Abstract
Dioscin is a typical saponin with multiple pharmacological activities. The past few years have seen an emerging interest in and growing research on this pleiotropic saponin. Here, we review the emerging pharmacological activities reported recently, with foci on its antitumor, antimicrobial, anti-inflammatory, antioxidative, and tissue-protective properties. The potential use of dioscin in therapies of diverse clinical disorders is also discussed.
1. Introduction
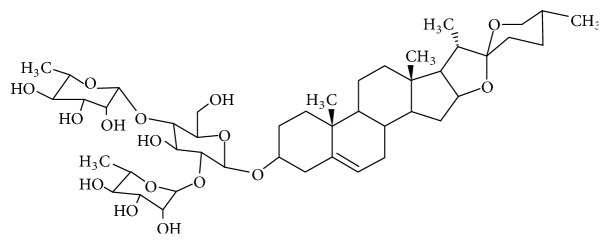
Natural products have been an important source of drugs for antifungal, antibacterial, antitumor, and other pharmacological interventions [1, 2]. Recent years have witnessed an increase in the researches on biological activities of compounds from plants. Those from medicinal and edible plant are of special interest, considering their relative safety profiles. One of these compounds is dioscin (Figure 1), the pharmacological activities of which have been renewed a lot in the past decade; therefore, we reviewed here the recent advances of dioscin.
Figure 1.
The chemical structure of dioscin. Mw=869.05 g/mol.
2. Sources
As a steroidal saponin, dioscin (diosgenyl 2,4-di-O-α-L-rhamnopyranosyl-β-D-glucopyranoside) could be isolated from various kinds of vegetables and herbs, most of which belong to the family of Dioscoreaceae (Table 1). Some edible plants of Dioscore genus (such as Dioscorea opposita, Dioscorea alata, and Dioscorea japonica) serve as starchy staple food in undeveloped countries around the world [3]. The rhizomes of these medicinal herbs are employed to treat atherosclerosis [4], burns [5], fever [5], myocardial ischemia [6], angina pectoris [6], asthma [7], rheumatoid arthritis [7, 8], bronchitis [7], anthrax [8], rheumatic heart disease [8–10], born injuries [9], and gastric disorders [8, 9], to lower cholesterol and triglyceride [11, 12], and to improve circulation [13] in multiple countries.
Table 1.
Occurrence of dioscin in plants.
| Family | Species | Plant parts | References |
|---|---|---|---|
| Dioscoreaceae | Dioscorea nipponica | Rhizomes | [7, 69] |
| Dioscoreaceae | Dioscorea panthaica | Rhizomes | [6, 8] |
| Dioscoreaceae | Dioscorea spongiosa | Rhizomes | [29] |
| Dioscoreaceae | Dioscorea alata | Rhizomes | [70] |
| Dioscoreaceae | Dioscorea bulbifera | Tubers | [17] |
| Dioscoreaceae | Dioscorea cayenensis | Rhizomes | [5, 71, 72] |
| Dioscoreaceae | Dioscorea zingiberensis | Rhizomes | [73, 74] |
| Dioscoreaceae | Dioscoreae septemlobae | Rhizomes | [30] |
| Dioscoreaceae | Dioscorea villosa | Roots | [18] |
| Dioscoreaceae | Dioscorea mangenotiana | Tubers | [71] |
| Dioscoreaceae | Dioscorea rotundata | Tubers | [71] |
| Dioscoreaceae | Dioscorea villosa | Roots | [75, 76] |
| Dioscoreaceae | Dioscorea pseudojaponica | Tubers | [77, 78] |
| Dioscoreaceae | Dioscorea batatas | Rhizomes | [79, 80] |
| Dioscoreaceae | Dioscorea parviflora | Rhizomes | [81] |
| Dioscoreaceae | Dioscorea futschauensis | Rhizomes | [82] |
| Dioscoreaceae | Dioscorea collettii | Rhizomes | [43, 44] |
| Dioscoreaceae | Dioscorea tokoro Makino | Rhizomes | [83] |
| Dioscoreaceae | Tamus communis | Rhizomes | [84, 85] |
| Liliaceae | Allium ampeloprasum | Bulbs | [86] |
| Liliaceae | Asparagus cochinchinensis | Roots | [87] |
| Liliaceae | Ophiopogon japonicus | Flowers | [88] |
| Liliaceae | Paris Chinensis | Unspecified | [89] |
| Liliaceae | Paris polyphylla | Rhizomes | [90, 91] |
| Liliaceae | Polygonatum sibiricum | Rhizomes | [19] |
| Liliaceae | Polygonatum Zanlanscianense Pamp | Roots | [50, 92, 93] |
| Liliaceae | Smilax bockii Warb | Roots | [94] |
| Liliaceae | Smilax china | Rhizomes | [95] |
| Liliaceae | Smilax excelsa | Rhizomes | [96, 97] |
| Liliaceae | Smilax menispermoidea | Rhizomes | [98] |
| Liliaceae | Trillium Tschonoskii Maxim | Rhizomes | [99] |
| Solanaceae | Solanum heteracanthum | Roots | [100] |
| Solanaceae | Solanum incanum | Roots | [100] |
| Solanaceae | Solanum indicum | Whole plant | [101] |
| Solanaceae | Solanum melongena L. Usukawamarunasu | Fruits | [16] |
| Solanaceae | Solanum rostratum | Aerial parts | [102] |
| Arecaceae | Borassus flabellifer | Male flowers | [103] |
| Convallariaceae | Smilacina atropurpurea | Rhizomes | [40] |
| Fabaceae | Trigonella foenum-graecum | Seeds | [104] |
| Zygophyllaceae | Tribulus terrestris | Arial parts | [96, 105] |
3. Safety Profiles
The dioscin-containing DA-9801 has completed phase II clinical trials for treating diabetic neuropathy in US in 2015 [14], indicating its acceptable safety profiles. Also, dioscin-containing Di-Ao-Xin-Xue-Kang capsules have been employed to treat cardiovascular disease for more than 20 years in China and have been approved for therapeutic use in Netherland since 2012 [15]. Without citing any other toxicity data, the entry for clinical trials and the approval for access into drug market are sufficient to clarify the safety profiles of dioscin for therapeutic use.
As an ingredient of Usukawamarunasu that has been eaten for centuries in Japan, the cytotoxicity of dioscin against HaCaT cells was low, with an IC50 of about 100 μM [16]. Dioscin at a concentration of 35 μM did not induce significant apoptosis increase or viability loss in HepG2 2.215 and 293 cells [17]. However, the toxicity of dioscin to many cancer cells was relatively high, with IC50 ranging from 2 to 20 μM [18–20]. Although dioscin has shown hepatotoxicity in cultured cells, at the level of μg/mL [21–23], it did demonstrate hepatoprotective effects on liver cells at level of ng/mL [21, 24]. Despite the presence of edema and magnocellular nucleus in hepatocytes of mice given a large dose of dioscin by tail vein injection [25], indicating its potential hepatoxicity, dioscin did exert hepatoprotective effects on mice and rats in many reports, which will be elaborated in the later part [21, 24, 26]. Nonetheless, the toxicity of dioscin must be considered, if administered in large dose. The maximal safe dose of dioscin in rats was reported to be 300 mg/kg/day in a subchronic toxicological assessment [27].
Using pooled human liver microsomes (H161) as the reaction system, dioscin, at a high concentration of 200 μM, showed no inhibitory activities against four UDP-glucuronosyltransferases (UGTs, UGT1A1, UGT1A4, UGT1A9, and UGT2B7) and six of eight Cytochrome P450 (CYP450) enzymes tested (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, and CYP3A4). Even for the two enzymes (CYP2C19 and CYP2D6) inhibited by dioscin, the IC50 values were higher than 150 μM [28]. Since CYP 450 and UGTs, besides drug transporters, are associated with drug-drug interactions, these results indicate that dioscin may hardly or very weakly influence the metabolism of other drugs, if used in combination with dioscin, although much more work are needed to be done.
4. Anti-Hyperuricemia
As the end product with poor solubility of purine catabolism catalyzed by xanthine oxidase (XO) in human, uric acid in blood is mainly excreted through urine and feces [29–31]. Resulting from overproduction (10%) or aberrantly low excretion (90%) of uric acid through kidney, hyperuricemia is responsible for a majority of gout patients and are associated with diabetes and chronic kidney disease [29, 32, 33]. Thus, besides the classic target XO that plays a critical role in uric acid production, transporters, such as organic anion transporter 1 (OAT1, SLC22A6) and urate transporter 1 (URAT1, SLC22A12) in kidney, might be promising targets for treating hyperuricemia. In rodent models of hyperuricemia induced by potassium oxonate, dioscin could reduce the uric acid level in blood and facilitate the urate clearance, thus ameliorating the renal injuries caused by hyperuricemia [29, 30]. In these studies, dioscin increased the expression of OAT1 and decreased URAT1 and GLUT-9 in kidney, whose function are secretion and re-absorption of urate, respectively [29, 30]. In addition, dioscin has showed weak inhibition on XO activity, which might contributes to its antihyperuricemic effects [30].
And this recently published study identified that it is the metabolite of dioscin, tigogenin, that contributes to the uric acid-lowering effects of dioscin through suppressing URAT1 and promoting ABCG2 (the primary intestinal transporter for uric acid excretion) [29]. Considering that P-glycoprotein transporter (P-gp) is also associated with urate transport in intestine [31] and dioscin has been reported to inhibit P-gp in cancer cells [34–37], dioscin may be a promising candidate for developing antihyperuricemic therapies.
5. Antifungal and Antiviral
As an important human fungal pathogen, Candida albicans could cause a series of infections that are associated with oral cavity, urogenital tracts, as well as the increasingly used indwelling devices. In some cases, C. albicans could cause life-threatening bloodstream infections [38]. The antifungal activity of dioscin against C. albicans was first reported by Sautour et al. in 2004 [5, 39] and confirmed later [40], and inhibitory activities against C. glabrata, C. tropicalis were also reported simultaneously [5, 39, 40]. Later in 2013, the antifungal activity of dioscin against C. albicans was attributed to its ability to induce damage to plasma membrane [7]. The cell membrane-disruptive activity of dioscin may be due to the penetration into and accumulation in membrane lipid and the binding to ergosterol, a counterpart of cholesterol in fungal cells [41]. Recently, dioscin was found to be effective against the formation and development of C. albicans biofilms, a microbial lifestyle with complex structures and high resistance to antifungal agents [38, 42].
