Abstract
Background:
With ageing world populations, multimorbidity (presence of two or more chronic diseases in the same individual) becomes a major concern in public health. Although multimorbidity is associated with age, its prevalence varies. This systematic review aimed to summarise and meta-analyse the prevalence of multimorbidity in high, low- and middle-income countries (HICs and LMICs).
Methods:
Studies were identified by searching electronic databases (Medline, Embase, PsycINFO, Global Health, Web of Science and Cochrane Library). The term ‘multimorbidity’ and its various spellings were used, alongside ‘prevalence’ or ‘epidemiology’. Quality assessment employed the Newcastle-Ottawa scale. Overall and stratified analyses according to multimorbidity operational definitions, HICs/LMICs status, gender and age were performed. A random-effects model for meta-analysis was used.
Results:
Seventy community-based studies (conducted in 18 HICs and 31 LMICs) were included in the final sample. Sample sizes ranged from 264 to 162,464. The overall pooled prevalence of multimorbidity was 33.1% (95% confidence interval (CI): 30.0–36.3%). There was a considerable difference in the pooled estimates between HICs and LMICs, with prevalence being 37.9% (95% CI: 32.5–43.4%) and 29.7% (26.4–33.0%), respectively. Heterogeneity across studies was high for both overall and stratified analyses (I 2 > 99%). A sensitivity analysis showed that none of the reviewed studies skewed the overall pooled estimates.
Conclusion:
A large proportion of the global population, especially those aged 65+, is affected by multimorbidity. To allow accurate estimations of disease burden, and effective disease management and resources distribution, a standardised operationalisation of multimorbidity is needed.
Keywords: Multimorbidity, prevalence, HICs, LMICs
Introduction
As the world’s populations are ageing rapidly, multimorbidity is becoming a major concern in public health. According to a recent report by the Academy of Medical Science,1 in most high-income countries (HICs), multimorbidity is considered the norm, not the exception. Multimorbidity also appears to be increasingly prevalent in low- and middle-income countries (LMICs).1 Patients experiencing multiple chronic conditions often have poorer health outcomes, such as declined physical and mental health functioning,2 higher mortality rates3 and frailty.4 Their needs for medical care are also different. Instead of a highly specialised but isolated approach, as used for single disease treatment, multimorbidity patients need a complex and structured care plan.1,5 This has serious impact on disease management, healthcare utilisation and costs.6–8 To assess the impact of multimorbidity on public health and to project medical care needs for patients with multimorbidity, an accurate estimation of its prevalence is critical. Yet, the complex nature of multimorbidity poses great difficulties for research into this topic area. This is due partly to inconsistencies in the conceptualisation and definition of multimorbidity. For instance, despite being distinct clinical entities, multimorbidity and comorbidity are still used interchangeably. While the former is defined as the co-occurrence of two or more chronic diseases, the latter is perceived as ‘the occurrence of medical conditions additional to an index disease’.9 The ambiguity in the conceptualisation of multimorbidity leads to a lack of a consensus about its operationalisation. The number of diseases used as cut-off points, disease combination and measure of multimorbidity vary across studies.
Although multimorbidity prevalence and its variations have been examined and summarised in a number of systematic reviews,10,11 these reviews usually only included studies in HICs. Only one review synthesised evidence on the prevalence and outcomes of multimorbidity in South Asia.12 Nonetheless, there has not been a review that systematically assessed the variations of multimorbidity prevalence estimates at a global level. Our aims were therefore to (1) summarise the available evidence in the literature on the global prevalence of multimorbidity in the context of community settings, (2) carry out a meta-analysis of the prevalence estimates to provide a pooled estimate and (3) assess how multimorbidity was operationalised across the different studies to examine whether this factor could explain the heterogeneity of the prevalence estimates.
Methods
We conducted a systematic review and meta-analysis (registered on PROSPERO Ref no. CRD42018087435), which followed the PRISMA statement for systematic review and analysis13 (Online Supplement 1).
Inclusion and exclusion criteria
Eligible studies were original, peer-reviewed articles (published either online or as hard copy, with available abstracts in English). Opinion pieces, conference presentations, books, letters, editorials, dissertations/theses or abstracts were not included. Studies with an index disease (e.g. multimorbidity among HIV-infected individuals) were also not eligible for inclusion, because they were deemed comorbidity studies. Only studies that clearly stated that their participants were community-based adults were considered. In other words, studies that recruited participants from communal establishments, such as hospitals, hospices, nursing homes or prisons, were ineligible. Those that used solely medical records from general practice as data source were also excluded to avoid selection bias. The study designs were restricted to cross-sectional and longitudinal studies. Where the design was longitudinal, only prevalence at baseline was included. Case-control and interventional studies (such as randomised controlled trials) were removed from consideration. There were no further restrictions regarding demographic characteristics of the population under study, for instance, age, sex, or socioeconomic status. Since the outcome of interest was the prevalence of multimorbidity, only studies that reported on this were selected.
Search strategy and study selection
We conducted an online literature search on Medline (Ovid interface), Embase (Ovid interface), PsycINFO (Ovid interface), Global Health (Ovid interface), Web of Science and Cochrane Library electronic databases, from inception up to May 2019. The term ‘multimorbidity’ and its various spellings (e.g. ‘multi-morbidity’, ‘multimorbidities’, ‘multi-morbidities’, ‘multi morbidity’, ‘multi morbidities’, ‘multiple morbidities’, ‘multiple-morbidities’) and ‘prevalence’ or ‘epidemiology’ were used (Online Supplement 2). We were interested in how multimorbidity was defined so deliberately excluded ‘comorbidity’ and other synonyms in our search strategy.
The titles and abstracts of all hits returned by the search were screened initially by the first reviewer (HN). The second reviewer (CD) tested a 10% random sample of all references to ensure that eligible studies were not missed out. Studies that satisfied all the eligibility criteria specified above were kept for full-text screening. The full-text screening was done independently by two reviewers (HN and GM). Where there were disagreements, HN and GM discussed to resolve them. AMP was consulted when agreement could not be reached. Disagreements were finally resolved by consensus.
Data extraction and quality assessment
We extracted all potentially eligible studies to EndNote (EndNote X8, Thomson Reuters). Duplicate articles were removed using EndNote X8 auto-deduplication function. Those that were not detected by this function were removed manually during the first screening.
A data extraction sheet was developed, pilot-tested on five randomly selected eligible studies and refined accordingly. We extracted the following information: year of study, study design, country of study, data source, sample size, mean age (men/women), definition of multimorbidity, measure of multimorbidity, prevalence of multimorbidity, number of diseases, ascertainment of diseases and combination of diseases. In case this information could not be retrieved from the included studies, the corresponding authors of such studies were contacted. Six authors were contacted and three provided the requested information.
We adopted the age-related adjustment method developed by Fortin et al11 to enable comparisons of age-specific prevalence across studies. If prevalence was reported for an age range, we calculated mean age between the lower and upper limits to present that range.11 If prevalence was reported for an age range with an upper or lower limit only, we adjusted the age to 10 years above the lower limit and 10 years below the upper limit.11 Exception of this rule was when the prevalence was reported for age groups such as 65+ or 75+, we used this prevalence without making any age adjustments.
To assess the risk of bias for individual studies, the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies was used. NOS uses eight items, categorised into three domains of potential bias, namely selection (representativeness of the sample, sample size, non-respondents, ascertainment of the exposure), comparability (the subjects in different outcome groups are comparable, based on the study design or analysis; and confounding factors are controlled) and outcome (assessment of outcome and statistical test).14 A study can be given a maximum of one star for each item within the selection and outcome categories. A maximum of two stars can be given for comparability. Thresholds for converting the NOS to Agency for Healthcare Research and Quality standards (good, fair and poor)15 are as follows:
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Poor quality: 0 or 1 star in selection domain OR 0 star in comparability domain OR 0 or 1 star in outcome/exposure domain.
Eligible studies after full-text screening were assessed (Online Supplement 3). Both good and poor quality studies were retained for sensitivity analysis at a later stage.
