Abstract
In esophageal squamous cell carcinoma, an elevated preoperative absolute monocyte count (Pre-AMC) is reported to be a predictor of survival, but the clinical application of postoperative absolute monocyte count change (AMCc) remains unknown. The present study was designed to investigate the prognostic value of AMCc in ESCC. 686 patients of ESCC after radical surgery without preoperative adjuvant therapy were enrolled. The Pre-AMC and AMCc were recorded within one week before the operation and one week after surgery. We considered the median of Pre-AMC as the optimal cut-off value to evaluate the relationship between Pre-AMC and patient survival. AMCc was defined as AMCc increased (higher than Pre-AMC) and AMCc decreased (lower than Pre-AMC). Demographic and clinical characteristics, disease-free survival (DFS), and overall survival (OS) were statistically analyzed. Multivariate analysis revealed that AMCc was a better independent prognostic factor for both OS (P = 0.002, HR = 0.614, 95% CI 0.450-0.837) and DFS (P = 0.023, HR = 0.656, 95% CI 0.456-0.943) than Pre-AMC which was only an independent prognostic factor for OS (P = 0.033, HR = 2.031, 95% CI 1.058-3.898). AMCc could be a better prognostic factor than Pre-AMC in patients with ESCC. AMCc decrease predicts worse OS and DFS in ESCC undergoing curative resection.
1. Introduction
Worldwide, esophageal cancer ranks seventh in incidence with 572,000 new cases and sixth in terms of mortality with 509,000 deaths, which suggests that this disease will be responsible for an estimated 1 in every 20 cancer deaths by 2018. In China, this disease ranks fifth in terms of incidence and fourth in mortality overall [1]. Esophageal squamous cell carcinoma usually comprises over 90% of all esophageal cancer cases in China [2, 3]. With the development of multimodality therapies, however, patients with ESCC still face with worse prognosis [4, 5]. Several factors including TNM stage and tumor differentiation have been reported that have relationship with the prognosis of ESCC. Nevertheless, patients with the same TNM stage have inconsistent prognosis [6]. Therefore, it is urgent to investigate new and suitable prognosis biomarkers.
A growing number of evidence indicates that inflammation might play a critical role in carcinogenesis of cancer [7, 8]. Many systemic inflammation based factors including tumor-associated macrophages (TAMs) and neutrophil-to-lymphocyte ratio (NLR) are independent prognostic factors of various cancers [9–12]. TAMs are key regulators of the tumor microenvironment and derived from myeloid progenitor cells and monocytes [13].
Several studies have shown that an increased preoperative absolute monocyte count (Pre-AMC) could predict unfavorable survival in patients with various carcinomas, such as lung adenocarcinoma [14], hepatocellular carcinoma [15], prostate cancer [16] and esophageal cancer [17]. These studies focused primarily on Pre-AMC, while the dynamic change of absolute monocyte count (AMC) after therapy was not considered. The postoperative absolute monocyte count change (AMCc) might be a meaningful parameter to assess survival after therapy, because the therapy including surgery and chemotherapy could cause a change. However, the AMCc might dynamically reflect the systemic inflammatory response against cancer after therapy, its significance is unknown. Therefore, this retrospective study aimed to investigate the association between AMCc and clinical features, and to evaluate the prognostic value of AMCc in patients with ESCC.
2. Results
2.1. Patient Characteristics
We enrolled 686 patients with ESCC in the present research, including 583 (85.0%) males and 103 (15.0%) females. The median age at therapy initiation was 61 years (range from 39 to 84 years). The median value of Pre-AMC was 0.5 (range from 0.2 to 1.6). We chose the median value of Pre-AMC as the cut-off value. One week after curative resection, the median value of postoperative AMC (Post-AMC) was 0.7 (range from 0.1 to 2.1). When comparing the Pre-AMC and Post-AMC, AMCc was increased in 573 (83.5%) patients and decreased in 113 (16.5%) patients. The 1-year OS and 1-year DFS of all patients were 48.3%, 46.2%. The 3-year OS and 3-year DFS of all patients were 25.7%, 24.5%. In addition, the 5-year OS and 5-year DFS were 12.1%, 11.4%. The baseline characteristics of patients with ESCC in the two AMCc groups were shown in Table 1. There was no significant difference between the two groups in the baseline features, except the females were more (P = 0.045), the median of Pre-AMC was lower (P <0.001) and the median of Post-AMC was higher (P <0.001) in AMCc increased group than in AMCc decreased group.
