Abstract
Aortopulmonary window (APW) is a rare form of congenital heart disease seen in isolation or with complex cardiac lesions. APW has been associated with other cardiac defects such as interrupted aortic arch and Tetralogy of Fallot, but few cases have been reported of APW associated with transposition of the great arteries (TGA). In a newborn with TGA and intact ventricular septum, diagnosis of APW requires a high index of suspicion. This article reviews the literature on TGA with APW and illustrates the importance of additional evaluation in neonates with TGA when oxygen saturation and PaO2 do not match predicted clinical values.
Keywords: aortopulmonary septal defect, aortopulmonary window, congenital heart disease, echocardiography, transposition of the great arteries
1 |. INTRODUCTION
Isolated aortopulmonary window (APW) constitutes a very rare type of congenital heart disease defect, with an incidence ranging from0.1–0.6%.1,2 Less rare in the APW population is the association with other cardiac anomalies, with an incidence reported between 25–75%.1,3,4 These associated cardiac anomalies include: tetralogy of Fallot, coarctation of the aorta, interrupted aortic arch (commonly type A), coronary artery anomalies, and in the rarest of cases an APW can be seen with transposition of the great arteries (TGA).1,5,6 On review of the current literature, there have been 18 reported cases of TGA with APW documented between 1988–20167–21 (Table 1). Given its rare occurrence, diagnosing an APW in association with other cardiac anomalies can be challenging. This is particularly true for TGA, where the orientation of the great arteries can lead to a missed or misclassified diagnosis.
TABLE 1.
Review of reported cases of transposition of the great arteries with aortopulmonary window
| No. | Author | Cardiac diagnosis | Date of surgery | Age at surgery | Diagnosis timing/GA at birth | Anomalies and clinical symptoms | Surgery | Post-operative outcomes/complications |
|---|---|---|---|---|---|---|---|---|
| 1 | Tiraboschi et al. | TGA/VSD, double APW | 1988 | 7 mo | Postnatal/-- | - | Division/patch repair of APW, no ASO | Died due to PHTN crisis, severe pulmonary vascular disease at autopsy |
| 2 | Krishnan et al. | TGA/VSD, type I APW | 1991 | 3 mo | Postnatal at 3 mo/- | - | Senning atrial switch, VSD closure, APW repair | Died within 24 h due to LCOS and severe PHTN |
| 3 | Tirado et al. | TGA/VSD, type I APW | 1993 | 7 d | Postnatal at 7 d/- | CHF, murmur | APW direct suture, pulmonary artery band (off CPB) | Died on POD 22 due to PTHN |
| 4 | McElhinney et al. | TGA/IVS, ASD, type I APW | 1995 | 8 d | Postnatal/-- | - | ASO, APW repair | Massive post-operative bleeding, coagulopathy, died on POD 1 |
| 5 | Marangi et al. | TGA/IVS, PDA, type I APW | 1996 | 7 d | Postnatal/-- | Goldenhar syndrome, cyanosis | ASO, APW repair | Doing well at 12-mo follow-up |
| 6 | Backer et al. | TGA/IVS, type I APW, LSVC | 1996 | 3 y | Postnatal/-- | ASO, no Lecompte with 22 mm homograft RV-PA conduit, transaortic APW patch repair | Doing well at 1-y follow-up | |
| 7 | Duca et al. | TGA/IVS, PFO, type I APW | 2002 | 26 d | Postnatal/Term | Tachypnea, tachycardia, murmur | ASO, fenestrated (4 mm) PFO closure, APW repair | PHTN crisis on POD 2, post-hemorrhagic hydrocephalus s/p VP shunt placement. Doing well at 9 mo follow-up, no neurologic deficits |
| 8 | Marwah et al. | TGA/IVS, ASD, restrictive APW | 2005 | Postnatal at 11 mo/- | Poor weight gain, cyanosis | ASO, transaortic APW patch repair | Survived post-operatively. No long-term follow-up data available | |
| 9 | Adluri et al. | TGA/IVS, type I APW | 2005 | 8 d | Postnata I/term | Cyanosis, PHTN | ASO, single coronary button modification, APW 2-patch repair | Open chest closed on POD 1, discharged home POD 8. Doing well at 6-mo follow-up |
| 10 | Najm et al. | Situs inversus, dextrocardia, TGA/IVS, PDA, PFO, bicuspid pulmonary valve, type I APW | 2008 | 32 d | Postnatal/term | Tachypnea, cyanosis | ASO, single coronary button modification and coronary to neo-aortic tunnel, PDA ligation, APW repair | Discharged home POD 9. Doing well at 1-y follow-up |