Data analysis
Overall and stratified analyses according to multimorbidity operational definitions (2+ and 3+ diseases cut-off points) and HIC–LMIC status were performed. HICs and LMICs were determined using the World Bank classification list of economies.16 Where possible, prevalence of multimorbidity was also stratified by age and gender. Studies that reported multimorbidity prevalence, both standardised and non-standardised, using the 2+ chronic diseases cut-off point as definition, were included in the meta-analysis. To perform the meta-analysis, we used the metaprop command.17 Multimorbidity prevalence was calculated as the quotient of the number of people with multimorbidity (numerator) and sample size (denominator). Where not available, the numerator was converted from the percentage of people with multimorbidity. Using absolute numbers to generate prevalence estimates enabled the calculation of standard error. Heterogeneity across studies was evaluated using I 2 statistic.18 It was expected that the I 2 statistic would be high, due to the heterogeneous operationalisation of multimorbidity. Hence, a random-effects model was used. Finally, a sensitivity analysis was carried out to test the influence of a single study in meta-analysis estimation of the pooled prevalence. All quantitative synthesis of this review was done in STATA version 15.19
Results
Overview of studies
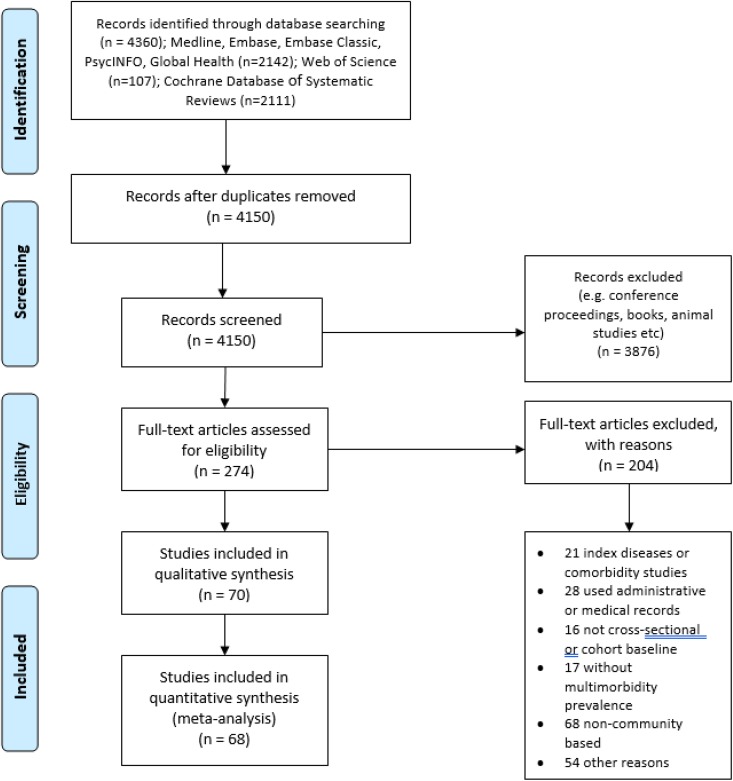
We identified 4360 studies from the initial search. After removing duplicates and records that were not original articles, there were 274 studies eligible for full-text screening. Two hundred four studies were further excluded after full-text screening, leaving 70 for final qualitative and quantitative synthesis. The PRISMA flow diagram in Figure 1 shows the exact process of studies selection.
Figure 1.
PRISMA flow diagram of studies selection.
Study quality and characteristics
Based on the NOS data quality assessment system, 63 studies were rated good quality (with a score of 6 or 7), 2 studies were rated fair quality (with a score of 5) and 5 studies were rated poor quality (with a score of 2 or 4). Those deemed poor quality were so because they omitted information about the representativeness of their data, sampling strategy or response rates (see Online Supplement 3). The total number of participants across 70 studies was 1,180,111 (men: 47.5%, women: 52.5%), with sample size ranging from 264 to 162,464. The mean age varied between 36 years and 75 years old. Thirty-seven studies were conducted in HICs (5 in Australia,20–24 4 in Canada,25–28 4 in Spain,29–32 3 in Germany,33–35 3 in Portugal,36–38 3 in the United Kingdom,39–41 3 in Hong Kong,42–44 2 in Singapore,45,46 2 in the United States47,48 and 1 each in Cyprus,49 Czech Republic,29 Denmark,50 Estonia,29 Finland,30 France,51 Hungary,29 Latvia,29 Poland,30 Ireland,52 South Korea,53 the Netherlands,54 Sweden55 and Switzerland56) and 35 in LMICs (9 in China,30,57–64 8 in India,30,65–71 10 in Brazil,29,72–80 3 in South Africa,29,30,81 2 in Ghana,29,30 2 in Pakistan,29,71 2 in Bangladesh,29,82 2 in Burkina Faso,29,83 and 1 each in Bosnia and Herzegovina,29 Colombia,84 Dominican Republic,29 Egypt,85 Georgia,29 Iran,86 Kazakhstan,29 Kenya,29 Kosovo,87 Laos,29 Malaysia,29 Mauritius,29 Mexico,30 Morocco,29 Myanmar,29 Namibia,29 Nepal,29 Paraguay,29 the Philippines,29 Russia,30 Serbia,88 Sri Lanka,29 Ukraine,29 Uruguay29 and Vietnam89) over a period of 25 years, from 1992 to 2017. Most of them (63 studies) were cross-sectional design, and seven21,23,39,43,54,57,60 had a longitudinal design, from which we used data from the baseline assessment. Only 5 out of 7022,47,75,76,80 studies focused solely on subgroups of either men or women. The number of diseases included in eligible studies ranged from 4 to 40 (with hypertension, diabetes, arthritis and stroke being four most frequent conditions). Disease count was the most common measure of multimorbidity, based on self-reported data. Details of studies’ characteristics are provided in Table 1.
Table 1.
