Abstract
Previously we have shown in rats a new method of urine collection, hydrophobic sand, to be an acceptable alternate in place of the traditional method using metabolic cages. Hydrophobic sand is non-toxic, induces similar or lower levels of stress in the rat, and does not contaminate clinical urine markers nor metal concentrations in collected samples (Hoffman et al., 2017 and 2018). Urine is often used in humans and many animal models as a readily-attainable biosample which contains proteins and microRNAs (miRNAs) within extracellular vesicles (EVs) that can be isolated to indicate changes in health. In order to ensure hydrophobic sand did not in any way contaminate or disrupt the extraction and analysis of these EVs and miRNAs, we used urine samples from the same 8 rats in the within-subjects crossover experiment comparing hydrophobic sand and metabolic cage collection methods. We isolated EVs and miRNAs from the urine set and examined their quantity and quality between the urine collection methods. We found no significant differences in particle size, particle concentration, total RNA, or the type and abundance of miRNAs contained within the urine EVs due to urine collection method, suggesting hydrophobic sand represents an easy-to-use, non-invasive method to collect rodent urine for EVs and biomarker studies.
Keywords: Extracellular vesicles, Urine, Urine collection, Hydrophobic sand, Metabolic cage, miRNA
Abbreviations: EVs, extracellular vesicles; LS, LabSand; MC, metabolic cage; miRNAs, microRNAs; PKD, polycystic kidney disease
1. Introduction
Exosomes are a type of small, membrane-bound extracellular vesicles (EVs) that may contain protein, mRNA, and microRNA (miRNA). Exosomes are released by most cells and can be isolated from biofluids such as serum and urine, which has sparked interest in their use as potential non-invasive diagnostic biomarkers for various diseases [[1], [2], [3], [4], [5]]. In humans, exosomes have recently been linked to a number of diseases, including nephronophthisis-related ciliopathies [6], diabetic kidney disease [7], bladder cancer [8,9], liver disease [10], and amyotrophic lateral sclerosis from cerebrospinal fluid [11]. Rat models of renal disease, such as polycystic kidney disease (PKD), show similar deviations in kidney health biomarkers as humans with PKD. In one study this included increases of urea, guanidinosuccinic acid, creatinine, guanidine, methylguanidine, and N(G)N(G)-dimethylarginine [12], and another study found elevated expression levels of activator of G-protein signaling 3 (AGS3) in urine exosomes for rat and humans with PKD compared to controls [13]. These studies highlight the importance of improving methods of isolating and using extracellular vesicles from humans and animals for the study of disease.
In humans, urine collection is straightforward and standard. In rodents, there are several available methods, but one acceptable standard is the use of a metabolic cage, where rats are isolated in a small, circular plastic cage with a wire mesh bottom that allows urine and feces to pass through into separate collection tubes. Although isolation and collection times can vary, they are usually limited to 24 h. Habituation of the animal to the metabolic cage is often required and its use must be justified due to its potential for causing stress to the animal [[14], [15], [16], [17]]. Previously we investigated the use of a new method for rat urine collection, hydrophobic sand (brand name LabSand for scientific use). Originally developed for home urine collection in the cat, LabSand is a biodegradable material with a non-toxic hydrophobic coating that causes urine to pool on its surface, making it easy to collect. We reported no significant differences in stress induction, toxicity to the animals, urine volume collection, or urine quality with several common clinical biomarkers [18], as well as sample integrity when assessing potential contamination during urinary metal concentration analyses [19]. Unlike the metabolic cage, hydrophobic sand is similar to home cage bedding, reducing animal stress and does not require as much time for collection or habituation [discussed in depth in our previous publications, 18 and 19]. Thus we concluded hydrophobic sand has the potential to become a valuable new method for urine collection in the rodent. Given the potential for EVs and miRNAs collected from urine to act as important biomarkers for health conditions in a multitude of research areas, our goal in the work presented here was to take the method comparison a step further and determine if the quantity and quality of EVs collected from rat urine by hydrophobic sand were comparable to that of urine collected by metabolic cage. If we did not find any differences in characterization of EVs and miRNAs collected from urine via these two methods, it would indicate that hydrophobic sand does not contaminate biosamples and thus would be an appropriate alternate method applicable to a broad range of research utilizing urine samples.
