Abstract
Background:
Extensive research shows that virtual reality (VR) enhances motor learning and has advantages in balance and gait rehabilitation of neurological patients. There is still uncertainty, however, as for the practicality and efficacy of VR in long-term clinical routine. The objective of this study was to report on 3 years of clinical practice conducting VR-based rehabilitation of balance and gait in a large medical center.
Methods:
This retrospective study systematically analyzed clinical records of patients who received VR-based rehabilitation in a large rehabilitation center during 3 years. We evaluated the effect of VR-based rehabilitation treatments on balance and gait, cognitive dual-task load, patient’s balance confidence (ABC-scale) and perception of suitability. Patients were either neurological patients, allocated to five groups: Parkinson’s disease (PD), poststroke (PS), multiple sclerosis, traumatic brain injury, and ‘other conditions’, or non-neurological patients.
Results:
Records of 167 patients were analyzed. The availability of multiple VR systems and environments contributed to highly personalized interventions that tailored specific deficits with therapeutic goals. VR-based rehabilitation significantly improved balance and gait (measured by 10-Meter Walk Test, Timed-Up-and-Go, Berg Balance Scale, and Mini BESTest). Patients with PD and PS decreased dual-task cost while walking. Patients increased balance confidence and deemed VR suitable for rehabilitation.
Conclusions:
Our results suggest that VR-based rehabilitation is practicable and effective in clinical routine. Functional measures of balance and gait show significant improvements following VR-based interventions. Clinical approaches should exploit VR advantages for promoting motor learning and motivation. This study serves to aid transition to long-term clinical implementation of VR.
Keywords: posture, walking, virtual reality, Parkinson’s disease, stroke, multiple sclerosis, brain injury, rehabilitation
Introduction
Balance and gait deficits are a major health problem that compromise quality of life and independence in activities of daily living.1–3 Amongst the various medical conditions commonly associated with balance and gait deficits, neurological diseases represent a major subgroup, including disorders such as Parkinson’s disease (PD),4 multiple sclerosis (MS), and poststroke (PS).5 Clinical interventions adopted for the rehabilitation of balance and gait usually rely on principles of neuroplasticity and motor learning, sometimes referred as motor learning strategies.6,7 These interventions aim to promote personalized training tailoring individual impairments in order to improve sensory, motor, and cognitive skills through intensive, task-oriented repetitive training.8 In particular, enhanced motor-cognitive dual-task ability appears to improve balance, gait, and cognition.9,10 Clinicians make use of different tools and training methods to facilitate the incorporation of motor learning strategies and to optimize the rehabilitation process. Virtual reality (VR) is one such emerging tool that has proved to have additional benefits in the rehabilitation of balance and gait.11
A growing body of literature recognizes the advantages of VR-based rehabilitation in neurological conditions.10,11 Such advantages stem from the capacity of VR to increase motivation and enhance motor learning.12,13 For instance, VR has the ability to provide real-time intrinsic and extrinsic multisensory feedback, and facilitates task variation through the application of various virtual environments that simulate real and daily life tasks.12,13 Despite extensive research, the transition to clinical implementation of VR-based rehabilitation is still in its initial steps. To our knowledge, only one study has reported on the clinical experience of using VR in a medical center.14 Moreover, recent joint multicenter efforts are setting the basis for the clinical development and advance of VR as a standardized approach.15 This indicates a need to understand and overcome the challenge surrounding the long-term implementation of VR in clinical practice. For instance, the suitability of VR as a therapeutic tool, the patients’ adaptability towards the technology, and clinician-selection of VR environments and tasks for targeting individual therapeutic goals need to be developed.
To contribute to the accomplishment of this objective, this study gives a retrospective account of the initial 3 years of clinical experience using VR-based rehabilitation in a large medical center, by examining clinical records of patients with distinct neurological conditions, including PD, PS, MS, traumatic brain injury (TBI), myelopathy, and cerebral palsy. Here, we describe the design and methods of the VR-based interventions, and the instruments to measure balance and gait; the characteristics of the VR systems and virtual environments used across interventions; the effects on balance and gait; and patient perception of the suitability of VR for their rehabilitation process. The main aim of this study is to provide clinicians and researchers with a critical examination of the challenges and outcomes involved with the implementation of VR in clinical practice. The study research questions were whether VR training is practicable in clinical routine, and whether it is effective for improving balance and gait.
Methods
This is a retrospective analysis of clinical records of patients who received routine VR-based rehabilitation in a large rehabilitation center. The study evaluated balance and gait outcomes from neurological and non-neurological (NN) patients exposed to VR-based rehabilitation treatments during 3 years (from November 2014 until March 2018), in the Center of Advanced Technologies in Rehabilitation (CATR) at the Sheba Medical Center, Ramat Gan, Israel. The Sheba Medical Center Institutional Review Board (IRB) approved the collection and use of patients’ records according to regulations for clinical trials in humans (IRB approval No. 3962-17-SMC). The need for informed consent was waived by IRB, since the study was based on a retrospective charts review of clinical data.
VR-based rehabilitation and medical record analysis
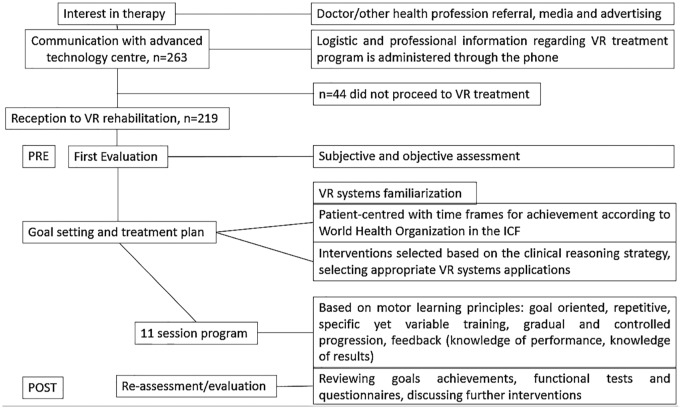
The flow diagram of the 263 patients referred to VR-based rehabilitation is shown in Figure 1. As part of the routine health service, the patients self-approached the VR facilities of CATR or were referred by health professionals. Referral sources were the various in- and out-patient clinics of the Sheba Medical Center, or external physicians with previous knowledge about the VR-based rehabilitation conducted in CATR. A team of four physical therapists that applied treatments and two technicians for operation of VR systems were involved at all stages of the VR-based treatments. Each rehabilitation treatment comprised 12 sessions; a full assessment session (PRE) was performed at session 1 and session 12 (POST). The number of treatments varied between patients.
Figure 1.
Flow diagram showing treatment steps as part of the routine health service. Each session starts and progresses from the point and achievement of any previous recorded session in each patient. Patients sit or stand when they need to rest during the session. Subjective assessment consists of chart review for medical history, interview patient/family: social history, level of functioning and personal needs, patient’s goals and concerns. Objective assessment comprises observation and examination, functional tests and questionnaires, identifying impairments contributing to loss of function and movement.
ICF: International Classification of Functioning, Disability and Health.
