Abstract
Purpose
BrightBrainer integrative cognitive rehabilitation system evaluation in a Medical Adult Day Program by a subject with Primary Progressive Aphasia (PPA) assumed to be of the mixed nonfluent/logopenic variant, and the determination of potential benefits.
Methods
Subject was a 51 year old Caucasian male diagnosed with PPA who had attended a Medical Adult Day Program for 18 months prior to BrightBrainer training. The subject interacted with therapeutic games using a controller that measured 3D hand movements and flexion of both index fingers. The computer simulations adapted difficulty level based on task performance; results were stored on a remote server. The clinical trial consisted of 16 sessions, twice/week for 8 weeks. Subject was evaluated through neuropsychological measures, therapy notes and caregiver feedback forms.
Results
Neuropsychological testing indicated no depression (BDI 0) and severe dementia (BIMS 1 and MMSE 3). The 6.5 hours of therapy consisted of games targeting Language comprehension; Executive functions; Focusing; Short-term memory; and Immediate/working memory. The subject attained the highest difficulty level in all-but-one game, while averaging 1,300 arm task-oriented active movement repetitions and 320 index finger flexion movements per session. While neuropsychological testing showed no benefits, the caregiver reported strong improvements in verbal responses, vocabulary use, speaking in complete sentences, following one-step directions and participating in daily activities. This corroborated well with therapy notes.
Conclusions
Preliminary findings demonstrate a meaningful reduction of PPA symptoms for the subject, suggesting follow up imaging studies to detail neuronal changes induced by BrightBrainer system and controlled studies with a sufficiently large number of PPA subjects.
Keywords: Primary Progressive Aphasia, Virtual Rehabilitation, bimanual game controller, BrightBrainer, caregiver feedback
1. Introduction
Dementia is a disorder that affects several cognitive domains such as memory and executive functions1, and language. The number of Americans affected by Alzheimer’s disease (the most prevalent form of dementia) is currently estimated at 5.2 million2. This number is expected to double by 2050. While generally dementia affects the elderly, its fronto-temporal lobar degeneration (FTLD) sub-group has been found in disproportionally higher numbers in those younger than 60 (estimated at 4–15 cases per 100,0003). Individuals affected by FTLD have behavioral abnormalities such as poor impulse control, inability to persist in a task, loss of social awareness (inappropriate comments to others) and rigidity of mental attitudes4.
Primary Progressive Aphasia (PPA) is a rare form of FTLD. Subjects with PPA have some of the behavioral abnormalities described above, and in addition have difficulty in naming, word-finding, have non-fluent speech and impaired language comprehension. Based on specific speech and language features, PPA has been subdivided into three variants: progressive nonfluent/agrammatic aphasia (PNFA), semantic dementia and logopenic (LPA)5. The core clinical diagnosis features are agramatism of language production and halting speech with sound errors in the nonfluent variant6. For the semantic variant of PPA core diagnostic features are confrontation naming and single-word comprehension, and some other features are impaired object knowledge as well as spared speech production. A clinical diagnostic of logopenic PPA is based on findings of impaired single-word retrieval in naming or speech, as well as impaired repetition of sentences. While speech is affected in all three variants, individuals with PPA have less involved visual spatial functions and motor abilities.
In view of the communication difficulties and behavioral issues of individuals with PPA as well as their younger age, participation in traditional Adult Day Programs may be challenging. Some intriguing questions then present themselves: Can such programs integrate virtual reality systems7 as a modality of non-verbal motor interaction that would benefit individuals with PPA? Can virtual reality games be used to improve their focusing abilities, reduce impulsiveness, even improve language comprehension and vocabulary? This article describes a subject with PPA who attended a Medical Adult Day Program in a multi-site aging service provider in central New Jersey where he trained on a virtual reality system, and the resulting outcomes.
2. Materials and Methods
2.1 Case characteristics and medical history
The subject was a 51 year old Caucasian male who had been High School Valedictorian and had a total of 16 years of education, resulting in a BS college degree. Subsequently he had been gainfully employed until his mid-40s, at which point he was placed on disability due to language impairments hindering communication and difficulty performing assigned tasks in a timely manner.