Characteristics of included studies.a
| Study (country of study) | Data collection period | Data source | Sample size | Age | Gender (% men) | Prevalence (%) (95% CI) |
|---|---|---|---|---|---|---|
| Afshar 2015 (28 countries) | 2003 | WHO World Health Survey | 125404 | 18+ | 48.5 | 7.8 (6.5–9.1) |
| Agborsangaya 2013 (Canada) | 2012 | Health Quality Council of Alberta (HQCA) 2012 Patient Experience Survey | 4803 | 18+ | 44.2 | 36.1 (34.7–37.3) |
| Alaba 2013 (South Africa) | 2008 | South Africa National Income Dynamic Survey (SA-NIDS) | 11638 | 18+ | 39.0 | 4.0 (3.6–4.4) |
| Alimohammadian 2018 (Iran) | 2004–2008 | Golestan Cohort Study (GCS) | 49946 | 40–75 | 42.4 | 19.4 (19.1–19.8) |
| Amaral 2018 (Brazil) | 2010 | Population-based study | 264 | 60–102 | 39.0 | 66.3 (60.4–71.7) |
| Araujo 2018 (Brazil) | 2015 | Population-based study | 4001 | 60+ | 47.2 | 29.0 (27.6–30.5) |
| Banjare 2014 (India) | 2011–2012 | Cross-sectional survey | 310 | 60+ | 49.4 | 56.8 (51.2–62.2) |
| Buttery 2016 (Germany) | 1997–1999 | German National Health Interview and Examination Survey 1998 (GNHIES98) | 2884 | 50–79 | 47.6 | M: 36.1 (33.6–38.7) F: 40.5 (38.1–43.0) |
| Camargo-Casas 2018 (Colombia) | 2012 | The SABE-B study | 2000 | 60+ | 36.6 | 40.4 (38.3–42.6) |
| Chen 2018 (China) | 2011 | China Health and Retirement Longitudinal Study (CHARLS) | 3737 | 45+ | 51.9 | 45.5 (41.4–49.7) |
| Cheung 2018 (Hong Kong) | 2016–2017 | Jockey Club Community eHealth Care project | 2618 | 60+ | 47.5 | 41.8 (39.9–43.7) |
| de Carvalho 2017 (Brazil) | 2013 | National Health Survey | 60202 | 18+ | N/A | 23.6 (22.9–24.3) |
| de Souza Santos Machado 2012 (Brazil) | 2005 | Population-based study | 377 | 40–65 | Women only |
39.3 (34.5–44.3) |
| de Souza Santos Machado 2013 (Brazil) | 2011 | Population-based study | 622 | 50+ | Women only |
58.2 (54.3–62.0) |
| Dhawalni 2016 (England) | 2002–2003 | English Longitudinal Study of Ageing (ELSA) | 11212 | 50+ | 46.4 | 31.7 (30.9–32.6) |
| El Lawindi 2019 (Egypt) | 2016–2017 | Community-based study | 2317 | 18–85 | 54.9 | 19.6 (18.0–21.3) |
| Fuchs 2012 (Germany) | 2008–2009 | German Health Update (GEDA) | 21262 | 18–100 | 48.5 | M: 36.3 (35.4–37.2) F: 43.9 (43.0–44.8) |
| Garin 2016 (9 countries) | 2008–2012 | WHO study on Global AGEing and Adult Health (SAGE) and the Collaborative Research on Ageing in Europe (COURAGE) survey | 41909 | 50+ | 46.5 | 62.7 (55.2–70.1) |
| Ge 2018 (Singapore) | 2015–2016 | The Population Health Index Survey | 1940 | 21+ | 44.3 | 36.9 (34.7–39.0) |
| Gu 2017 (China) | 2013 | Cluster random sampling survey | 2452 | 60–93 | 51.5 | 49.4 (47.4–51.4) |
| Hameed 2015 (India) | 2013 | Community-based study | 375 | 60+ | 57.9 | 79.4 (75.1–83.3) |
| Hien 2014 (Burkina Farso) | 2012 | Cluster random sampling survey | 389 | 60+ | 55.3 | 65.0 (59.9–69.4) |
| Humphreys 2018 (UK) | 2007–2008 | The Hertfordshire Cohort study | 2299 | 64–68 | 51.0 | 43.4 (41.4–45.5) |
| Islam 2014 (Australia) | 2009 | Stratified random sampling survey | 4574 | 50+ | N/A | 52.0 (50.5–53.4) |
| Jankovic 2018 (Serbia) | 2013 | 2013 Serbian National Health Survey | 13765 | 20+ | 46.0 | 30.2 (29.4–30.9) |
| Jerliu 2013 (Kosovo) | 2011 | Nationwide cross-sectional study | 1890 | 65+ | 50.2 | 51.1 (48.8–53.3) |
| Johnston 2019 (UK) | 2001 | The Aberdeen Children of the 1950s (ACONF) | 7184 | 50+ | 47.7 | 5.4 (4.9–6.0) |
| Khanam 2011 (Bangladesh) | 2003–2004 | Poverty and Health in Ageing study | 452 | 60–92 | 45.1 | 53.7 (49.2–58.3) |
| Kiliari 2014 (Cyprus) | 2008 | Nationally based survey | 465 | N/A | 43.2 | 28.5 (24.7–32.9) |
| Kirchberger 2012 (Germany) | 2008–2009 | KORA-AGE study | 4127 | 65–94 | 48.8 | 58.6 (50.7–60.2) |
| Kshipra 2018 (India) | 2012–2013 | Cross-sectional study | 400 | 50+ | N/A | 31.0 (26.7–35.7) |
| Kumar 2015 (India) | 2012–2013 | Household survey | 55091 | N/A | 52.3 | 0.7 (0.6–0.7) |
| Lai 2019 (Hong Kong) | 1999 | Thematic Household Survey (THS) | 17229 | 35+ | 49.5 | 3.5 (3.2–3.8) |
| Laires 2019 (Portugal) | 2014 | The Portuguese National Health Interview Survey (Inquerito Nacional de Saude, INS) | 15196 | 25–79 | 44.0 | 43.9 (43.1–44.7) |
| Lalitha 2016 (India) | 2009 | Household survey | 815 | 40+ | 51.3 | 44.1 (40.6–47.5) |
| Lang 2015 (US) | 2012–2013 | EuroQol 5 dimensions (EQ-5D) study | 3058 | 40–64 | Women only |
30.6 (29.0–32.3) |
| Larsen 2017 (Denmark) | 2013 | Danish National Health Survey | 162283 | 16+ | 49.0 | 39.7 (39.4–39.9) |
| Le Cossec 2016 (France) | 2008 | Disability Healthcare Household Section Survey (HSM – Enquete Handicap Sante - Menages) | 11089 | 55+ | 45.1 | M: 18.7 (17.6–19.9) F: 15.2 (14.3–16.1) |
| Li 2019 (China) | 2017 | Community-based survey | 4833 | 60+ | 45.5 | 16.1 (15.1–17.1) |
| Loprinzi 2015 (US) | 2005–2006 | 2005–2006 National Health and Nutrition Examination Survey (NHANES) | 2048 | 20+ | 50.9 | 58.4 (55.3–61.5) |
| Loza 2009 (Spain) | 1999–2000 | EPISER study | 2192 | N/A | N/A | 30.0 (25.0–34.0) |
| Lujic 2017 (Australia) | 2005–2009 | 45 and up study | 90352 | 45+ | 44.3 | 37.4 (37.1–37.7) |
| Maregoni 2016 (Sweden) | 2001–2004 | Swedish National Study on Ageing and Care in Kungsholmen (SNAC-K) | 3155 | 60+ | 35.7 | 52.4 (50.6–54.2) |
| Mini 2017 (India) | 2011 | UNFPA funded national survey | 9852 | 60+ | 47.0 | 30.7 (29.8–31.6) |
| Ninh 2015 (Vietnam) | 2010 | Population-based study | 2400 | 60+ | 34.8 | 41.6 (39.5–43.8) |
| Noguchi 2016 (Australia) | 2005–2007 | Concord Health and Ageing in Men Project (CHAMP) | 1705 | 70–99 | 100.0 | 69.3 (67.1–71.5) |
| Nunes 2016 (Brazil) | 2012 | Population-based cross-sectional study | 2927 | 20+ | 41.1 | 29.1 (27.1–31.1) |
| Nunes 2019 (Brazil) | 2015–2016 | The Brazilian Longitudinal Study of Ageing (ELSI-Brazil) | 9412 | 50+ | 46.0 | 67.8 (65.6–69.9) |
| Nunes 2015 (Brazil) | 2008 | Population-based survey | 1593 | 60+ | 37.2 | 81.3 (79.3–83.3) |
| Pache 2015 (Switzerland) | 2003–2006 | Cohorte Lausannoise (CoLaus) study | 3714 | 35–75 | 47.0 | 34.8 (33.3–36.4) |
| Park 2018 (Korea) | 2013–2014 | The sixth Korean National Health and Nutritional Examination Survey (KNHANES) | 5996 | 50+ | 46.6 | 26.8 (25.7–27.9) |
| Picco 2016 (Singapore) | 2012–2013 | Well-being of the Singapore Elderly (WiSE) study | 2565 | 50+ | N/A | 55.4 (53.4–57.3) |
| Ramond-Roquin 2016 (Canada) | 2010 | PRECISE study | 1710 | 18+ | 48.3 | 63.8 (61.5–6.1) |