2. Materials and methods
2.1. Animals
All animals in this study are the same as those previously reported in detail [18]. No changes to animal manipulation or urine collection were made for the purposes of this paper. Briefly, 8 male Sprague Dawley rats (Envigo, Frederick, MD, USA) were maintained on a 12:12 light:dark cycle with access to food and water ad libitum and pair-housed except during urine collection periods. Rats underwent no treatment or experimental conditions beyond exposure to both urine collection methods. All procedures involving animals were approved by the AFRRI Institutional Animal Care and Use Committee under protocol 2016–05-006.
2.2. Urine collection and experimental design
A crossover within-subjects design was used to compare urine collection from traditional metabolic cages and hydrophobic sand (LabSand, Coastline Global, Palo Alto, CA, USA) [full design and method details can be found in 18]. Briefly, rats were randomly assigned to two groups: (A) metabolic cage followed by LabSand or (B) LabSand followed by the metabolic cage. Both groups were run simultaneously under the same testing conditions. There was a total of 5 collection sessions: at 2 h, at 4 h, and three separate 6 h sessions, each separated by a rest period of at least 48 h. The method crossover occurred after the last session and the entire pattern was repeated. Food and water were not provided to any animal during urine collection sessions, but each animal was provided with a water replacement gel in a plastic cup (HydroGel®, Clear H2O, Westbrook, ME, USA) to avoid dilution from a water bottle drip.
For the metabolic cage method, animals were individually housed in a standard circular metabolic cage (Nalgene Nunc, Rochester, NY, USA) with a wire mesh floor where urine collected into a Nalgene tube at the bottom of a funnel system. Urine could only be collected at the end of the session due to the cage design. For the hydrophobic sand method, animals were individually housed in a rectangular microisolator cage with the sand lining the bottom of the cage in place of regular bedding; urine pools on top of the sand, which is then collected with a pipette. For each rat, we collected urine every half hour which was subsequently pooled at the end of the session. The urine collected in [18] was also analyzed in [19] and an aliquot of the same urine samples were used here for EV isolation and analysis.
2.3. Extracellular vesicle isolation and characterization
The experimental design of n = 8 rats with a within-subjects crossover design (4 in hydrophobic sand, 4 in metabolic cages for 5 collection sessions, then the groups switched for an additional 5 sessions) provided an n = 8 for each session of each collection method. In order to have enough volume to collect extracellular vesicles from each rat, we created a pooled sample (3 mL total) of all 5 sessions within each individual rat's method of urine collection; this gives an n = 8 total urine samples collected by each method. Extracellular vesicles were isolated using ExoQuick-TC kit according to the manufacturer's protocol (System Biosciences, Palo Alto, CA, USA). Urine EV size and abundance were measured using nanoparticle tracking analysis (NTA) with ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and corresponding software 8.04.02 sp1. After calibration using 100 nm polystyrene particles, EVs were appropriately diluted using Dulbecco's Phosphate-Buffered Saline (Thermo Fisher, Waltham, MA, USA) to measure the particle size and concentration. NTA measurements were analyzed at 11 positions at a constant temperature of 23 °C.
2.4. miRNA microarray analysis and quantification of select miRNA expression by qPCR
A total of 150 ng of RNA isolated from the EVs was pooled from all samples within each urine collection method to conduct a survey of microRNA abundance using microarray analysis. The microarray hybridization and processing were performed at the University of Kentucky Genomic Core Laboratory using Affymetrix miRNA 4.0 array chips (Santa Clara, CA, USA). Raw signal intensity data were normalized with robust multi-array average (RMA) from the Affymetrix data bank and sorted from highest to lowest signal using the LabSand group.