Dioscin could also cause increased membrane permeability and excessive ROS generation in Saprolegnia parasitica, a pathogenic fungus in freshwater ecosystem [43]. In addition, dioscin has also been reported to induce abnormal mycelial morphologies of Pyricularia oryzae, one famous fungal pathogen of rice [44]. In a word, dioscin has showed significant antifungal activities.
Viruses are important human pathogens that could cause a broad spectrum of diseases. Recently, dioscin was reported to have in vitro antiviral activities against adenovirus, hepatitis B virus and vesicular stomatitis virus, although the efficacy varies in different phases of infections [17].
6. Antitumor
Dioscin has demonstrated antitumor activities against many kinds of tumors, such as lung cancer, esophageal cancer, gastric cancer, colon cancer, glioblastoma, cervix carcinoma, ovarian cancer, breast cancer, prostate cancer, and leukemia. The target tissues listed can be referred to the review on saponins in 2016 [45]. Recently, the antitumor spectrum of dioscin has been further expanded [46–49]. In most studies, dioscin exerts antitumor effects through intrinsic mitochondrial apoptosis, involving activation of caspase-9 and caspase-3 and reduce in antiapoptotic proteins such as Bcl-2, Bcl-xl, cIAP-1, and Mcl-1 [50–52]. At the same time, the levels of pro-apoptotic proteins (Bak, Bax and Bid) are increased. In the apoptosis induced by dioscin, Ca2+ release and increased endogenous reactive oxygen species (ROS) production are common to be found [51, 53–56]. PI3K/Akt/mTOR and p38 MAPK and JNK signaling pathways are also involved in the antitumor activities of dioscin [19, 48, 57, 58]. In pancreatic cancer PANC-1 and ASPC-1 cells, dioscin-induced apoptosis is caused by inhibition of Akt1 signaling, mediated by miR-149-3P, which is one of the 107 microRNAs affected by dioscin [49]. In some cancer cells, extrinsic death receptor pathway was activated by dioscin in addition to mitochondrial pathway [19, 59], although both signaling pathways could converge on downstream pro-apoptotic proteins such as caspase-3, caspase-7 and downstream Bid and Bak [59].
Besides, the apoptosis-inducing effects of dioscin also involves the activation of ERK1/2 and AIF pathway [60, 61], the increase in the levels of NO and inducible NO synthase [20], and demethylation of DAPK1 and RASSF-1α gene [47], potentiation of TRAIL-induced apoptosis in human renal cancer cells by downregulation of c-FLIPL [62]. Meanwhile, dioscin was found to inhibit cell proliferation by causing DNA damage and DNA hypodiploidy, and then cell cycle arrest [51, 53, 63]. G2/M cell cycle arrest was found in multiple kinds of tumor cells through downregulation of cyclin B1, checkpoint kinase CHK2 and cyclin-dependent kinase CDK1 [19, 20, 63, 64], while S-phase arrest in cells, by way of regulating DNA Topo I, p53, CDK2, and Cyclin A expression [53, 54].
Antitumor effects of dioscin also involves inhibition of migration, invasion and angiogenesis. Dioscin could decrease the CAP-1 related cell migration and invasion, and markedly down-regulate MMP2 and MMP9 expression [53, 58]. DNMT3A, TET2, TET3, ZFPM2 and E-cad were increased while TET1, VIM and MMP9 were decreased by dioscin in breast cancer cells [18]. Angiogenesis is important in the solid tumor growth and migration. Dioscin could suppress the VEGF-induced blood vessel formation and downregulate VEGFR2 and its downstream protein kinases phosphoinositide 3-kinase (PI3K), Src, FAK, AKT, phosphorylated p38 mitogen-activated protein kinase (MAPK), and Erk1/2 expression. Phosphorylated P38 MAPK was upregulated in the human umbilical vein endothelial cells (HUVECs) [65] but decreased in ovarian cancer SKOV3 cells [66], the difference may be related to the different cell types. The in vivo studies also confirmed that dioscin could attenuate invasion and migration, decrease tumor size, and extend the lifespan of rats [53, 54].
Dioscin could overcome multidrug resistance and enhance antitumor activity of other drugs. Multi-Drug Resistance (MDR) gene encodes p-glycoprotein (p-170), which is located on the cell membrane and pumps drugs out of cancer cells, resulting in drug resistance. Dioscin was found to significantly inhibit MDR1 mRNA and protein expression and inhibit the NF-κB signaling pathway (via blocking inhibitor κB-α (IκB-α) degradation), thus strengthening drug absorption in adriamycin- (ADR-) resistant erythroleukemic K562/ADR cells, HepG2/ADR cells [35] and human breast cancer MCF-7/ADR cells, as well as methotrexate- (MTX-) resistant Caco-2 colon cancer cells [34, 36, 37]. Indeed, in rat intestine, the absorption of MTX could be enhanced by dioscin treatment [36]. In lung adenocarcinoma, tyrosine kinase inhibitors (TKI) resistance could be inhibited by dioscin, which acts as dual inhibitor of the MEK/ERK and PI3K/AKT signaling pathways, via suppressing SHP2 expression [67]. Another strategy in melanoma tumors is to utilize the bystander effect of gene therapy, and the main method is introducing the suicide gene into tumor cells and giving drugs. In melanoma cells and in vivo samples, dioscin could upregulate the expression of the major components of gap junctions connexins Cx26 and Cx43, resulting in more efficient herpes simplex virus thymidine kinase/ganciclovir- (HSV-tk/GCV-) induced bystander killing [46].
Autophagy also participates in dioscin-induced apoptosis, which could be detected 12 hours after low-dose dioscin exposure and earlier than apoptosis in human lung cancer A549 and H1299 cells and hepatoma Huh7 cells. Dioscin-induced autophagy via ERK1/2 and JNK1/2 pathways. When autophagy was inhibited, dioscin-induced cell apoptosis was significantly enhanced, hence dioscin may provide some benefits for cell survival and act as a cytoprotector [60, 68]. It can also induce autophagy to ameliorate cytotoxicity of ADR by inhibiting PI3K/AKT pathways in MCF-7 and MCF-7/ADR cells [37].
Dioscin also induces differentiation of promyelocytes and melanogenesis in melanoma cells [16, 59]. In AML, dioscin could increase the expression of CCAAT/enhancer-binding protein α (C/EBPα), which is a critical factor for myeloid differentiation and induce the differentiation of promyelocytes to granulocytes and monocytes [59]. Dioscin was found to reduce expression of tyrosinase, TRP-1, and TRP-2, which could lead to inhibition of intracellular production of melanin, and at the same time, it could decrease the expression of MITF via inhibition of phosphorylation of CREB in the α-MSH-induced melanogenesis [16].
Given its significant antitumor activities, dioscin may be a promising candidate for developing anticancer therapies. Moreover, its resistance-sensitizing activity may make dioscin an adjunctive to current anticancer pharmacological therapies.
7. Hepatoprotective
As the major organ in human for metabolism (detoxification and assimilation), liver often faces multiple stresses and thus can undergo various pathogenesis in response to those stresses. These pathological changes can manifest as fibrosis, injuries, cholestasis, and fatty liver diseases.
7.1. Fibrosis
Hepatic fibrosis, featured by excessive accumulation of extracellular matrix (ECM, mainly collagen and fibronectin), is a long-term process that gradually causes damaged architecture and impaired function of liver, resulting in cirrhosis associated with high mortality and morbidity [106]. Via PI3K/Akt signaling pathway, dioscin can significantly inhibit the synthesis of proteins of ECM, such as Col1a1, Col1a2, Col2a1, Col5a1 and Col6a1 [107]. The degradation of ECM could also be enhanced by dioscin through regulating MMPs and their inhibitors, TIMP [108]. In alcoholic fibrosis, dioscin could mitigate this process through Toll-like receptor 4 (TLR4)/MyD88/NF-kB signaling [109]. Through suppressing p38 MAPK signaling mediated by Sirt1/Nrf2, this saponin could also ameliorate liver fibrosis caused by BDL or DMN [110]. Dioscin could prevent the activation and induce the senescence and apoptosis of hepatic stellate cells, which are critical in liver fibrosis. Besides, the expression of PPAR-γ, Nrf2, HO-1, and SOD was increased, while TGF-β1/Smad, Wnt/β catenin, and MAPK signaling pathways could be suppressed by dioscin to mitigate oxidative stress and alleviate hepatic fibrosis [108, 111], suggesting that dioscin could be a promising candidate with multiple targets in liver fibrosis.
7.2. Acute Liver Damage
Dioscin could attenuate the acute liver injury induced by DMN and CCl4 through inhibiting apoptosis, necrosis, oxidative stress and inflammation [112, 113]. The acute liver injury induced by thioacetamide could also be rescued by treatment with dioscin, and this protection involves inhibition on oxidative stress and inflammation through FXR/AMPK signaling pathway [114]. The protective effects of dioscin against liver injury induced by ethanol and acetaminophen are associated with mitochondrial function adjustment, besides apoptosis and inflammation inhibition [115, 116]. Acute inflammatory liver injury induced by LPS could also be attenuated through suppressing TLR4/MyD88 signaling [21]. Virus infections caused by HBV also compromise the function of liver, while the antiviral activity of dioscin implies another kind of protection against damage upon liver [17].
7.3. Nonalcoholic Fatty Liver Disease (NAFLD)
Featured by abundant fat storage in liver, NAFLD can progress into cirrhosis and hepatocarcinoma [117]. In NAFLD model of rodents, dioscin could activate the SIRT1/AMPK signaling pathways to regulate genes (such as SREBP-1c and CPT) to attenuate lipid accumulation, suggesting the therapeutic effect of dioscin on NAFLD [24].
7.4. Cholestasis
Cholestasis, caused by excessive accumulation of bile salts in liver and featured by primary sclerosing cholangitis, biliary cirrhosis and atresia, could result in increased oxidative stress and apoptosis [26, 118]. In cholestasis models induced by α-naphthylisothiocyanate (ANIT), dioscin could clean toxic bile constituents from the liver through upregulation of multidrug resistance-associated protein 2 (Mrp2) and bile salt export pump (Bsep) [26, 118]. Aberrant changes in the expression of other transporters of bile salts (organic anion transporting polypeptides (OATPs), organic cation transporters (OCTs) and Na+-taurocholate cotransporting polypeptide (Ntcp)) caused by ANIT could also be prevented by dioscin treatment. Moreover, dioscin could increase the levels of antioxidative powers (such as GSH, GSH-Px, and SOD) and antiapoptotic proteins in animals, as well as in primary cultured cells, to mitigate oxidative stress and to reduce apoptosis in cholestasis [118].