| Roberts 2015 (Canada) | 2011–2012 | Canadian Community Health Survey (CCHS) | 105416 | 25–75 | 40.5 | 12.9 (12.6–13.2) |
| Rodrigues 2018 (Portugal) | 2013–2015 | EpiDoc 2 study | 2393 | 65+ | 44.2 | 67.9 (66.0–9.7) |
| Romana 2019 (Portugal) | 2015 | Inquerito Nacional de Saude com Exame Fisico (INSEF) | 4911 | 25–74 | 47.5 | 38.4 (37.0–39.8) |
| Ruel 2014 (Australia) | 2000–2002 | North West Adelaide longitudinal Health Study (NWAHS) | 1854 | 20+ | 44.1 | 32.0 (30.0–4.0) |
| Ruel 2014 (China) | 2002 | Jiangsu longitudinal Nutrition Study (JIN) | 1020 | 18+ | 48.0 | 14.0 (12.0–16.3) |
| Ryan 2018 (Ireland) | 2010 | The Irish Longitudinal Study on Ageing (TILDA) | 4823 | 50+ | N/A | 53.7 (52.3–55.1) |
| Sakib 2019 (Canada) | 2015 | The Canadian Longitudinal Study of Ageing (CLSA) | 29841 | 45–64 | 49.4 | 39.6 (38.4–40.7) |
| Singh 2019 (India and Pakistan) | 2010–2011 | The Cardiometabolic Risk Reduction in South Asia Surveillance Study (CARRS Surveillance Study) | 16287 | 20+ | 47.3 | 9.4 (8.7–10.1) |
| Su 2016 (China) | 2013 | Multistage cluster study | 2058 | 80+ | 42.1 | 49.2 (47.0–51.3) |
| Timmermans 2019 (the Netherlands) | 1992–1993 | The Longitudinal Ageing Study Amsterdam (LASA) | 2199 | 64–84 | 44.9 | 43.6 (41.6–45.7) |
| Valadares 2015 (Brazil) | 2012–2013 | Cross-sectional study | 736 | 45–60 | Women only |
53.0 (49.4–56.6) |
| Violan 2013 (Spain) | 2006 | Health Survey for Catalonia database 2006 | 15926 | 15+ | 49.5 | 59.6 (58.8–60.4) |
| Wang 2014 (China) | 2011 | Cross-sectional community household survey | 162464 | All | 51.4 | 11.1 (10.6–11.6) |
| Wang 2017 (Australia) | 2007 | 2007 Australian National Survey of Mental Health and Wellbeing | 8841 | 16–85 | 49.7 | 28.7 (27.8–9.7) |
| Wang 2015 (China) | 2010–2011 | Confucious Hometown Aging Project (CHAP) | 1480 | 60+ | 40.6 | 90.5 (88.9–91.9) |
| Wang 2015 (China) | 2012 | Jilin Provincial chronic Disease Survey | 21435 | 18–79 | N/A | 24.7 (24.1–25.4) |
| Wong 2008 (Hong Kong, China) | N/A | Cross-sectional study | 3394 | 65+ | 56.0 | 68.0 (66.4–9.5) |
M: male; F: female; CI: confidence interval.
a Multimorbidity was defined as the presence of two or more chronic diseases in the same individual; 95% CI as reported in original studies was presented in Table 1. Where this was not available, we used the 95% CI generated by STATA for the meta-analysis. For studies that investigated multimorbidity prevalence in several countries, the prevalence presented in Table 1 was the pooled country prevalence estimates.
Prevalence of multimorbidity
Among 37 nationally representative studies in HICs, one conducted in Hong Kong reported the lowest prevalence (3.5%)43 while another conducted in Russia reported the highest prevalence (70%).30 Of those that were conducted in LMICs, one study which used data from a household survey in 26 villages in India reported the lowest multimorbidity prevalence (1%).68 Another study with data derived from the Confucius Hometown Project in China, on the contrary, reported the highest prevalence estimate (90%).64
In addition to the common cut-off point of 2+ chronic diseases (2+MM) used in 68 studies, 20 studies also investigated the prevalence estimate when multimorbidity was defined as ‘the co-occurrence of three or more chronic diseases’. As the number of diseases included in the definition increased, the prevalence decreased (Table 2). The biggest difference was observed in Khanam et al.’s study,82 where the prevalence of 3+ morbidities (3+MM) was reported to be 19.5%, which represented a decrease of 34.3 percentage points compared to the prevalence of 2+ morbidities (53.8%). On average, the weighted difference between the prevalence of 2+MM and 3+MM was 12.9 percentage points.
Table 2.
Prevalence of multimorbidity by two or more diseases and three or more diseases cut-off points.
| Study | 2+ MM (%) | 3+ MM (%) | Difference | Sample size |
|---|---|---|---|---|
| Araujo (2018) | 29.0 | 15.2 | 13.8 | 4001 |
| Banjare and Pradhan (2016) | 56.8 | 30.0 | 26.8 | 310 |
| Dhawalni (2016) | 31.7 | 11.7 | 20.0 | 11212 |
| Garin (2014) | 9.7 | 5.4 | 4.3 | 4583 |
| Humphreys (2018) | 47.6 | 21.6 | 26.0 | 2299 |
| Khanam (2011) | 53.8 | 19.5 | 34.3 | 452 |
| Lang (2015) | 17.9 | 12.7 | 5.2 | 3058 |
| Lujic (2017) | 37.4 | 8.7 | 28.7 | 90352 |
| Nunes (2016) | 29.1 | 14.3 | 14.8 | 2927 |
| Nunes (2019) | 67.8 | 47.1 | 20.7 | 9412 |
| Nunes (2015) | 81.3 | 64.0 | 17.3 | 1593 |
| Ramond-Roquin (2016) | 63.8 | 48.9 | 14.9 | 1710 |
| Roberts (2015) | 12.9 | 3.9 | 9.0 | 105416 |
| Ruel (2014) | 32.0 | 9.0 | 23.0 | 1854 |
| Su (2016) | 49.2 | 18.5 | 30.7 | 2058 |
| Wang (2014) | 11.1 | 6.1 | 5.0 | 162464 |
| Wang (2015) | 90.5 | 76.5 | 14.0 | 1480 |
| Wang (2015) | 24.8 | 12.0 | 12.8 | 21430 |
| Wang (2017) | 26.0 | 10.1 | 15.9 | 8820 |
| Wong (2008) | 68.0 | 42.4 | 25.6 | 3394 |
| Average difference | 18.4 | |||
| Weighted average difference | 12.9 | |||
2+MM: two or more disease cut-off point; 3+MM: three or more disease cut-off point.
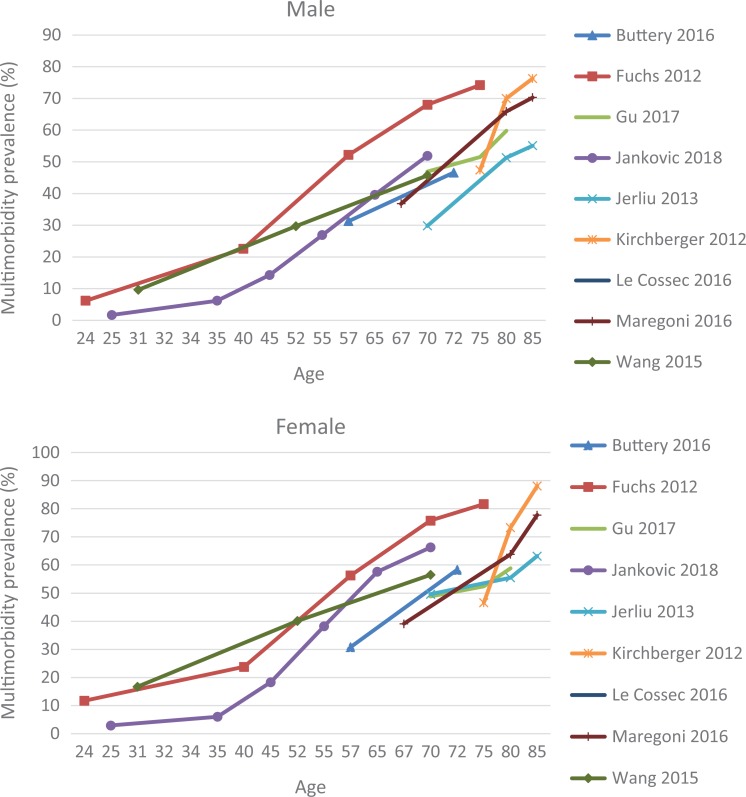
Among 25 studies that explored gender variations, the majority (21 studies) reported a higher prevalence in women than in men. Alaba and Chola,81 in particular, found that multimorbidity was almost double in females (74% in females vs. 26% in males). The reverse trend was observed in only four studies.51,65,67,68 Nevertheless, when men were reported to have higher multimorbidity prevalence, the differences in prevalence estimates between the two sexes were relatively small (Online Supplement 4). Figure 2 shows that the prevalence of multimorbidity invariably increased for both men and women as they aged. There was an upward trend, where multimorbidity prevalence was positively associated with age. Of nine studies that reported age–sex specific prevalence, Kirchberger et al.35 found that the highest prevalence was in the 85+ group (men: 76.3%; women: 88.1%). Although this association was supported by 24 other studies that investigated age-specific prevalence, three studies observed a lower prevalence estimate among the oldest old (80–85 age group). Kiliari et al,49 in particular, found that after the age of 85, the prevalence of multimorbidity in Cyprus decreased to 33.3% (from 80% in the previous age bracket). The highest prevalence estimate was reported for the 75+ age group in Spain (92.9%).32 The largest variation, with prevalence of multimorbidity ranging from 16.3% to 87.5%, was around the age of 70.
