Figure 1.
Primary hMSCs Can Be Genetically Engineered without Altering Their Capacity to Form Hypertrophic Cartilage
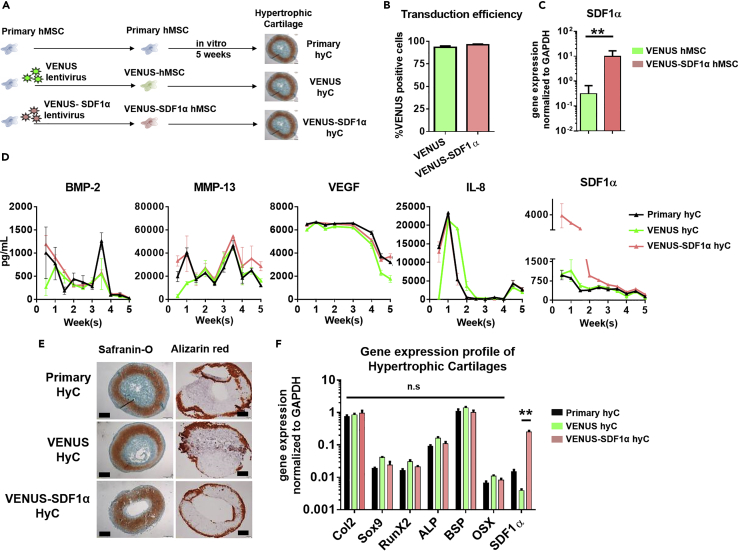
(A) Experimental design for generation of hypertrophic cartilage (hyC). SDF1α, stromal-derived factor 1 alpha.
(B) Primary hMSCs were successfully transduced with the VENUS and VENUS-SDF1α lentiviruses, as assessed by flow cytometry. n ≥ 4 biological replicates.
(C) The VENUS-SDF1α transduction led to a significantly higher expression of SDF1α levels in corresponding cells before hyC formation. ∗∗p < 0.01, using non-parametric Mann-Whitney t test. n ≥ 5 biological replicates.
(D) All hyC display similar protein secretion patterns during in vitro culture time, but VENUS-SDF1α hyC releases higher amounts of SDF1α. n ≥ 3 biological replicates. BMP-2, bone morphogenetic protein-2; MMP-13, matrix metalloproteinase-13; VEGF, vascular endothelial growth factor; IL-8, interleukin-8.
(E) VENUS and VENUS-SDF1α successfully displayed features of mature hypertrophic cartilage tissue following 5 weeks of in vitro culture, as assessed by histological analysis. Safranin O staining reveals the presence of glycosaminoglycans (red), whereas alizarin red reveals the presence of mineralized tissue (red). Scale bars, 500 μm.
(F) After 5 weeks of in vitro culture, VENUS-SDF1α hyC successfully displayed a typical hypertrophic molecular profile while exhibiting a significant SDF1α increase.
∗∗p < 0.01, using one-way ANOVA. n ≥ 4 biological replicates. Col2, collagen type 2; RunX2, Runt-related transcription factor 2; ALP, alkaline phosphatase; BSP, bone sialoprotein; OSX, osterix. Data are represented as mean ± SEM.