7.5. Hepatic Ischemia/Reperfusion (I/R) Injury
Ischemia/reperfusion (I/R) can engender damage to tissues in surgery, transplantation and stroke, while the underlying mechanism involves oxidative stress, inflammation and apoptosis [119, 120]. Although dioscin exerts apoptosis-inducing effects in various kinds of tumor cell lines in vitro [19, 48, 51, 54, 61], dioscin did also show protective effects on hepatic I/R injury through suppressing apoptosis in rats [119]. The in vivo antiapoptotic activity was exerted through upregulating Bcl-2 and Bcl-x and downregulating Bak, CYP2E1, caspase 3, caspase 9, p53, and PARP [119]. The protection was also associated with decreased ROS, RNS and inflammation [119]. In a word, dioscin has multiple protecting effects on liver. Besides, dioscin can also ameliorate gastric and intestinal I/R injury through inhibiting inflammation and apoptosis, although the signaling pathways affected are different [121–123].
8. Lung-Protective
In mouse model of pulmonary fibrosis induced by crystalline silica, dioscin could inhibit TGF-β/Smad3 signaling to delay fibrosis [124]. Through regulating immune response, dioscin could reduce the secretion of pro-inflammatory cytokines, which could also be seen in LPS-induced acute lung damage [125]. In addition, dioscin could inhibit the expression and production of MUC5AC mucin by airway epithelial cells, thus contributing to treating inflammatory pulmonary diseases which are associated with mucus hypersecretion [87].
9. Nephroprotective
Chronic kidney disease (CKD) severely influences the people's health, with 10-15% of the adults in the world affected, imposing a heavy global burden [126]. CKD also increases the risk for other ailments (such as cardiovascular disease, diabetes, and tumor) and can be triggered by chemicals and food additives including fructose [127, 128]. Renal injury induced by excessive fructose consumption is a well-established rat model for studying the pathogenesis of renal injury [128, 129]. This induction involves decrease in SOD, abnormalities in lipid metabolism and the presence of inflammatory response and fibrosis [128–130]. Fructose-induced renal histopathological changes in rats, such as epithelial cell swelling and vacuolar degeneration of vacuoles and brush border, could be rescued significantly by dioscin treatment [128]. In renal tissue of rat, the inhibitory effects of dioscin against fibrosis, oxidative stress and abnormal lipid metabolism were via restoring the normal expression of Sirt3, the reduced expression of which would result in NF-kB activation and cell death [128, 131]. The expression of TGF-β1, which plays an important role in renal fibrosis, could also be inhibited by dioscin via Sirt3 to mitigate renal fibrosis [128].
In inflammatory kidney injury induced by LPS, TLR4/MyD88 pathways was suppressed by dioscin via upregulating miR-let-7i [132]. Moreover, apoptosis and oxidative stress were also inhibited. While in I/R renal injury, TLR4/MyD88 signaling was suppressed by dioscin through upregulating Hsp70 to alleviate damage [133]. Inflammation, as well as oxidative stress, caused by nephrotoxic doxorubicin could be attenuated by dioscin through farnesoid X receptor- (FXR-) mediated signaling [134]. In contrast, cisplatin-induced nephrotoxicity can be suppressed by dioscin through regulating miR-34a/sirtuin 1 (Sirt1) signaling pathways [135].
Since renal damage could be caused directly by hyperuricemia, given the anti-hyperuricemic effect of dioscin, dioscin may offer another kind of nephroprotection [29, 30]. The increased concentration of creatinine in blood (an indicator of renal function) caused by potassium oxonate could be lowered through increased excretion by treatment with dioscin [30]. The pathological lesions caused by potassium oxonate and adenine during hyperuricemia induction in rodent animals could also be mitigated by dioscin [29]. The effects of dioscin on uptake transporters in kidney, such as OATP1B1, OATP1B3, OAT3, and OATP2B1, are different. Dioscin weakly inhibits the activity of OAT3 (major uptake transporter) and OATP1B3 while it stimulates OATP1B1 and OATP2B1, thus facilitating the uptake of steviol glucuronide (natural sweetener used in beverages) mediated by OATP1B1 and OATP2B1 [136]. In addition, through regulating OAT1, URAT1 and OCT2, dioscin shows uricosuric and nephroprotective effects in mice [30].
10. Cardioprotective
Clinically, the therapeutic use of doxorubicin for cancer is impeded by its cardiotoxicity, while treatment with dioscin (at the level of ng/mL) could increase the viability of cardiocytes that were exposed to doxorubicin. This kind of protective effects was associated with decreased oxidative insults mediated by downregulation of miR-140-5p in H9c2 cells and rodents [137]. Moreover, apoptosis and oxidative damage in H9c2 cells induced by I/R could also be suppressed by dioscin, despite that dioscin could induce apoptosis in various cancer cells [138]. In vivo protective effects of dioscin against myocardial I/R injury in rats were exerted through upregulation of connexin 43 [139]. In addition, dioscin could also suppress cardiac hypertrophy induced by angiotensin II infusion through downregulating MAPK and Akt/GSK3β/mTOR pathways, improving the impaired cardiac function [140].
11. Cerebral Protection
During treatment of ischemic stroke, which causes a large proportion of morbidity and mortality worldwide, reperfusion can cause damage to brain [141]. Dioscin could attenuate cerebral I/R injury through inhibiting TLR4/MyD88/TRAF6 signaling mediated by HGMB-1 suppression [141]. In this process, cerebral inflammation can also be inhibited by dioscin [141, 142]. Combined with baicalein, dioscin could mitigate the malfunction of spatial learning memory caused by cerebral I/R in mice [143]. This combination could also contribute to hippocampal plasticity through multiple signaling pathways, thus facilitating post-ischemic rehabilitation associated with stroke [144]. One of the latest researches also showed that dioscin could protect hippocampus from endotoxemia induced neuroinflammation [145].
12. Antiatherosclerosis
Adhesion molecules-mediated interaction between monocytes and endothelial cells plays an important role in the initial phase of atherosclerosis, which is an inflammation-associated vascular disease [146]. Dioscin could suppress the expression of adhesion molecules (such as VCAM-1 and ICAM-1) and the synthesis of endothelial lipase in HUVECs stimulated by inflammatory cytokine TNF-α, through inhibiting NF-kB nuclear translocation and activation [146].
Meanwhile, the secretory inhibition of IL-1β, IL-6, and TNF-α via modulation of NK-kB can attenuate the inflammatory response in atherosclerotic plaque [147]. Another consequence of NF-kB inhibition is that LOX-1 expression would be inhibited by blocking the binding of LOX-1 promoter with NF-kB [147]. The formation of macrophage-derived foam cells, which constitute the lipid core and is a characteristic of early atherosclerosis, could also be inhibited by dioscin treatment [147]. In rat model of atherosclerosis induced by high-fat diet, dioscin could decrease the lipid level in blood and aortic vessels [147]. Dioscin has also been used as a positive control for its lipid-lowering effect [77]. This makes dioscin a promising molecule of anti-atherosclerotic and lipid-lowering property.
13. Anti-Inflammatory
Inflammation, as the host response to detrimental stimuli, are mediated by pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6, which are secreted by immune cells recruited to injury sites. Sustained inflammatory cytokines release and reaction result in chronic inflammation, which is featured by excessive immune cell infiltration and is the major cause of many diseases [148].
TNF-α, IL-1β, and IL-6 secretion in HUVECs, macrophages, NRK-52E and HK-2 cells, can be inhibited by dioscin [135, 146, 147]. The mRNA levels of TNF-α, IL-1β and IL-6 can also be suppressed in mice and rat model of multiple diseases, such as acute liver injury [112], liver fibrosis [108], obesity [149], cerebral and intestinal I/R injury [121, 141], and inflammatory injuries of kidney, liver, and lung [21, 125, 132], thus attenuating inflammatory damage.
Acute neuroinflammation caused by endotoxemia could be mitigated by dioscin treatment, which is associated with improved 5-HT metabolism [145]. Protecting roles of dioscin against systemic inflammatory response syndrome has also been demonstrated, though inhibiting TLR2/MyD88/NF-kB signaling pathway in mice and rats [150].
In adipocytes, dioscin could also increase the expression of adiponectin, a hormone known to own anti-inflammatory effects, thus possibly exerting another kind of indirect anti-inflammatory effect [151].
14. Antiarthritic
As a chronic inflammatory disease caused by disorder of individual immune system and featured by overgrowth of synoviocytes and fibroblast-like synoviocytes, rheumatoid arthritis may engender articular degeneration and damage, affecting heavily public health given its prevalence [152, 153]. The in vitro inhibitory effects of dioscin on synovial cell hyperplasia induced by IL-1β has been reported [154]. In collagen induced arthritis in mice, dioscin could also palliate the synovial hyperplasia and mitigate the inflammatory response, through increasing the expression levels of p-STAT6 and GATA3 and decreasing p-STAT4 in synovium, which is mediated by regulating the balance between Th1 and Th2 cells [152].
In gouty arthritis induced by monosodium urate crystal, the in vivo therapeutic effects of dioscin may be accomplished by inhibiting the increase in stromal cell-derived factor-1 (SDF-1), CXCR4 (receptor of SDF-1) and p38 MAPK signaling in synovial tissue of rats [154]. Considering that SDF-1/CXCR4 signaling is involved in the pathogenesis of rheumatoid arthritis (synovium-derived SDF-1 was increased over 10-fold in RA patients), dioscin may exert multiple protective effects on arthritis [152, 154, 155].
Osteoarthritis, emanating from damage to cartilage and underlying bones, can cause pain, stiffness, and deformity and affect millions of people all around the world [156, 157]. In monosodium iodoacetate induced osteoarthritis, the degradation of ECM by MMP13, CHOP-mediated apoptosis of chondrocytes, inflammation, ER stress and oxidative stress could be inhibited by dioscin. Wnt/β-catenin signaling, which activates catabolism in chondrocytes, could also be downregulated by dioscin, while PPARγ, whose activation can lower the production of catabolic factors and ameliorate the oxidative stress, could be upregulated, thus contributing to the protecting effects of dioscin against osteoarthritis [156].