Analysis of medical records for the present study
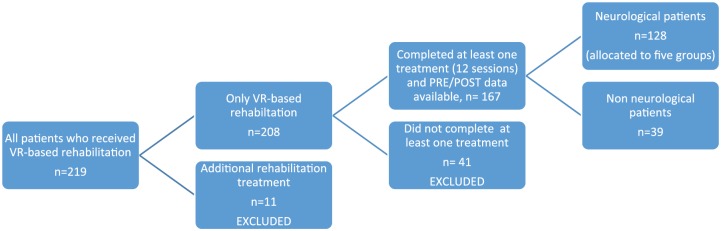
The flow diagram of medical records inclusion/exclusion to analysis is summarized in Figure 2. In order to reach homogeneity for outcome analysis, inclusion/exclusion criteria for patient records to be analyzed were set as follows (Figure 1): Inclusion criteria for records were: completion of at least one rehabilitation treatment (12 sessions), and presence of a PRE–POST clinical evaluation. Exclusion criteria for records were: no completion of at least one rehabilitation treatment, or already under additional, conventional, rehabilitation treatment. In order to detect those records to be included/excluded, we first reviewed the medical records of all patients, focusing on the number of received treatments and presence of PRE/POST data in the records.
Figure 2.
Flow diagram showing medical record inclusion/exclusion for analysis. Of the 263 initially eligible persons, 44 did not proceed to treatment and 11 received additional treatments. Thus, only 208 patients initiated VR treatments, 41 of which were excluded because they did not complete at least one treatment.
Included records were allocated to patient groups according to most frequent neurological conditions: PD, Parkinsonism, PS, MS, and TBI. Patients with PD and Parkinsonism formed a combined group: ‘PD’. A group of other neurological (ON) conditions allocated patients with neurological conditions such as myelopathy and cerebral palsy. NN patients, for example, with lower limb injury, above-knee amputation, or low back pain, were included as additional group for comparison purposes.
Equipment
Four VR systems were used: the Computer Assisted Rehabilitation Environment (CAREN) High-End, or CAREN Dome, the CAREN Base, the V-Gait, and the C-Mill (all from Motekforce Link, Amsterdam, The Netherlands). Clinicians had 23 rehabilitative applications (virtual environments) available to apply in each treatment session according to therapeutic goals. Table e1 summarizes the characteristics of the virtual environments applied most frequently during the reported VR treatments. A motion capture system (Vicon, Oxford, UK) tracked the movement of passive markers located in body regions relevant for the function of each virtual environment.
Exposure
Each rehabilitation treatment comprised 12 sessions: a full assessment session (PRE) and 11 tailored training sessions of 30–45 min each (Figure 1). Each session focused on balance and gait rehabilitation, and on the application of cognitive load. Treatment sessions could include more than one VR environment. A reassessment took place after session 12 of treatment (POST). Only outcomes from the first treatment were used for data analysis (some patients had more than one rehabilitation treatment, as detailed in Results).
Treatments were personalized and intended to promote motor learning. Irrespective of the VR system or virtual environment, clinicians aimed to exploit the advantages of VR for promoting task-oriented repetitive training and other motor learning principles such as sensory feedback, task variation, and progression (Table e1).
Outcome measures
To evaluate the effects of the treatments, we report on standardized gait and balance assessments performed both before (PRE) and after (POST) treatment as part of clinical monitoring. These included the 10 Meter Walk Test (10MWT);16 the Timed Up and Go (TUG);17 the Four Square Step Test (FSST);18 the Berg Balance Scale (BBS),19 and the Mini Balance Evaluation systems Test (Mini BESTest).20
To evaluate the effect of cognitive load, single-task (ST) and motor-cognitive dual-task (DT) modalities of the TUG and 10MWT were used. During the 10MWT assessment, using body-worn sensors, the APDM Opal wireless system (APDM, Portland, OR, USA)21 evaluated spatiotemporal gait parameters in both ST and DT conditions. The dual-task cost was calculated according to the equation DT-cost = 100*(ST outcome – DT outcome)/ST outcome.22
To evaluate changes in confidence on performing balance and locomotion tasks and the suitability of VR systems in the rehabilitation of balance and gait, the Activities-Specific Balance Confidence Scale (ABC Scale)23 and the Suitability Evaluation Questionnaire (SEQ),24,25 respectively, were used.
To recognize the most frequently applied virtual tasks among neurological cohorts, we counted the VR systems and the virtual environments performed per patient during treatment sessions.
Statistical analysis
Statistical analysis was performed on data obtained from the first 12 training sessions (i.e. first treatment) for each patient. We computed one-way ANOVA for comparison of outcome change [i.e. 100*(POST – PRE)/PRE] among groups, and paired two-tailed t-tests for comparisons PRE versus POST treatment. Data are presented as mean ± standard deviation unless otherwise stated. We conducted analyses for all combined groups as well as for each subgroup. To evaluate clinically significant changes, we followed previously published values of Minimal Detectable Change or Minimal Clinically Important Difference (MCID) (Table e2). Statistical tests were performed using a numerical computing software (Matlab; The Mathworks, Natick, MA). Statistical significance was set to p < 0.05.
Results
The demographic data of patients whose records were included in the analysis (see Figures 1 and 2) are depicted in Table 1. The neurological group included 128 patients (allocated to five groups). The non-neuroloical group (NN) included 39 persons with increased risk of falls (n = 15), cancer (n = 11), non-neurological gait abnormality (n = 5), lower limb injury (n = 3), above-knee amputation (n = 3), low back pain (n = 1), and polymyalgia rheumatica (n = 1) (Table 1).
Table 1.
Demographic characteristics of the patients.
| Cohort | N | Age mean ± SD | Male/Female | Patients (%) |
|---|---|---|---|---|
| Parkinson’s disease and Parkinsonism | 36 | 72.1 ± 8.8 | 26/10 | 22 |
| Poststroke | 31 | 68.2 ± 14.1 | 24/7 | 19 |
| Multiple Sclerosis | 9 | 53.1 ± 12.2 | 2/7 | 5 |
| Traumatic Brain Injury | 10 | 40.3 ± 20.1 | 6/4 | 6 |
| Other neurological | 42 | 65.1 ± 18.2 | 23/19 | 25 |
| Non-neurological | 39 | 65.5 ± 21.0 | 18/21 | 23 |
| Total | 167 | 63.8 ± 18.9 | 99/68 | 100 |
Data analysis was based only on initial treatments for each patient (167, highlighted). Other neurological conditions included patients with myelopathy (n = 19), polyneuropathy (n = 5), cerebral palsy (n = 4), polio (n = 4), Guillain-Barre syndrome (n = 2), vertigo (n = 3), ataxia (n = 2), cerebellar ataxia (n = 1), vestibular Schwannoma (n = 1), and hyperekplexia (n = 1). Non-neurological conditions included patients with recurrent falls (n = 15), cancer (n = 11), non-neurological gait abnormality (n = 5), lower limb injury (n = 3), above-knee amputation (n = 3), low back pain (n = 1), and polymyalgia rheumatica (n = 1).
Efficacy of VR-based treatments
For all patients combined, VR-based rehabilitation significantly improved gait speed during single and dual-task conditions (10MWT-ST, p = 0.0011 and 10MWT-DT, p = 0.0025), functional mobility with cognitive load (TUG-DT, p = 0.0142), dynamic balance and risk of falling (BBS, p = 0.0003 and Mini BESTest, p = 0.0000) (Table 2). No differences were observed in TUG-ST and FSST in the comparison PRE versus POST treatment.
Table 2.
