice daily 50 mg/kg doses for 7 days. Details are in the study by Green et al.68 (D) Trp was administered orally to normal female subjects in a 50 mg/kg dose. Details are in the study by Green et al.69
As shown in Figure 3, an initial decrease in the [Kyn]/[Trp] ratio occurs, which is of no significance, as it simply reflects the influx of the Trp load into the circulation. In Figure 3A, the small Trp dose (1.15 g or ~16 mg/kg) with low leucine does not increase the ratio, except only moderately (by 19%) at 7 h. By contrast, the mixture containing the same Trp dose, but with a larger Leu dose (HL) increases the ratio by 21% to 113% from 3 h onwards. This is consistent with Leu enhancing the flux of Trp through TDO by activating the enzyme.65 Of the 2 large Trp doses, the 5.15 g dose (~ 74 mg/kg) appears to induce maximum activation of TDO and the maximum increases in the [Kyn]/[Trp] ratio (by 47%-140% from 4 h onwards). Doubling this Trp dose and also that of Leu does not cause a further increase in the ratio (48%-148% at 4-7 h). The absence of an additive effect of Trp and Leu suggests that both act by the same mechanism, namely TDO enhancement. In the other 3 studies, the oral 10 mg/kg Trp dose exerts only a minimal effect on the ratio (Figure 3 C), whereas a 14 mg/kg intravenous dose appears to increase the ratio at 4-5 h (Figure 3B). The ratio is further increased by Trp doses of ⩾25 mg/kg (Figure 3A to D). Based on the observation68 that the plasma Trp clearance rate is decreased with increasing dose of Trp, which suggests the saturable nature of Trp metabolism, it was suggested that doses of Trp below 50 mg/kg do not cause a strong TDO activation. In rats, a 50 mg/kg dose of Trp also does not activate TDO, but protects it against degradation.62 This stabilisation with a consequent increase in TDO catalytically active protein may explain the large increase in the [Kyn]/[Trp] ratio (Figure 3C) in subjects previously treated daily for 7 days with 2 50 mg/kg doses of Trp. Taken together, it is likely that, in humans, a 25-50 mg/kg Trp dose may activate TDO only moderately, although the plasma Trp elevation by the 25 mg/kg dose is large enough (from a baseline value of 49 µM to 98-279 µM)68 to inhibit IDO activity. Thus, the flux of Trp down the KP most likely involves activation of TDO, rather than simple passage down the pathway.
Other Potential Determinants: Microbial Trp Breakdown and Plasma Protein Binding of Kyn
The gut flora plays an important role in Trp synthesis and degradation, with the latter producing tryptamine and the less familiar metabolites skatole, indole, and its various indole derivatives.70 The question of how much gut Trp synthesis and degradation contribute to circulating [Trp] in host is difficult to ascertain in humans, but can be addressed in animal models, namely germ-free mice and those treated with antibiotics. In germ-free mice, plasma [Trp] is ~50%71 to 70%72 higher than in conventional mice. These rises are limited to male mice. Plasma [Trp] is already higher in conventional female mice.71 The [Kyn]/[Trp] ratio is, however, lower in both male and female germ-free mice, compared with conventional controls.71 In fasting normal human subjects, however, women (n = 60) have a 15% lower plasma total [Trp], a 31% higher plasma free [Trp], and a 16% higher [Kyn]/[Trp] ratio, in comparison with men (n = 54).66 It is still possible that normal variations in plasma [Trp] can be explained in part by gut flora Trp-metabolising activity in addition to dietary factors.
Kynurenine undergoes albumin binding, with an association constant of 2.8 × 105(M−1).73 In rat serum, 27% of a tracer dose of Kyn is bound to proteins,74 whereas a greater binding (65%) was reported after administration of a 16.2 µmol/kg dose (3.37 mg/kg) of l-Kyn.75 In another study in rats, Kyn (5 mg/kg intraperitoneally) was reported to bind to albumin to a similar extent as does Trp.76 In pregnant women, 20% of plasma Kyn is albumin-bound, compared with 19% of Trp.77 As an increase in plasma [Kyn] is associated with increased cerebral uptake, it is unlikely that Kyn binding will present a significant barrier to Kyn entry into the brain (or peripheral organs). At large concentrations (5 µmol/mL), Kyn has been reported to displace Trp from albumin-binding sites.78 However, such a high concentration is unlikely to be reached even after Trp loading or under pathological conditions. For example, a large oral loading dose of Trp (10.3 g) increases plasma [Trp] from a zero-time value of 61 µM maximally to 410 µM at 4 h, whereas [Kyn] rises to only 27 µM.66 In rats, plasma [Kyn] is increased from 2.5 to 9.6 µM at 3 h after administration of the KYNU inhibitor m-nitrobenzoylalanine,54 whereas targeted KMO gene deletion in mice raises plasma [Kyn] from 0.5 to ~8 µM.52 In normal human subjects, a plasma [Kyn] of ~ 2 µM is unlikely to compete successfully with a circulating [Trp] of ~60 µM.