At age 46 the subject was diagnosed with fronto-temporal dementia (PPA type), without a specific variant specification. Based on clinical observation during the present study, it is believed that the subject had a mixed nonfluent/logopenic variant of PPA. After diagnosis the subject was treated pharmacologically for about 9 months (until medication effect leveled off). Subsequently he commenced weekly sessions with a speech therapist at a local major medical center, supplemented by home therapy 5 days/week (words, reading, and language work sheets provided by the therapist). Despite the therapy undertaken, the subject’s condition gradually worsened, affecting both emotive (depression, inability to connect emotionally to family), and cognitive domains (long and short-term memory, loss of ability to read, behavioral disturbances).
Eighteen months prior to training in virtual reality the subject started attending a Medical Adult Day Program (MADP) in Central New Jersey (USA). The Program offered activities for individuals with cognitive or physical impairments, either as a group (for individuals with similar abilities) or individual activities for those who did not benefit from group programs. The activities were physical, cognitive (games, mind joggers, puzzles, reminiscing, news), social (celebrations, live entertainment) and creative arts (painting, guided drawing, crafts etc.). Many participants helped out with small tasks so that they contributed to the program on a daily basis. The environment was highly structured so that participants were able to get involved in programs they enjoyed. Individuals with PPA, such as the subject in this article, function best with structure but have a hard time when things change. Built into the program was an element of flexibility as the population of the MADP changed daily and others needs change as their illnesses progressed. The subject had difficulty when the routine differed from the original daily plan and often was challenged, for example when “his seat” had been moved or he sat with new people. Due to his cognitive impairment the subject lacked the ability to understand the needs of others and often would try to help them when in fact he was disrupting the normal routine of other participants. During the 18 months participation in the MADP the subject’s rate of cognitive decline seemed to have been reduced. Improvements were also noted in his emotive state (happy with no depression), and to some degree in his dependency on routine and inability to follow directions, prior to the virtual reality experimental intervention.
A group of 9 other members of the same MADP underwent the same VR therapy, as part of a feasibility study for the novel system. Their outcomes will be briefly and qualitatively mentioned in the Discussion section, while detailed data on this “control” group will be published elsewhere.
2.2 BrightBrainer virtual rehabilitation system
The BrightBrainer™ (Figure 1)8 is a portable computerized system which consisted of an HP ENVY 17 laptop, a Razer Hydra game controller, a projector, screen, custom games and remote server. The laptop was a “gamer” model with an NVidia graphics card necessary to render the high resolution graphics (1920 × 1080 pixels) used in therapy.
Figure 1.
Subject with Primary Progressive Aphasia training on the BrightBrainer system. Subject holds a bimanual game controller and sees the games projected on a screen. All interaction is through whole arm movement and index fingers flexion. © Bright Cloud International Corp. Reprinted by permission.
The Razer Hydra9 has two very light game pendants held in each hand, which measure many times per second the position of the subject’s hands, and pendant triggers which detect the amount of index finger flexion. The game controllers thus allow the subject to interact with the laptop through whole arm movements, in combination with index finger flexion. Optional wrist weights may be added for increased upper body exertion.
The LG WXGA DPL projector (1280×800 pixel resolution) presents the simulation scenes used in therapy on a screen that covers a substantial portion of the subject’s field of view. The large display size combined with the real time response of the laptop graphics increase the subject’s feeling of immersion in the game, which in turn facilitates increased focus on the virtual reality tasks10.
The BrightBrainer laptop uses a wireless Internet connection to transmit data to an HP blade server that stores it in an Oracle MySql database11. The server acts as a clinical data repository where the subject’s game performance is secured under password protection and stored under a code. At the start of a session the laptop retrieves the subject’s high scores for each of the games previously played and adjusts the difficulty of the games accordingly. This is done automatically, without therapist intervention.
2.3 Custom BrightBrainer integrative games
BrightBrainer has a library of therapeutic games that each target one, or several cognitive domains and also induce a high level of arm movement repetitions. Unlike other videogames on the market, BrightBrainer games are adaptable in the motor domain, such that arm disability does not impede playing the games. Individuals with weak arms and small arm reach can support their limb on a tabletop using a small towel to reduce friction and still play the games. This takes advantage of a baseline-dependent mapping between (reduced) physical arm movement and normal movement of the corresponding avatar12. Furthermore, some individuals’ lack of volitional finger movement could interfere with their ability to control index flexion/extension when using the game pendant. To account for such situations, games have settings which do not require pressing the hand controller trigger. For example in Card Island, turning a card face up (with the appropriate settings) can be accomplished simply by levitating the hand avatar over that card for a certain amount of time.