Table 1.
Demographic and clinical data of 686 ESCC patients accroding to AMC change.
| Charateristics | Total (N=686), % | AMCc | P value | ||
|---|---|---|---|---|---|
| Increased (N=573), % | Decreased (N=113), % | ||||
| Sex | Male | 583 (85.0) | 480 (83.8) | 103 (91.2) | 0.045 |
| Female | 103 (15.0) | 93 (16.2) | 10 (8.8) | ||
| Age (years) | ⩽60 | 316 (46.1) | 270 (47.1) | 46 (40.7) | 0.211 |
| >60 | 370 (53.9) | 303 (52.9) | 67 (59.3) | ||
| Pathology grade | Well differentiated | 49 (7.1) | 44 (7.7) | 5 (4.4) | 0.335 |
| middle differentiated | 454 (66.2) | 381 (66.5) | 73 (64.6) | ||
| Poorly differentiated | 167 (24.3) | 133 (23.2) | 34 (30.1) | ||
| Undifferentiated | 2 (0.3) | 2 (0.3) | 0 (0) | ||
| Missing | 14 (2.0) | 13 (2.3) | 1 (0.9) | ||
| Depth of tumor | T1a–1b | 64 (9.3) | 53 (9.2) | 11 (9.7) | 0.983 |
| T2 | 132 (19.2) | 110 (19.2) | 22 (19.5) | ||
| T3 | 490 (71.4) | 410 (71.6) | 80 (70.8) | ||
| Lymph node metastasis | N0 | 298 (43.4) | 245 (42.8) | 53 (46.9) | 0.686 |
| N1 | 215 (31.3) | 185 (32.3) | 30 (26.5) | ||
| N2 | 119 (17.3) | 98 (17.1) | 21 (18.6) | ||
| N3 | 54 (7.9) | 45 (7.9) | 9 (8.0) | ||
| Pathological stage | 1a–1b | 117 (17.1) | 94 (16.4) | 23 (20.4) | 0.565 |
| 2a–2b | 230 (33.5) | 195 (34.0) | 35 (31.0) | ||
| 3a–3c | 339 (49.4) | 284 (49.6) | 55 (48.7) | ||
| Vessel invasive | Yes | 210 (30.6) | 173 (30.2) | 37 (32.7) | 0.591 |
| No | 476 (69.4) | 400 (69.8) | 76 (67.3) | ||
| Nerve infiltration | Yes | 256 (37.3) | 208 (36.3) | 48 (42.5) | 0.215 |
| No | 430 (62.7) | 365 (63.7) | 65 (57.5) | ||
| Treatment regimen | S | 464 (67.6) | 384 (67.0) | 80 (70.8) | 0.710 |
| S + postoperative C | 157 (22.9) | 133 (23.2) | 24 (21.2) | ||
| S + postoperative CRT | 65 (9.5) | 56 (9.8) | 9 (8.0) | ||
| Hospital time (days) | ⩽14 | 542 (79.0) | 460 (80.3) | 82 (72.6) | 0.066 |
| >14 | 144 (21.0) | 113 (19.7) | 31 (27.4) | ||
| Pre-AMC | Median | 0.5 (0.4-0.6) | 0.5 (0.4-0.6) | 0.7 (0.5-0.9) | <0.001 |
| Post-AMC | Median | 0.7 (0.6-0.9) | 0.8 (0.6-1.0) | 0.5 (0.3-0.6) | <0.001 |
| Pre-Platelet | Median | 201 (160-238) | 206.5 (149.5-244.0) | 200.0 (162.8-237.3) | 0.500 |
| Post-Platelet | Median | 261 (200-331) | 263.0 (202.0-332.3) | 251.5 (177.0-324.0) | 0.074 |
S, surgery; C, chemotherapy; CRT, chemoradiotherapy; Pre-AMC: preoperative absolute monocyte count; Post-AMC: postoperative absolute monocyte count; AMCc: postoperative absolute monocyte count change; Pre-platelet: preoperative platelet; Post-platelet: postoperative platelet.