Figure 2.
Age- and sex-specific prevalence of multimorbidity.
Meta-analysis
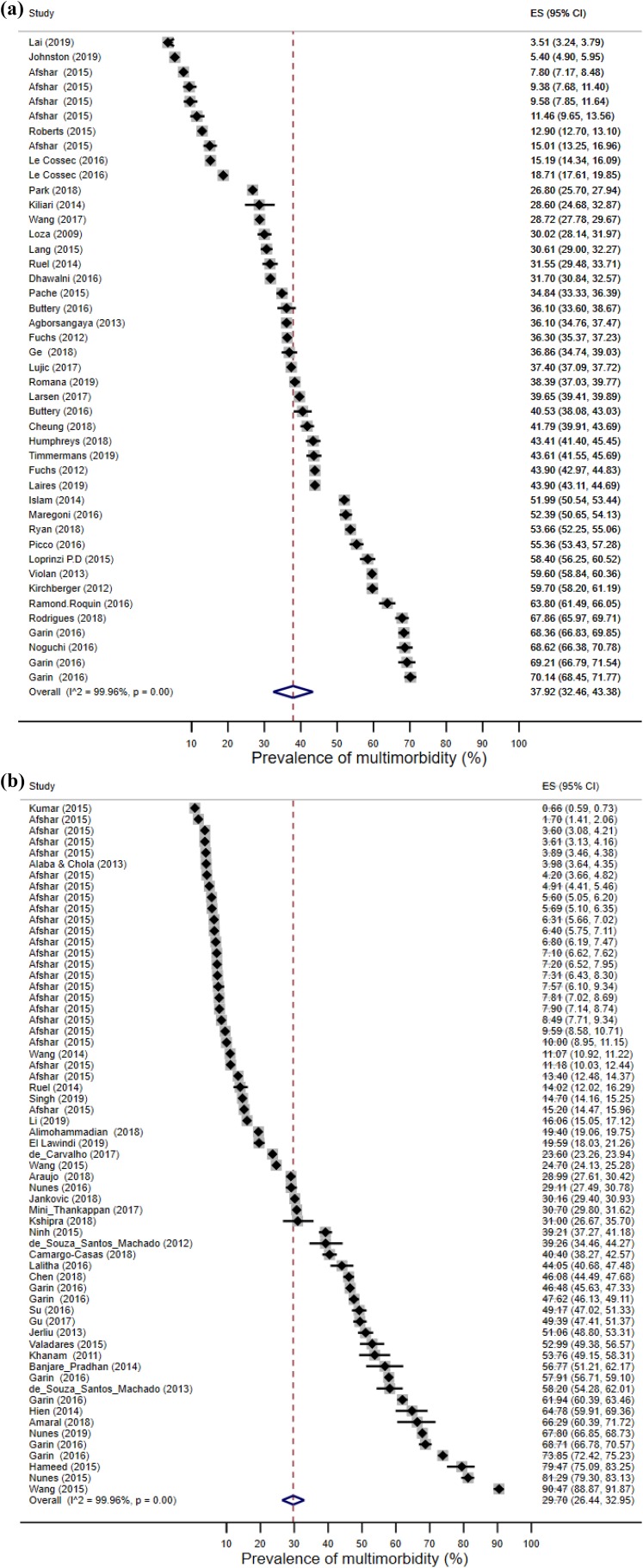
Data combined from 106 prevalence estimates (from 68 studies) showed that the overall random-effect pooled prevalence of multimorbidity was 33.1% (95% CI): 30.0–36.3%). There was a considerable difference in the pooled prevalence estimates between HICs (44 estimates) and LMICs (62 estimates). Specifically, the pooled prevalence of multimorbidity among HICs was 37.9% (95% CI: 32.5–43.4%), while the pooled prevalence estimate among LMICs was 29.7% (26.4–33.0%; Figures 3(a) and (b), Online Supplement 5).
Figure 3.
(a) Forest plot showing multimorbidity prevalence in HICs. (b) Forest plot showing multimorbidity prevalence in LMICs. HIC: high-income country; LMIC: low-income country.
However, when standardised prevalence was removed (34 estimates), LMICs’ prevalence was found to be higher than that of HICs. This difference, nonetheless, was marginal, with HICs prevalence being 41.3% (95% CI: 35.2–47.4%) and LMICs 43.5% (95% CI: 38.4–48.6%). The overall pooled estimate of non-standardised prevalence rose to 42.4% (95% CI: 38.1–46.6%; Online Supplement 6). Heterogeneity across studies, both before and after adjusting for standardisation, was very high (I 2 > 99%). A sensitivity analysis showed that none of the studies included in our review skewed the overall pooled estimates.
Discussion
This systematic review provides an up-to-date and comprehensive analysis of multimorbidity prevalence at a global level. It shows that prevalence estimates varied substantially according to age, gender and operational definitions of multimorbidity. This was due to wide variations in sample size, characteristics and how prevalence was reported across studies. Nevertheless, the main findings from our review were consistent with those in previous studies and systematic reviews.10,90 Specifically, our data suggested that multimorbidity increases with age. While the prevalence estimates varied between and within age groups, most studies in our sample indicated that a large proportion (more than 50% in many cases) of individuals over the age of 65 had multimorbidity. Where prevalence estimates by gender were reported, females appeared to have higher multimorbidity prevalence rates than males. This is indicative of an association between sex and multimorbidity (evidence of which was provided in multiple studies74,86). Although there was no uniformity in disease combinations and cut-off points, it followed that the higher the cut-off point, the lower the prevalence. This finding supported an observation by Harrison et al.,91 where it was found that from 44% (when multimorbidity was defined as 2+ diseases), the prevalence reduced to 27% (for 3+ diseases), 15% (for 4+ diseases), 7% (for 5+ diseases) and only 3% (for 6+ diseases). The highest prevalence estimates in our sample were reported in studies that used the 2+MM definition. Harrison et al.91 also ascertained that the combination of diseases may make multimorbidity prevalence differ significantly. In the existing literature, a range of different combinations have been proposed from a list of 16 chronic diseases92 to a list of 291 diseases93 and anything in between.94 Ferrer et al.92 argued that an open list of diagnoses should be used, since it gave the highest prevalence estimate. In our sample, the number of diseases ranged from 4 to 40, but a similar trend (i.e. the higher the disease number, the higher the prevalence) was not found. The prevalence of multimorbidity estimated using a list of 40 diseases was, in fact, lower than the prevalence estimated using a list of six diseases. The concern perhaps should be shifted to the fact that there were no specific criteria for disease inclusion in these studies. They were often determined by the authors’ experience and expertise rather than a standardised list. Most commonly, conditions included were those with the highest prevalence or clinical relevance.12
Our quantitative synthesis of the data generated a pooled prevalence estimate of 33.1% (95% CI: 30.0–36.3%), which must be interpreted with some caveats. When both age–sex standardised and non-standardised prevalence estimates were included, stratified analysis suggested that there were differences between HICs and LMICs multimorbidity prevalence. Specifically, multimorbidity prevalence was higher in HICs than LMICs. However, when only non-standardised prevalence estimates were taken into consideration, the prevalence of multimorbidity was shown to be marginally higher in LMICs than in HICs. This reverse trend could be due to the fact that of the 34 standardised prevalence estimates removed, 27 of them were from LMICs. The inclusion of these 27 standardised estimates might have deflated the original pooled prevalence estimated for LMICs. Nonetheless, it was not yet clear whether the geographical variation in multimorbidity prevalence was genuine (i.e. multimorbidity prevalence was higher in HICs than LMICs) or whether it simply reflected the differences in diagnostic and data management systems between HICs and LMICs. Xu et al.95 suggested that the difference between HICs and LMICs prevalence estimates might also be due to the comparatively limited knowledge on multimorbidity from LMICs compared with HICs, which, consequently, led to fewer publications on multimorbidity prevalence in LMICs.95
The reduction in heterogeneity level as a result of stratified analysis was not considerable (all I 2 statistics were greater than 99%). In this review, attempts have been made to ensure consistency of prevalence estimates by including in the meta-analysis only studies that used 2+MM definition, disease count measure and self-reported data in community settings.
