Abstract
Background
The POP Trial was a phase 1, open-label, rising-dose, randomised study that explored the safety and tolerability of calmangafodipir (superoxide dismutase mimetic) co-treatment with n-acetylcysteine (NAC) for paracetamol overdose.
Methods
Patients were recruited at the Royal Infirmary of Edinburgh (8th June 2017-10th May 2018). Inclusion criterion: adults within 24 h of a paracetamol overdose that required NAC. Within each of 3 sequential cohorts, participants were randomly assigned, with concealed allocation, to NAC and a single intravenous calmangafodipir dose (n = 6) or NAC alone (n = 2). Calmangafodipir doses were 2, 5, or 10 μmol/kg. Participants, study and clinical teams were not blinded. The primary outcome was safety and tolerability. Secondary outcomes were alanine transaminase (ALT), international normalised ratio (INR), keratin-18, caspase-cleaved keratin-18 (ccK18), microRNA-122, and glutamate dehydrogenase (GLDH). (Clinicaltrials.gov:NCT03177395).
Findings
All 24 participants received their allocated drug doses and were analysed. Primary endpoints: all participants experienced ≥1 adverse event (AE), most commonly gastrointestinal. Patients experiencing ≥1 serious adverse event (SAE): NAC alone, 2/6; NAC + calmangafodipir (2 μmol/kg), 4/6; NAC + calmangafodipir (5 μmol/kg), 2/6; NAC + calmangafodipir (10 μmol/kg), 3/6. No AEs or SAEs were probably or definitely calmangafodipir-related. Secondary safety outcomes demonstrated no differences between groups. With NAC alone, 2/6 had ALT > 100 U/L; with NAC + calmangafodipir, 0/18. No INR difference. Keratin-18 and ccK18 increased in the NAC alone group more than with calmangafodipir (baseline to 20 h fold change, NAC + calmangafodipir (5 μmol/kg) compared to NAC alone: 0.48 (95%CI 0.28–0.83)). microRNA-122 changes were similar to K18, GLDH was frequently undetected.
Interpretation
Calmangafodipir was tolerated when combined with NAC and may reduce biomarkers of paracetamol toxicity.
Research in context.
Evidence before this study
Paracetamol overdose is a common reason for emergency admission to hospital and the commonest cause of acute liver failure in Europe and North America. Acetylcysteine (NAC) is effective at preventing liver injury if administered promptly, but it is substantially less effective if started later than around 8 h after overdose. We have previously demonstrated that a shorter 12 h NAC regimen (the ‘SNAP’ regimen) produces fewer adverse reactions than the conventional 21 h protocol. The following Pubmed search strategy “phase 1 AND trial AND acetaminophen AND overdose” (10 March 2019) yielded no papers in this clinical space aside from the protocol paper for our trial. The search “acetaminophen overdose” “phase 1” in clinicaltrials.gov only identified our trial (10 March 2019). These search results demonstrate that paracetamol overdose is neglected as a target for new drug development despite being common and having a clear unmet clinical need. In this phase 1 trial we combine a novel therapeutic agent with the SNAP regimen.
Added value of this study
In this phase 1 study we explored the safety and tolerability of calmangafodipir (a superoxide dismutase mimetic) co-treatment with the SNAP NAC regimen. The combination of NAC and calmangafodipir was safe and tolerated. There was evidence from measurement of conventional and exploratory biomarkers that the combination treatment may reduce liver injury biomarkers more than NAC alone after paracetamol overdose.
Implications of all the available evidence
The current licenced treatment for paracetamol overdose is NAC. When a patient develops liver injury there is no further therapeutic option other than liver transplantation in severe cases. This trial supports further development of calmangafodipir in robust clinical trials that determine whether it is effective (clinically and economically) in patients at risk of paracetamol toxicity.
Alt-text: Unlabelled Box
1. Introduction
Paracetamol/acetaminophen (N-acetyl-p-aminophenol) is the most common drug taken in overdose in the United Kingdom (UK). Annually, overdose directly leads to around 100,000 hospital attendances in the UK with around half of these patients being admitted to hospital for emergency antidote treatment [1]. Paracetamol is directly responsible for the deaths of 100–150 people per year in the UK [2]. In the USA, paracetamol overdose accounts for >56,000 hospital attendances and around 450 deaths due to acute liver failure each year [3].
In overdose, the normal paracetamol detoxification pathways are overwhelmed which allows cellular injury to be produced by the reactive intermediate metabolite, N-acetyl-p-benzoquinoneimine (NAPQI). Animal data demonstrate that paracetamol-induced toxicity broadly occurs in two phases: an initial metabolic phase followed by an oxidative phase. In human patients the metabolic phase is predominantly during the first 8 h after overdose with the oxidative phase dominating thereafter. During the metabolic phase, paracetamol metabolites are conjugated in the liver. In the oxidative phase hepatocyte glutathione (GSH) stores are depleted and NAPQI binds to intra-cellular proteins causing increased oxidative stress, mitochondrial injury and cell death [4].