Balance and gait measures PRE and POST treatments.
| Measure | No. patients | PRE-treatment | POST-treatment | p* |
|---|---|---|---|---|
| 10MWT-ST Gait speed (m/s) | 155 | 0.88 ± 0.36 | 0.93 ± 0.38 | 0.0011 |
| 10MWT-DT Gait speed (m/s) | 127 | 0.78 ± 0.29 | 0.83 ± 0.30 | 0.0025 |
| TUG - ST (s) | 157 | 19.56 ± 28.84 | 18.72 ± 31.77 | 0.3753 |
| TUG - DT (s) | 124 | 19.24 ± 11.55 | 17.87 ± 12.10 | 0.0142 |
| FSST (s) | 118 | 13.63 ± 6.99 | 13.65 ± 8.47 | 0.5863 |
| BBS (score) | 31 | 33.52 ± 14.74 | 37.48 ± 15.29 | 0.0003 |
| Mini BESTest (score) | 120 | 16.48 ± 4.94 | 18.38 ± 5.34 | 0.0000 |
Values and statistical analysis relate to patients in which each measure was assessed both PRE and POST. *: significant (p <0.05) values are shown in bold.
10MWT, 10 Meter Walk Test; BBS, Berg Balance Scale; DT, dual task; FSST, Four Square Step Test; Mini BESTest, Mini Balance Evaluation systems; ST, single task; TUG, Timed Up and Go.
Table 3 shows the PRE and POST treatment outcomes for each variable within each subgroup. There were no statistically significant differences of change among groups (p > 0.05, one-way ANOVA). Figure 3 depicts the treatment effects as score changes PRE versus POST treatment for each subgroup for analysis of clinical outcomes. Thresholds for minimal detectable change or MCID were taken from references published in the literature for each measure, and were found only for some measures and only for PD, PS, and TBI (for threshold values and references, see Table e2). Comparison of balance and gait measures showed that most PD, PS, and TBI patients had gains after treatments. For example, 60% of TBI, 33% of PS, and 20% of PD patients surpassed MCID in BBS. Additionally, TUG scores improved in 28% and 26% among patients with PD and PS, respectively, while the rate of clinical improvement in measures of 10MWT ranged from 10% to 14%.
Table 3.
Comparison of balance and gait measures PRE and POST treatments within subgroups.
| Measure | PD |
PS |
MS |
TBI |
ON |
NN |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | PRE | POST | N | PRE | POST | N | PRE | POST | N | PRE | POST | N | PRE | POST | N | PRE | POST | |
| 10MWT-ST Gait speed (m/s) |
35 | 1.01 ± 0.05 | 1.03 ± 0.05 | 28 | 0.72 ± 0.07 | 0.77 ± 0.08 | 9 | 0.93 ± 0.16 | 0.90 ± 014 | 10 | 0.68 ± 0.14 | 0.74 ± 0.15 | 37 | 0.90 ± 0.05 | 0.92 ± 0.05 | 36 | 0.90 ± 0.06 | 0.97 ± 0.06* |
| 10MWT-DT Gait speed (m/s) |
30 | 0.86 ± 0.05 | 0.92 ± 0.06 | 22 | 0.71 ± 0.06 | 0.76 ± 0.07 | 8 | 0.74 ± 0.15 | 0.77 ± 0.14 | 5 | 0.76 ± 0.14 | 0.75 ± 0.10 | 31 | 0.76 ± 0.05 | 0.78 ± 0.04 | 30 | 0.77 ± 0.05 | 0.87 ± 0.05* |
| TUG - ST (s) | 35 | 14.34 ± 1.79 | 13.16 ± 1.46 | 29 | 29.03 ± 8.15 | 27.56 ± 6.37 | 9 | 22.85 ± 9.58 | 17.70 ± 5.95 | 9 | 27.62 ± 11.70 | 26.63 ± 11.05 | 38 | 12.88 ± 0.76 | 12.07 ± 0.69* | 36 | 15.36 ± 1.68 | 13.47 ± 1.31 |
| TUG - DT (s) | 29 | 17.94 ± 1.25 | 17.80 ± 2.35 | 20 | 25.98 ± 4.10 | 23.00 ± 3.37 | 8 | 24.17 ± 8.47 | 24.73 ± 9.86 | 5 | 18.52 ± 3.21 | 17.16 ± 1.97 | 31 | 17.22 ± 1.20 | 15.79 ± 1.00* | 27 | 16.62 ± 1.29 | 14.72 ± 0.90* |
| FSST (s) | 21 | 11.25 ± 0.71 | 10.44 ± 0.78* | 12 | 13.87 ± 1.55 | 14.60 ± 2.31 | 6 | 12.92 ± 4.92 | 13.38 ± 4.60 | 2 | 19.13 ± 6.32 | 15.36 ± 4.87 | 19 | 14.35 ± 2.08 | 15.79 ± 2.53 | 20 | 14.63 ± 1.33 | 14.00 ± 1.31 |
| BBS (score) | 5 | 33.20 ± 8.92 | 37.40 ± 8.88 | 6 | 26.33 ± 5.74 | 29.67 ± 6.52 | 1 | 37 | 36 | 5 | 26.00 ± 5.94 | 29.00 ± 9.55 | 9 | 44.56 ± 2.96 | 48.22 ± 2.29* | 5 | 29.40 ± 6.38 | 36.00 ± 6.88 |
| Mini BESTest (score) |
28 | 17.96 ± 0.86 | 20.43 ± 0.70* | 22 | 16.77 ± 1.19 | 18.59 ± 1.23* | 7 | 15.86 ± 2.50 | 17.00 ± 2.47* | 6 | 14.83 ± 2.21 | 15.5 ± 2.83 | 27 | 14.89 ± 0.80 | 17.11 ± 1.15* | 30 | 16.80 ± 0.86 | 18.33 ± 0.89* |
Data are presented as mean ± error.
p < 0.05 in pre versus post comparison.
10MWT, 10 Meter Walk Test; BBS, Berg Balance Scale; DT, dual task; FSST, Four Square Step Test; Mini BESTest, Mini Balance Evaluation systems; N, number of patients; ST, single task; TUG, Timed Up and Go.
Figure 3.
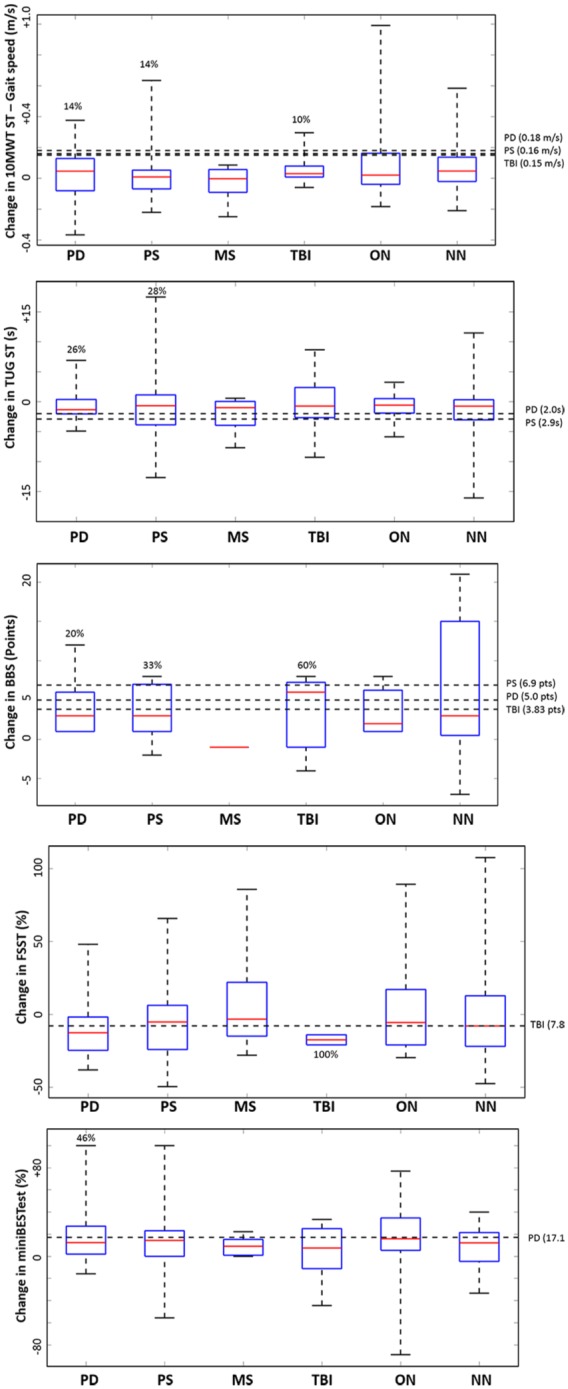
Treatment effects on balance and gait. Boxplots represent subgroup data. The central red line in each box indicates the median, and the upper and lower edges third and first quartile, respectively. Horizontal dashed lines indicate clinical thresholds, that is, MCID or MDC. Thresholds were taken from references published in the literature for each measure, and were found only for some measures and only for PD, PS, and TBI (for threshold values and references see Table e2). Percentages accompanying boxplots indicate the number of patients that, over completing the first treatment, successfully surpassed clinical thresholds. Values >0 indicate improvements for 10MWT, BBS, and miniBEST. Values <0 indicate improvements for TUG and FSST.