General Conclusions, Comments, and Recommendations
From the above discussion, it is reasonable to conclude that IDO induction is not the only determinant of the increase in the plasma [Kyn]/[Trp] ratio in vivo. Other determinants are liver TDO activity, increased flux of Trp through TDO, and KP enzymes influencing [Kyn], especially KMO, KYNU, and to a lesser extent KAT. Thus, in the absence of direct proof of induction of IDO activity, it is necessary to establish the potential role(s) of these other determinants and explore the contributions of various factors influencing them. This approach is likely to help establish clearly the underlying mechanism(s). Adopting the following recommendations may help achieve this clarity.
Indoleamine 2,3-dioxygenase induction. This requires primarily demonstration of increased enzyme catalytic activity in suitable cells, eg PBMC. If this is not possible, supporting evidence should be sought in an increase in plasma neopterin and proinflammatory cytokines, especially IFN-γ, IL-6, and/or TNF-α, a decrease in plasma [Trp] and an increase in [Kyn] at least at high IDO induction levels. Tryptophan 2,3-dioxygenase induction, increased flux of free Trp and inhibition of KMO and KYNU should be excluded using the criteria described below.
Tryptophan 2,3-dioxygenase induction. This requires demonstration of decreased plasma [Trp] and increased plasma [Kyn], [cortisol], and/or flux of free Trp. In addition, induction of TDO and possibly also IDO should lead to decreased plasma-free [Trp] and there may be a case for using the plasma [Kyn]/[free Trp] ratio, which appears to be more informative regarding Trp oxidation.65
Increased flux of plasma-free Trp down the KP. This requires demonstration of increased plasma-free [Trp] and [Kyn] and increased [NEFA] and/or decreased [albumin], with total [Trp] preferably decreased.
Kynurenine monooxygenase. KMO inhibition has not been encountered in disease states, but, if suspected, it will require demonstration of increased plasma [Kyn], [KA], and [AA], decreased plasma [3-HK], [3-HAA] and [QA] and absence of a decrease in plasma [Trp]. Upregulation of KMO, however, will, among others (Table 2), lower the ratio.
Kynureninase inhibition. This will increase the ratio, lower [AA], [3-HAA], and [QA] and possibly increase the transamination products KA and XA.
It is hoped that measurements of these additional experimental parameters will contribute to a clearer understanding of the Trp status in immune studies and in relation to IDO involvement in immune and related diseases, and improve and strengthen the diagnostic value of the [Kyn]/[Trp] ratio.
Acknowledgments
AA-BB held an honorary professorship at Cardiff Metropolitan University, Wales, UK during the 10-year period September 2006-September 2016. Prof Guillemin is supported by the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC) and Macquarie University.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AA-BB wrote the first draft. Both authors revised it and approved the final version.
ORCID iDs: Abdulla A-B Badawy  https://orcid.org/0000-0002-8743-2804
https://orcid.org/0000-0002-8743-2804
Gilles Guillemin  https://orcid.org/0000-0001-8105-4470
https://orcid.org/0000-0001-8105-4470
References
- 1. Badawy AA-B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yasui H, Takai K, Yoshida R, Hayaishi O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: its possible occurrence in cancer patients. Proc Natl Acad Sci USA. 1986;83:6622-6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond). 2015;129:601-672. [DOI] [PubMed] [Google Scholar]
- 4. Ozaki Y, Edelstein MP, Duch DS. The actions of interferon and antiinflammatory agents on induction of indoleamine 2,3-dioxygenase in human peripheral blood monocytes. Biochem Biophys Res Commun. 1987;144:1147-1153. [DOI] [PubMed] [Google Scholar]
- 5. Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-γ action: characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-γ and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041-2048. [PubMed] [Google Scholar]
- 6. Green AR, Woods HF, Joseph MH. Tryptophan metabolism in the isolated perfused liver of the rat: effects of tryptophan concentration, hydrocortisone and allopurinol on tryptophan pyrrolase activity and kynurenine formation. Br J Pharmacol. 1976;57:103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Badawy AA-B, Namboodiri AMA, Moffett JR. The end of the road for the tryptophan depletion concept in pregnancy and infection. Clin Sci (Lond). 2016;130:1327-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Connor JC, Lawson MA, André Cet al. Induction of IDO by Bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edelstein MP, Ozaki Y, Duch DS. Synergistic effects of phorbol ester and IFN-γ on the induction of indoleamine 2,3-dioxygenase in THP-1 monocytic leukemia cells. J Immunol. 1989;143:2969-2973. [PubMed] [Google Scholar]