| 11 | Mishra et al. | TGA/IVS, type I APW | 2009 | 6 mo | - | ASO, transaortic APW patch repair | Died on POD 21 due to severe PHTN (present preoperative) and sepsis | |
| 12 | Reddy et al. | TGA, multiple VSDs, PDA, type I APW | 2010 | 5 mo | - | - | ASO, APW repair | Doing well at 18-mo follow-up |
| 13 | Mishra et al. | TGA/VSD, type I APW | 2011 | 28 d | - | - | ASO, VSD closure, transaortic APW patch repair | Severe PHTN preoperatively. Doing well on outpatient follow-up |
| 14 | Mishra et al. | TGA/VSD, type I APW | 2012 | 35 d | - | - | ASO, VSD closure, transaortic APW patch repair | Severe PHTN preoperatively. Doing well on outpatient follow-up |
| 15 | Mishra et al. | TGA/VSD, type I APW | 2012 | 40 d | - | - | ASO, VSD closure, transaortic APW patch repair | Severe PHTN preoperatively. Doing well on outpatient follow-up |
| 16 | Singh et al. | TGA/IVS, ASD, type I APW | 2013 | - | Postnatal at 26 d/Term | Tachypnea, cyanosis, murmur, hepato-megaly | ASO, transaortic APW patch repair | Discharged home POD 11. No long-term follow-up data available |
| 17 | Gopalan et al. | TGA/IVS, ASD, APW | 2014 | - | Postnatal at 3 mo/-- | Poor feeding | ASO, APW repair | No outcome or long-term follow-up data available |
| 18 | Fotaki et al. | TGA/IVS, PFO, PDA, type II APW | 2016 | 7 d | Postnatal/-- | -- | ASO, APW repair | Discharged on POD 15. Doing well at 3-y follow-up with only mild bilateral branch pulmonary artery stenosis. |
| Our case | Dhillon et al. | Mesocardia, TGA/IVS, PFO, large type II APW | 2016 | 7 d | Postnatal at 3 d/Term (38 + 6 wk) | Isolated unilateral clubfoot, cyanosis | ASO, PA patch augmentation, transaortic APW patch repair | Discharged home POD 13. Doing well on 1-y follow-up |
Abbreviations: GA, gestational age; TGA, transposition of the great arteries; VSD, ventricular septal defect; d, days old; mo, months old; y, years old; APW, aortopulmonary window; ASO, arterial switch operation;PHTN, pulmonary hypertension; IVS, intact ventricular septum; LCOS, low cardiac output syndrome; CHF, congestive heart failure; POD, post-operative day; ASD, atrial septal defect; PDA, patent ductus arteriosus; BAS, balloon atrial septostomy; LSVC, persistent left superior vena cava; PVR, pulmonary vascular resistance (in Woods units/m2); RV, right ventricle; PA, pulmonary artery; PFO, patent foramen ovale; VP, ventriculo-peritoneal; RA, right atrium; LV, left ventricle; Ao, aorta. (–) = data unavailable
2 |. CASE REPORT
A term male neonate was born at 38 and 6/7 weeks gestation and 3325 g at a referring hospital following a normal pregnancy. The mother received standard prenatal care in a low-risk setting with a prenatal ultrasound that was reportedly normal. At birth, the child had normal oxygen saturations and Appearance, Pulse, Grimace, Activity, Respiration (APGAR) scores were nine at 1 and 5 minutes. The infant was noted to have an isolated, unilateral clubfoot at birth without other anomalies. The following morning in the newborn nursery, the infant was found to be cyanotic with oxygen saturations in the low 70s. Due to concern for surfactant deficiency or primary pulmonary infectious process, the child was transferred to the referring hospital neonatal intensive care unit (NICU). Evaluation in the NICU demonstrated a murmur on exam, dextrocardia on x-ray (mesocardia on further imaging), reverse differential cyanosis (a lower oxygen saturation in the upper extremities compared to the lower extremities), and a saturation of 80% with no improvement on oxygen provided by CPAP. Given these findings, a call was made to our tertiary care center NICU to discuss transfer to a higher level of care given the concern for congenital heart disease based on cyanosis that was nonresponsive to oxygen. The neonatologist on-call at our institution recommended transfer after intubation and initiation of prostaglandin (PGE).