2.2. Differences in Overall Survival and Disease-Free Survival according to AMCc
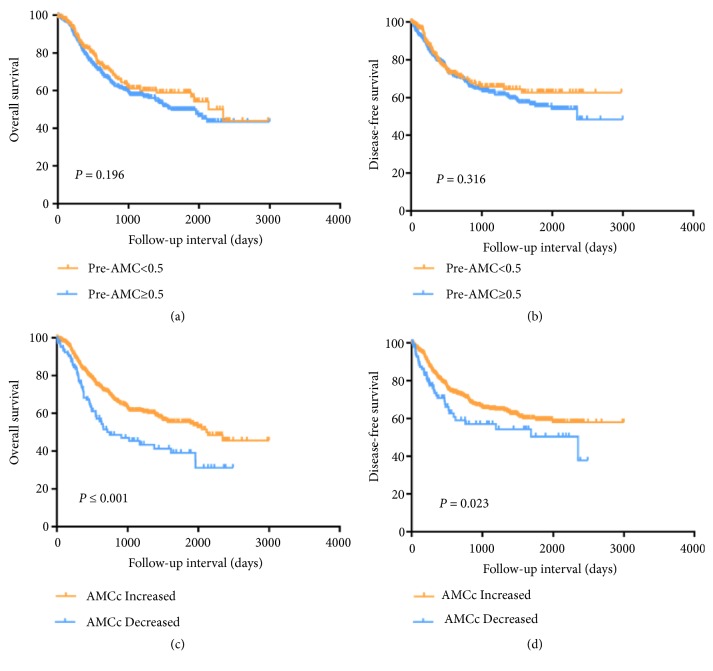
We chose the median of Pre-AMC as the cutoff value and divided the patients into low Pre-AMC group (Pre-AMC <0.5) and high Pre-AMC group (Pre-AMC ≥0.5). The Pre-AMC was significantly associated with overall survival (OS) (HR, 2.145; 95% CI, 1.124-4.095; P = 0.021), but not to disease-free survival (DFS) (HR, 1.853; 95% CI, 0.883-3.889; P = 0.103). After adjustment for confounders, there was significant relationship between Pre-AMC and OS (HR, 2.031; 95% CI, 1.058-3.898; P = 0.033), but not to DFS (Tables 2 and 3). As shown in Figure 1, the Kaplan–Meier curves indicated that there was no significant difference between low Pre-AMC group and high Pre-AMC group both in OS (P=0.196, Figure 1(a)) and DFS (P=0.316, Figure 1(b)).
Table 2.
Overall survival analyses according to Pre-AMC in 686 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Pre-AMC | 2.145 | 1.124-4.095 | 0.021 | 2.031 | 1.058-3.898 | 0.033 |
| Sex | 1.175 | 0.809-1.705 | 0.398 | |||
| Age (years) | 1.001 | 0.984-1.018 | 0.926 | |||
| Depth of tumor | 1.508 | 1.177-1.932 | 0.001 | 1.344 | 0.970-1.862 | 0.075 |
| Lymph node metastasis | 1.758 | 1.548-1.996 | <0.001 | 1.777 | 1.449-2.180 | <0.001 |
| Pathological stage | 1.905 | 1.548-2.344 | <0.001 | 0.813 | 0.561-1.179 | 0.275 |
| Vessel invasive | 1.753 | 1.348-2.280 | <0.001 | 1.186 | 0.892-1.576 | 0.24 |
| Nerve infiltration | 1.838 | 1.422-2.376 | <0.001 | 1.543 | 1.177-2.023 | 0.002 |
| Treatment regimen | 1.009 | 0.899-1.131 | 0.883 | |||
| Hospital time (days) | 1.007 | 0.995-1.019 | 0.24 | |||
Table 3.