- 10. Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Neopterin formation and tryptophan degradation by a human myelomonocytic cell line (THP-1) upon cytokine treatment. Cancer Res. 1990;50:2863-2867. [PubMed] [Google Scholar]
- 11. Habara-Ohkubo A, Takikawa O, Yoshida R. Cloning and expression of a cDNA encoding mouse indoleamine 2,3-dioxygenase. Gene. 1991;105:221-227. [DOI] [PubMed] [Google Scholar]
- 12. Saito K, Chen CY, Masana M, Crowley JS, Markey SP, Heyes MP. 4-Cloro-3- hydroxyanthranilate, 6-chlorotryptophan and norharmane attenuate quinolinic acid formation by interferon-stimulated monocytes (THP-1 cells). Biochem J. 1993;291:11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res. 2009;2:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gleissenthall GV, Geisler S, Malik Pet al. Tryptophan metabolism in post-withdrawal alcohol-dependent patients. Alcohol Alcohol. 2014;49:251-255. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Xie Z, Xiao Cet al. Peripheral kynurenine/tryptophan ratio is not a reliable marker of systemic indoleamine 2,3-dioxygenase: a lesson drawn from patients on hemodialysis. Oncotarget. 2017;8:25261-25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. 2003;54:175-189. [PubMed] [Google Scholar]
- 17. Saito K, Fugigaki S, Heyes MPet al. Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol. 2000;279:F565-F572. [DOI] [PubMed] [Google Scholar]
- 18. Clària J, Moreau R, Fenaille Fet al. Orchestration of tryptophan-kynurenine pathway, acute decompensation and acute-on-chronic liver failure in cirrhosis. J Hepatol. 2018;69:1686-1701. [DOI] [PubMed] [Google Scholar]
- 19. Lyon DE, Walter JM, Starkweather AR, Schubert CM, McCain NL. Tryptophan degradation in women with breast cancer: a pilot study. BMC Res Notes. 2011;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao J. Plasma kynurenic acid/tryptophan ratio: a sensitive and reliable biomarker for the assessment of renal function. Ren Fail. 2013;35:648-653. [DOI] [PubMed] [Google Scholar]
- 21. Kurz K, Fiegl M, Holzner Bet al. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE. 2012;7:e36956. doi: 10.1371/journal.pone.0036956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang A, Fuchs D, Widner B, Glover C, Henderson DC, Allen-Mersh TG. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br J Cancer. 2002;86:1691-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998;44:858-862. [PubMed] [Google Scholar]
- 24. Liu X, Shin N, Koblish HKet al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520-3530. [DOI] [PubMed] [Google Scholar]
- 25. Darcy CJ, Davis JS, Woodberry Tet al. An observational cohort study of the kynurenine to tryptophan ratio in sepsis: association with impaired immune and microvascular function. PLoS ONE. 2011;6:e21185. doi: 10.1371/journal.pone.0021185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188:719-726. [DOI] [PubMed] [Google Scholar]
- 27. Debnath S, Velagapudi C, Redus Let al. Tryptophan metabolism in patients With chronic kidney disease secondary to type 2 diabetes: relationship to inflammatory markers. Int J Tryptophan Res. 2017;10:1178646917694600. doi: 10.1177/1178646917694600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Møller SE. Pharmacokinetics of tryptophan, renal handling of kynurenine and the effect of nicotinamide on its appearance in plasma and urine following L-tryptophan loading of healthy subjects. Eur J Clin Pharmacol. 1981;21:137-142. [DOI] [PubMed] [Google Scholar]
- 29. Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids. 2011;41:1173-1183. [DOI] [PubMed] [Google Scholar]
- 30. Badawy AA-B. Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J Psychopharmacol. 2013;27:878-893. [DOI] [PubMed] [Google Scholar]
- 31. Badawy AA-B. Effects of pregnancy on tryptophan metabolism and disposition in the rat. Biochem J. 1988;255:369-372. [PMC free article] [PubMed] [Google Scholar]
- 32. Badawy AA-B. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809-815. [DOI] [PubMed] [Google Scholar]
- 33. Gessa GL, Tagliamonte A. Possible role of free serum tryptophan in the control of brain tryptophan level and serotonin synthesis. Adv Biochem Psychopharmacol. 1974;11:119-131. [PubMed] [Google Scholar]