Each game, when won provides positive feedback (congratulatory text, applause), which addresses the subject’s emotive side. When combined with the upper body motor exercise this creates an integrative therapy environment which addresses the cognitive-motor-emotive triad. Each game has a scoring formula which is game-dependent and used to quantify subject progress (higher scores correspond to better performance). Task completion time, number of errors, and the playing mode (unimanual or bimanual) are among elements used to determine the score of a game.
The cognitive domains targeted by BrightBrainer (and corresponding game titles) are: a) language comprehension (Submarine Rescue, Pick-and-Place); b) executive function (Submarine Rescue); c) focusing (Breakout 3D, Musical Drums); d) short term visual and auditory memory (Card Island, Xylophone); and e) immediate/working memory (Pick-and-Place, Avalanche).
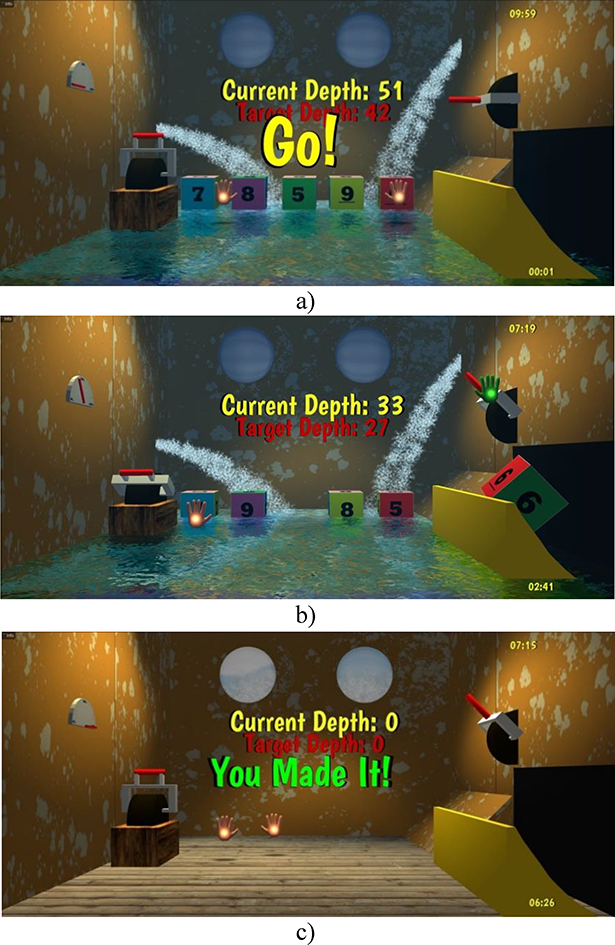
Pick-and-Place, Breakout 3D, Musical Drums, Card Island and Xylophone have been previously described8, 11. The Submarine Rescue game asks the subject to remove crates from a damaged submarine, one-at-a-time, so to gradually lift it to the surface (Figure 2a,b,c). Crates are numbered and the submarine depth is reduced if the correct crate is removed from an array of possible crates. Selecting the correct crate involves solving an arithmetic equation, with the solution indicating the number shown on the crate to be removed. Crates float in the water gushing into the submarine and are removed by picking them up with the right hand avatar and placing them in a chute. The subject has then to pull a lever to evacuate the crate. At higher levels of difficulty there are more crates to select from, and the level of water rises faster, masking the numbers on the crates. Thus the subject has to use his left hand avatar to pump the water out and make the crates visible again. Therefore, the game trains not only language comprehension, and executive function but also dual-tasking. After repeated crate removals (by repeatedly finding correct solutions for a sequence of subtraction equations) the submarine reaches the surface, at which point the subject is rewarded with texts such as “You saved the submarine!” or “You made it!” or “Good job!”
Figure 2.
Screen images of Submarine Rescue: a) game start; b) game in progress; c) game completion. Submarine Rescue trains the problem solving (subtraction), part of the executive function domain, as well as language reading and comprehension. © Bright Cloud International Corp. Reprinted by permission.