15. Antiobesity and Diabetes
Obesity is often associated with increased risk for a series of diseases including type 2 diabetes mellitus (T2DM), certain cancers, osteoarthritis, cardiovascular, and respiratory diseases. Worldwide, the incidence of obesity is growing while the drug is limited, highlighting the necessity for developing new drugs [158].
Dioscin inhibits porcine pancreatic lipase in vitro with an IC50 of 20 μg/ml (thus inhibiting the absorption of fat) while Dioscorea nipponica Makino powders containing dioscin inhibits the rise of blood triacylglycerol concentration induced by oral gavage of corn oil emulsion in mice [158]. In rats, this powder also lowers the increase in body weight and adipose tissue caused by high-fat diet (HFD), through enhanced excretion of fat through feces other than decreased food intake [158]. This effect was also confirmed by the later researches, which reported that HFD-induced obesity, as well as NAFLD, could be suppressed by dioscin [149, 151]. In the later research, the anti-obesity effect of dioscin was due to the elevated energy expenditure rather than the increased physical activity in obese and HFD mice [149].
At concentrations without obvious cytotoxicity, dioscin can inhibit insulin exposure-initiated differentiation by delaying cell cycle progression and suppressing the phosphorylation of ERK1/2 and p38 rather than c-JNK in differentiating preadipocytes. Dioscin can also inhibit adipogenesis through inducing phosphorylation of AMPK, which stimulates the catabolic process of fatty acid to facilitate the energy production [151].
Besides its anti-obesity effects, dioscin might exert beneficial effects on diabetes, which represents a heavy burden on public health system. In a study published in 2018, dioscin has been shown to promote β cell proliferation through Wnt/β-catenin pathway and attenuate the impairments (decreased viability and increased apoptosis) of β cells induced by high-glucose treatment [159]. Moreover, in HFD-fed mice, the insulin resistance in adipose tissues could be mitigated by treatment with dioscin through insulin receptor substrate 1 (IRS-1)/PI3K/Akt pathway [160]. Taken together, dioscin may has potential for treating obesity and diabetes, although it has a very long way to go.
16. Antioxidative Stress
Oxidative stress plays a considerable role in the pathogenesis of a variety of diseases and injuries such as pulmonary fibrosis [161], CKD [128], inflammatory lung injury [125], and cisplatin- and doxorubicin-induced kidney injury [134, 135].
SOD2, which contributes to removing superoxide during oxidative stress [162], could be activated by Sirt3, which could be upregulated by dioscin in renal tissue [128]. The inducing effects of TGF-β1 on ROS production and myofibroblast differentiation could also be suppressed by overexpression of Sirt3 [163]. In cardiac myoblast H9c2 cells, dioscin treatment could attenuate oxidative stress through increasing SOD expression [138]. Dioscin could also increase the in vivo expression of other antioxidant enzymes such as HO-1, Nrf2, and GSH-Px in rats and lower the levels of MDA, NO and iNOS [108, 119, 125, 149]. In a word, the inhibitory effects of dioscin on oxidative stress can also be seen in a variety of disease models, such as hepatic fibrosis, I/R injuries, cardiac hypertrophy, and drugs-induced toxicity [110, 112, 119, 137, 140, 141].
17. Antiosteoporosis
Osteoporosis, featured with loss of bone tissue that associated with raised risk of fracture, is caused by imbalanced bone homeostasis towards bone resorption by osteoclasts [164]. This imbalance could be restored by induction of osteoblast activity and suppression of osteoclast function [164, 165].
Dioscin could facilitate osteoblastic proliferation and differentiation through low-density lipoprotein receptor related protein 5 (Lrp5) and estrogen receptor (ER) pathways in mouse pre-osteoblast-like MC3T3-E1 cells and in human osteoblast-like MG-63 cells [164]. Apoptosis in osteoblasts could also be suppressed by dioscin through increasing the expression of the anti-apoptotic Bcl-2, thus promoting the bone mass maintenance [164]. Another beneficial effect of dioscin on osteoporosis is decreasing the expression of RANKL (receptor activator of NF-kB ligand, secreted by osteoblast and stromal cells) which induces NF-kB signaling pathway through binding to and activating osteoclast surface receptor RANK and subsequent Akt phosphorylation [164–166]. Phosphorylated Akt stimulates NF-kB and NFATc1 activity, which are critical for osteoclastic survival and differentiation. Therefore, dioscin could also inhibit bone resorption by osteoclasts though down-regulating the Akt signaling cascades [165]. The protecting effect was also confirmed in bone destruction mice model induced by LPS [165]. The TRAF6 (tumor necrosis factor receptor-associated factor 6) signaling, which initiates pathways associated with osteoclast activation (such as MAPKs and Akt) and can be activated by LPS/TLR4, could also be inhibited by dioscin [166]. The decrease in osteoprotegerin (OPG, the decoy receptor for RANKL) mRNA level and OPG/RANKL ratio in femurs induced by ovariectomy could be rescued by dioscin in rodents, albeit the effects on reversing osteoclastogenesis were in vain [166].
18. Other Bioactivities
Dioscin could synergize with oxyresveratrol in suppressing tyrosinase (the enzyme critical in melanogenesis), using L-tyrosine and L-DOPA as substrates in vitro, suggesting its potential use as cosmetic whitening agent and for preventing melanin hyperpigmentation [95]. Later, in B16 murine melanoma cells, dioscin was shown to inhibit the melanin production induced by α-melanocyte-stimulating hormone (MSH), and this inhibition was due to the decreased expression of tyrosinase, tyrosinase-related protein-1 (TRP-1) and TRP-2 via downregulation of phosphor-CREB and microphthalmia-related transcription factor (MITF) [16]. In vitro inducing effect of dioscin on growth hormone release in rat pituitary cells has been reported to be strong, with an approximately 17-fold stimulation of release [104]. Dioscin has also showed anti-depressant effects through increasing 5-hydroxytryptamine (5-HT) in hippocampus in mice [145].
Dioscin also showed strong anthelmintic activity against the aquacultural ectoparasite Dactylogyrus intermedius, with an EC50 of 0.44 mg/L, which is more effective than the positive used [167].
19. Conclusions
Recent years have seen a lot of reports on the beneficial effects of dioscin, including antitumor, hepatoprotective, antiviral, nephroprotective, cardioprotective, antifungal, anti-hyperuricemia activities. Although dioscin falls out of the rule of five due to its large molecular weight, it does fall into the sweet scope where the balance between protein-protein interaction (PPI) inhibition and oral bioavailability may be found [168]. Moreover, many derivatives with diverse modifications have been synthesized, leading to great ease to explore the structure-activity relationship for optimization [169–171]. Considering the safety profiles shown by this compound, dioscin could be a promising candidate for developing therapies against multiple disorders.
Acknowledgments
This work is supported by the Natural Science Foundation of Jilin Province [20180520118JH], the Education Department of Jilin Province [JJKH20190052KJ], and National Natural Science Foundation of China [no. 81800849].
Contributor Information
Xin Liu, Email: dr_liuxin@jlu.edu.cn.
Lufei Wang, Email: 13404356065@163.com.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Moloney M. G. Natural products as a source for novel antibiotics. Trends in Pharmacological Sciences. 2016;37(8):689–701. doi: 10.1016/j.tips.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Roemer T., Xu D., Singh S. B., et al. Confronting the challenges of natural product-based antifungal discovery. Chemistry & Biology. 2011;18(2):148–164. doi: 10.1016/j.chembiol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Adedayo C., Oboh G., Ademiluyi A., Akindaahunsi A. Comparative studies on antioxidant properties of some tropical Nigerian yam varieties (Dioscorea spp.) Advances in Food Research. 2011;33:28–33. [Google Scholar]
- 4.Solomonova L. N. The use of polysponin in patients with coronary atherosclerosis. Kardiologiya . 1968;8(2):71–74. [PubMed] [Google Scholar]
- 5.Sautour M., Miyamoto T., Dongmo A. Antifungal steroid saponins from dioscorea cayenensis. Planta Medica. 2004;70(1):90–92. doi: 10.1055/s-2004-815467. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y., Yi T., Chen H., Zhao Z., Liang Z., Chen H. Quantitative comparison of multiple components in dioscorea nipponica and D. Panthaica by ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Phytochemical Analysis. 2013;24(4):413–422. doi: 10.1002/pca.2428. [DOI] [PubMed] [Google Scholar]
- 7.Cho J., Choi H., Lee J., Kim M.-S., Sohn H.-Y., Lee D. G. The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochimica et Biophysica Acta. 2013;1828(3):1153–1158. doi: 10.1016/j.bbamem.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Dong M., Feng X.-Z., Wang B.-X., Wu L.-J., Ikejima T. Two novel furostanol saponins from the rhizomes of Dioscorea panthaica prain et burkill and their cytotoxic activity. Tetrahedron. 2001;57(3):501–506. doi: 10.1016/S0040-4020(00)01024-3. [DOI] [Google Scholar]
- 9.Wang W., Zhao Y., Jing W., et al. Ultrahigh-performance liquid chromatography-ion trap mass spectrometry characterization of the steroidal saponins of Dioscorea panthaica Prain et Burkill and its application for accelerating the isolation and structural elucidation of steroidal saponins. Steroids. 2015;95:51–65. doi: 10.1016/j.steroids.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y.-N., He X.-C., Ye M., et al. Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. Journal of Ethnopharmacology. 2015;175:451–455. doi: 10.1016/j.jep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Ma H., Zhou Q., Wang B. Antagonistic effect of DX and diosgenin on hyperlipidemia induced by cholesterol in vivo and on blood platelet aggregation in vitro. Chinese Journal of Hospital Pharmacy. 2002;22:323–325. [Google Scholar]