10MWT, 10 Meter Walk Test; BBS, Berg Balance Scale; FSST, Four Square Step Test; MiniBESTest, Mini Balance Evaluation Systems Test; MDC, minimal detectable change; MCID, minimal clinically important difference; MS, Multiple Sclerosis; NN, non-neurological conditions; ON, other neurological; PD, Parkinson Disease; PS, Poststroke; TBI, Traumatic Brain Injury; TUG, Timed Up and Go.
Table 4 reports on cognitive dual-task cost examined in 32 patients who completed the OPAL assessment. PS patients successfully decreased cognitive DT-cost while walking at POST versus PRE treatment for stride time (p = 0.0229) and for step time (p = 0.0053). There has been a statistically marginal (p = 0.0936) decrease in DT-cost within patients with PD post-treatment as well for stride time. No significant reduction in DT-cost for step time or stride time was observed in other populations.
Table 4.
Comparison of single-task, cognitive dual-task and dual-task cost while walking PRE and POST treatment.
| Parameter | ST PRE | ST POST | DT PRE | DT POST | DT-cost PRE | DT-cost POST | p* |
|---|---|---|---|---|---|---|---|
| Stride time (s) | |||||||
| PD and Parkinsonism | 1.17 ± 0.09 | 1.14 ± 0.11 | 1.32 ± 0.16 | 1.25 ± 0.17 | –13.41 ± 11.69 | –8.77 ± 8.25 | 0.0936 |
| Poststroke | 1.27 ± 0.16 | 1.26 ± 0.23 | 1.38 ± 0.15 | 1.32 ± 0.20 | –9.13 ± 5.51 | –4.86 ± 3.73 | 0.0229 |
| Other neurological | 1.21 ± 0.21 | 1.24 ± 0.22 | 1.39 ± 0.31 | 1.43 ± 0.38 | –13.82 ± 12.99 | –14.34 ± 13.36 | 0.7997 |
| Non-neurological | 1.25 ± 0.15 | 1.17 ± 0.12 | 1.41 ± 0.17 | 1.31 ± 0.17 | –13.11 ± 5.86 | –11.75 ± 8.39 | 0.8309 |
| Step time (s) | |||||||
| PD & Parkinsonism | 0.59 ± 0.05 | 0.58 ± 0.05 | 0.66 ± 0.07 | 0.63 ± 0.08 | –12.43 ± 11.26 | –8.27 ± 8.45 | 0.1957 |
| Poststroke | 0.63 ± 0.12 | 0.62 ± 0.13 | 0.69 ± 0.12 | 0.63 ± 0.12 | –10.16 ± 9.44 | –3.90 ± 5.73 | 0.0053 |
| Other neurological | 0.60 ± 0.13 | 0.61 ± 0.13 | 0.67 ± 0.17 | 0.70 ± 0.18 | –11.52 ± 9.97 | –14.90 ± 14.12 | 0.3154 |
| Non-neurological | 0.62 ± 0.08 | 0.58 ± 0.06 | 0.71 ± 0.10 | 0.65 ± 0.08 | –13.51 ± 5.93 | –11.64 ± 7.04 | 0.7638 |
Significant (p <0.05) values are shown in bold. p values refer to DT-cost (PRE versus POST). DT-cost = 100×(ST outcome – DT outcome)/ST outcome.
Parkinson Disease (PD) and Parkinsonism (n = 9 patients), Poststroke (n = 9), ‘Other neurological’ conditions (n = 10; comprising four with myelopathy, two with multiple sclerosis, one with traumatic brain injury, one with cerebral palsy, one with vertigo, and one with vestibular Schwannoma) and non-neurological conditions (n = 4).
All patients presented an overall improvement in the confidence for performing balance and ambulatory activities PRE and POST VR treatment (ABC scores: 68.82 ± 20.38 versus 72.04 ± 20.56, p = 0.0002) and 29% of PD patients surpassed MCID (Figure 4). Perception on VR suitability (Table e3) was particularly positive for levels of enjoyment (e.g. median = 5 in the response to question 1; i.e. highest score) and immersion (e.g. median = 5 and 4 in response to question 2 and 5, respectively; i.e. highest and second highest scores). On the other hand, suitability perception was less conclusive in regards to perception on task difficulty and successfulness in the system (questions 3 and 12; median = 3 in each).
Figure 4.
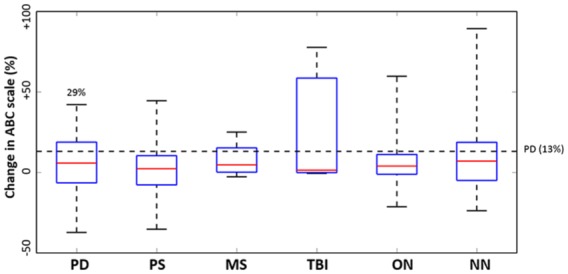
Treatment effects on the Activities-Specific Balance Confidence (ABC) Scale. The central red line in each box indicates the median, and the upper and lower edges third and first quartile, respectively. Subgroups include MS, multiple sclerosis; NN, non-neurological conditions; ON, other neurological; PD, Parkinson disease; PS, poststroke; TBI, traumatic brain injury. The horizontal dashed line represents the minimal detectable change (MDC) in PD. Percentages accompanying PD boxplot indicate patients that, over completing the first treatment, successfully surpassed clinical threshold (MDC); 29% of PD patients surpassed the clinical threshold of 13% (for cohorts with known MCID/MDC, for published values see Table e2).
Use of VR systems and environments
We observed heterogeneity in the use of VR systems and choices of treatments. The most-used VR systems were the CAREN Base and CAREN Dome, followed by the V-Gait, and, to a lesser extent, the C-mill (Table 5). However, the C-mill was unavailable to the clinical team during large extent of the assessed period, and, to a lesser extent, the CAREN Dome was unavailable during some periods. Moreover, VR environments more frequently used were Boat, Road walk, Road obstacle, Road stand, and Cradle reach. They represented 78.9% of all VR environments applied during sessions (Table 5). Table e1 describes the most relevant VR environments and their therapeutic goals.