Disease-free survival analyses according to Pre-AMC in 686 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Pre-AMC | 1.853 | 0.883-3.889 | 0.103 | |||
| Sex | 1.177 | 0.778-1.782 | 0.440 | |||
| Age (years) | 0.993 | 0.9974-1.011 | 0.425 | |||
| Depth of tumor | 1.137 | 0.898-1.439 | 0.286 | |||
| Lymph node metastasis | 1.601 | 1.382-1.855 | <0.001 | 1.596 | 1.283-1.987 | <0.001 |
| Pathological stage | 1.557 | 1.257-1.928 | <0.001 | 0.825 | 0.603-1.131 | 0.232 |
| Vessel invasive | 1.273 | 0.935-1.732 | 0.125 | |||
| Nerve infiltration | 1.608 | 1.205-2.145 | 0.001 | 1.506 | 1.113-2.036 | 0.008 |
| Treatment regimen | 1.368 | 1.219-1.535 | <0.001 | 1.264 | 1.118-1.428 | <0.001 |
| Hospital time (days) | 0.998 | 0.982-1.013 | 0.759 | |||
Figure 1.
Overall survival and disease-free survival analysis according the preoperative absolute monocyte count (Pre-AMC) (a, b) and postoperative absolute monocyte count change (c, d).
In univariate analyses of AMCc, there was a significant difference in OS (HR, 0.581; 95% CI, 0.428-0.789; P = 0.001) and DFS (HR, 0.671; 95% CI, 0.468-0.962; P = 0.030) between the AMCc increased group and AMCc decreased group. In multivariate analyses, AMCc decreased was associated with worse OS (HR, 0.614; 95% CI, 0.450-0.837; P = 0.002) and DFS (HR, 0.656; 95% CI, 0.456-0.943; P = 0.023) (Tables 4 and 5). The Kaplan–Meier curves suggested that AMCc decreased could be predict worse OS (P⩽0.001, Figure 1(c)) and DFS (P=0.023, Figure 1(d)). Above all, AMCc was a better independent prognostic factor than Pre-AMC in patients with ESCC after esophageal radical surgery.
Table 4.
Overall survival analyses according to AMC change in 686 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| AMCc (increased vs. decreased) | 0.581 | 0.428-0.789 | 0.001 | 0.614 | 0.450-0.837 | 0.002 |
| Sex | 1.175 | 0.809-1.705 | 0.398 | |||
| Age (years) | 1.001 | 0.984-1.018 | 0.926 | |||
| Depth of tumor | 1.508 | 1.177-1.932 | 0.001 | 1.339 | 0.970-1.849 | 0.076 |
| Lymph node metastasis | 1.758 | 1.548-1.996 | <0.001 | 1.766 | 1.441-2.165 | <0.001 |
| Pathological stage | 1.905 | 1.548-2.344 | <0.001 | 0.838 | 0.578-1.217 | 0.354 |
| Vessel invasive | 1.753 | 1.348-2.280 | <0.001 | 1.184 | 0.892-1.573 | 0.243 |
| Nerve infiltration | 1.838 | 1.422-2.376 | <0.001 | 1.479 | 1.129-1.936 | 0.004 |
| Treatment regimen | 1.009 | 0.899-1.131 | 0.883 | |||
| Hospital time (days) | 1.007 | 0.995-1.019 | 0.24 | |||
Table 5.