- 34. Curzon G, Knott PJ. Effects on plasma and brain tryptophan in the rat of drugs and hormones that influence the concentration of unesterified fatty acids in the plasma. Br J Pharmacol. 1974;50:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gentil V, Lader MH, Kantamaneni BD, Curzon G. Effects of adrenaline injection on Human plasma tryptophan and non-esterified fatty acids. Clin Sci Mol Med. 1977;53:227-232. [DOI] [PubMed] [Google Scholar]
- 36. Badawy AA-B, Evans M. The effects of ethanol on tryptophan pyrrolase activity and their comparison with those of phenobarbitone and morphine. Adv Exp Med Biol. 1975;59:229-251. [DOI] [PubMed] [Google Scholar]
- 37. Badawy AA-B. Tryptophan metabolism, dispositions and utilisation in pregnancy. Biosci Rep. 2015;35:e00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sherlock S. Chapter 2. Biochemical assessment of liver function. In: Sherlock S, ed. Diseases of the Liver and Biliary System. 6th ed Oxford: Blackwell;1981:14-27. [Google Scholar]
- 39. Record CO, Buxton B, Chase RA, Curzon G, Murray-Lyon IM, Williams R. Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Eur J Clin Invest. 1976;6:387-394. [DOI] [PubMed] [Google Scholar]
- 40. Hijikata Y, Hara K, Shiozaki Y, Murata K, Sameshima Y. Determination of free tryptophan in plasma and its clinical applications. J Clin Chem Clin Biochem. 1984;22:291-299. [DOI] [PubMed] [Google Scholar]
- 41. Rössle M, Herz R, Klein B, Gerok W. Tryptophan-metabolismus bei lebererkrankungen: eine pharmakokinetische und enzymatische untersuchung. Klin Wochenschr. 1986;64:590-594. [DOI] [PubMed] [Google Scholar]
- 42. Sullivan PA, Murnaghan D, Callaghan N, Kantamaneni BD, Curzon G. Effect of dialysis on plasma and CSF tryptophan and CSF 5-hydroxyindoleacetic acid in advanced renal disease. J Neurol Neurosurg Psychiatry. 1980;43:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walser M, Hill SB. Free and protein-bound tryptophan in serum of untreated patients with chronic renal failure. Kidney Int. 1993;44:1366-1371. [DOI] [PubMed] [Google Scholar]
- 44. Badawy AA-B. Targeting tryptophan availability to tumors: the answer to immune escape? Immunol Cell Biol. 2018;96:1026-1034. [DOI] [PubMed] [Google Scholar]
- 45. Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14-21. [DOI] [PubMed] [Google Scholar]
- 46. Ronit A, Sharma S, Baker JVet al. Serum albumin as a prognostic marker for serious non-AIDS endpoints in the Strategic Timing of Antiretroviral Treatment (START) Study. J Infect Dis. 2018;217:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lindegaard B, Frøsig C, Petersen AMet al. Inhibition of lipolysis stimulates peripheral glucose uptake but has no effect on endogenous glucose production in HIV lipodystrophy. Diabetes. 2007;56:2070-2077. [DOI] [PubMed] [Google Scholar]
- 48. Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr. 2003;77:490-494. [DOI] [PubMed] [Google Scholar]
- 49. Mody A, Bartz S, Hornik CPet al. Effects of HIV infection on the metabolic and hormonal status of children with severe acute malnutrition. PLoS ONE. 2014;9:e102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Castell JV, Gómez-Lechón MJ, David Met al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237-239. [DOI] [PubMed] [Google Scholar]
- 51. Ramadori G, Van Damme J, Rieder Het al. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988;18:1259-1264. [DOI] [PubMed] [Google Scholar]
- 52. Giorgini F, Huang S-Y, Sathyasaikumar KVet al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554-36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clark CJ, Mackay GM, Smythe GA, Bustamante S, Stone TW, Phillips RS. Prolonged survival of a murine model of cerebral malaria by kynurenine pathway inhibition. Infect Immun. 2005;73:5249-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176-1183. [DOI] [PubMed] [Google Scholar]
- 55. Wonodi I, Stine OC, Sathyasaikumar KVet al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665-674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Peripheral kynurenine-3- monooxygenase deficiency as a potential risk factor for metabolic syndrome in schizophrenia patients [published online ahead of print May 10, 2017]. Integr Clin Med. doi: 10.15761/ICM.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parrott JM, O’Connor JC. Kynurenine 3-monooxygenase: an influential mediator of neuropathology. Front Psych. 2015;6:116. doi: 10.3389/fpsyt.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. doi:10.3389/ fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harden JL, Lewis SM, Lish SR, Suárez-Fariñas M, Gareau D, Lentini T. The tryptophan metabolism enzyme L-kynureninase is a novel inflammatory factor in psoriasis and other inflammatory diseases. J Allergy Clin Immunol. 2016;137:1830-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Christensen M, Duno M, Lund AM, Skovby F, Christensen E. Xanthurenic aciduria due to a mutation in KYNU encoding kynureninase. J Inherit Metab Dis. 2007;30:248-255. [DOI] [PubMed] [Google Scholar]