Table 5.
Virtual reality systems and environments per group.
| Group | PD | PS | MS | TBI | ON | NN | Total |
|---|---|---|---|---|---|---|---|
| VR System (percentage within population, %) | |||||||
| Base | 25.9 | 30.0 | 45.2 | 31.1 | 39.5 | 44.4 | 35.7 |
| Dome | 39.0 | 37.2 | 27.1 | 41.3 | 27.9 | 28.4 | 33.3 |
| V-gait | 28.7 | 26.6 | 24.0 | 22.3 | 31.4 | 24.5 | 26.9 |
| CMILL | 6.4 | 6.2 | 3.7 | 5.3 | 1.2 | 2.7 | 4.1 |
| VR Environment (percentage within VR environment, %) | |||||||
| Boat | 14.6 | 17.9 | 28.8 | 18.3 | 19.9 | 21.5 | 19.5 |
| Road walk | 17.1 | 22.0 | 11.5 | 22.6 | 18.2 | 16.1 | 18.4 |
| Road obstacle | 26.1 | 15.4 | 15.8 | 14.5 | 16.8 | 13.6 | 16.8 |
| Road stand | 13.0 | 17.3 | 19.7 | 8.7 | 10.8 | 14.1 | 13.8 |
| Cradle reach | 11.0 | 9.2 | 8.8 | 9.4 | 11.2 | 11.2 | 10.4 |
| Surf | 0.7 | 1.7 | 0.6 | 11.2 | 8.7 | 2.5 | 4.2 |
| Cradle balls | 3.6 | 3.9 | 2.7 | 2.7 | 3.2 | 5.6 | 3.9 |
| Forest | 3.0 | 4.2 | 1.2 | 2.2 | 3.8 | 4.8 | 3.6 |
| Active balance | 1.6 | 2.0 | 3.3 | 2.7 | 2.1 | 2.1 | 2.2 |
| Counter balance | 0.9 | 0.3 | 3.3 | 3.4 | 0.9 | 2.8 | 1.5 |
| Cradle shape | 2.1 | 0.7 | 1.8 | 2.2 | 1.4 | 1.1 | 1.4 |
| Endless road | 1.8 | 1.0 | 0.6 | 0.0 | 0.8 | 1.7 | 1.1 |
| CMILL* | 1.4 | 2.3 | 1.2 | 1.1 | 0.0 | 0.0 | 1.0 |
| Cradle feet balls | 0.3 | 0.8 | 0.0 | 0.0 | 0.5 | 2.2 | 0.8 |
| Road avoid | 0.4 | 0.1 | 0.6 | 0.7 | 1.0 | 0.7 | 0.6 |
| Road obstacle hide | 0.0 | 0.9 | 0.0 | 0.0 | 0.7 | 0.2 | 0.4 |
| Corridor | 1.6 | 0.3 | 0.0 | 0.2 | 0.0 | 0.0 | 0.3 |
| Pert Train | 0.4 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 |
| Road gain | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
The count represents the ratio percent in which each VR system/environment was used.
VR environments performed in the C-Mill (Stepping-stones, Obstacles, Track, Tandem, Slalom).
MS, multiple sclerosis; NN, non-neurological conditions; ON, other neurological; PD, Parkinson’s disease; PS, poststroke; TBI, traumatic brain injury; VR, virtual reality.
For informative purposes, we report on the number of received treatments. Included patients underwent one rehabilitation treatment (n = 114) or more treatments: two (n = 28), three (n = 9), four (n = 6), five (n = 3), six (n = 3), seven (n = 1), eight (n = 1), or nine (n = 2). In total, 287 treatments were conducted. Exceptionally, 10 patients conducted five or more treatments, with 2 patients with PD and polio having nine treatments within 3 years. Among neurological cohorts, patients with PS and PD had the highest number of treatments (62 and 52, respectively), while 18 and 26 treatments were conducted in patients with MS and TBI. ON and NN patients underwent 73 and 56 treatments, respectively. As mentioned in the Methods, PRE and POST evaluations relate to the first treatment only.
Discussion
This study reports on the use of advanced VR technologies in routine clinical practice and describes the implementation of VR-based rehabilitation of balance and gait across patients with different neurological conditions, and patients without neurological diagnosis as well. Our results suggest that using VR is viable and effective when implemented in a clinical routine manner. This builds upon a large body of literature concluding that VR-based rehabilitation brings advantages over conventional rehabilitation for incorporating motor learning principles.11 There are many challenges related to the incorporation of VR technology as a routine therapeutic tool,15 mainly due to the relatively large number of degrees of freedom that the technology allows, for example, types of VR environments and feedback scenarios. In light of this heterogeneity, this naturalistic study reports on the effects of VR-based treatments on balance and gait, the design and application of these clinical treatments, the use of VR as a rehabilitation tool, and patients’ perception of VR usability and effectiveness.
Effects on balance and gait
Our results suggest that VR-based rehabilitation led to improvements in balance and gait. In particular, walking speed (with and without cognitive load) and functional mobility with (BBS, Mini BESTest) and without cognitive dual tasks (TUG-DT) (Table 2). In the latter case, we observed a significant post-treatment reduction in DT-cost while walking among patients with PS (Table 4). Patients with PD showed a tendency to decrease DT-cost as well.
We observed clinically significant progress in patients with TBI (e.g. BBS and FSST assessments), PD (e.g. BBS, miniBESTest and TUG), and PS (e.g. BBS and TUG) (Figure 3). Due to the unavailability of clinical thresholds, we were unable to determine specific rates of significant clinical changes for the remaining neurological and NN conditions; however, most patients had positive treatment effects marked by an overall improvement (over 50%) in functional measures (see Median values in Figure 3). Our findings also suggest that VR-based rehabilitation increases patients’ confidence to perform balance and gait tasks (Figure 4). Nearly one-third of patients with PD had a clinical significant change after treatment.
The biological plausibility of VR-based rehabilitation lies in the ability to induce cortical reorganization (e.g. in chronic PS),26 and to enhance experience-dependent neural plasticity through the incorporation of such motor learning principles as real-time feedback, focus of attention and implicit learning.8,13,27 VR also facilitates objective progression and task variation and specificity, subsequently better allowing the application of motor learning strategies during rehabilitation treatments.7,12 However, small samples could compromise finding statistically significant differences in some cases. For instance, although no difference PRE versus POST was seen in TBI patients for changes in BBS and FSST (Table 3), 60% and 100% of these patients, respectively, showed clinically significant results for these outcomes after treatment (Figure 3).
There might be several reasons to find no outcome differences within some subgroups. For instance, muscle weakness and fatigue are known to affect walking speed (and gait in general) during rehabilitation treatments in PD patients.28 Variables including age and disease severity highly influence balance and gait rehabilitation in TBI patients.29 Similarly, there are also PS conditions in which balance outcomes such as TUG tend to deteriorate.30 In MS patients, other studies using VR-based rehabilitation did not find significant differences in BBS outcomes,31 and one study concluded that VR (i.e. Nintendo Wii Fit) does not provide significant improvements.32 Moreover, the high heterogeneity of the sample could have hindered the finding of statistically significant differences in the comparison of outcomes among groups.14 We deem VR as a rehabilitation tool that can be complemented with other methods of treatment. Therefore, we suggest having a rigorous individualized evaluation, seeking to incorporate the best possible motor learning strategy prior to rehabilitation, regardless of the method of treatment.