- 12.Zhang Z., Zhang J., Liu Z., Ge H. Clinical study on “Dunye Guanxinning Tablet” in treating hyperlipemia. Chinese Journal of Integrative Medicine on Cardio/ Cerebrovascular Disease. 2009:255–256. [Google Scholar]
- 13.Si Q., Zhou D. Clinic observation of anti-platelet aggregation of dioscin. Chinese Journal of Clinical Pharmacy. 2006;15:238–239. [Google Scholar]
- 14.Kang K. B., Ryu J., Cho Y., Choi S.-Z., Son M., Sung S. H. Combined application of UHPLC-QTOF/MS, HPLC-ELSD and 1H–NMR spectroscopy for quality assessment of DA-9801, a standardised dioscorea extract. Phytochemical Analysis. 2017;28(3):185–194. doi: 10.1002/pca.2659. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z., Zou W., Wang R., Zhou Z. The review and therapeutic effect analysis for ten-years clinical application of Di-ao-xin-xue-kang. China Journal of Traditional Chinese Medicine and Pharmacy. 2004;19:620–622. [Google Scholar]
- 16.Nishina A., Ebina K., Ukiya M., et al. Dioscin derived from Solanum melongena L. “Usukawamarunasu” attenuates α-MSH-induced melanogenesis in B16 murine melanoma cells via downregulation of phospho-CREB and MITF. Journal of Food Science. 2015;80(10):H2354–H2359. doi: 10.1111/1750-3841.13068. [DOI] [PubMed] [Google Scholar]
- 17.Liu C., Wang Y., Wu C., et al. Dioscin’s antiviral effect in vitro. Virus Research. 2013;172(1-2):9–14. doi: 10.1016/j.virusres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Aumsuwan P., Khan S. I., Khan I. A., et al. The anticancer potential of steroidal saponin, dioscin, isolated from wild yam (Dioscorea villosa) root extract in invasive human breast cancer cell line MDA-MB-231 in vitro. Archives of Biochemistry and Biophysics. 2016;591:98–110. doi: 10.1016/j.abb.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Ma Y., Thakur K., et al. Molecular mechanism and inhibitory targets of dioscin in HepG2 cells. Food and Chemical Toxicology. 2018;120:143–154. doi: 10.1016/j.fct.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Xu L., Yin L., et al. iTRAQ-based proteomic analysis of dioscin on human HCT-116 colon cancer cells. Proteomics. 2014;14(1):51–73. doi: 10.1002/pmic.201300101. [DOI] [PubMed] [Google Scholar]
- 21.Yao H., Hu C., Yin L., et al. Dioscin reduces lipopolysaccharide-induced inflammatory liver injury via regulating TLR4/MyD88 signal pathway. International Immunopharmacology. 2016;36:132–141. doi: 10.1016/j.intimp.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y. X., Wang Y. G., Ma Z. C., et al. Preliminary study on hepatotoxicity induced by dioscin and its possible mechanism. China Journal of Chinese Materia Medica. 2015;40:2748–2752. doi: 10.4268/cjcmm20151413. [DOI] [PubMed] [Google Scholar]
- 23.Yang F., Liang Y., Xu L., et al. Exploration in the cascade working mechanisms of liver injury induced by total saponins extracted from Rhizoma Dioscorea bulbifera. Biomed Pharmacother. 2016;83:1048–1056. doi: 10.1016/j.biopha.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Yao H., Tao X., Xu L., et al. Dioscin alleviates non-alcoholic fatty liver disease through adjusting lipid metabolism via SIRT1/AMPK signaling pathway. Pharmacological Research. 2018;131:51–60. doi: 10.1016/j.phrs.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q., Yao G., Jin R. Preliminary study on hepatotoxicity induced by dioscin. Pharmacology and Clinics of Chinese Materia Medica. 2013;29:29–32. [Google Scholar]
- 26.Zhang A., Jia Y., Xu Q., et al. Dioscin protects against ANIT–induced cholestasis via regulating Oatps, Mrp2 and Bsep expression in rats. Toxicology and Applied Pharmacology. 2016;305:127–135. doi: 10.1016/j.taap.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Xu T., Zhang S., Zheng L., Yin L., Xu L., Peng J. A 90-day subchronic toxicological assessment of dioscin, a natural steroid saponin, in Sprague–Dawley rats. Food and Chemical Toxicology. 2012;50(5):1279–1287. doi: 10.1016/j.fct.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Ji H. Y., Liu K. H., Kong T. Y., et al. Evaluation of DA-9801, a new herbal drug for diabetic neuropathy, on metabolism-mediated interaction. Archives of Pharmacal Research. 2013;36(1):1–5. doi: 10.1007/s12272-013-0014-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Jin L., Liu J., et al. Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. Journal of Ethnopharmacology. 2018;214:29–36. doi: 10.1016/j.jep.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Su J., Wei Y., Liu M., et al. Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Archives of Pharmacal Research. 2014;37(10):1336–1344. doi: 10.1007/s12272-014-0413-6. [DOI] [PubMed] [Google Scholar]
- 31.Hosomi A., Nakanishi T., Fujita T., Tamai I., Singh S. R. Extra-renal elimination of uric acid via intestinal efflux transporter BCRP/ABCG2. Plos One. 2012;7(2) doi: 10.1371/journal.pone.0030456.e30456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichida K. What lies behind serum urate concentration? Insights from genetic and genomic studies. Genome Medicine. 2009;1(12) doi: 10.1186/gm118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L., Yin H., Lan Z., et al. Anti-hyperuricemic and nephroprotective effects of Smilax china L. Journal of Ethnopharmacology. 2011;135(2):399–405. doi: 10.1016/j.jep.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Meng Q., Wang C., et al. Dioscin restores the activity of the anticancer agent adriamycin in multidrug-resistant human leukemia K562/adriamycin cells by down-regulating MDR1 via a mechanism involving NF-κB signaling inhibition. Journal of Natural Products. 2013;76(5):909–914. doi: 10.1021/np400071c. [DOI] [PubMed] [Google Scholar]
- 35.Sun B. T., Zheng L. H., Bao Y. L., et al. Reversal effect of Dioscin on multidrug resistance in human hepatoma HepG2/adriamycin cells. European Journal of Pharmacology. 2011;654(2):129–134. doi: 10.1016/j.ejphar.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Wang C., Peng J., et al. Dioscin enhances methotrexate absorption by down-regulating MDR1 in vitro and in vivo. Toxicology and Applied Pharmacology. 2014;277(2):146–154. doi: 10.1016/j.taap.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Wang C., Huo X., Wang L., et al. Dioscin strengthens the efficiency of adriamycin in MCF-7 and MCF-7/ADR cells through autophagy induction: More than just down-regulation of MDR1. Scientific Reports. 2016;6(1) doi: 10.1038/srep28403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Ma Z., Zhang J., Yang L. Antifungal compounds against candida infections from traditional Chinese medicine. BioMed Research International. 2017;2017:12. doi: 10.1155/2017/4614183.4614183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sautour M., Mitaine-Offer A., Miyamoto T., Dongmo A., Lacaille-Dubois M. A new steroidal saponin from Dioscorea cayenensis. Chemical & Pharmaceutical Bulletin. 2004;52(11):1353–1355. doi: 10.1248/cpb.52.1353. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Li H., Zhang Y., et al. Atropurosides A–G, new steroidal saponins from smilacina atropurpurea. Steroids. 2006;71(8):712–719. doi: 10.1016/j.steroids.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Lin F., Wang R. Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. Journal of Molecular Modeling. 2010;16(1):107–118. doi: 10.1007/s00894-009-0523-0. [DOI] [PubMed] [Google Scholar]
- 42.Yang L., Liu X., Zhong L., et al. Dioscin inhibits virulence factors of candida albicans. BioMed Research International. 2018;2018:9. doi: 10.1155/2018/4651726.4651726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu L., Shen Y., Liu G., et al. Inhibition of dioscin on Saprolegnia in vitro. FEMS Microbiology Letters. 2015;362(24) doi: 10.1093/femsle/fnv196.fnv196 [DOI] [PubMed] [Google Scholar]
- 44.Hu K., Dong A., Yao X., Kobayashi H., Iwasaki S. Antineoplastic agents; I. Three spirostanol glycosides from rhizomes of Dioscorea collettii var. hypoglauca. Planta Medica. 1996;62(06):573–575. doi: 10.1055/s-2006-957978. [DOI] [PubMed] [Google Scholar]
- 45.Xu X., Li T., Fong C., et al. Saponins from Chinese medicines as anticancer agents. Molecules. 2016;21(10):p. 1326. doi: 10.3390/molecules21101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao J., Zhang G., Li B., et al. Dioscin augments HSV-tk-mediated suicide gene therapy for melanoma by promoting connexin-based intercellular communication. Oncotarget. 2017;8(1):798–807. doi: 10.18632/oncotarget.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q., Song W., Xiao W. Dioscin induces demethylation of DAPK-1 and RASSF-1α genes via the antioxidant capacity, resulting in apoptosis of bladder cancer T24 cells. EXCLI Journal. 2017;16:101–112. doi: 10.17179/excli2016-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song X., Wang Z., Liang H., et al. Dioscin induces gallbladder cancer apoptosis by inhibiting ros-mediated PI3K/AKT signalling. International Journal of Biological Sciences. 2017;13(6):782–793. doi: 10.7150/ijbs.18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Si L., Xu L., Yin L., et al. Potent effects of dioscin against pancreatic cancer via miR-149-3P-mediated inhibition of the Akt1 signalling pathway. British Journal of Pharmacology. 2017;174(7):553–568. doi: 10.1111/bph.13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai J., Liu M., Wang Z., Ju Y. Apoptosis induced by dioscin in Hela cells. Biological & Pharmaceutical Bulletin. 2002;25(2):193–196. doi: 10.1248/bpb.25.193. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X., Tao X., Xu L. Dioscin induces apoptosis in human cervical carcinoma HeLa and SiHa cells through ROS-mediated DNA damage and the mitochondrial signaling pathway. Molecules. 