On arrival, the first transthoracic echocardiogram preliminary findings at 48 h of life demonstrated transposition of the great arteries with intact ventricular septum (TGA/IVS), patent foramen ovale (PFO) with minimal atrial level shunting, and a large tortuous patent ductus arteriosus (PDA). Over the following 12 h on ventilatory support, the oxygen saturations improved to > 85% on PGE. Given the limited atrial level shunt demonstrated on echocardiogram, the cardiac catheterization team was consulted for a balloon atrial septostomy (BAS). Prior to BAS, the patient’s oxygen saturations continued to improve to the mid-90s and the degree of oxygenation was difficult to explain in the setting of a minimal atrial level communication for mixing. Review of the initial echocardiogram confirmed almost no atrial level communication and the previously identified PDA were noted to arise from the aortic arch at an unusually proximal location. Given discordance between the clinical picture and initial transthoracic echocardiogram (TTE) findings, a repeat TTE was performed at approximately 72 h of life.
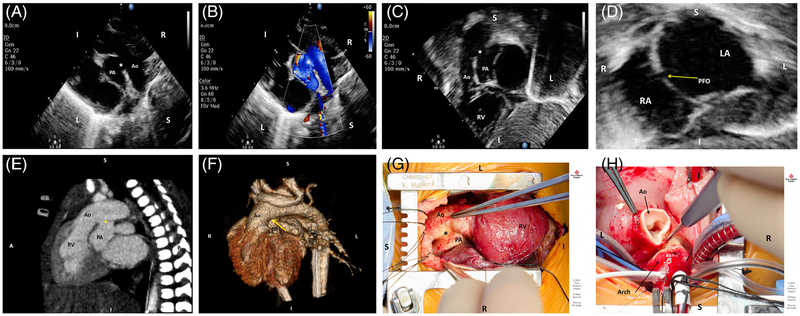
Parasternal long axis views and focused subcostal imaging revealed a large aortopulmonary window (APW) type II between the transverse aortic arch and main pulmonary artery, which had previously been described as a large PDA (Figures 1 A,B,C) and a PFO (Figure 1D). Given this finding along with high saturations, it was felt that there was an appropriate mixing lesion at the arterial level, and a BAS was deemed unnecessary. PGE was discontinued and oxygen saturations remained >90% until the patient was taken to the operating room for cardiac surgery on day of life seven. Preoperative computerized tomographic (CT) imaging was also performed which confirmed great arterial origins consistent with TGA, confluent branch pulmonary arteries, and a large type II APW measuring 10 mm in width that began approximately 9–10 mm distal to the aortic valve (Figure 1 E,F). A comprehensive chromosomal microarray panel was drawn which did not reveal any copy number changes associated with known microdeletion or microduplication syndromes.
FIGURE 1.
A, Two-dimensional transthoracic echocardiographic parasternal long-axis view demonstrating the aortopulmonary window (asterisk) in relation to a rightwards aorta (Ao) and leftwards pulmonary artery (PA). B, Parasternal long-axis color Doppler view revealing shunting at the aortopulmonary window (asterisk), shunting systemic-to-pulmonary in this view. C, Two-dimensional transthoracic echocardiographic subcostal view showing the rightwards aorta (Ao) arising off the anterior right ventricle (RV) and dropout of the great vessel wall at the level of the aortopulmonary window (asterisk). D, Two-dimensional transthoracic echocardiographic subcostal view at the level of the atrial septum demonstrating a patent foramen ovale (arrow) with an otherwise intact atrial septum, with left atrium (LA) on the left and right atrium (RA) on the right. E, Cardiac computerized tomographic sagittal view demonstrating transposition anatomy with the aorta (Ao) anterior and arising off a rightwards RV, a posterior PA, and the aortopulmonary window (asterisk) measuring 10 mm in diameter and originating 10 mm distal to the aortic valve leaflets. F, Cardiac computerized tomographic 3D reconstruction demonstrating transposition anatomy with the area of the aortopulmonary window (arrow). G, Intra-operative findings of a type B aortopulmonary window (asterisk) between the anterior aorta (Ao) and posterior PA. The RV is noted to be the anterior ventricle. H, A trans-aortic approach was undertaken for repair of the aortopulmonary window. The transected aorta (Ao) and proximal transverse arch can be seen here in the arterial switch component of the operation
Intraoperatively, the patient was confirmed to have TGA with a large type II aortopulmonary window in the mid/distal ascending aorta at the level of the pulmonary artery bifurcation (Figure 1G). An arterial switch operation (Figure 1H) with pulmonary artery patch augmentation and aortopulmonary window repair using a trans-aortic approach under cardiopulmonary bypass was undertaken (total myocardial ischemic time 118 minutes, total cardiopulmonary bypass time 244 minutes). The patient had an uneventful postoperative course, was discharged home on postoperative day 13, and is currently doing well with a good surgical result.