Disease-free survival analyses according to AMC change in 686 patients with ESCC.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| AMCc (increased vs. decreased) | 0.671 | 0.468-0.962 | 0.030 | 0.656 | 0.456-0.943 | 0.023 |
| Sex | 1.177 | 0.778-1.782 | 0.440 | |||
| Age (years) | 0.993 | 0.9974-1.011 | 0.425 | |||
| Depth of tumor | 1.137 | 0.898-1.439 | 0.286 | |||
| Lymph node metastasis | 1.601 | 1.382-1.855 | <0.001 | 1.572 | 1.263-1.957 | <0.001 |
| Pathological stage | 1.557 | 1.257-1.928 | <0.001 | 0.849 | 0.620-1.165 | 0.311 |
| Vessel invasive | 1.273 | 0.935-1.732 | 0.125 | |||
| Nerve infiltration | 1.608 | 1.205-2.145 | 0.001 | 1.462 | 1.081-1.978 | 0.014 |
| Treatment regimen | 1.368 | 1.219-1.535 | <0.001 | 1.274 | 1.128-1.440 | <0.001 |
| Hospital time (days) | 0.998 | 0.982-1.013 | 0.759 | |||
3. Discussion
The systemic inflammatory reaction in the tumor microenvironment plays a critical part in tumorigenesis and progression [18]. The systemic inflammatory reaction increases the circulating counts of monocytes, neutrophils and platelets [19–21]. Preoperative absolute monocyte count (Pre-AMC) has been demonstrated to be associated with the prognosis of various cancers. However, all studies reported the role of Pre-AMC without the evaluation of the AMCc. These researches focused only on the Pre-AMC, not on the dynamic changes in AMC after therapy. Moreover, no universal cut-off value of Pre-AMC exists, which was typically set by the receiver operating characteristics curve (ROC) [14], or mean value [22]. Therefore, the optimal cut-off value of Pre-AMC varied in different researches, no matter there were the real variations between different clinical laboratories and races [14–17]. We think that an optimal cut-off value of Pre-AMC or Post-AMC that satisfies all of the clinical laboratories in worldwide does not exist. In this study, for the first time, we evaluated AMCc that was not affected by the cut-off value of AMC and might reflect the dynamic change of the systemic inflammatory reaction from preoperative to postoperative. We demonstrated that AMCc could be a better prognosis factor in patients with ESCC after curative resection than Pre-AMC.
In this study, multivariate analysis indicated that AMCc was an independent prognosis biomarker both for OS and DFS. While Pre-AMC was only an independent prognosis biomarker for OS, not for DFS. This findings were inconsistent with the previous study, which reported Pre-AMC could predict worse outcomes both in OS and DFS in patients with ESCC [17]. The reason for this inconsistent was that we used different manners to choose optimal cut-off value, and that different numbers of patients were enrolled. Interestingly, when comparing the demographic and clinical features of the AMCc increased and decreased group, we found that the median Pre-AMC was lower and the median Post-AMC was higher in the AMCc increased group than AMCc decreased group. This finding suggested that the balance between the systemic inflammatory reaction and immune reaction might change after the curative surgery. This change might lead to a different prognosis. However, the precise mechanism that monocyte might predict clinical suivival are not fully understood. One proposed postulation is as follows: monocytes are recruited by some cytokines and chemokines around the cancer. Then monocytes differentiate into tumor-associated macrophages (TAMs), which facilitate numerous pro-tumorigenic mechanisms. Macrophages are classified into the two states: the classically activated type 1 macrophages (M1) and the alternatively activated type 2 macrophages (M2) [23]. M1 produces type I proinflammatory cytokines, present antigen to T cells for an adaptive immune response, and partake anti-tumor function. While M2 produces type II cytokines and contributes to pro-tumorigenic effect including tumor initiation, invasion, angiogenesis and metastasis [24, 25]. TAM infiltration was related to overall worse outcome and poor responses to chemotherapy in patients with ESCC [26–28]. In the present study, AMCc increased is significantly associated with worse outcome in ESCC. When the relationship between systemic inflammatory reaction and immune reaction is out of balance, this might partake the pro-tumor function that leads to worse prognosis. AMCc could accurately represent the dynamic change of the relationship between systemic inflammatory reaction and immune reaction from preoperative to postoperative.
There were several limitations of this study: first, it was the single-center design and retrospective analysis. Multi-center design and prospective trials are needed to prove these findings. Second, our data were not divided into a training set and a validation set for statistical validation. In the future, we are looking forward to the similar results of other types of cancer.