2016;21(6):p. E730. doi: 10.3390/molecules21060730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Li H., Zhang X., Xiong C., Ruan J. Dioscin-induced apoptosis of human LNCaP prostate carcinoma cells through activation of caspase-3 and modulation of Bcl-2 protein family. Journal of Huazhong University of Science and Technology (Medical Sciences) 2014;34(1):125–130. doi: 10.1007/s11596-014-1243-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhao X., Xu L., Zheng L., et al. Potent effects of dioscin against gastric cancer in vitro and in vivo. Phytomedicine. 2016;23(3):274–282. doi: 10.1016/j.phymed.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Lv L., Zheng L., Dong D., et al. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food and Chemical Toxicology. 2013;59:657–669. doi: 10.1016/j.fct.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 55.Zhiyu W., Yue C., Neng W., et al. Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biology & Therapy. 2014;13(3):138–147. doi: 10.4161/cbt.13.3.18693. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Che C.-M., Chiu J.-F., He Q.-Y. Dioscin (saponin)-induced generation of reactive oxygen species through mitochondria dysfunction: a proteomic-based study. Journal of Proteome Research. 2007;6(12):4703–4710. doi: 10.1021/pr070399r. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., He Q., Chiu J. Dioscin induced activation of p38 MAPK and JNK via mitochondrial pathway in HL-60 cell line. European Journal of Pharmacology. 2014;735:52–58. doi: 10.1016/j.ejphar.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Si L., Zheng L., Xu L., et al. Dioscin suppresses human laryngeal cancer cells growth via induction of cell-cycle arrest and MAPK-mediated mitochondrial-derived apoptosis and inhibition of tumor invasion. European Journal of Pharmacology. 2016;774:105–117. doi: 10.1016/j.ejphar.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Chan S., Liang P., Guh J. An integrated approach to elucidate signaling pathways of dioscin-induced apoptosis, energy metabolism and differentiation in acute myeloid leukemia. Naunyn-Schmiedeberg's Archives of Pharmacology. 2018;391(6):587–602. doi: 10.1007/s00210-018-1484-6. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh M. J., Yang S. F., Hsieh Y. S., et al. Autophagy inhibition enhances apoptosis induced by dioscin in Huh7 cells. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11. doi: 10.1155/2012/134512.134512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim E., Jang J., Lee Y., et al. Dioscin induces caspase-independent apoptosis through activation of apoptosis-inducing factor in breast cancer cells. Apoptosis. 2014;19(7):1165–1175. doi: 10.1007/s10495-014-0994-z. [DOI] [PubMed] [Google Scholar]
- 62.Kim Y., Kim E., Park K., et al. Dioscin sensitizes cells to TRAIL-induced apoptosis through downregulation of c-FLIP and Bcl-2. Oncology Reports. 2012;28(5):1910–1916. doi: 10.3892/or.2012.1962. [DOI] [PubMed] [Google Scholar]
- 63.Liu M.-J., Wang Z., Ju Y., Zhou J.-B., Wang Y., Wong R. N.-S. The mitotic-arresting and apoptosis-inducing effects of diosgenyl saponins on human leukemia cell lines. Biological & Pharmaceutical Bulletin. 2004;27(7):1059–1065. doi: 10.1248/bpb.27.1059. [DOI] [PubMed] [Google Scholar]
- 64.Gao L.-L., Li F.-R., Jiao P., et al. Paris chinensis dioscin induces G2/M cell cycle arrest and apoptosis in human gastric cancer SGC-7901 cells. World Journal of Gastroenterology. 2011;17(39):4389–4395. doi: 10.3748/wjg.v17.i39.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tong Q., Qing Y., Wu Y., Hu X., Jiang L., Wu X. Dioscin inhibits colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicology and Applied Pharmacology. 2014;281(2):166–173. doi: 10.1016/j.taap.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 66.Guo X., Ding X. Dioscin suppresses the viability of ovarian cancer cells by regulating the VEGFR2 and PI3K/AKT/MAPK signaling pathways. Oncology Letters. 2018 doi: 10.3892/ol.2018.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Wu D., Wu T., Wang L., Chen C., Lee H. Dioscin overcome TKI resistance in EGFR-mutated lung adenocarcinoma cells via down-regulation of tyrosine phosphatase SHP2 expression. International Journal of Biological Sciences. 2018;14(1):47–56. doi: 10.7150/ijbs.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsieh M., Tsai T., Hsieh Y., Wang C., Chiou H. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Archives of Toxicology. 2013;87(11):1927–1937. doi: 10.1007/s00204-013-1047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin L., Xu L., Wang X., Lu B., Liu Y., Peng J. An economical method for isolation of dioscin from Dioscorea nipponica Makino by HSCCC coupled with ELSD, and a computer-aided UNIFAC mathematical model. Chromatographia. 2010;71(1-2):15–23. doi: 10.1365/s10337-009-1407-2. [DOI] [Google Scholar]
- 70.Wu Z.-G., Jiang W., Wei Y.-H., Tao Z.-M. New variety breeding of Dioscorra alata, cultivar “wenshanyao No. 1”. Zhongguo Zhongyao Zazhi. 2015;40(9):1705–1709. doi: 10.4268/cjcmm20150914. [DOI] [PubMed] [Google Scholar]
- 71.Kwon Y., Jie E. Y., Sartie A., et al. Rapid metabolic discrimination and prediction of dioscin content from African yam tubers using Fourier transform-infrared spectroscopy combined with multivariate analysis. Food Chemistry. 2015;166:389–396. doi: 10.1016/j.foodchem.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 72.Ali Z., Smillie T. J., Khan I. A. Two spirostan steroid glycoside fatty esters from Dioscorea cayenensis. Natural Product Communications (NPC) 2013;8:323–326. doi: 10.1177/1934578X1300800311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J., Liang Q., Li C., Liu M., Zhang Y. Comparative transcriptome analysis identifies putative genes involved in dioscin biosynthesis in Dioscorea zingiberensis. Molecules. 2018;23(2):p. 454. doi: 10.3390/molecules23020454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun W., Tu G., Zhang Y. A new steroidal saponin from dioscorea zingiberensis wright. Natural Product Research (Formerly Natural Product Letters) 2003;17(4):287–292. doi: 10.1080/1478641031000136997. [DOI] [PubMed] [Google Scholar]
- 75.Yoon K. D., Kim J. Preparative separation of dioscin derivatives from Dioscorea villosa by centrifugal partition chromatography coupled with evaporative light scattering detection. Journal of Separation Science. 2008;31(13):2486–2491. doi: 10.1002/jssc.200800136. [DOI] [PubMed] [Google Scholar]
- 76.Manda V. K., Avula B., Ali Z., et al. Characterization of in vitro ADME properties of diosgenin and dioscin from dioscorea villosa. Planta Medica. 2013;79(15):1421–1428. doi: 10.1055/s-0033-1350699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pan C.-H., Tsai C.-H., Liu F.-C., et al. Influence of different particle processing on hypocholesterolemic and antiatherogenic activities of yam (Dioscorea pseudojaponica) in cholesterol-fed rabbit model. Journal of the Science of Food and Agriculture. 2013;93(6):1278–1283. doi: 10.1002/jsfa.5882. [DOI] [PubMed] [Google Scholar]
- 78.Yang D., Lu T., Hwang L. S. Isolation and identification of Steroidal saponins in Taiwanese yam cultivar ( Dioscorea pseudojaponica Yamamoto) Journal of Agricultural and Food Chemistry. 2003;51(22):6438–6444. doi: 10.1021/jf030390j. [DOI] [PubMed] [Google Scholar]
- 79.Ho Y. L., Dae Y. J., Ha H., Son K.-H., Jeon S.-J., Kim C. Induction of growth hormone release by dioscin from Dioscorea batatas DECNE. Journal of Biochemistry and Molecular Biology. 2007;40(6):1016–1020. doi: 10.5483/bmbrep.2007.40.6.1016. [DOI] [PubMed] [Google Scholar]
- 80.Jeon J. R., Lee J. S., Lee C. H., Kim J. Y., Kim S. D., Nam D. H. Effect of ethanol extract of dried Chinese yam (Dioscorea batatas) flour containing dioscin on gastrointestinal function in rat model. Archives of Pharmacal Research. 2006;29(5):348–353. doi: 10.1007/BF02968583. [DOI] [PubMed] [Google Scholar]
- 81.Yang S. L., Ma Y. H., Liu X. K. Steroidal constituents from Dioscorea parviflora. Acta pharmaceutica Sinica. 2005;40:145–149. [PubMed] [Google Scholar]
- 82.Liu H. W., Hu K., Zhao Q. C., Cui C. B., Kobayashi H., Yao X. S. Bioactive saponins from Dioscorea futschauensis. Die Pharmazie. 2002;57(8):570–572. [PubMed] [Google Scholar]
- 83.Kawasaki T., Yamauchi T. Structures of prosapogenin-B and -A of dioscin and cooccurrence of B with dioscin in the rhizoma of Dioscorea tokoro Makino. Chem Pharm Bull (Tokyo) 1968;16:1070–1075. doi: 10.1248/cpb.16.1070. [DOI] [PubMed] [Google Scholar]
- 84.Aquino R., Conti C., De Simone F., Orsi N., Pizza C., Stein M. Antiviral activity of constituents of Tamus communis. Journal of Chemotherapy. 2016;3(5):305–309. doi: 10.1080/1120009X.1991.11739110. [DOI] [PubMed] [Google Scholar]
- 85.Aquino R., Behar I., De Simone F., Pizza C. Steroidal glycosides of Tamus communis. Bollettino della Societa Italiano di Biologia Sperimentale. 1984;60:2229–2235. [PubMed] [Google Scholar]
- 86.Sata N., Matsunaga S., Fusetani N., Nishikawa H., Takamura S., Saito T. New antifungal and cytotoxic steroidal saponins from the bulbs of an elephant garlic mutant. Bioscience, Biotechnology, and Biochemistry. 1998;62(10):1904–1911. doi: 10.1271/bbb.62.1904. [DOI] [PubMed] [Google Scholar]
- 87.Lee H. J., Park J. S., Yoon Y. P., et al. Dioscin and methylprotodioscin isolated from the root of Asparagus cochinchinensis suppressed the gene expression and production of airway MUC5AC mucin induced by phorbol ester and growth factor. Phytomedicine. 2015;22(5):568–572. doi: 10.1016/j.phymed.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Zhu Y.-H., Zhao M., Ren L., Tian D., Dou F., Wang J.-X. Studies on the chemical constituents from the flowers of Ophiopogon japonicus. Journal of Chinese Medicinal Materials. 2011;34(5):720–723. [PubMed] [Google Scholar]
- 89.Gao L. L., Li F. R., Jiao P., Yao S. T., Sang H., Si Y. H. Apoptosis of human ovarian cancer cells induced by Paris chinensis dioscin via a Ca(2+)-mediated mitochondrion pathway. Asian Pacific Journal of Cancer Prevention. 2011;12:1361–1366. [PubMed] [Google Scholar]
- 90.Xiao X., Yuan Z., Li G. Separation and purification of steroidal saponins from Paris polyphylla by microwave-assisted extraction coupled with countercurrent chromatography using evaporative light scattering detection. Journal of Separation Science. 2014;37(6):635–641. doi: 10.1002/jssc.201301341. [DOI] [PubMed] [Google Scholar]