Our findings are mostly consistent with a recent systematic review that evaluated 97 studies reporting the efficacy of VR-based rehabilitation in improving balance and gait in patients with neurological conditions, especially regarding specific gains observed in BBS, TUG, and 10MWT. 11 It is important to highlight that (unlike the treatments reported here) some of those studies complemented VR with conventional and alternative therapies. Thus, the present study reinforces the potential of VR alone for rehabilitation in clinical settings. Our results are comparable with those of another clinical study, which found significant improvements in TUG and FSST measures after 5 weeks of treadmill training combined with VR.14 However, the authors did not find differences in gait speed or TUG change among subgroups with different pathologies, and further reported no significant improvements on gait speed (i.e., with reference to the comparison before and after training). Our study further contributes to the literature by providing tools for the design and planning of theory-driven VR-based rehabilitation.
Design and planning of VR-based interventions
A major objective of this study was to identify the main characteristics involved in the design and methods used for VR-based rehabilitation. We observed a wide spectrum of treatment choices and a lack of standardized VR subprotocols. Key aspects for a therapeutically valid rehabilitation approach are theoretically driven planning of training intensity, and a personalized treatment plan adapted for each individual.11,33 Thus, in the first session of the reported VR-based treatments, clinicians conduct a meticulous evaluation of the sensory, motor, and cognitive abilities and deficits of each individual (Figure 1). The evaluation considers prior clinical diagnoses, previous treatments, recent falls, medication, and physical activity. Familiarization activities also take place in the VR facilities. Based on this first (evaluation) session, intervention parameters are defined and determine the specific contextual needs of the patient: the content of the VR-based intervention (e.g. suitable VR environments, dosage); and training components that will be adjusted during and between sessions as part of the treatment progression.34 Therapeutic goals are also set before treatment and based on the International Classification of Functioning, Disability and Health (ICF),35 according to impaired activity and participation. Setting the right training intensity should incorporate at least two variables: progression (i.e. adjustments on the level of difficulty) and motivation.11 Such strategy is in accordance with the flow channel theory, in which the right challenge in a therapeutic session is considered a balance to avoid frustration (challenges that are too hard) or boredom (challenges that are too easy).36
Clinicians have a battery of instruments to measure balance and gait. Selecting a proper measure depends on the clinical condition of the patient and the therapeutic goal of the VR-based intervention. The measures mostly used in the reported VR-based interventions are consistent with those commonly found in the literature to assess balance (e.g. TUG, BBS) and gait (e.g. 10MWT).11 However, we observed a lack of homogeneity in applied balance and gait measures (Table 2). The fact that applied balance and gait measures were inconsistent among patients and treatments may suggest a lack of standardization of the clinical assessment protocol. This is not surprising since most of these functional measures are commonly used in clinical research protocols rather than in the physician’s office. However, given the complexity involved in planning and designing personalized VR-based treatment protocols, we call on the community to standardize the assessments and tests according to the personalized interventions. Longitudinal follow-ups at patient level, as well as at the level of the healthcare provider, will also benefit from standardization of assessments to monitor clinical efficacy within and across centers.
Prior to each VR-based intervention and additional to treatment steps (see Figure 1), a high-level protocol for the incorporation of theory-driven VR-based rehabilitation11 should be designed. The protocol should follow the therapeutic validity requirements general to exercise interventions,33 and should be specific to VR, to comprise (but not be limited to) the following work items:
(1) Identifying both clinician and patients’ acceptability to VR: to examine the risk of cybersickness (e.g. dizziness) and the level of visual dependency.37,38
(2) Recognizing VR tools: to determine the therapeutic and motor learning characteristics offered by the VR systems and environments available for treatment (e.g. see Table e1).39
(3) Matching VR tools with individual rehabilitation goals: to identify suitable VR systems/environments for the treatment of each individual. If no suitable VR tool is identified, consider rehabilitation alternatives (e.g. conventional therapy).
(4) Regulating VR intensity/dosage: we recommend following the flow theory and balance task difficulty according to patients’ motivation.40,41 Such VR properties as objective progression and external feedback (e.g. see Table e1) are particularly useful for this aim.11
(5) Adherence: to evaluate patients’ satisfaction with VR-based rehabilitation at the end of each session. Compare suitability with that of other rehabilitation tools (e.g. conventional therapy).
VR systems and environments
We aimed to describe the application of VR. Medical centers or laboratories intending to undergo clinical implementation of VR-based rehabilitation should have a precise understanding of the systems and environments available, and their functionality.15 Both clinical and research teams periodically analyze the VR systems and environments reported here (see Tables 5 and e1), and discuss their characteristics and suitability for rehabilitation according to therapeutic goals. For example, one VR environment may address specific gait deficits and be suitable to conduct obstacle negotiation tasks (e.g. ‘road obstacle’), another can be useful for weight-shifting tasks (e.g. ‘boat’, ‘surf’), and other may focus on visual-spatial training (e.g. ‘road stand’). Our clinical team has access to four VR systems and 23 VR environments, certainly permitting a large number of degrees of freedom. While it is impractical to design a priori ‘algorithms’ that refer to all possibilities, a rationalized outlined approach is encouraged. It is possible to use a spectrum of ‘small’ VR tasks aimed at specific deficits, and to use their different combinations to individually tailor the algorithm according to affected neurological systems. For example, let us consider the case of a person with balance deficits evidenced by a history of falls. A potential therapeutic goal is improving the control of center-of-mass variations. Virtual tasks from the environments ‘road stand’, ‘boat’ or ‘cradle reach’ may be assigned to such patient. Another example is a patient with difficulties for sorting obstacles out; in such a case the patient may be assigned to environments such as ‘road walk’, ‘road obstacle’ or ‘forest’ (or a combination thereof).
As the benefits of VR rely on its capacity to facilitate incorporation of motor learning principles,12,13 we recommend recognizing and evaluating the characteristics of the VR systems and environments that can potentially promote such principles as extrinsic and intrinsic feedback, task variation, and implicit learning. Another important VR characteristic is immersion. For example, owing to its 360° room-size shaped screen, the CAREN Dome allows a more enhanced level of immersion than the rest of VR systems in our facilities. Hence, taking therapeutically valid and theoretically driven choices in VR-based rehabilitation relies on understanding the potential of VR for exploiting motor learning. Thus, one of the recommendations of the present study is that professional specialization is needed to those clinicians treating with VR-based interventions. Further research is needed to better understand the effects of specific VR systems and environments on individual outcome measures.
Patients’ perception of VR usability and effects
One interesting finding is that some patients enrolled to multiple VR-based rehabilitation treatments (Table 1). We interpret this as an indication of acceptability. For instance, between 40% and 50% of patients with PS, TBI, and other neurological conditions (i.e. the ON group) attended more than one full treatment, while among patients with PD, MS, and NN conditions, it was between 17% and 23%.
On the other hand, while the reasons for not completing at least 12 sessions (i.e. one treatment) were not available in the records, the fact that 41 persons out of the 208 who initiated VR (41/208, 19%), did not complete at least one treatment may point to a subgroup that may not be suitable for VR-treatments for clinical/acceptability bias. Further studies should also focus on reasons for such drop-outs in order to remediate possible bias.
The SEQ24,25 questionnaire is particularly relevant since it evaluates the perception of every individual towards VR as a rehabilitation tool, and the effect of VR in the rehabilitation process. The most positive feedback obtained was in regards to topics such as enjoyment, immersion, clarity, and easiness of the VR system, and lack of discomfort or adverse events (e.g. dizziness) (Table e3). Some aspects identified to be insufficient were, namely, feeling of successfulness and of difficulty in completing the task in the VR environment.