The Avalanche game depicts a scene in which people are trapped in a mountain cabin by thick layers of ice. The subject is asked to reach the cabin using a pick-axe and a shovel, and free those inside. The layers of ice, once broken by the pick-axe, change color (visual cue), and can then be scooped up with the shovel through repeated moves. Thus the game trains immediate/working memory, as well as task sequencing. Immersion in the game is enhanced by the sounds of those trapped in the cabin and the barking of their dog. The closer the subject is to the cabin, the louder these sounds. Similar to the Submarine Rescue game, congratulatory text is displayed, together with applause, at the successful completion of the task. At higher levels of difficulty there are more layers of ice to dig through, and the amount scooped out by the shovel gets smaller, thus requiring more arm moves to complete the rescue within a set time.
2.4 Virtual rehabilitation experimental protocol
The experimental protocol, as well as two consent forms, were approved by Western Institutional Review Board. One consent form was used to consent the subject’s spouse so she could provide feedback on changes in the subject’s behavior, emotional state and participation in ADLs as a result of the experimental therapy. The second consent form was used by the spouse to consent the subject as his Legally Authorized Representative.
A BrightBrainer training session consisted of a multitude of games, which could be selected by the subject using a game controller. A game was selected when the subject’s hand avatar overlapped the game icon among several presented on the screen and the subject pressed the controller trigger. Once a game had been selected, BrightBrainer presented another scene which graphically showed the level difficulty achieved, prior to game start. Each game was timed, and the subject’s ability to play was disabled once the scheduled session duration had been reached.
The subject underwent 16 sessions, twice/week for 8 weeks. The duration of a session started at 20 minutes and increased by 5 minutes every two weeks, such that by the end of therapy, sessions lasted 40 minutes of actual play. To maintain the subject’s interest, new games were periodically introduced, thus the array of icons to select games from increased over the therapy weeks. The difficulty of each game was increased automatically based on subject performance, as well as by switching from unimanual play in the first 6 sessions to bimanual play for the remainder of therapy and by wearing wrist weights. A quiet room was reserved for the duration of the 8 weeks experiment, and an assistant was present in the room to help the subject with questions he may have. In addition, the assistant took the subject’s blood pressure before and at the end of each session so to monitor for possible overexertion/over excitement effects.
2.5 Data Collection instruments
The study used an ABAA protocol, data being collected pre- (A), during training (B), post-trials (A) and at 8 week follow up (A). An independent neuropsychologist administered standardized neuropsychological measures of depression and cognition pre-, post- and at follow up. The instruments used were the Beck Depression Inventory, Revised13, the Mini Mental State Examination (MMSE)14 and the Brief Interview for Mental Status (BIMS)15. Raw scores were utilized in all data analysis.
The system was not subjectively evaluated by the subject. However a custom feedback form was used to sample the caregiver feedback, consisting of 8 questions provided by the Director of the Medical Adult Day Program (also a co-author). Each question was scored on a 5-point Likert scale, with 1 meaning the least desirable outcome (Strongly Disagree) and 5 the most desirable one (Strongly Agree). The questions asked for feedback regarding the changes observed in the subject as a result of the computer therapy, namely: 1) Improved ability to focus on a task ; 2) Improvement in verbal responses; 3) Improved ability to follow one-step directions; 4) Improvement in ability to participate in activities of daily living (Grooming, Dressing, etc.); 5) Able to share their experience with the computer games; 6) Appears more willing/ready to attend the program; 7) Open to trying new things, interacting with others; 8) Introduced them to more technology in the Home. The form was mailed to the caregiver mid-therapy (B), at the end of therapy (A) and at 8 week follow up (A).
The collected game performance data (B) consisted of number of times a particular game had been played (to see whether the subject had preferred some games over others), level of difficulty achieved in each game, game scores, number of arm movement and index finger flexion repetitions in each session (as a measure of intensity of motor actions), whether wrist weights were worn and their value, time spent exercising and resting (Session time – exercise time = rest time), success or failure in a given game, as well as blood pressure and pulse.