Acetylcysteine (N-acetylcysteine; NAC) was developed as an antidote for paracetamol poisoning in the 1970s [5]. It acts by replenishing hepatocellular GSH to increase the detoxification of NAPQI during the metabolic phase of toxicity. Most commonly, patients receive an intravenous (IV) 21 h NAC regimen of 150 mg/kg over 1 h, then 50 mg/kg over 4 h, then 100 mg/kg over 16 h (total dose 300 mg/kg) [5]. Although highly effective at preventing paracetamol-induced hepatotoxicity when used within 8 h of overdose, this regimen is associated with the following challenges: (i) Reduced efficacy when administered later than around 8 h after overdose ingestion. However, there are data demonstrating benefit when administered to patients later than 8 h after overdose [6]. (ii) Adverse drug reactions (ADRs): nausea/vomiting occurs in more than half of recipients and anaphylactoid reactions in about a third [7]. (iii) Prolonged duration: The regime is time consuming, taking at least 21 h, leading to significant hospital bed occupancy (around 47,000 bed days per year in England) [8].
To address the high incidence of ADRs and prolonged duration of the standard NAC regimen, a shorter 12 h intravenous regimen has been developed (the ‘SNAP’ regimen) [9]. In this regimen the initial loading dose (NAC 100 mg/kg in 200 mL) is given over 2 h, followed by a second dose (200 mg/kg in 1000 mL) infused over 10 h (total dose of NAC same as 21 h regimen). The SNAP regimen has been demonstrated to be effective at reducing the incidence of vomiting and anaphylactoid reactions, compared with the standard intravenous acetylcysteine schedule [9]. The SNAP regimen is as effective as the standard NAC regimen with regard to preventing liver injury after paracetamol overdose [10].
In this study we explored the safety and tolerability of combining the SNAP regimen with a new therapeutic agent. Mangafodipir was originally developed as a MRI contrast agent and approved for that indication by the USA Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Mangafodipir has been demonstrated to prevent paracetamol-induced liver injury in mice by acting as a superoxide dismutase (SOD) mimetic which prevents mitochondrial injury during the oxidative phase of toxicity [11]. Consistent with this mechanism of action, in mice, this protection is at a time point when NAC is no longer active [11]. Calmangafodipir (Ca4Mn(DPDP)5), is derived from mangafodipir, with 80% of the manganese in mangafodipir being replaced with calcium. Based on the similarities between calmangafodipir and mangafodipir, it is anticipated that calmangafodipir would also exhibit SOD-mimetic pharmacologic actions similar to those of mangafodipir [12]. Calmangafodipir has been studied in a Phase 2 safety and efficacy study of chemotherapy-induced peripheral neuropathy in patients with advanced metastatic colorectal cancer (PLIANT Trial). As is the case with paracetamol toxicity, a central mechanism of this neuropathy is oxidative stress. In the PLIANT Trial calmangafodipir was well tolerated across three doses (2, 5 and 10 μmol/kg) and prevented the development of chemotherapy-induced peripheral neuropathy [13].
The primary objective of the POP Trial was to assess the adverse events (AEs) and serious adverse events (SAEs) associated with calmangafodipir co-treatment with the SNAP NAC treatment regime in patients with paracetamol overdose. A secondary study objective was the measurement of clinical and exploratory biomarkers of acute liver injury.
2. Methods
2.1. Trial design
This study, EudraCT number 2017-000246-21, ClinicalTrials.gov Identifier NCT03177395, was funded by the Sponsor, PledPharma AB, Stockholm, Sweden and was approved by the UK medicines regulator, MHRA (25th April 2017) and West of Scotland Research Ethics Committee 1, Glasgow, UK (11th April 2017). The trial rationale and full protocol have been published [14]. The following is a summary of the study methods as per the CONSORT Guidelines. The study was an open label, randomised, exploratory, rising dose design, NAC controlled, phase 1 safety and tolerability study in patients treated with NAC following paracetamol overdose. There were no significant changes to the protocol after trial commencement. This was a single centre study.