RNA from individual samples was then used to validate the five most abundant miRNAs as determined by microarray. Reverse transcription reactions for let-7b, let-7c, miR-3473, miR-23b, miR-200b and cel-miR-39 were performed with 3 ng of total RNA using Taqman MicroRNA Reverse Transcription Kit (ThermoFisher Scientific) according to the manufacturer's directions. qPCR was carried out with Taqman Gene Expression Master Mix (2×) (ThermoFisher Scientific) and TaqMan gene expression assays (let-7b, #000378; let-7c, #000379; miR-3473, #475642_mat; miR-23b, #000400; miR-200b, #001800; cel-miR-39, #000200) using cDNA in a 10 μL reaction volume. The cel-miR-39 was used to normalize miRNA expression; 1 nM of a 5′-phosphorylated sequence (#478293_mir) was spiked into each cDNA synthesis reaction.
qPCR reactions were performed in the ABI 7500 qPCR system (Applied Biosystems, Santa Clara, CA, USA). qPCR efficiency was calculated by linear regression from fluorescence increase in the exponential phase in the LinRegPCR software v11.1 [20]. The comparison of urine EV miRNA expression between LabSand and metabolic cage was determined following normalization with cel-miR-39. The relative microRNA expression was measured by using the comparative CT method [21].
2.5. Statistics
All comparisons between metabolic cage and LabSand urine collection methods were performed by paired t-test (EV size, EV concentration, and total RNA) or unpaired t-test (all 5 PCR validations due to lack of enough volume for a few samples to complete the pair) using GraphPad Prism Software (version 8.01, La Jolla, CA, USA). P values less than 0.05 were considered significant.
3. Results
3.1. Characterizing extracellular vesicles
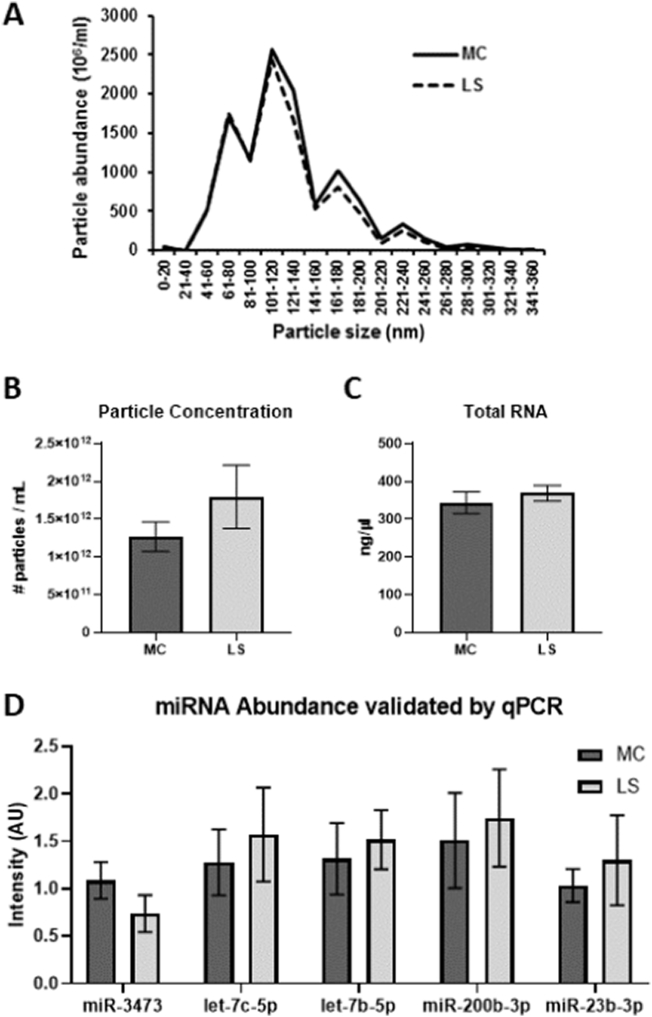
Exosomes are typically 50–150 nm in diameter [3,5]. Extracellular vesicles in our samples had a size range between 15 and 345 nm, with the largest peaks falling within 41–160 nm, as can be seen in the size spectrum analysis in Fig. 1A. There were no significant differences between characteristics of EVs isolated from urine collected with the metabolic cage (MC) method versus the LabSand (LS) method. The size of the particles extracted from the MC urine samples were not significantly different from the size of the particles extracted from the LS urine samples (Fig. 1A, entire particle size distribution: MC mean 122 nm, STD 6.46, min 114, max 134; LS mean 125 nm, STD 9.69, min 107, max 136; t7 = 0.8685, p = .4139). Similarly, the particle concentration (number of particles per mL of urine) was not significantly different between the two