3. Results
3.1 Game performance
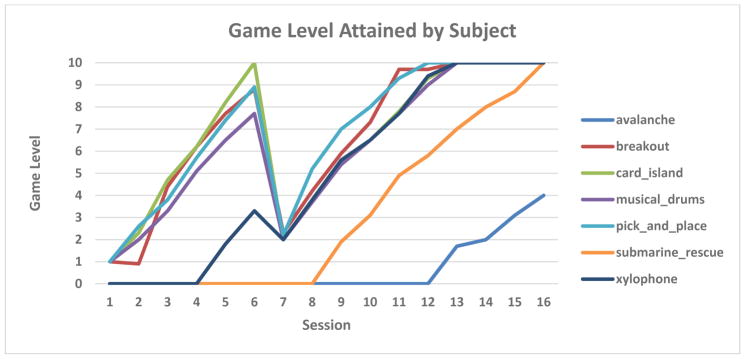
Figure 3 shows the difficulty level the subject was able to play in each of the 7 games used in therapy. The subject started with 4 games in unimanual mode, for which he achieved the maximum level (10) by week 6 of therapy. The Submarine Rescue game was introduced later (week 5) yet the subject attained maximum level before the 8 weeks ended. The only game he did not max out was Avalanche (introduced after Submarine Rescue), yet he progressed to level 4 in the two weeks the game was available for play. Session 7 was the one where play went from unimanual to the more difficult bimanual setting which explains the temporary drop in game level, followed by a steady increase. By the final session of therapy (session 16) the subject achieved an average game difficulty of 9.5. This dropped slightly to 9.1 during a follow up game play session 8 weeks later.
Figure 3.
Subject with PPA progression of therapeutic games difficulty level. Each color represents a different game, which had difficulty levels between 1 (easiest) and 10 (hardest). Subject attained the highest level in all but one game. © Bright Cloud International Corp. Reprinted by permission.
Of the 6.5 hours of therapy the dose was 28% for Language comprehension; 15% Executive functions; 37% Focusing; 30% Short-term memory; and 18% Immediate/working memory. As explained in Section 2.2, language comprehension was trained in games which also trained other domains so the percentages add to 128%.
3.2 Motor training intensity and vital signs
The subject played the games through whole arm movement and index flexion. The real-time interaction nature of the games and the duration of play lead to an average of over 1,300 arm task-oriented active movement repetitions and an average of 320 index finger flexion repetitions per session. The intensity of motor training was further increased by the wrist weights that progressed in size from 0.5 lb in week 5, to 1 lb in week 6 and 2 lb in weeks 7 and 8. The subject tolerated well the weights and they did not adversely affect his game play. Wrist weights may be seen as disturbances to motor control, and the subject’s ability to maintain game performance despite these disturbances are indicative of superior motor control and hand-eye coordination.
The intensity of play (both cognitive and motor) did not produce an increased pulse for the subject. On the contrary, his pulse taken at the end of each session went from 81 beats/minute in session 1 to 55 beats/minute in session 16. His systolic blood pressure at the end of the session started at 110 mmHg in the first session and ended at 106 mmHg at the completion of the last session. Notably, his systolic blood pressure was high (155 mmHg) at the start of session 7, which was the one where bimanual play was introduced, but dropped to 114 mmHg by the end of that session. For that same session his pulse averaged 70 beats/minute.
3.3 Cognitive and depression evaluation outcomes
The neuropsychological testing at pre- and post-therapy showed that the subject endorsed no depression (BDI score of 0), although the examiner noted the subject had difficulty understanding some questions. This poor comprehension may have skewed the data. Pre-intervention his BIMS score was 1, and MMSE score was 3, indicative of severe dementia. Post-therapy the subject had a score of 0 for BIMS and 2 on MMSE. At 8 week follow-up his cognitive evaluations had improved slightly (BIMS of 3 from 1 at baseline, and MMSE of 4 vs. 3 at baseline). His depression at follow-up was mild (BDI score of 2).
3.4 Caregiver subjective evaluation of therapy outcomes
After the first 4 weeks of therapy the subject’s caregiver agreed that he had “Improved ability to focus on a task,” had “Improved in verbal responses,” had “Improved ability to follow one-step directions,” and “Improved ability to participate in activities of daily living.” The caregiver also agreed that the subject was “Open to trying new things, to interact with others,” and that the therapy “Introduced him to more technology in the Home.” By the end of therapy the caregiver strongly agreed with the above statements. Additionally, she agreed that the subject was “Able to share his experience with the computer games,” something she had disagreed with at mid-therapy.
3.5 Observational evidence of subject’s progress in reversing PPA symptoms
The therapy coach (also a co-author of this manuscript) was asked to log in his observations of the subject’s interaction with the BrightBrainer system and general behavior during the virtual rehabilitation sessions. He observed that in week 1 the subject showed impulsive behavior, moving his arms around with poor control, stopping performing a task whenever he did not understand it, and was not enjoying the games.