These findings are pertinent given the novelty of VR in clinical routine for rehabilitation, and for a successful transition to clinical implementation of VR.
Strengths and limitations
The strength of our study is the large number of VR treated patients and our vast experience in a variety of clinical conditions and treatments. This study analyzed the implementation of VR-based rehabilitation of balance and gait in 167 patients with different neurological and NN conditions. Our additional strength stems from our focus on VR-related rehabilitation, which represents a relevant and timely theme in the world scenario of chronic diseases, and the increase of therapeutic options for the rehabilitation of neurological dysfunctions.
Indeed, VR-based rehabilitation in ecologically valid environments has been suggested to be more effective than conventional training, for example, for cognitive training in stroke patients.42 However, one technical limitation observed stems from the fact that the VR system does not exactly expose patients to real life situations (i.e. VR tasks are still game-like and not realistic enough). This affects the ability to treat actual patients’ goals. We believe that with the advance and development of computer science, more realistic and oriented VR environments and tasks will be possible in the short term.
Several limitations relate to the study design and data analyses. While randomized controlled trials and cross-sectional studies may present with a rigorous design and control of experimental stages, routine clinical practice is subject to hospital organization and patient availability.
The retrospective nature of the present study presents limitations. Ideally, subjects should be recruited to VR treatments according to inclusion criteria posed before treatments, thus generating homogenous clinical groups for outcome measures. In the present study, we analyzed retrospective data on heterogeneous patients.
To overcome the fact that the number of treatments was unequal between patients, we opted to analyze only the first completed 12 sessions (first treatment) for each patient. We also observed some heterogeneity of the treatments themselves, for example, the combination of different VR environments, a fact that may influence outcomes. Nevertheless, the reported VR-based rehabilitation treatments followed therapeutic validity methodologies based on a-priori aims and intentions, having a rationale for the content and intensity (which can be monitored and adjusted when necessary), and, maybe most importantly, being personalized and contextualized to the individuals.33 This implies that therapeutic valid treatments (i.e. VR-based or others) will be heterogeneous in many cases; particularly, in routine clinical practice in which interventions are not bound to specific procedures and methodologies.
For an effective transition to (and understanding of) implementing VR in a clinical routine manner, we advocate a fully detailed report on the clinical aspects occurring during (and between) sessions as well as adverse events such as falls, which were not monitored in the present study.
Another deficient aspect concerns the lack of quantification for feasibility. Our conclusion concerning the practicability of VR refers mainly on the demonstration that (based on our experience described in the present study) VR-based rehabilitation is clinically practicable and might be incorporated within routine interventions in hospital rehabilitation units. Future studies using protocols including quantifiable methods of feasibility will be contributory.
Based on clinical trials, cohort studies and case reports, a recent systematic review suggests that an optimal use of VR for rehabilitation might be in combination with conventional therapies.11 The review included studies that failed to prove strong methodological quality and did not incorporate theory-driven protocols to promote motor learning. Still, the results of the present study suggest that the implementation of VR alone in routine clinical practice is viable and effective for the rehabilitation of balance and gait. We found that a variety of VR systems and environments facilitates personalized and contextualized treatments because it allows simulating different tasks with specific therapeutic goals. Furthermore, through intrinsic characteristics such as multisensory feedback and task variation, VR-based rehabilitation can promote motor learning and enables a meticulous control of progression within, and among, rehabilitation sessions.
This study can serve as reference for clinicians willing to incorporate VR as part of their clinical service, and is oriented to contribute to the transition from short-term VR application (e.g. in research studies) to long-term VR implementation in the clinical area.
Supplemental Material
Supplemental material, Table_e1._Virtual_Environments.Description_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease
Supplemental Material
Supplemental material, Table_e2._Clinical_Thresholds_EN_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease
Supplemental Material
Supplemental material, Table_e3._Suitability_Evaluation_Questionnaire_-_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors thank Dr. Orit Elion and Mr. Hanania Sharon for their advice on clinical training planning.
Footnotes
Author contributions: D.C.P. analyzed data and is the primary author. M.P., H.S., Y.Z.N. and G.Z. contributed to the design and plan of clinical treatments. H.S. leaded the clinical team conducting VR-based treatments and participated in data acquisition. D.C.P., M.P., G.Z. and R.I. iteratively revised the manuscript. M.P., R.I. and G.Z. supervised the study. All authors discussed the results and critically reviewed the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The international training network Perception and Action in Complex Environments (PACE) supported this study, in part. The PACE Project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 642961. This study was also supported in part by the Israel Science Foundation grant # 1657-16, and the Israel Ministry of Science Technology and Space grant # 3-12072.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics committee approval: The Sheba Medical Center Institutional Review Board (IRB) approved the collection and use of patients’ records according to regulations for clinical trials in humans (IRB approval No. 3962-17-SMC). The need for informed consent was waived by IRB, since the study was based on a retrospective charts review of clinical data.
ORCID iD: Meir Plotnik  https://orcid.org/0000-0003-2637-3457
https://orcid.org/0000-0003-2637-3457
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Desiderio Cano Porras, Center of Advanced Technologies in Rehabilitation, Sheba Medical Center, Ramat Gan, Tel HaShomer, Israel; Sackler Faculty of Medicine, Tel Aviv University, Israel; Perception and Action in Complex Environments, Marie Curie International Training Network, European Union’s Horizons 2020 Research and Innovation Program.
Hadar Sharon, Center of Advanced Technologies in Rehabilitation, Sheba Medical Center, Ramat Gan, Tel HaShomer, Israel.
Rivka Inzelberg, Center of Advanced Technologies in Rehabilitation, Sheba Medical Center, Tel HaShomer, Israel; Sagol School of Neuroscience, Tel Aviv University, Israel; Department of Neurology and Neurosurgery, Sackler Faculty of Medicine, Tel Aviv University, Israel; Department of Applied Mathematics and Computer Science, The Weizmann Institute of Science, Rehovot, Israel.
Yitzhak Ziv-Ner, Department of Orthopedic Rehabilitation, Sheba Medical Center, Tel HaShomer, Israel.
Gabriel Zeilig, Department of Neurological Rehabilitation, Sheba Medical Center, Tel HaShomer, Israel; Department of Physical and Rehabilitation Medicine, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Meir Plotnik, Center of Advanced Technologies in Rehabilitation, Sheba Medical Center, Derech Sheba 2, Ramat Gan, Tel HaShomer 52621, Israel; Department of Physiology and Pharmacology, Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel; Sagol School of Neuroscience, Tel Aviv University, Tel Aviv, Israel.