- 91.Negi J. S., Bisht V. K., Bhandari A. K., Bhatt V. P., Singh P., Singh N. Paris polyphylla: chemical and biological prospectives. Anti-Cancer Agents in Medicinal Chemistry. 2014;14(6):833–839. doi: 10.2174/1871520614666140611101040. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y., Cheung Y. H., Yang Z., Chiu J., Che C., He Q. Proteomic approach to study the cytotoxicity of dioscin (saponin) Proteomics. 2006;6(8):2422–2432. doi: 10.1002/pmic.200500595. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z., Zhou J., Ju Y., Zhang H., Liu M., Li X. Effects of two saponins extracted from the polygonatum Zanlanscianense pamp on the human leukemia (HL-60) cells. Biological & Pharmaceutical Bulletin. 2000;24(2):159–162. doi: 10.1248/bpb.24.159. [DOI] [PubMed] [Google Scholar]
- 94.Xu J., Li X., Zhao C. C., Wang Y. Pregnane glycosides and steroid saponins from Smilax bockii Warb. and their NGF-potentiating activity. Natural Product Research (Formerly Natural Product Letters) 2008;22(10):884–889. doi: 10.1080/14786410701642557. [DOI] [PubMed] [Google Scholar]
- 95.Liang C., Lim J.-H., Kim S.-H., Kim D.-S. Dioscin: a synergistic tyrosinase inhibitor from the roots of Smilax china. Food Chemistry. 2012;134(2):1146–1148. doi: 10.1016/j.foodchem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Ivanova A., Serly J., Dinchev D., Ocsovszki I., Kostova I., Molnar J. Screening of some saponins and phenolic components of Tribulus terrestris and Smilax excelsa as MDR modulators. In Vivo. 2009;23(4):545–550. [PubMed] [Google Scholar]
- 97.Ivanova A., Mikhova B., Klaiber I., Dinchev D., Kostova I. Steroidal saponins from Smilax excelsa rhizomes. Natural Product Research (Formerly Natural Product Letters) 2009;23(10):916–924. doi: 10.1080/14786410802624827. [DOI] [PubMed] [Google Scholar]
- 98.Ju Y., Jia Z.-J. Steroidal saponins from the rhizomes of Smilax menispermoidea. Phytochemistry. 1992;31(4):1349–1351. doi: 10.1016/0031-9422(92)80288-p. [DOI] [PubMed] [Google Scholar]
- 99.Gao X., Sun W., Fu Q., Niu X. Rapid identification of steroidal saponins in trillium tschonoskii maxim by ultraperformance liquid chromatography coupled to electrospray ionisation quadrupole time-of-flight tandem mass spectrometry. Phytochemical Analysis. 2015;26(4):269–278. doi: 10.1002/pca.2560. [DOI] [PubMed] [Google Scholar]
- 100.Manase M. J., Mitaine-Offer A., Pertuit D., et al. Solanum incanum and S. heteracanthum as sources of biologically active steroid glycosides: Confirmation of their synonymy. Fitoterapia. 2012;83(6):1115–1119. doi: 10.1016/j.fitote.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 101.Chiang H.-C., Tseng T.-H., Wang C.-J., Chen C.-F., Kan W.-S. Experimental antitumor agents from Solanum indicum L. Anticancer Reseach. 1991;11(5):1911–1917. [PubMed] [Google Scholar]
- 102.Hao L. J., Wang S., Zhu J. J., Wang Z. M., Wei S. H. Chemical constituents from Solanum rostratum. China Journal of Chinese Materia Medica. 2014;39:2034–2038. [PubMed] [Google Scholar]
- 103.Yoshikawa M., Xu F., Morikawa T., et al. Medicinal flowers. XII. New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chemical & Pharmaceutical Bulletin. 2007;55(2):308–316. doi: 10.1248/cpb.55.308. [DOI] [PubMed] [Google Scholar]
- 104.Shim S. H., Lee E. J., Kim J. S. Rat growth-hormone release stimulators from fenugreek seeds. Chemistry Biodiversity. 2008;5(9):1753–1761. doi: 10.1002/cbdv.200890164. [DOI] [PubMed] [Google Scholar]
- 105.Dinchev D., Janda B., Evstatieva L., Oleszek W., Aslani M. R., Kostova I. Distribution of steroidal saponins in Tribulus terrestris from different geographical regions. Phytochemistry. 2008;69(1):176–186. doi: 10.1016/j.phytochem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 106.Oh C. J., Kim J.-Y., Min A.-K., et al. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radical Biology & Medicine. 2012;52(5):671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 107.Xu L., Yin L., Tao X., et al. Dioscin, a potent ITGA5 inhibitor, reduces the synthesis of collagen against liver fibrosis: Insights from SILAC-based proteomics analysis. Food and Chemical Toxicology. 2017;107:318–328. doi: 10.1016/j.fct.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Han X., Yin L., et al. Potent effects of dioscin against liver fibrosis. Scientific Reports. 2015;5(1) doi: 10.1038/srep09713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu M., Xu Y., Han X. Dioscin alleviates alcoholic liver fibrosis by attenuating hepatic stellate cell activation via the TLR4/MyD88/NF-kappaB signaling pathway. Scientific Reports. 2015;5, article 18038 doi: 10.1038/srep18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gu L., Tao X., Xu Y., et al. Dioscin alleviates BDL- and DMN-induced hepatic fibrosis via Sirt1/Nrf2-mediated inhibition of p38 MAPK pathway. Toxicology and Applied Pharmacology. 2016;292:19–29. doi: 10.1016/j.taap.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 111.Zhang X., Xu L., Yin L., et al. Quantitative chemical proteomics for investigating the biomarkers of dioscin against liver fibrosis caused by CCl 4 in rats. Chemical Communications. 2015;51(55):11064–11067. doi: 10.1039/C4CC09160D. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W., Yin L., Tao X., et al. Dioscin alleviates dimethylnitrosamine-induced acute liver injury through regulating apoptosis, oxidative stress and inflammation. Environmental Toxicology and Pharmacology. 2016;45:193–201. doi: 10.1016/j.etap.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 113.Lu B., Xu Y., Xu L., et al. Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Environmental Toxicology and Pharmacology. 2012;34(2):127–135. doi: 10.1016/j.etap.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Zheng L., Yin L., Xu L., et al. Protective effect of dioscin against thioacetamide-induced acute liver injury via FXR/AMPK signaling pathway in vivo. Biomedicine & Pharmacotherapy. 2018;97:481–488. doi: 10.1016/j.biopha.2017.10.153. [DOI] [PubMed] [Google Scholar]
- 115.Xu T., Zheng L., Xu L., et al. Protective effects of dioscin against alcohol-induced liver injury. Archives of Toxicology. 2014;88:739–753. doi: 10.1007/s00204-013-1148-8. [DOI] [PubMed] [Google Scholar]
- 116.Zhao X., Cong X., Zheng L., Xu L., Yin L., Peng J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicology Letters. 2012;214(1):69–80. doi: 10.1016/j.toxlet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 117.Friedman S. L., Neuschwander-Tetri B. A., Rinella M., Sanyal A. J. Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao H., Xu Y., Yin L., et al. Dioscin protects ANIT-induced intrahepatic cholestasis through regulating transporters, apoptosis and oxidative stress. Frontiers in Pharmacology. 2017;8 doi: 10.3389/fphar.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tao X., Wan X., Xu Y., et al. Dioscin attenuates hepatic ischemia-reperfusion injury in rats through inhibition of oxidative-nitrative stress, inflammation and apoptosis. Transplantation. 2014;98(6):604–611. doi: 10.1097/TP.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 120.Bouhlel A., Ben Mosbah I., Hadj Abdallah N., et al. Thymoquinone prevents endoplasmic reticulum stress and mitochondria-induced apoptosis in a rat model of partial hepatic warm ischemia reperfusion. Biomedicine & Pharmacotherapy. 2017;94:964–973. doi: 10.1016/j.biopha.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 121.Zheng L., Han X., Hu Y., et al. Dioscin ameliorates intestinal ischemia/reperfusion injury via adjusting miR-351-5p/MAPK13-mediated inflammation and apoptosis. Pharmacological Research. 2019;139:431–439. doi: 10.1016/j.phrs.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 122.Hu Y., Tao X., Han X., et al. Dioscin attenuates gastric ischemia/reperfusion injury through the down-regulation of PKC/ERK1/2 signaling via PKCα and PKCβ2 inhibition. Chemico-Biological Interactions. 2016;258:234–244. doi: 10.1016/j.cbi.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 123.Hu Y., Mao Z., Xu L., et al. Protective effect of dioscin against intestinal ischemia/reperfusion injury via adjusting miR-351-5p-mediated oxidative stress. Pharmacological Research. 2018;137:56–63. doi: 10.1016/j.phrs.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 124.Li C., Lu Y., Du S., et al. Dioscin exerts protective effects against crystalline silica-induced pulmonary fibrosis in mice. Theranostics. 2017;7(17):4255–4275. doi: 10.7150/thno.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao H., Sun Y., Song S., et al. Protective effects of dioscin against lipopolysaccharide-induced acute lung injury through inhibition of oxidative stress and inflammation. Frontiers in Pharmacology. 2017;8, article no. 120 doi: 10.3389/fphar.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Levin A., Tonelli M., Bonventre J., et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. The Lancet. 2017;390(10105):1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 127.Chronic Kidney Disease Prognosis C., Matsushita K., van der Velde M., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qiao Y., Xu L., Tao X., et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicology Letters. 2018;284:37–45. doi: 10.1016/j.toxlet.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 129.Prince P. D., Lanzi C. R., Toblli J. E., et al. Dietary (–)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats. Free Radical Biology & Medicine. 2016;90:35–46. doi: 10.1016/j.freeradbiomed.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 130.Dornas W. C., Cardoso L. M., Silva M., et al. Oxidative stress causes hypertension and activation of nuclear factor-κB after high-fructose and salt treatments. Scientific Reports. 2017;7(1) doi: 10.1038/srep46051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen C., Fu Y., Yu W., Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-κB. Biochemical and Biophysical Research Communications. 2013;430(2):798–803. doi: 10.1016/j.bbrc.2012.11.066. [DOI] [PubMed] [Google Scholar]