Implications of findings
Inconsistencies in the prevalence of multimorbidity may lead to an over-/underestimation of healthcare costs, hospital admissions, resources distribution and general disease burden. This, subsequently, hinders the effects of health interventions. The need for a uniform method to estimate multimorbidity prevalence, therefore, becomes more and more urgent. Future research on multimorbidity is urged to follow a standardised protocol, using a consistent disease classification system, disease cut-off point and measure of multimorbidity. Since the age structures of HICs and LMICs are different, both crude and standardised prevalence should be reported. Results from prevalence studies should also be stratified by gender and age. Age groups, where possible, should be categorised using standardised intervals.
Strengths and limitations
The strengths of this review lie in the fact that our study selection and screening processes were vigorous. Our search strategy and inclusion criteria were comprehensive, which, subsequently led to our review being the largest systematic review on multimorbidity prevalence to date. Results after the initial screening were double-checked by a second reviewer, and the full-text screening that followed was carried out independently. Our data extraction and quality assessment were also cross-checked and very few disagreements arose. The studies included in the analysis were mainly of high quality, all community-based and covered both HICs and LMICs. This enabled the findings to be extrapolated to the global population.
This review, however, was not without limitations. Notwithstanding effort made to ensure eligible studies were included, there were still possibilities that potentially eligible articles (especially those not in English) were missed out. This might contain studies that focused on two or more chronic conditions without using the term multimorbidity in their titles or abstracts. Most of the studies in our sample reported multimorbidity prevalence based on self-reported data (though some also used medical examinations such as blood test). Results of such studies were therefore prone to response bias (due to misunderstanding of survey questions or recall timeframe). The majority of studies in this review were cross-sectional, which only allowed estimation of multimorbidity at a certain point in time. In addition, the measures of multimorbidity used in these studies were mostly disease count, with only one exception of the functional comorbidity index (FCI) in one study.56 Disease count and FCI were only 2 of nearly 20 different measures available to date. Fortin et al.96 reported a much higher prevalence of multimorbidity when using the Cumulative Illness Rating Scale, compared with the prevalence measured by disease count in other studies.96 A simple count of chronic diseases, despite being the most common method to estimate multimorbidity prevalence, may sometimes be considered too crude a measure.97 That being said, in a cross-sectional, population-based study conducted in Switzerland by Pache et al.,56 the prevalence of multimorbidity measured by disease count was found to be higher than that measured by the FCI.56 The lack of consistency in measuring and reporting the prevalence of multimorbidity in the included studies was a factor that needs to be taken into account when interpreting findings from our analyses. However, as discussed above, given that there is no consensus about multimorbidity, heterogeneity across studies is inevitable.
Finally, for our meta-analysis, we used absolute numbers (i.e. the number of people reporting multimorbidity and sample sizes) to generate multimorbidity prevalence estimates. However, this strategy did not take into account the weights that were applied to the prevalence estimates in some studies. Our results, therefore, need to be interpreted with this caveat in mind.
Conclusion
Investigating multimorbidity prevalence is of great importance in the study of ageing. This systematic review of 70 studies reveals that a large proportion of the global population, especially those above the age of 65, are affected by multiple chronic diseases. The prevalence estimates of multimorbidity differ among studies. The need for a consistent operationalisation of multimorbidity is evident. It will enable more accurate estimations of disease burden and, consequently, more effective disease management and resources distribution.
Supplemental material
Supplemental Material, Supplement_materials_revised for Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies by Hai Nguyen, Gergana Manolova, Christina Daskalopoulou, Silia Vitoratou, Martin Prince and A Matthew Prina in Journal of Comorbidity
Acknowledgements
We would like to thank the authors of studies who were contacted and provided further details for this manuscript. We would also like to thank Christina Blanner for her statistical support with the meta-analysis and Melissa Co for her help with the translation of a Portuguese paper included in this review. We extend our thanks also to ATHLOS (Ageing Trajectories of Health: Longitudinal Opportunities and Synergies) project.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This systematic review was funded by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement number 635316, as part of the ATHLOS Consortium (Ageing Trajectories of Health: Longitudinal Opportunities and Synergies, http://athlosproject.eu/).
ORCID iD: Hai Nguyen  https://orcid.org/0000-0003-2171-1955
https://orcid.org/0000-0003-2171-1955
Supplemental material: Supplemental material for this article is available online.
References
- 1. Academy of Medical Sciences. Multimorbidity: a priority for global health research. 2018. https://acmedsci.ac.uk/policy/policy-projects/multimorbidity (accessed 12 May 2018).
- 2. Ryan A, Wallace E, O’Hara P, et al. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes 2015; 13: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nunes BP, Flores TR, Mielke GI, et al. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67: 130–138. [DOI] [PubMed] [Google Scholar]
- 4. Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2019; 74(5): 659–666. [DOI] [PubMed] [Google Scholar]
- 5. Salisbury C, Johnson L, Purdy S, et al. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61(582): e12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huntley AL, Johnson R, Purdy S, et al. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012; 10(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US veterans affairs health care system. BMJ Open 2015; 5(4): e007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JT, Hamid F, Pati S, et al. Impact of noncommunicable disease multimorbidity on healthcare utilisation and out-of-pocket expenditures in middle-income countries: cross sectional analysis. PLoS One 2015; 10(7): e0127199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akker MV, Buntinx F, Knottnerus A. Comorbidity or multimorbidity. What’s in a name: a review of literature. Eur J Gen Pract 1996; 2(2): 65–70. [Google Scholar]
- 10. Violan C, Foguet-Boreu Q, Flores-Mateo G, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLos One 2014; 9(7): e102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012; 10(2): 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pati S, Swain S, Hussain MA, et al. Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open 2015; 5(10): e007235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ-Brit Med J 2015; 350: g7647. [DOI] [PubMed] [Google Scholar]
- 14. Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One 2016; 11: e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Rockville: Aency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews, 2012. [PubMed] [Google Scholar]
- 16. World Bank. World Bank list of economies. 2017. http://iccmoot.com/wp-content/uploads/2017/07/World-Bank-List-of-Economies.pdf (accessed 30 August 2017).