3 |. DISCUSSION
In this case report, we describe the presentation of a neonate with a postnatal diagnosis of TGA with intact ventricular septum and a small PFO in whom unexpectedly high saturations led to review of echocardiographic images and eventual diagnosis of a type II APW, which has not been previously reported, making it an important contribution to the TGA/APW literature. A type I is defined as a proximal APW that begins just above the sinus of Valsalva and semilunar valve, a type II is a distal APW located in the uppermost portion of the ascending aorta, a type III APW is a larger defect that involves the majority of the ascending aorta, and type IV APWs are intermediate defects.10 Given the rare occurrence of diagnosing an APW in association with complex congenital heart lesions, a high index of suspicion is needed on initial echocardiograms. This is true for TGA, where the orientation of the great arteries can lead to a missed or misclassified diagnosis of a PDA instead of an APW. APWs can also be missed on echocardio-gram in the early neonatal period from parasternal long-axis views due to a lack of significant shunting across the defect secondary to high pulmonary vascular resistance (PVR) or a presumption of artifactual dropout between the great vessel walls.4,22
Expected saturations for various cardiac lesions can be skewed by the presence of an APW and hemodynamic effects on the pulmonary vasculature and left ventricle can be altered, making management particularly challenging.2,3,10,23 With normal cardiac structural anatomy, in the early neonatal period when PVR is highest and transient pulmonary hypertension is present, pulmonary-to-systemic shunting at the APW level may lead to lower extremity systemic desaturations; however, as the PVR falls, shunting direction will become systemic-to-pulmonary leading to pulmonary overcirculation. When transposition of the great arteries is present concomitantly with an APW, this can lead a reverse differential saturation early in the neonatal period due to shunting of oxygen-rich blood from pulmonary-to-systemic at the APW level distal to the head/neck arch vessels and resulting higher saturations in the lower extremities while the head/neck vessels proximal to the APW receive deoxygenated blood from the right ventricle and ascending aorta. As the PVR falls, shunting at the APW becomes systemic-to-pulmonary and improved mixing occurs at this large lesion resulting in improved oxygen saturations. Depending on the size and type of APW, failure to make a timely diagnosis can result in the pulmonary vascular system experiencing significant over-circulation leading to congestive heart failure, increased pulmonary arterial pressures, and eventual irreversible pulmonary vascular changes.3,10,19,23 Depending on the size and location of an APW with the associated complex congenital heart lesion, early surgical repair may be prudent. Numerous studies have documented lower mortality with early closure of an APW, while mortality after late closure is generally higher and dependent on the pulmonary vascular resistance and presence of complex associated lesions.3,4,7,10,19
As the systemic and pulmonary circulations are in parallel in TGA, mixing of the two circulations is necessary to allow for oxygenated pulmonary venous blood to mix with deoxygenated systemic venous blood to provide adequate oxygen delivery to the systemic circulation. Oxygenated umbilical venous blood during fetal life entering the right heart can still enter the systemic circulation via the right heart and across the PFO/PDA. Postnatally once the pulmonary vascular resistance drops and the PFO closes, oxygenated blood from the pulmonary venous bed no longer has a direct route to the systemic circulation and an area of mixing is needed, without which profound desaturation and cardiopulmonary collapse can result. Mixing of these parallel circulations is most successful at the atrial level. These parallel circulations highlight the importance of prenatal diagnosis, as early postnatal intervention is often necessary and a missed prenatal diagnosis can lead to delay in care of a child postnatally if there is not ready access to a congenital heart surgery or pediatric cardiac catheterization team. Furthermore, prenatal diagnosis can reduce the impact on neurodevelopmental outcomes for vulnerable TGA patients, a population that has been shown to have improved postnatal brain development and less preoperative brain injury when a prenatal cardiac diagnosis is made24. The decision to perform a BAS in a neonate with TGA/IVS to improve intracardiac mixing is commonly dictated by the following factors: evidence of a restrictive atrial septum on echocardiogram, hypoxemia, signs of inadequate tissue oxygen delivery, hemodynamic instability, presence of left atrial hypertension, and as a measure to discontinue prostaglandin infusion.25,26 In our patient, although the presence of minimal atrial mixing was noted, the type II APW proved to be an adequate source of mixing of two parallel circulations which allowed for discontinuation of prostaglandin infusion while maintaining hemodynamic stability, adequate tissue oxygen delivery, and minimized the need for an additional invasive procedure. While having an APW assists mixing of two parallel circulations, not recognizing an APW and having a child unnecessarily undergo a balloon atrial septostomy would not have been in the best interest of the patient and could have resultant unnecessary morbidity associated with it (from known potential complications of balloon atrial septostomies26).