- 132.Qi M., Yin L., Xu L. Dioscin alleviates lipopolysaccharide-induced inflammatory kidney injury via the microRNA let-7i/TLR4/MyD88 signaling pathway. Pharmacological Research. 2016;111:509–522. doi: 10.1016/j.phrs.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 133.Qi M., Zheng L., Qi Y. Dioscin attenuates renal ischemia/reperfusion injury by inhibiting the TLR4/MyD88 signaling pathway via up-regulation of HSP70. Pharmacological Research. 2015;100:341–352. doi: 10.1016/j.phrs.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Y., Xu Y., Qi Y., et al. Protective effects of dioscin against doxorubicin-induced nephrotoxicity via adjusting FXR-mediated oxidative stress and inflammation. Toxicology. 2017;378:53–64. doi: 10.1016/j.tox.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 135.Zhang Y., Tao X., Yin L., et al. Protective effects of dioscin against cisplatin-induced nephrotoxicity via the microRNA-34a/sirtuin 1 signalling pathway. British Journal of Pharmacology. 2017;174(15):2512–2527. doi: 10.1111/bph.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang M., Qi H., Li J., Xu Y., Zhang H. Transmembrane transport of steviol glucuronide and its potential interaction with selected drugs and natural compounds. Food and Chemical Toxicology. 2015;86:217–224. doi: 10.1016/j.fct.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 137.Zhao L., Tao X., Qi Y., Xu L., Yin L., Peng J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biology. 2018;16:189–198. doi: 10.1016/j.redox.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin J., Kang Y., Xu Z., Zang C., Fang B., Liu X. Dioscin prevents the mitochondrial apoptosis and attenuates oxidative stress in cardiac H9c2 cells. Drug Research. 2014;64(01):47–52. doi: 10.1055/s-0033-1349101. [DOI] [PubMed] [Google Scholar]
- 139.Cheng J., Sun C., Zhang J., Zou Q., Hao Q., Xue Y. The protective effects of preconditioning with dioscin on myocardial ischemia/reperfusion-induced ventricular arrhythmias by increasing connexin 43 expression in rats. Journal of Cardiovascular Pharmacology and Therapeutics. 2019;24(3):262–268. doi: 10.1177/1074248418801567. [DOI] [PubMed] [Google Scholar]
- 140.Chen L., Li Q., Lei L., Li T. Dioscin ameliorates cardiac hypertrophy through inhibition of the MAPK and Akt/GSK3β/mTOR pathways. Life Sciences. 2018;209:420–429. doi: 10.1016/j.lfs.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 141.Tao X., Sun X., Yin L., et al. Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radical Biology & Medicine. 2015;84:103–115. doi: 10.1016/j.freeradbiomed.2015.03.003.12343 [DOI] [PubMed] [Google Scholar]
- 142.Zhu S., Tang S., Su F. Dioscin inhibits ischemic stroke-induced inflammation through inhibition of the TLR4/MyD88/NF-κB signaling pathway in a rat model. Molecular Medicine Reports. 2018;17(1):660–666. doi: 10.3892/mmr.2017.7900. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z., Du Q., Wang F., et al. Microarray analysis of gene expression on herbal glycoside recipes improving deficient ability of spatial learning memory in ischemic mice. Journal of Neurochemistry. 2004;88(6):1406–1415. doi: 10.1046/j.1471-4159.2003.02258.x. [DOI] [PubMed] [Google Scholar]
- 144.Wang Z., Du Q., Wang F., et al. Large scale analysis of genes contributing to the herbal preparation dependent hippocampal plasticity in postischemic rehabilitation. Vascular Pharmacology. 2007;47(5-6):319–327. doi: 10.1016/j.vph.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 145.Yang R., Chen W., Lu Y., et al. Dioscin relieves endotoxemia induced acute neuro-inflammation and protect neurogenesis via improving 5-HT metabolism. Scientific Reports. 2017;7(1) doi: 10.1038/srep40035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu S., Xu H., Peng J., et al. Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1and EL expression via the NF-κB pathway. Biochimie. 2015;110:62–72. doi: 10.1016/j.biochi.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 147.Wang P., He L., Shen G., Li R., Yang J. Inhibitory effects of Dioscin on atherosclerosis and foam cell formation in hyperlipidemia rats. Inflammopharmacology. 2017;25(6):633–642. doi: 10.1007/s10787-017-0341-4. [DOI] [PubMed] [Google Scholar]
- 148.Dvorakova M., Landa P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacological Research. 2017;124:126–145. doi: 10.1016/j.phrs.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 149.Liu M., Xu L., Yin L. Potent effects of dioscin against obesity in mice. Scientific Reports. 2015;5, article 7973 doi: 10.1038/srep07973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao X., Yin L., Fang L., et al. Protective effects of dioscin against systemic inflammatory response syndrome via adjusting TLR2/MyD88/NFkappab signal pathway. International Immunopharmacology. 2018;65:458–469. doi: 10.1016/j.intimp.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 151.Poudel B., Lim S., Ki H., Nepali S., Lee Y., Kim D. Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. International Journal of Molecular Medicine. 2014;34(5):1401–1408. doi: 10.3892/ijmm.2014.1921. [DOI] [PubMed] [Google Scholar]
- 152.Guo Y., Xing E., Song H., et al. Therapeutic effect of dioscin on collagen-induced arthritis through reduction of Th1/Th2. International Immunopharmacology. 2016;39:79–83. doi: 10.1016/j.intimp.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 153.Rahmati M., Moosavi M. A., McDermott M. F. ER stress: a therapeutic target in rheumatoid arthritis? Trends in Pharmacological Sciences. 2018;39(7):610–623. doi: 10.1016/j.tips.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Lu F., Liu L., Yu D., Li X., Zhou Q., Liu S. Therapeutic effect of Rhizoma Dioscoreae Nipponicae on gouty arthritis based on the SDF-1/CXCR 4 and p38 MAPK pathway: an in vivo and in vitro study. Phytotherapy Research. 2014;28(2):280–288. doi: 10.1002/ptr.4997. [DOI] [PubMed] [Google Scholar]
- 155.Kanbe K., Takagishi K., Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis & Rheumatism. 2002;46(1):130–137. doi: 10.1002/1529-0131(200201)46:160;130::aid-art1002062;3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 156.Lu J., Zhang T., Sun H., Wang S., Liu M. Protective effects of dioscin against cartilage destruction in a monosodium iodoacetate (MIA)-indcued osteoarthritis rat model. Biomedicine & Pharmacotherapy. 2018;108:1029–1038. doi: 10.1016/j.biopha.2018.09.075. [DOI] [PubMed] [Google Scholar]
- 157.Jones I. A., Togashi R., Wilson M. L., Heckmann N., Vangsness C. T. Intra-articular treatment options for knee osteoarthritis. Nature Reviews Rheumatology. 2019;15(2):77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kwon C.-S., Sohn H. Y., Kim S. H., et al. Anti-obesity effect of Dioscorea nipponica makino with lipase-inhibitory activity in rodents. Bioscience, Biotechnology, and Biochemistry. 2003;67(7):1451–1456. doi: 10.1271/bbb.67.1451. [DOI] [PubMed] [Google Scholar]
- 159.Yu F., Bing L., Xie Y., Yu W. Dioscin promotes proliferation of pancreatic beta cells via Wnt/β-catenin signaling pathways. Clinical Laboratory. 2018;64(5):785–791. doi: 10.7754/Clin.Lab.2018.171136. [DOI] [PubMed] [Google Scholar]
- 160.Li H., Yu L., Zhao C. Dioscin attenuates highfat dietinduced insulin resistance of adipose tissue through the IRS1/PI3K/Akt signaling pathway. Molecular Medicine Reports. 2018 doi: 10.3892/mmr.2018.9700. [DOI] [PubMed] [Google Scholar]
- 161.Anathy V., Lahue K. G., Chapman D. G., et al. Reducing protein oxidation reverses lung fibrosis. Nature Medicine. 2018;24(8):1128–1135. doi: 10.1038/s41591-018-0090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ratliff B. B., Abdulmahdi W., Pawar R., Wolin M. S. Oxidant mechanisms in renal injury and disease. Antioxidants & Redox Signaling. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bindu S., Pillai V. B., Kanwal A., et al. SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017;312(1):L68–L78. doi: 10.1152/ajplung.00188.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang C., Peng J., Wu S., et al. Dioscin promotes osteoblastic proliferation and differentiation via Lrp5 and ER pathway in mouse and human osteoblast-like cell lines. Journal of Biomedical Science. 2014;21, article 30 doi: 10.1186/1423-0127-21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qu X., Zhai Z., Liu X., et al. Dioscin inhibits osteoclast differentiation and bone resorption though down-regulating the Akt signaling cascades. Biochemical and Biophysical Research Communications. 2014;443(2):658–665. doi: 10.1016/j.bbrc.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 166.Tao X., Qi Y., Xu L., et al. Dioscin reduces ovariectomy-induced bone loss by enhancing osteoblastogenesis and inhibiting osteoclastogenesis. Pharmacological Research. 2016;108:90–101. doi: 10.1016/j.phrs.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 167.Wang G.-X., Han J., Zhao L.-W., Jiang D.-X., Liu Y.-T., Liu X.-L. Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine. 2010;17(14):1102–1105. doi: 10.1016/j.phymed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 168.Iqbal E. S., Hartman M. C. Shaping molecular diversity. Nature Chemistry. 2018;10(7):692–694. doi: 10.1038/s41557-018-0095-7. [DOI] [PubMed] [Google Scholar]
- 169.Zhu S., Zhang Y., Li M., et al. Synthesis and cytotoxicities of dioscin derivatives with decorated chacotriosyl residues. Bioorganic & Medicinal Chemistry Letters. 2006;16(21):5629–5632. doi: 10.1016/j.bmcl.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 170.Kaskiw M. J., Tassotto M. L., Mok M., et al. Structural analogues of diosgenyl saponins: Synthesis and anticancer activity. Bioorganic & Medicinal Chemistry. 2009;17(22):7670–7679. doi: 10.1016/j.bmc.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 171.Chen P., Wang P., Song N., Li M. Convergent synthesis and cytotoxic activities of 26-thio- and selenodioscin. Steroids. 2013;78(9):959–966. doi: 10.1016/j.steroids.2013.05.018. [DOI] [PubMed] [Google Scholar]