By the end of week 2 the subject started enjoying the BrightBrainer 3D games, and gradually stopped moving around impulsively. About the same time the subject began speaking in complete sentences, something also observed by his caregiver as communicated to the Director of the Medical Adult Day Program (also a co-author to this manuscript). The subject on his way home began to read words associated with one of the games, off vehicles. Previously he had not been reading anything for a year.
By the end of week 3 the subject began recognizing colors, and game performance improved. In week 4 the interaction mode was switched to bimanual, and the subject at first complained of the change in games. But subsequently he adapted and stopped complaining. About the same time the caregiver reported that the subject was introduced to non-violent online 2D video games at home, which he liked and quickly mastered. The subject was subsequently playing these games three to four times/week, spending 30 to 45 minutes each time for the remainder of 4 weeks of the study. During activities such as shopping he showed better self-control of a compulsion to rearrange things. In the words of his caregiver, the subject’s cognition – including his awareness and judgment – seemed to have been “sparked” by the virtual reality therapy.
In week 6 the subject appeared to become a bit bored with the BrightBrainer games and thought they became too easy. During weeks 7 and 8 the caregiver remarked that the subject’s language skills had improved significantly showing an ability to speak and converse in sentences, and his vocabulary had increased significantly as well. The subject was much more alert and lively, often initiating a conversation, and he seemed more aware and interested in the world around him.
At 8 week follow up the subject remembered the room he had trained in as well as the BrightBrainer games he had played. His enjoyment with the games did not seem to be diminished by 8 weeks of no play. The subject was able to play all the games at levels close to where he had left at the end of therapy, except for Submarine Rescue where his performance had degraded.
Discussion
Garcia and colleagues16 in their review of the use of virtual reality in dementia stressed that it is very important to explore brain-stimulating activities targeted at various regions of the brain damaged by the disease. They argued that studying such targeted activities could uncover the neuroplasticity they may induce in persons with dementia, and help determine which brain regions undergo such plasticity. Greenwood and Parasuraman17 offer in fact a neurocognitive framework in which plasticity at the neuronal level underpins cognitive plasticity, namely adaptive changes in patters of cognition related to brain activity. It follows that studying activities that induce brain plasticity at the neuronal level may offer a way to improve cognition and behavior.
A very recent review18 argues the superiority of game-based therapies that induce brain plasticity to both pharmaceutical and behavioral therapies which represent the standard of care today. The authors argue that the new game-based computer therapies, which can be done without a clinician preset, are disruptive to today’s standard of care, but face a lack of support in current medical education and practice.
The present study used games as activities targeted at executive function, memory, focusing and language understanding, all cognitive areas affected by fronto-temporal dementia (including PPA). With regards to neuroplasticity, it is likely that the subject’s brain rewired motor neurons, in view of: a) the 1300 average targeted arm repetitions per session. Previous studies had shown that upwards of 600 targeted arm repetitions are needed to induce brain plasticity;19 b) the bimanual nature of the tasks he performed in the last 5 weeks of therapy. Bimanual tasks are known to increase cross-lobe connections, which improve one’s ability to multitask20; Games such as Submarine Rescue required dual-tasking, something problematic in dementia populations21, but which the subject mastered.
During the second half of the study the subject was introduced to, and started playing 2D online videogames at home. He played three to four times/week, for 30 to 45 minutes each time. At first glance playing online videogames may seem like a confounding factor to this study. One should note, however that the games the subject played on the BrightBrainer at the MADP were of significantly higher complexity (bimanual vs. unimanual and 3D vs. 2D at home) and higher intensity compared to those played at home. Even if the home games resulted in some improvements, they could not explain the subject’s gains in the first 3 weeks of therapy when he played no games at home.
Studies have shown that motor training (as in physical exercise) in older healthy adults benefits cognition (memory) (see recent reviews22,23). It is possible that the integrative nature of BrightBrainer therapy, not only the intensity of play, was a reason for the substantial improvements observed in the subject. An earlier study of the system on 10 elderly subjects with dementia residents of a local Skilled Nursing Facility, with the same intensity and duration of therapy resulted in statistically significant improvements in executive function and reduction in depression for the group8.
Improving cognition using a computerized system that induces brain plasticity was studied in the IMPACT controlled study24 which used the Posit Science “Brain Fitness Program.” Researchers targeted auditory memory in an intensive program where experimental subjects were healthy adults age 65 and over. They trained for 40 hours (1 hour/day, 5 days/week for 8 weeks). Results showed that gains in the auditory domain generalized to other, non-trained memory domains, as well as to processing speed, indicative of brain plasticity in the cognitive areas.