A trans-aortic approach and closure of the APW affecting the great arteries with two separate patches is the commonly preferred approach to APW repair and allows for better visualization of the coronary ostia and minimizes distortion of the pulmonary arterial architecture.3,4,7 For our case, a transaortic approach to repair the APW was performed prior to the arterial switch; the arterial switch was subsequently performed, including visualization of the coronary ostia and coronary button reimplantation.
APWs can be seen in association with complex congenital heart disease, but are rarely seen with TGA.1,3–5 Based on the available literature, type I APW appears to be more common than type II APW in the setting of TGA. The sonographer and cardiologist should evaluate for an APW in patients with TGA when significant intracardiac mixing is not apparent despite high oxygen saturations and in cases of unusual ductus arteriosus morphology or location. Part of the echocardiogram evaluation should be focused on interrogation of the great arteries to evaluate for vessel wall defects and communications. As evidenced in this case, APWs can be misdiagnosed as PDAs; hence, close attention must be paid to identifying the location, course and communication of the great arteries, and dedicated subcostal imaging must be performed to assure an accurate diagnosis. The diagnosis of an APW requires a high index of suspicion for the primary clinician and must be considered in complex cardiac lesions when physiology and intracardiac mixing cannot explain the clinical presentation or saturations. This case is highly relevant from both clinical and imaging standpoints as a diagnosis of APW with TGA by echocardiography may avoid an unnecessary BAS, eliminate the need for PGE, and changes surgical timing and approach. Failure to make the correct diagnosis in a timely manner could result in unnecessary procedures and a modified or delayed surgical approach, with potential for increased morbidity.
Footnotes
FINANCIAL DISCLOSURE
The authors have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kuehn A, Oberhoffer R, Vogt M, Lange R, Hess J. Aortopulmonary window with ventricular septal defect and pulmonary atresia: prenatal diagnosis and successful early surgical correction. Ultrasound Obstet Gynecol. 2004. December;24(7):793–796. [DOI] [PubMed] [Google Scholar]
- 2.Allen Hugh D, Driscoll David J, Shaddy Robert E, Feltes TF. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents Including the Fetus and Young Adult. Vol 31 8th ed Philadelphia: Lippincott Williams & Wilkins Chapter; 2013:740–743. [Google Scholar]
- 3.Tkebuchava T, von Segesser LK, Vogt PR, et al. Congenital aortopulumonary window: diagnosis, surgical technique and long-term results. Eur J Cardiothorac Surg. 1997. February;11(2):293–297. [DOI] [PubMed] [Google Scholar]
- 4.Bagtharia R, Trivedi KR, Burkhart HM, et al. Outcomes for patients with an aortopulmonary window, and the impact of associated cardiovascular lesions. Cardiol Young. 2004. October;14(5):473–480. [DOI] [PubMed] [Google Scholar]
- 5.Kutsche LM, Van Mierop LH. Anatomy and pathogenesis of aorticopulmonary septal defect. Am J Cardiol. 1987. February 15;59(5):443–447. [DOI] [PubMed] [Google Scholar]
- 6.Gangana CS, Malheiros AF, Alves EV, et al. Aortopulmonary window—impact of associated lesions on surgical results. Arq Bras Cardiol. 2007. April;88(4):402–407. [DOI] [PubMed] [Google Scholar]
- 7.McElhinney DB, Reddy VM, Tworetzky W, et al. Early and late results after repair of aortopulmonary septal defect and associated anomalies in infants <6 months of age. Am J Cardiol. 1998. January 15;81(2):195–201. [DOI] [PubMed] [Google Scholar]
- 8.Fotaki A, Novaes J, Jicinska H, et al. Fetal aorto-pulmonary window: case series and review of the literature. Ultrasound Obstet Gynecol. 2016. April 10;49(4):533–539. [DOI] [PubMed] [Google Scholar]