- 61. Badawy AA-B, Bano S. Tryptophan metabolism in rat liver after administration of tryptophan, kynurenine metabolites, and kynureninase inhibitors. Int J Tryptophan Res. 2016;9:51-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Badawy AA-B, Evans M. Regulation of rat liver tryptophan pyrrolase by its cofactor haem: experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem J. 1975;150:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Badawy AA-B, Bano S, Steptoe A. Tryptophan in alcoholism treatment II: inhibition of the rat liver mitochondrial low Km aldehyde dehydrogenase activity, elevation of blood acetaldehyde concentration and induction of aversion to alcohol by combined administration of tryptophan and benserazide. Alcohol Alcohol. 2011;46:661-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith SA, Pogson CI. The metabolism of L-tryptophan by isolated rat liver cells: effect of albumin binding and amino acid competition on oxidation of tryptophan by tryptophan 2,3-dioxygenase. Biochem J. 1980;186:977-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Badawy AA-B, Lake SL, Dougherty DM. Mechanisms of the pellagragenic effect of leucine: stimulation of hepatic tryptophan oxidation by administration of branched-Chain amino acids to healthy human volunteers and the role of plasma free tryptophan and total kynurenines. Int J Tryptophan Res. 2014;7:23-32. doi: 10.4137/IJTR.S18231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Badawy AA-B, Dougherty DM. Assessment of the human kynurenine pathway: comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int J Tryptophan Res. 2016;9:31-49. doi: 10.4137/IJTR.S38189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heuther G, Hajak G, Reimer Aet al. The metabolic fate of infused L-tryptophan in men: possible clinical implications of the accumulation of circulating tryptophan and tryptophan metabolites. Psychopharmacology (Berl). 1992;109:422-432. [DOI] [PubMed] [Google Scholar]
- 68. Green AR, Aronson JK, Curzon G, Woods HF. Metabolism of an oral tryptophan load I: effects of dose and pretreatment with tryptophan. Br J Clin Pharmacol. 1980;10:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Green AR, Bloomfield MR, Woods HF, Seed M. Metabolism of an oral tryptophan load by women and evidence against the induction of tryptophan pyrrolase by oral contraceptives. Br J Clin Pharmacol. 1978;5:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ. Microorganisms, tryptophan metabolism, and kynurenine pathway: a complex interconnected loop influencing human health status. Int J Tryptophan Res. 2019;12:1178646919852996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clarke G, Grenham S, Scully Pet al. Themicrobiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666. [DOI] [PubMed] [Google Scholar]
- 72. Wikoff WR, Anfora AT, Liu Jet al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Churchich JE. L-Kynurenine: a fluorescent probe of serum albumins. Biochim Biophys Acta. 1972;285:91-98. [DOI] [PubMed] [Google Scholar]
- 74. Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007-2017. [DOI] [PubMed] [Google Scholar]
- 75. Fukushima T, Sone Y, Mitsuhashi S, Tomiya M, Toyo’oka T. Alteration of kynurenic acid concentration in rat plasma following optically pure kynurenine administration: a comparative study between enantiomers. Chirality. 2009;21:468-472. [DOI] [PubMed] [Google Scholar]
- 76. Joseph MH, Kadam BV. Kynurenine: penetration to the brain, effect on brain tryptophan and 5-hydroxytryptamine metabolism and binding to plasma albumin. Br J Pharmacol. 1979;66:483P-484P. [PMC free article] [PubMed] [Google Scholar]
- 77. Morita I, Kawamoto M, Yoshida H. Difference in the concentration of tryptophan metabolites between maternal and umbilical foetal blood. J Chromatogr. 1992;576:334-339. [DOI] [PubMed] [Google Scholar]
- 78. Cangiano C, Cardelli P, Peverini Pet al. Effect of kynurenine on tryptophan-albumin binding in human plasma. Adv Exp Med Biol. 1999;467:279-282. [DOI] [PubMed] [Google Scholar]