- 17. Nyaga V, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19. StataCorp. Stata statistical software: release 15. College Station: StataCorp LLC, 2017. [Google Scholar]
- 20. Islam MM, Valderas JM, Yen L, et al. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS One 2014; 9(1): e83783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lujic S, Simpson JM, Zwar N, et al. Multimorbidity in Australia: comparing estimates derived using administrative data sources and survey data. PLoS One [Electronic Resource] 2017; 12(8): e0183817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noguchi N, Blyth FM, Waite LM, et al. Prevalence of the geriatric syndromes and frailty in older men living in the community: the concord health and ageing in men project. [Erratum appears in Australas]. J Ageing 2017; 36(1): 80 PMID: 28326691 Australasian Journal on Ageing 2016; 35(4): 255–261. [DOI] [PubMed] [Google Scholar]
- 23. Ruel G, Levesque JF, Stocks N, et al. Understanding the evolution of multimorbidity: evidences from the North West Adelaide Health Longitudinal Study (NWAHS). PLoS One [Electronic Resource] 2014; 9(5): e96291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang L, Palmer AJ, Cocker F, et al. Multimorbidity and health-related quality of life (HRQoL) in a nationally representative population sample: implications of count versus cluster method for defining multimorbidity on HRQoL. Health & Qual Life Outcomes 2017; 15(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agborsangaya CB, Ngwakongnwi E, Lahtinen M, et al. Multimorbidity prevalence in the general population: the role of obesity in chronic disease clustering. BMC Public Health 2013; 13: 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramond-Roquin A, Haggerty J, Lambert M, et al. Different Multimorbidity measures result in varying estimated levels of physical quality of life in individuals with multimorbidity: a cross-sectional study in the general population. BioMed Res Int 2016; 2016: 7845438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts KC, Rao DP, Bennett TL, et al. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can 2015; 35(6): 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakib MN, Shooshtari S, St John P, et al. The prevalence of multimorbidity and associations with lifestyle factors among middle-aged Canadians: an analysis of Canadian Longitudinal Study on Aging data. BMC Public Health 2019; 19(1): 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Afshar S, Roderick PJ, Kowal P, et al. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the world health surveys. BMC Public Health 2015; 15: 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A-Bio Sci Med Sci 2016; 71(2): 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loza E, Jover JA, Rodriguez L, et al. Multimorbidity: prevalence, effect on quality of life and daily functioning, and variation of this effect when one condition is a rheumatic disease. Semin Arthritis Rheum 2009; 38(4): 312–319. [DOI] [PubMed] [Google Scholar]
- 32. Violan C, Foguet-Boreu Q, Hermosilla-Perez E, et al. Comparison of the information provided by electronic health records data and a population health survey to estimate prevalence of selected health conditions and multimorbidity. BMC Public Health 2013; 13: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buttery AK, Du Y, Busch MA, et al. Changes in physical functioning among men and women aged 50-79 years in Germany: an analysis of National Health Interview and Examination Surveys, 1997-1999 and 2008-2011. BMC Geriat 2016; 16: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuchs J, Busch M, Lange C, et al. Prevalence and patterns of morbidity among adults in germany. Results of the German telephone health interview survey German Health Update (GEDA) 2009. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012; 55(4): 576–586. [DOI] [PubMed] [Google Scholar]
- 35. Kirchberger I, Meisinger C, Heier M, et al. Patterns of multimorbidity in the aged population results from the KORA-age study. PLoS One 2012; 7(1): e30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laires P, Perelman J. The current and projected burden of multimorbidity: a cross-sectional study in a southern Europe population. Eur J Ageing 2018; 16: 181–192. No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodrigues AM, Gregorio MJ, Sousa RD, et al. Challenges of ageing in Portugal: data from the EpiDoC cohort. Acta Med Port 2018; 31(2): 80–93. [DOI] [PubMed] [Google Scholar]
- 38. Quinaz Romana G, Kislaya I, Salvador MR, et al. Multimorbidity in Portugal: results from the first national health examination survey. [Portuguese]. Acta Med Port 2019; 32(1): 30–37. [DOI] [PubMed] [Google Scholar]
- 39. Dhalwani NN, O’Donovan G, Zaccardi F, et al. Long terms trends of multimorbidity and association with physical activity in older English population. Int J Behav Nutr Phys Act 2016; 13: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humphreys J, Jameson K, Cooper C, et al. Early-life predictors of future multi-morbidity: results from the Hertfordshire cohort. Age Ageing 2018; 47(3): 474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnston MC, Black C, Mercer SW, et al. Impact of educational attainment on the association between social class at birth and multimorbidity in middle age in the Aberdeen children of the 1950s cohort study. BMJ Open 2019; 9(1): e024048 (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cheung JTK, Yu R, Wu Z, et al. Geriatric syndromes, multimorbidity, and disability overlap and increase healthcare use among older Chinese. BMC Geriat 2018; 18(1): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai FTT, Guthrie B, Wong SYS, et al. Sex-specific intergenerational trends in morbidity burden and multimorbidity status in Hong Kong community: an age-period-cohort analysis of repeated population surveys. BMJ Open 2019; 9(1): e023927 (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong SYS, Mercer SW, Woo J, et al. The influence of multi-morbidity and self-reported socio-economic standing on the prevalence of depression in an elderly Hong Kong population. BMC Public Health 2008; 8: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ge L, Wei Yap C, Heng BH. Sex differences in associations between multimorbidity and physical function domains among community-dwelling adults in Singapore. PLoS One 2018; 13(5): e0197443 (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Picco L, Achilla E, Abdin E, et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res 2016; 16: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang K, Alexander IM, Simon J, et al. The impact of multimorbidity on quality of life among midlife women: findings from a U.S. nationally representative survey. J Women’s Health 2015; 24(5): 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loprinzi PD. Sedentary behavior and medical multimorbidity. Physiol Behav 2015; 151: 395–397. [DOI] [PubMed] [Google Scholar]
- 49. Kiliari N, Theodosopoulou E, Papanastasiou E. Multimorbidity and unmet citizens’ needs and expectations urge for reforms in the health system of Cyprus: a questionnaire survey. JRSM Open 2014; 5(1): 2042533313515860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larsen FB, Pedersen MH, Friis K, et al. A latent class analysis of multimorbidity and the relationship to socio-demographic factors and health-related quality of life. a National population-based study of 162,283 Danish adults. PLoS One [Electronic Resource] 2017; 12(1): e0169426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le Cossec C, Perrine AL, Beltzer N, et al. Pre-frailty, frailty, and multimorbidity: prevalences and associated characteristics from two French national surveys. J Nutr Health Aging 2016; 20(8): 860–869. [DOI] [PubMed] [Google Scholar]
- 52. Ryan A, Murphy C, Boland F, et al. What is the impact of physical activity and physical function on the development of multimorbidity in older adults over time? A population-based cohort study. J Gerontol A, Biol Sci Med Sci 2018; 73(11): 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park B, Ock M, Lee HA, et al. Multimorbidity and health-related quality of life in Koreans aged 50 or older using KNHANES 2013-2014. Health & Qual Life Outcomes 2018; 16(1): 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Timmermans EJ, Hoogendijk EO, Broese van Groenou MI, et al. Trends across 20 years in multiple indicators of functioning among older adults in the Netherlands. Eur J Public Health 2019: pii: ckz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marengoni A, Angleman S, Meinow B, et al. Coexisting chronic conditions in the older population: variation by health indicators. Eur J Intern Med 2016; 31: 29–34. [DOI] [PubMed] [Google Scholar]
- 56. Pache B, Vollenweider P, Waeber G, et al. Prevalence of measured and reported multimorbidity in a representative sample of the Swiss population. BMC Public Health 2015; 15: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen H, Cheng M, Zhuang Y, et al. Multimorbidity among middle-aged and older persons in urban China: prevalence, characteristics and health service utilization. Geriatr Gerontol Int 2018; 18(10): 1447–1452. [DOI] [PubMed] [Google Scholar]