4. Methods
4.1. Patient Selection
748 patients newly diagnosed with ESCC from Feb 2008 to Feb 2015 at the Zhejiang Cancer Hospital were enrolled in this study. The World Health Organization classification criteria were the standard for the determination of the histological grades. These included patients were pathologically confirmed, and received operation after diagnosis without preoperative adjuvant therapy including chemotherapy or radiotherapy. There are 62 patients were excluded: 45 patients with preoperative chemotherapy, 9 patients with preoperative radiotherapy, 3 patients without Pre-AMC available, and 5 patients without Post-AMC available. As a result, 686 patients with ESCC were chosen in the present study.
The Pre-AMC was examined within 1 week prior to surgery, and the Post-AMC was checked after 1 week after curative operation. The present study was approved by the Ethics Committee of Zhejiang Cancer Hospital. All included patients completed written informed consent.
4.2. Blood-Routine Markers
Blood (2 mL) was drawn into the EDTA-K2 anticoagulative tubes for a routine fasting blood sample. The peripheral monocyte and platelet were checked by the SYSMEX XE-2100 (Sysmex, Kobe, Japan) Automatic Blood Cell Analyzer.
4.3. Statistical Analysis
The Pre-AMC, Post-AMC, Pre-platelet and Post-platelet were analyzed as continuous variables and all clinical features were counted as categorical variables. Categorical data are expressed as numbers and percentage, and continuous data, which do not meet a normal distribution, are presented as median and interquartile range. The relationship between AMCc and clinical features of ESCC was analyzed using chi-square tests. The median of Pre-AMC was chosen to determine the optimal cut-off value. The Kaplan-Meier method was shown overall survival (OS) and disease-free survival (DFS). The Kaplan-Meier curve was analyzed by GraphPad Prism 7 software. The effect of clinical features on prognosis was calculated by the log-rank test. Univariate and multivariate COX regression analyses were used to evaluate the predictors, which were expressed as hazard ratio, 95% confidence interval and P value. P <0.05 was considered to be statistically significant. The SPSS software (version 19.0) was used for statistical analysis.
Acknowledgments
The authors thank the included patients and all the investigators, including the clinicians and laboratory technicians in our study. This study was funded by National Natural Science Foundation of China (Contract/Grant no. 81602615) and General research program of Health Department of Zhejiang Province (Contract/Grant no. 2016KYB048) and Zhejiang Youth Talents Project (Contract/Grant no. 2019RC026).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All procedures in the present study were performed in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. The study approval was obtained from ethics committee at Zhejiang Cancer Hospital.
Consent
Informed consent was informed from all participants.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Qian Song and Sheng Wang contributed to conception and design; Qian Song and Jun-zhou Wu managed data acquisition; Qian Song and Jun-zhou Wu conducted analysis and interpretation of data (e.g., statistical analysis); Qian Song and Sheng Wang did the writing, review, and/or revision of the manuscript. All authors read and approved the final manuscript.
References
- 1.Bray F., Ferlay J., Soerjomataram I. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Laversanne M., Brown L. M., Devesa S. S., Bray F. Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. American Journal of Gastroenterology. 2017;112(8):1247–1255. doi: 10.1038/ajg.2017.155. [DOI] [PubMed] [Google Scholar]
- 3.Tran G. D., Sun X.-D., Abnet C. C., et al. Prospective study of risk factors for esophageal and gastric cancers in the linxian general population trial cohort in China. International Journal of Cancer. 2005;113(3):456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 4.Gertler R., Stein H. J., Langer R., et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: evaluation of the new union internationale contre le cancer/American joint cancer committee staging system. Annals of Surgery. 2011;253(4):689–698. doi: 10.1097/sla.0b013e31821111b5. [DOI] [PubMed] [Google Scholar]
- 5.Allum W. H., Stenning S. P., Bancewicz J., et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. Journal of Clinical Oncology. 2009;27:5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 6.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Annals of Surgical Oncology. 2010;17(12):3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 7.Dupre A., Malik H. Z. Inflammation and cancer: what a surgical oncologist should know. European Journal of Surgical Oncology. 2018;44:566–570. doi: 10.1016/j.ejso.2018.02.209. [DOI] [PubMed] [Google Scholar]