urine collection methods (Fig. 1B: MC mean 1.27 × 1012 particles/mL, STD 0.553 × 1012, min 6.0 × 1011, max 1.9 × 1012; LS mean 1.79 × 1012 particles/mL, STD 1.18 × 1012, min 4.0 × 1011, max 3.9 × 1012 t7 = 1.871, p = .1035). Further, total RNA collected from the EVs was also not significantly different between urine from the two methods (Fig. 1C: MC mean 344 ng/μl, STD 80.7, min 257, max 513; LS mean 369 ng/μl, STD 59.9, min 290, max 477; t7 = 0.6986, p = .5073). For all samples, the bioanalyzer electropherogram showed a single peak at the 25 nt location, indicating that the type of RNA in EVs collected from urine samples were primarily miRNAs. It is also important to note that without the presence of rRNA, there is no RIN value to indicate quality of RNA extracted beyond the size peak.
Fig. 1.
Extracellular vesicles (EVs) isolated from urine samples collected by either metabolic cage (MC) or LabSand (LS) urine collection methods were characterized for their (A) size, (B) particle concentration, (C) total RNA, and type and abundance of miRNAs expressed in the EVs to determine whether the LabSand urine collection method resulted in similar quantity and quality of EV collection as the traditional metabolic cage urine collection method. Relative expression of the top five miRNAs determined by microarray were verified by qPCR (D). Data is presented as the mean ± SEM.
3.2. qPCR validation of top 5 most abundant miRNA
To perform a “survey” analysis of the identification and relative abundance of the miRNAs present within the EVs, all 8 individual rat session-pooled samples were further pooled within each urine collection method and assayed via miRNA microarray (n = 1 for each collection method). This data is not shown, since it is just a list of abundant miRNAs, although the list is in the same order for both collection methods. The top 5 most abundant miRNAs as determined by microarray were validated by qPCR, going back to the 8 individual rat session-pooled samples (n = 7 for metabolic sand, n = 6 for lab sand due to not enough leftover volume for verification). Samples were analyzed by qPCR for expression of miR-3473, let-7c-5p, let 7b-5p, miR-200b-3p, and miR-23b-3p. Expression was normalized to a spike of cel-miR-39. There were no statistically significant differences in the level of expression (arbitrary units, AU) for any of these miRNAs between samples collected from the MC method compared to the LS method (Fig. 1D; see Table 1 for mean, STD, and statistical results).
Table 1.
Mean, standard deviation, and statistical results for graphs in Fig. 1D.