- 9.Gopalan Nair R, Kalathingathodika S, Bastian C. Aortopulmonary window: a rare mechanism of inter-circulatory mixing and prepared left ventricle in transposition of the great arteries with intact ventricular septum. Cardiol Young. 2014. August;24(4):762–763. [DOI] [PubMed] [Google Scholar]
- 10.Backer CL, Mavroudis C. Surgical management of aortopulmonary window: a 40-year experience. Eur J Cardiothorac Surg. 2002. May; 21(5):773–779. [DOI] [PubMed] [Google Scholar]
- 11.Marangi D, Peterson RJ, Ceithaml EL, Marvin WJ Jr. Surgical repair of d-transposition with aortopulmonary window: a case report. J Thorac Cardiovasc Surg. 1996. March;111(3):671–672. [DOI] [PubMed] [Google Scholar]
- 12.Marwah A, Maheshwari S, Suresh PV, Misri A, Sharma R. Transposition of great arteries with aortopulmonary window: an unusual cause of prepared left ventricle at 11 months. Indian Heart J. 2005. Jul-Aug; 57(4):353–354. [PubMed] [Google Scholar]
- 13.Duca V, Sulliotti G, Maggio C, Corsello G. Transposition of the great arteries and aortopulmonary window in the same patient: clinical report and follow-up. Pediatr Cardiol. 2002. Jul-Aug;23(4):474–475. [DOI] [PubMed] [Google Scholar]
- 14.Adluri K, Barron DJ, Brawn WJ. D-transposition of the great arteries with an aortopulmonary window: a new corrective technique. Ann Thorac Surg. 2005. March;79(3):1066–1067. [DOI] [PubMed] [Google Scholar]
- 15.Reddy SM, Bisoi AK, Sharma P, Chauhan S. Surgical repair of D-TGA with an aortopulmonary window and ventricular septal defects. Rev Bras Cir Cardiovasc. 2010. Oct-Dec;25(4):585–587. [DOI] [PubMed] [Google Scholar]
- 16.Tiraboschi R, Salomone G, Crupi G, et al. Aortopulmonary window in the first year of life: report on 11 surgical cases. Ann Thorac Surg. 1988. October;46(4):438–441. [DOI] [PubMed] [Google Scholar]
- 17.Najm HK, Jijeh AM, El Moazamy YM, et al. Dextrocardia, aortopulmonary window with transposition of the great arteries, case report. J Saudi Heart Assoc. 2010. April;22(2):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan P, Airan B, Sambamurthy, et al. Complete transposition of the great arteries with aortopulmonary window: surgical treatment and embryologic significance. J Thorac Cardiovasc Surg. 1991. April; 101(4):749–751. [PubMed] [Google Scholar]
- 19.Mishra A, Gandhi H, Sharma P, et al. Transposition of great arteries with aortopulmonary window: our surgical experience. Ann Thorac Surg. 2014. January;97(1):196–201. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Singh A, Mahrous DE. A rare case of transposition of great arteries with an intact septum and aorto-pulmonary window. Int J Surg. 2014;2(2):24–26. [Google Scholar]
- 21.Moruno Tirado A, Santos De Soto J, Grueso Montero J, et al. Aortopulmonary window: clinical assessment and surgical results. Rev Esp Cardiol. 2002. March;55(3):266–270. [DOI] [PubMed] [Google Scholar]
- 22.Chen CA, Chiu SN, Wu ET, et al. Surgical outcome of aortopulmonary window repair in early infancy. J Formos Med Assoc. 2006. October; 105(10):813–820. [DOI] [PubMed] [Google Scholar]
- 23.Alborino D, Guccione P, Di Donato R, et al. Aortopulmonary window coexisting with tetralogy of Fallot. J Cardiovasc Surg. 2001. April;42(2): 197–199. [PubMed] [Google Scholar]
- 24.Peyvandi S, De Santiago V, Chakkarapani E, et al. Association of Prenatal Diagnosis of critical congenital heart disease with postnatal brain development and the risk of brain injury. JAMA Pediatr. 2016. April; 170(4):e154450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorts A, Krawczeski CD. Perioperative care of a child with transposition of the great arteries. Curr Treat Options Cardiovasc Med. 2011. October;13(5):456–463. [DOI] [PubMed] [Google Scholar]
- 26.Mosca R Balloon atrial septostomy let’s take a closer look. J Am Coll Cardiol. May 12, 2009;53(19):1812–1813. [DOI] [PubMed] [Google Scholar]