References
- 1. Darekar A, McFadyen BJ, Lamontagne Aet al. Efficacy of virtual reality-based intervention on balance and mobility disorders post-stroke: a scoping review. J Neuroeng Rehabil 2015; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diehr PH, Thielke SM, Newman ABet al. Decline in health for older adults: five-year change in 13 key measures of standardized health. J Gerontol A Biol Sci Med Sci 2013; 68: 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kasser SL, Jacobs JV, Ford Met al. Effects of balance-specific exercises on balance, physical activity and quality of life in adults with multiple sclerosis: a pilot investigation. Disabil Rehabil 2015; 37: 2238–2249. [DOI] [PubMed] [Google Scholar]
- 4. Massano J, Bhatia KP. Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med 2012: a008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil 2005; 86: 1552–1556. [DOI] [PubMed] [Google Scholar]
- 6. Levac D, Missiuna C, Wishart Let al. Documenting the content of physical therapy for children with acquired brain injury: development and validation of the Motor Learning Strategy Rating Instrument. Phys Ther 2011; 91: 689–699. [DOI] [PubMed] [Google Scholar]
- 7. Levac DE, Glegg SM, Sveistrup Het al. Promoting therapists’ use of motor learning strategies within virtual reality-based stroke rehabilitation. PLoS One 2016; 11: e0168311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear R 2008; 51: S225–S239. [DOI] [PubMed] [Google Scholar]
- 9. Fritz NE, Cheek FM, Nichols-Larsen DS. Motor-cognitive dual-task training in neurologic disorders: a systematic review. J Neurol Phys Ther 2015; 39: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perez-Marcos D, Bieler-Aeschlimann M, Serino A. Virtual reality as a vehicle to empower motor-cognitive neurorehabilitation. Front Psychol 2018; 9: 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cano Porras D, Siemonsma P, Inzelberg Ret al. Advantages of virtual reality in the rehabilitation of balance and gait: systematic review. Neurology 2018; 90: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 12. Keshner EA, Fung J. The quest to apply VR technology to rehabilitation: tribulations and treasures. J Vestib Res 2017; 27: 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Levin MF, Weiss PL, Keshner EA. Emergence of virtual reality as a tool for upper limb rehabilitation: incorporation of motor control and motor learning principles. Phys Ther 2015; 95: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shema SR, Brozgol M, Dorfman Met al. Clinical experience using a 5-week treadmill training program with virtual reality to enhance gait in an ambulatory physical therapy service. Phys Ther 2014; 94: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 15. O’Neil O, Fernandez MM, Herzog Jet al. Virtual reality for neurorehabilitation: insights from three European clinics. Phys Med Rehab 2018; 10(9 Suppl. 2): S198–S206. [DOI] [PubMed] [Google Scholar]
- 16. Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 1997; 26: 15–19. [DOI] [PubMed] [Google Scholar]
- 17. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 18. Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehab 2002; 83: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 19. Berg KO, Wood-Dauphinee SL, Williams JIet al. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992; 83: S7–S11. [PubMed] [Google Scholar]
- 20. King LA, Priest KC, Salarian Aet al. Comparing the Mini-BESTest with the Berg Balance Scale to evaluate balance disorders in Parkinson’s disease. Parkinsons Dis 2012; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adusumilli G, Lancia S, Levasseur VAet al. Turning is an important marker of balance confidence and walking limitation in persons with multiple sclerosis. PLoS One. 2018; 13: e0198178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDowd JM. The effects of age and extended practice on divided attention performance. J Gerontol 1986; 41: 764–769. [DOI] [PubMed] [Google Scholar]
- 23. Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1995; 50: M28–M34. [DOI] [PubMed] [Google Scholar]
- 24. Gil-Gómez J-A, Gil-Gómez H, Lozano-Quilis J-Aet al. SEQ: suitability evaluation questionnaire for virtual rehabilitation systems. Application in a virtual rehabilitation system for balance rehabilitation. In: Proceedings of the 7th international conference on pervasive computing technologies for healthcare Vienna, Austria, 14–15 November 2013, pp. 335–338. Institute for Computer Sciences, Social-Informatics and Telecommunications Engineering. [Google Scholar]
- 25. Lozano-Quilis J-A, Gil-Gómez H, Gil-Gómez J-Aet al. Virtual rehabilitation for multiple sclerosis using a kinect-based system: randomized controlled trial. JMIR Serious Games 2014; 2: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. You SH, Jang SH, Kim Y-Het al. Virtual reality–induced cortical reorganization and associated locomotor recovery in chronic stroke: an experimenter-blind randomized study. Stroke 2005; 36: 1166–1171. [DOI] [PubMed] [Google Scholar]
- 27. Papegaaij S, Morang F, Steenbrink F. Virtual and augmented reality based balance and gait training. White Paper 2017. Amsterdam: Motek. [Google Scholar]
- 28. Huang Y-Z, Chang F-Y, Liu W-Cet al. Fatigue and muscle strength involving walking speed in Parkinson’s disease: insights for developing rehabilitation strategy for PD. Neural Plast 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenwald BD, Cifu DX, Marwitz JHet al. Factors associated with balance deficits on admission to rehabilitation after traumatic brain injury: a multicenter analysis. J Head Trauma Rehabil 2001; 16: 238–252. [DOI] [PubMed] [Google Scholar]
- 30. Persson CU, Danielsson A, Sunnerhagen KSet al. Timed Up & Go as a measure for longitudinal change in mobility after stroke–Postural Stroke Study in Gothenburg (POSTGOT). J Neuroeng Rehabil 2014; 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalron A, Fonkatz I, Frid Let al. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: a pilot randomized controlled trial. J Neuroeng Rehabil 2016; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nilsagård YE, Forsberg AS, von Koch L. Balance exercise for persons with multiple sclerosis using Wii games: a randomised, controlled multi-centre study. Mult Scler J 2013; 19: 209–216. [DOI] [PubMed] [Google Scholar]
- 33. Hoogeboom TJ, Oosting E, Vriezekolk JEet al. Therapeutic validity and effectiveness of preoperative exercise on functional recovery after joint replacement: a systematic review and meta-analysis. PLoS One 2012; 7: e38031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skjæret N, Nawaz A, Morat Tet al. Exercise and rehabilitation delivered through exergames in older adults: An integrative review of technologies, safety and efficacy. Int J Med Inform 2016; 85: 1–16. [DOI] [PubMed] [Google Scholar]
- 35. Stucki G, Cieza A, Melvin J. The international classification of functioning, disability and health: A unifying model for the conceptual description of the rehabilitation strategy. J Rehabil Med 2007; 39: 279–285. [DOI] [PubMed] [Google Scholar]
- 36. Lawrence AB. Csikszentmihalyi M. Flow: The Psychology of Optimal Experience. New York: Harper Perennial, 1990. [Google Scholar]
- 37. Slaboda J, Barton J, Maitin Iet al. Visual field dependence influences balance in patients with stroke. In: 2009 Proceedings of the 31st annual international conference of the IEEE engineering in medicine and biology society Hilton Minneapolis, Minnesota, 2–6 September 2009, pp.1147–1150. Piscataway, NJ: IEEE. [DOI] [PubMed] [Google Scholar]
- 38. Yu Y, Tucker CA, Lauer RTet al. Influence of visual dependence on inter-segmental coordination during upright stance in cerebral palsy. J Motor Behav 2019; 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kenyon RV, Leigh J, Keshner EA. Considerations for the future development of virtual technology as a rehabilitation tool. J Neuroeng Rehabil 2004; 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Belchior P, Marsiske M, Sisco Set al. Older adults’ engagement with a video game training program. Act Adapt Aging 2012; 36: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riva G, Mantovani F, Gaggioli A. Presence and rehabilitation: toward second-generation virtual reality applications in neuropsychology. J Neuroeng Rehabil 2004; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faria AL, Andrade A, Soares Let al. Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. J Neuroeng Rehabil 2016; 13: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Table_e1._Virtual_Environments.Description_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease
Supplemental material, Table_e2._Clinical_Thresholds_EN_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease
Supplemental material, Table_e3._Suitability_Evaluation_Questionnaire_-_Proof for Advanced virtual reality-based rehabilitation of balance and gait in clinical practice by Desiderio Cano Porras, Hadar Sharon, Rivka Inzelberg, Yitzhak Ziv-Ner, Gabriel Zeilig and Meir Plotnik in Therapeutic Advances in Chronic Disease