While the IMPACT study targeted healthy older individuals, a randomized pilot study targeted higher functioning dementia (Alzheimer’s disease) participants from an Adult Day Program25. Participants in the experimental group undertook 3 weekly 20-min sessions of Interactive Multimedia Internet-based System training, for 12 weeks. A control group had 8 hours/day of Integrated Psycho-stimulation Program (IPP) offered by the Adult Day Program, and a second control group had only medication in their home. Both interactive multimedia and IPP training resulted in improved cognition for the dementia participants after 12 weeks training (MMSE average difference of 2 points for the experimental group and 0.5 point for IPP group). There were no gains for the second control group which only took medication. The gains were better maintained at 12 week follow up by the experimental group.
The PPA subject in the study presented here was attending a Medical Adult Day Program. His participation in the program overlapped the BrightBrainer training, and this may be seen as a confounding factor. From his caregiver and his therapy coach accounts, it appears that the gains observed during virtual reality training where distinct from, and in addition to prior gains resulting from 1.5 years of participation in the Medical Adult Day Program. The subject trained as part of a larger feasibility study that involved 9 other members of the same Medical Adult Day Program. Eight of 9 other participants had various non-PPA dementias, with cognitive impairment ranging from mild cognitive impairment to early onset of Alzheimer’s disease. The subject with PPA was the youngest of the group with all 10 subjects undergoing the same therapy on the BrightBrainer system.
Compared to the dementia subjects in the previously mentioned Adult Day Program study, who had a group MMSE score at baseline of 20.6, the subject with PPA that trained on BrightBrainer had a much lower score at baseline (3). His MMSE score post-therapy was 2, and this negative change is in contrast with the improvements in language skills, self-control, and ability to focus on a task observed by his training coach and separately by his caregiver. The subject’s cognitive measures were slightly better at follow up (MMSE score of 4). One reason for the discrepancy between caregiver observations and the cognitive testing results may be the MMSE tendency to overestimate dementia severity in subjects with PPA26. The lack of an appropriate (and more detailed) cognitive standardized evaluation is a limitation in the present study. Another obvious limitation is the small sample (n=1), as is the lack of imaging studies to back up the authors’ belief that brain rewiring had occurred. Nonetheless the authors believe that this study findings merit dissemination to the Neuroscience community.
Conclusion
The changes witnessed by the research team and fully corroborated by the subject’s caregiver are indicative of a meaningful reduction of symptoms associated with PPA. Substantially more work is needed to determine what neuronal changes had been induced to cause such reduction of symptoms. Such research should include imaging studies, as well as controlled studies with a sufficiently large number of subjects.
The authors believe that the present study is the first time virtual reality integrative games-based training with bimanual interaction was tried by a PPA subject. They are eager to partner with other researchers so to explore how PPA can be reversed in the absence of pharmacological interventions. The use of the techniques pioneered in this study is very supportive of such a possibility and may someday benefit a larger number of subjects with fronto-temporal dementia as well as those with normal age-related cognitive decline.
Acknowledgments
Funding
Research reported here was supported in part by grant 9R44AG044639-02A1 from the National Institute of Health/NIA.
We thank Jasdeep Hundal PsyD for performing the cognitive and depression evaluations of the subject. We also thank the staff of the Medical Adult Day Program for facilitating this study, and the subject’s caregivers for providing detailed and timely feedback on his progress they witnessed outside the Medical Adult Day Program.
Footnotes
Declaration of Interests
Grigore Burdea PhD, is inventor on a patent related to the technology described in this article. He is Founder, President and majority shareholder of Bright Cloud International Corp (BCI).
Kevin Polistico and Gregory House, PhD are full time employees and Richard Liu, Assistant, was an intern of BCI.
Roberto Muñiz is President and CEO of the multisite aging-care organization, Natalie Macaro is Director of Adult Day Programs and Lisa Slater is Director of Professional Education at the same multisite aging-care organization.
References
- 1.Garcia L, Kartolo A, Methot-Curtis E. A discussion of the Use of Virtual Reality in Dementia. Eichenberg C, editor. [last accessed December 10, 2014];In Virtual Reality in Psychological, Medical and Pedagogical Applications. 2012 Chapter 6:14. Available from: http://cdn.intechopen.com/pdfs-wm/39040.pdf.