- 8.Lin C., Zhang J. Inflammasomes in inflammation-induced cancer. Frontiers in Immunology. 2017;8(271) doi: 10.3389/fimmu.2017.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan X., Zhang J., Li D., et al. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecologic Oncology. 2017;147(1):181–187. doi: 10.1016/j.ygyno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Becker M., Muller C. B., De Bastiani M. A., Klamt F. The prognostic impact of tumor-associated macrophages and intra-tumoral apoptosis in non-small cell lung cancer. Histology and Histopathology. 2014;29:21–31. doi: 10.14670/HH-29.21. [DOI] [PubMed] [Google Scholar]
- 11.Li M.-X., Liu X.-M., Zhang X.-F., et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. International Journal of Cancer. 2014;134(10):2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 12.Liu J., Yang X. J., Geng X. F., et al. Prognostic significance of tumor-associated macrophages density in gastric cancer: a systemic review and meta-analysis . Minerva Medica. 2016;107:314–321. [PubMed] [Google Scholar]
- 13.Farajzadeh Valilou S., Keshavarz-Fathi M., Silvestris N., Argentiero A., Rezaei N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine & Growth Factor Reviews. 2018;39:46–61. doi: 10.1016/j.cytogfr.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai S., Marumo S., Shoji T., et al. Prognostic impact of preoperative monocyte counts in patients with resected lung adenocarcinoma. Lung Cancer. 2014;85(3):457–464. doi: 10.1016/j.lungcan.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Shen S., Fu S., Huang X., et al. Elevated preoperative peripheral blood monocyte count predicts poor prognosis for hepatocellular carcinoma after curative resection. BMC Cancer. 2014;14, article 744(1) doi: 10.1186/1471-2407-14-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Zhu Y., Pan J., et al. Peripheral monocyte count: an independent diagnostic and prognostic biomarker for prostate cancer - a large Chinese cohort study. Asian Journal of Andrology. 2017;19(5):579–585. doi: 10.4103/1008-682X.186185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han L., Jia Y., Song Q., et al. Prognostic significance of preoperative absolute peripheral monocyte count in esophageal squamous cell carcinoma. Diseases of the Esophagus. 2016;29(7):740–746. doi: 10.1111/dote.12401. [DOI] [PubMed] [Google Scholar]
- 18.Crusz S. M., Balkwill F. R. Inflammation and cancer: advances and new agents. Nature Reviews Clinical Oncology. 2015;12(10):584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Valdes N., Basagoiti M., Dotor J., et al. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFbeta1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Research. 2011;71(3):812–821. doi: 10.1158/0008-5472.CAN-10-2698. [DOI] [PubMed] [Google Scholar]
- 20.Torisu H., Ono M., Kiryu H., et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. International Journal of Cancer. 2000;85(2):182–188. [PubMed] [Google Scholar]
- 21.Kuss I., Hathaway B., Ferris R. L., et al. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clinical Cancer Research. 2004;10:3755–3762. doi: 10.1158/1078-0432.CCR-04-0054. [DOI] [PubMed] [Google Scholar]
- 22.Huang S. H., Waldron J. N., Milosevic M., et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes in oropharyngeal cancer stratified by human papillomavirus status. Cancer. 2015;121(4):545–555. doi: 10.1002/cncr.29100. [DOI] [PubMed] [Google Scholar]
- 23.Biswas S. K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology. 2010;11(10):889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 24.Quail D. F., Joyce J. A. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Condeelis J., Pollard J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Shigeoka M., Urakawa N., Nakamura T., et al. Tumor associated macrophage expressing CD204 is associated with tumor aggressiveness of esophageal squamous cell carcinoma. Cancer Science. 2013;104(8):1112–1119. doi: 10.1111/cas.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin E. W., Karakasheva T. A., Hicks P. D., et al. The tumor microenvironment in esophageal cancer. Oncogene. 2016;35:5337–5349. doi: 10.1038/onc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y., Li M., Bo C., et al. Prognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinoma. Cancer Immunology, Immunotherapy. 2017;66(3):343–354. doi: 10.1007/s00262-016-1931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.