- 58. Gu J, Chao J, Chen W, et al. Multimorbidity in the community-dwelling elderly in urban China. Arch Gerontol Geriatr 2017; 68: 62–67. [DOI] [PubMed] [Google Scholar]
- 59. Li X, Cai L, Cui WL, et al. Association of socioeconomic and lifestyle factors with chronic non-communicable diseases and multimorbidity among the elderly in rural southwest China. J Public Health 2019; 12: 12. [DOI] [PubMed] [Google Scholar]
- 60. Ruel G, Shi Z, Zhen S, et al. Association between nutrition and the evolution of multimorbidity: the importance of fruits and vegetables and whole grain products. Clin Nutr 2014; 33(3): 513–520. [DOI] [PubMed] [Google Scholar]
- 61. Su P, Ding H, Zhang W, et al. The association of multimorbidity and disability in a community-based sample of elderly aged 80 or older in Shanghai, China. BMC Geriat 2016; 16(1): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang HH, Wang JJ, Wong SY, et al. Epidemiology of multimorbidity in China and implications for the healthcare system: cross-sectional survey among 162,464 community household residents in southern China. BMC Med 2014; 12: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang R, Yan Z, Liang Y, et al. Prevalence and patterns of chronic disease pairs and multimorbidity among older Chinese adults living in a rural area. PLoS One [Electronic Resource] 2015; 10(9): e0138521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang SB, D’Arcy C, Yu YQ, et al. Prevalence and patterns of multimorbidity in northeastern China: a cross-sectional study. Public Health 2015; 129(11): 1539–1546. [DOI] [PubMed] [Google Scholar]
- 65. Banjare P, Pradhan J. Socio-economic inequalities in the prevalence of multi-morbidity among the rural elderly in Bargarh District of Odisha (India). PLoS One [Electronic Resource] 2014; 9(6): e97832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shahul H, Nanjesh K, Naik PM, et al. Morbidity pattern among the elderly population in a rural area of Dakshina Kannada, Karnataka - a cross sectional study. Natl J Community Med 2015; 6(2): 222–225. [Google Scholar]
- 67. Kshipra J, Perianaygam A. Urbanization, multi-morbidities and preference for health care facility: an insight from Rajasthan, India. J Urban Regional Anal 2018; 10(2): 143–176. [Google Scholar]
- 68. Kumar D, Raithatha SJ, Gupta S, et al. Burden of self-reported Noncommunicable diseases in 26 villages of Anand district of Gujarat, India. Int J Chronic Dis 2015; 2015: 260143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lalitha V, Vinod K, Barun K. Rising challenge of multiple morbidities among the rural poor in India - a case of the Sundarbans in West Bengal. Int J Med Sci Public Health 2016; 5(2): 343–350. [Google Scholar]
- 70. Mini GK, Thankappan KR. Pattern, correlates and implications of non-communicable disease multimorbidity among older adults in selected Indian states: a cross-sectional study. BMJ Open 2017; 7(3): e013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh K, Patel SA, Biswas S, et al. Multimorbidity in South Asian adults: prevalence, risk factors and mortality. J Public Health (Oxf) 2019; 41(1): 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Amaral TLM, Amaral CA, Lima NS, et al. Multimorbidity, depression and quality of life among elderly people assisted in the family health strategy in Senador Guiomard, Acre, Brazil. Cien Saude Colet 2018; 23(9): 3077–3084. [DOI] [PubMed] [Google Scholar]
- 73. Araujo MEA, Silva MT, Galvao TF, et al. Prevalence and patterns of multimorbidity in amazon region of Brazil and associated determinants: a cross-sectional study. BMJ Open 2018; 8(11): e023398 (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Carvalho JN, Roncalli AG, Cancela MC, et al. Prevalence of multimorbidity in the Brazilian adult population according to socioeconomic and demographic characteristics. PLoS One 2017; 12(4): e0174322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Souza Santos MacHado V, Valadares ALR, Da Costa-Paiva LS, et al. Multimorbidity and associated factors in Brazilian women aged 40 to 65 years: a population-based study. Menopause 2012; 19(5): 569–575. [DOI] [PubMed] [Google Scholar]
- 76. Machado SSV, Valadares AL, Costa-Paiva LH, et al. Aging, obesity, and multimorbidity in women 50 years or older: a population-based study. Menopause 2013; 20(8): 818–824. [DOI] [PubMed] [Google Scholar]
- 77. Nunes BP, Batista SRR, Andrade FB, et al. Multimorbidity: the Brazilian longitudinal study of aging (ELSI-Brazil). Rev Saude Publica 2018; 52(Suppl 2): 10s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nunes BP, Camargo-Figuera FA, Guttier M, et al. Multimorbidity in adults from a southern Brazilian city: occurrence and patterns. Int J Public Health 2016; 61(9): 1013–1020. [DOI] [PubMed] [Google Scholar]
- 79. Nunes BP, Thume E, Facchini LA. Multimorbidity in older adults: magnitude and challenges for the Brazilian health system. BMC Public Health 2015; 15: 1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Valadares AL, Lui-Filho JF, Costa-Paiva L, et al. Middle-aged female sexual dysfunction and multimorbidity: a population-based study. Menopause 2016; 23(3): 304–310. [DOI] [PubMed] [Google Scholar]
- 81. Alaba O, Chola L. The social determinants of multimorbidity in South Africa. Int J Equity Health 2013; 12: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Khanam MA, Streatfield PK, Kabir ZN, et al. Prevalence and patterns of multimorbidity among elderly people in rural Bangladesh: a cross-sectional study. J Health Popul Nutr 2011; 29(4): 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hien H, Berthe A, Drabo MK, et al. Prevalence and patterns of multimorbidity among the elderly in Burkina Faso: cross-sectional study. Trop Med Int Health 2014; 19(11): 1328–1333. [DOI] [PubMed] [Google Scholar]
- 84. Camargo-Casas S, Suarez-Monsalve S, Zepeda MUP, et al. Multimorbidity, depressive symptoms, and self-reported health in older adults: a secondary analysis of the Sabe Bogota Study. Rev Inves Clin 2018; 70(4): 192–197. [DOI] [PubMed] [Google Scholar]
- 85. El Lawindi MI, Salem MR, Razik MMA, et al. Socioeconomic predictors of morbidities in a rural setting: a community based study. Int J Epidemiol 2019; 15(1): 1–7. [Google Scholar]
- 86. Alimohammadian M, Majidi A, Yaseri M, et al. Multimorbidity as an important issue among women: results of a gender difference investigation in a large population-based cross-sectional study in West Asia. BMJ Open 2017; 7(5): e013548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jerliu N, Toci E, Burazeri G, et al. Prevalence and socioeconomic correlates of chronic morbidity among elderly people in Kosovo: a population-based survey. BMC Geriatr 2013; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jankovic J, Mirkovic M, Jovic-Vranes A, et al. Association between non-communicable disease multimorbidity and health care utilization in a middle-income country: population-based study. Public Health 2018; 155: 35–42. [DOI] [PubMed] [Google Scholar]
- 89. Ninh Thi H, Ninh Hoang L, Khanal V, et al. Multimorbidity and its social determinants among older people in southern provinces, Vietnam. Int J Equity Health 2015; 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10(4): 430–439. [DOI] [PubMed] [Google Scholar]
- 91. Harrison C, Britt H, Miller G, et al. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 2014; 4(7): e004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ferrer A, Formiga F, Sanz H, et al. Multimorbidity as specific disease combinations, an important predictor factor for mortality in octogenarians: the Octabaix study. Clin Interv Aging 2017; 12: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Quinones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol a-Biol 2016; 71(6): 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. van den Bussche H, Koller KD, Kolonko T, et al. Which chronic diseases and disease combinations are specific to multimorbidity in the elderly? Results of a claims data based cross-sectional study in Germany. BMC Public Health 2011; 11: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xu X, Mishra GD, Jones M. Mapping the global research landscape and knowledge gaps on multimorbidity: a bibliometric study. J Glob Health 2017; 7(1): 010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fortin M, Bravo G, Hudon C, et al. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med 2005; 3(3): 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pathirana TI, Jackson CA. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust Nz J Public Health 2018; 42(2): 186–194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplement_materials_revised for Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies by Hai Nguyen, Gergana Manolova, Christina Daskalopoulou, Silia Vitoratou, Martin Prince and A Matthew Prina in Journal of Comorbidity