- 2.Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2014;10(2):e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovici GD, Miller BL. Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management. CNS Drugs. 2010;24:375–98. doi: 10.2165/11533100-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 5.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114(1):31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 7.Burdea G, Coiffet P. Virtual Reality Technology. 2nd. Wiley; 2003. [Google Scholar]
- 8.Burdea G, Polistico K, Krishnamoorthy A, House G, Rethage D, Hundal J, et al. A feasibility study of the BrightBrainer™ cognitive therapy system for elderly nursing home residents with dementia. Disab Rehab – Assist Tech. 2014 Mar 9;:12. doi: 10.3109/17483107.2014.900575. early online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sixense Entertainment. [last accessed 10 December 2014];Razer Hydra Master Guide. 2011 Available from: dl.razerzone.com/master-guides/Hydra/HydraOMG-ENG.pdf.
- 10.MySQL. [last accessed 10 Dec 2014];MySQL on Windows – why, where and how. 2010 Available from http://www.oracle.com/partners/en/most-popular-resources/mysql-on-windows-wwh-final-468247.pdf.
- 11.Burdea G, Coiffet P. Virtual Reality Technology. 2. Wiley; [Google Scholar]
- 12.Burdea G, Defais C, Wong K, Bartos J, Hundal J. Feasibility study of a new game-based bimanual integrative therapy. Proceedings 10th Int. Conference on Virtual Rehabilitation; Philadelphia, PA. August 26–29, 2013; pp. 101–108. [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory. 2. San Antonio (TX): Psychology Corporation; 1996. [Google Scholar]
- 14.Rosenzweig A. The Mini-Mental State Exam and Its Use as an Alzheimer’s Screening Test. 2014 Online at http://alzheimers.about.com/od/testsandprocedures/a/The-Mini-Mental-State-Exam-And-Its-Use-As-An-Alzheimers-Screening-Test.htm.
- 15.Mansbach W, Mace R, Clark K. Differentiating levels of cognitive functioning: a comparison of the Brief Interview for Mental Status (BIMS) and the Brief Cognitive Assessment Tool (BCAT) in a nursing home sample. Aging and Mental Health. 2014;18(7):921–28. doi: 10.1080/13607863.2014.899971. [DOI] [PubMed] [Google Scholar]
- 16.Garcia L, Kartolo A, Méthot-Curtis E. Virtual Reality in Psychological, Medical and Pedagogical Applications. Chapter 6. InTech; Rijeka, Croatia: 2012. A Discussion of the Use of Virtual Reality in Dementia; pp. 123–36. [Google Scholar]
- 17.Greenwood P, Parasuraman R. Neural and cognitive plasticity: a neurocognitive framework for ameliorating cognitive aging. Frontiers Aging Neuroscience. 2010;2:1–14. doi: 10.3389/fnagi.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merzenich M, VanVleet TM, Nahum M. Brain plasticity-based therapeutics. Frontiers in Human Neuroscience. 2014 Jun;8(385):1–16. doi: 10.3389/fnhum.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowdena MG, Woodbury ML, Duncan PW. Promoting Neuroplasticity and Recovery After Stroke. Current Opinion in Neurology. 2013;26(1):37–42. doi: 10.1097/WCO.0b013e32835c5ba0. [DOI] [PubMed] [Google Scholar]
- 20.Aramaki Y, Honda M, Okada T, Sadato N. Neural Correlates of the Spontaneous Phase Transition during Bimanual Coordination. Cereb Cortex. 2006;16(9):1338–48. doi: 10.1093/cercor/bhj075. [DOI] [PubMed] [Google Scholar]
- 21.Inasaridze K, Foley JA, Logie RH, Sala SD. Dual task impairments in vascular dementia. Behavioural Neurology. 2010;22(1/2):45–52. doi: 10.3233/BEN-2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Pinilla F, Hillman C. The Influence of Exercise on Cognitive Abilities. Comprehensive Physiology. 2013 Jan;3(1):403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai L, Chan J, Yan J, Peng K. Brain plasticity and motor practice in cognitive aging. Frontiers in Aging Neuroscience. 2014;6:1–12. doi: 10.3389/fnagi.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith G, Housen P, Yaffe K, et al. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J Am Ger Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tárraga L, Boada M, Modinos G, et al. A randomized pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1116–21. doi: 10.1136/jnnp.2005.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osher JE, Wicklund AH, Rademaker A, et al. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2007;22(6):468–73. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]