| miRNA | MC |
LS |
Statistics | ||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | ||
| miR-3473 | 1.09 | 0.514 | 0.738 | 0.476 | t11 = 1.2660, p = .2316 |
| let-7c-5p | 1.28 | 0.919 | 1.57 | 1.21 | t11 = 0.4926, p = .6320 |
| let-7b-5p | 1.32 | 0.998 | 1.52 | 0.760 | t11 = 0.4057, p = .6927 |
| miR-200b-3p | 1.51 | 1.33 | 1.75 | 1.25 | t11 = 0.3299, p = .7477 |
| miR-23b-3p | 1.03 | 0.461 | 1.30 | 1.16 | t11 = 0.5605, p = .5864 |
4. Discussion
We have previously shown that a hydrophobic sand material is a suitable alternate method for urine collection in the rat compared to the traditional metabolic cage because it does not increase stress or stress markers in the rats, nor does it contaminate or otherwise alter normal clinical urinary markers or the concentration of various metals. Here we wanted to take this a step further and determine whether the hydrophobic sand compromised the quantity or quality of EVs, and their miRNA cargo, which could be collected from rat urine samples compared to the use of the metabolic cage method. We found no significant differences in particle size or concentration, total RNA collected, or the types and abundance of miRNAs contained within urine EVs due to urine collection method. It is interesting to note that of the most abundant miRNAs detected in EVs isolated from these urine samples, three (let-7c, miR-23b and miR-200c) are associated with kidney function [[22], [23], [24], [25]]. The let-7 family is known to be highly abundant in different bodily fluids with let-7b being a proposed as a potential biomarker for kidney disease [26]. miRNA-3473 was the most abundant urine miRNA detected in the current study and has been reported to be associated with renal tubular injury [27]. We conclude that the use of hydrophobic sand in the collection of rodent urine for studies of changes in EVs as biomarkers of kidney health will not compromise the quality of the miRNAs examined, and is thus an acceptable alternate method of urine collection for an even broader range of studies.
Funding and acknowledgements
This work was supported by grant W81XWH-16-2-0058 from the Peer Reviewed Medical Research Program of the Congressionally Directed Medical Research Program. Authors have no financial conflicts of interest to disclose. The use of the LabSand brand of hydrophobic sand in this work does not represent an endorsement of the product or the company by the U.S. Government. The views expressed in the paper are those of the authors and do not reflect the official policy or position of the Armed Forces Radiobiology Research Institute, the Uniformed Services University, the Department of Defense, or the United States Government.
References
- 1.Knepper M.A., Pisitkun T. Exosomes in urine: who would have thought…? Kidney Int. 2007;72(9):1043–1045. doi: 10.1038/sj.ki.5002510. [DOI] [PubMed] [Google Scholar]
- 2.Street J.M. Urine exosomes: an emerging trove of biomarkers. Adv. Clin. Chem. 2017;78:103–122. doi: 10.1016/bs.acc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Gheinani A.H. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Nat. Sci. Rep. 2018;8:3945. doi: 10.1038/s41598-018-22142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdbrugger U., Le T.H. Extracellular vesicles in renal diseases: more than novel biomarkers? J. Am. Soc. Nephrol. 2016;27(1):12–26. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant M.L. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat. Rev. Nephrol. 2017;13(12):731–749. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokman M.F. Changes in the urinary extracellular vesicle proteome are associated with nephronophtisis-related ciliopathies. J. Proteome. 2019;192:27–36. doi: 10.1016/j.jprot.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Xu W.C. Urinary extracellular vesicle: a potential source of early diagnostic and therapeutic biomarker in diabetic kidney disease. Chin. Med. J. 2018;131(11):1357–1364. doi: 10.4103/0366-6999.232801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y.R., Ortiz-Bonilla C.J., Lee Y.F. Extracellular vesicles in bladder cancer: biomarkers and beyond. Int. J. Mol. Sci. 2018;19:2822. doi: 10.3390/ijms19092822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leiblich A. Recent developments in the search for urinary biomarkers in bladder cancer. Curr. Urol. Rep. 2017;18(12):100. doi: 10.1007/s11934-017-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo G., Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2017;14(8):455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otake K., Kamiguchi H., Hirozane Y. Identification of biomarkers for amyotrophic lateral sclerosis by comprehensive analysis of exosomal mRNAs in human cerebrospinal fluid. BMC Med. Genet. 2019;12(1):7. doi: 10.1186/s12920-019-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torremans A. Biochemical validation of a rat model for polycystic kidney disease: comparison of guanidine compound profile with the human condition. Kidney Int. 2006;69(11):2003–2012. doi: 10.1038/sj.ki.5000443. [DOI] [PubMed] [Google Scholar]
- 13.Keri K.C. Urinary exosomal expression of activator of G protein signaling 3 in polycystic kidney disease. BMC Res. Notes. 2018;11(1):359. doi: 10.1186/s13104-018-3467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Animal Research Review Panel [Internet]. Guideline 20: Guidelines for the Housing of Rats in Scientific Institutions. 2007. http://www.animalethics.org.au/__data/assets/pdf_file/0014/222512/housing-rats-scientific-institutions.pdf [Cited 26 May 2017]. Available at.
- 15.Eriksson E. Effect of metabolic cage housing on immunoglobin A and corticosterone excretion in faeces and urine of young rats. Exp. Physiol. 2004;89:427–433. doi: 10.1113/expphysiol.2004.027656. [DOI] [PubMed] [Google Scholar]
- 16.Gil M.C. Influence of age on stress responses to metabolic cage housing in rats. Cell. Mol. Neurobiol. 1999;19:625–633. doi: 10.1023/a:1006984402291. [DOI] [PubMed] [Google Scholar]
- 17.Kurien B.T., Everds N.E., Scofield R.H. Experimental animal urine collection; a review. Lab. Anim. 2004;38:333–361. doi: 10.1258/0023677041958945. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman J.F. Hydrophobic sand versus metabolic cages: a comparison of urine collection methods for the rat (Rattus norvegicus) J. Am. Assoc. Lab. Anim. Sci. 2018;57:1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman J.F. Hydrophobic sand is a non-toxic method of urine collection, appropriate for urinary metal analysis in the rat. Toxics. 2017;5(4):25. doi: 10.3390/toxics5040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruijter J.M. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Zaman M.S., Thamminana S., Shahryari V., Chiyomaru T., Deng G., Saini S., Majid S., Fukuhara S., Chang I., Arora S., Hirata H., Ueno K., Singh K., Tanaka Y., Dahiya R. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennan E.P., Nolan K.A., Borgeson E., Gough O.S., McEvoy C.M., Docherty N.G., Higgins D.F., Murphy M., Sadlier D.M., Ali-Shah S.T., Guiry P.J., Savage D.A., Maxwell A.P., Martin F., Godson C. Lipoxins attenuate renal fibrosis by inducing let-7c and suppressing TGFbetaR1. J. Am. Soc. Nephrol. 2013;24:627–637. doi: 10.1681/ASN.2012060550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long J.D., Sullivan T.B., Humphrey J., Logvinenko T., Summerhayes K.A., Kozinn S., Harty N., Summerhayes I.C., Libertino J.A., Holway A.H., Rieger-Christ K.M. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015;7:2500–2509. [PMC free article] [PubMed] [Google Scholar]
- 25.Ghai V., Wu X., Bheda-Malge A., Argyropoulos C.P., Bernardo J.F., Orchard T., Galas D., Wang K. Genome-wide profiling of urinary extracellular vesicle microRNAs associated with diabetic nephropathy in type 1 diabetes. Kidney Int. Rep. 2018;3:555–572. doi: 10.1016/j.ekir.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorko M., Juracek J., Stanik M., Svoboda M., Poprach A., Buchler T., Pacik D., Dolezel J., Slaby O. Detection of let-7 miRNAs in urine supernatant as potential diagnostic approach in non-metastatic clear-cell renal cell carcinoma. Biochem. Med. 2017;27:411–417. doi: 10.11613/BM.2017.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhayana S., Song F., Jacob J., Fadda P., Denko N.C., Xu-Welliver M., Chakravarti A., Jacob N.K. Urinary miRNAs as biomarkers for noninvasive evaluation of radiation-induced renal tubular injury. Radiat. Res. 2017;188:626–635. doi: 10.1667/RR14828.1. [DOI] [PMC free article] [PubMed] [Google Scholar]