2.2. Participants
The inclusion criteria were: 1. Any patient with capacity admitted to hospital within 24 h of either a single acute paracetamol overdose or more than one dose of paracetamol (staggered) and deemed to require treatment with NAC (as per contemporaneous UK guidelines provided by the National Poisons Information Service via the Toxbase website). 2. Provision of written informed consent. 3. Males and females of at least 16 years of age. The exclusion criteria were: 1. Patients that do not have the capacity to consent to participate in the study. 2. Patients detained under the Mental Health Act or deemed unfit by the Investigator to participate due to mental health. 3. Patients with known permanent cognitive impairment. 4. Patients who are pregnant or nursing. 5. Patients who have previously participated in this trial. 6. Unreliable history of overdose. 7. Patients presenting >24 h after overdose. 8. Patients who take anticoagulants (e.g. warfarin) therapeutically or have taken an overdose of anticoagulants. 9. Patients who, in the opinion of the responsible clinician/nurse, are unlikely to complete the full course of NAC e.g. expressing wish to self-discharge. 10. Prisoners. 11. Non-English speaking patients. Patients who took a mixed overdose that included medications in addition to paracetamol were included in the trial.
2.3. Interventions
Study participants were randomly assigned to receive NAC and a single dose of calmangafodipir, or NAC alone. This was performed in 3 sequential cohorts of 8 patients within which patients received NAC + calmangafodipir (n = 6) or NAC alone (n = 2). The dose of calmangafodipir used within each of the 3 cohorts was 2, 5, or 10 μmol/kg [13]. Overall, 24 patients were planned to be allocated as follows: NAC alone (n = 6), NAC and calmangafodipir (2 μmol/kg) (n = 6), NAC and calmangafodipir (5 μmol/kg) (n = 6), NAC and calmangafodipir (10 μmol/kg) (n = 6).
Treatment started with a NAC IV infusion of 100 mg/kg in 200 mL saline or 5% dextrose over 2 h. After this infusion was complete, calmangafodipir was administered as a bolus IV infusion over 5 min at the dose specified by the dosing cohort. Those patients randomised to the NAC alone group had no intravenous injection. In all patients the NAC regimen continued with 200 mg/kg NAC diluted in 1000 mL delivered IV over 10 h. As per local clinical practice, there is one blood sample taken 2 h before the end of the second NAC bag (the 10 h time-point) and a second blood sample taken 10 h later (the 20 h time-point). NAC (at 200 mg/kg in 1000 mL IV over a further 10 h) was continued if any of the following criteria were reached: ALT activity had more than doubled since the admission measurement, OR ALT activity was two times the upper limit of normal (100 U/L) or more, OR international normalised ratio (INR) was >1.3 OR paracetamol concentration > 20 mg/L.
2.4. Outcomes
Our primary objective was to determine the safety and tolerability of calmangafodipir add-on treatment to the SNAP NAC treatment regimen in patients treated for overdose. Therefore, the primary outcome was the occurrence of any AEs or SAEs. Patients were followed using their NHS Lothian electronic records. Primary outcome data were collected 7, 30 and 90 days after randomisation, as were events of special interest: representation to hospital (any reason), representation with liver injury, repeat overdose, death and transfer to liver transplantation unit.
Secondary outcomes included clinical observations (pulse rate, blood pressure, respiratory rate, pulse oximetry, temperature) and haematological and clinical biochemistry parameters. Liver injury was quantified by standard parameters (alanine transaminase (ALT) and the international normalised ratio (INR)) and also the following exploratory circulating biomarkers: keratin-18 (K18), caspase cleaved K18 (ccK18), microRNA-122 (miR-122) and glutamate dehydrogenase (GLDH). These outcomes were pre-defined in detail in our study protocol. There were no significant changes to the outcomes after trial commencement.
2.5. Sample size
There was no formal power calculation for this Phase 1 study. Six patients per group in this dose escalation study allowed initial exploration of potential dose limiting toxicity.
An independent safety data monitoring committee (SDMC) evaluated safety prior to each planned calmangafodipir dosing step increase and recommended the continuation or termination of the study. During recruitment, summary data of in-hospital mortality/morbidity and any other information available on major outcome events (including SAEs believed to be due to treatment) were supplied to the SDMC along with any other data that the committee requested. The stopping guidelines determined that all further patient enrolment would be paused pending advise if any of the following stopping rules were met: 1) Patient death, admission to a Critical Care Unit or admission to a Liver Transplantation Unit due to any reason, or 2) One suspected unexpected serious adverse reaction (SUSAR) that definitely or probably relates to either calmangafodipir or NAC or both.
2.6. Randomisation and blinding
The allocation sequence for each dosing cohort was created by an Edinburgh Clinical Trials Unit (ECTU) programmer (GM) using computer-generated random numbers, using blocking to ensure the required 6:2 ratio. The randomisation list was held centrally at ECTU in order to conceal treatment allocations until these were implemented via the secure web-based randomisation system. There was no blinding of participants or emergency department staff. The statistical analysis plan was written blinded to the treatment allocations.
2.7. Biomarker measurement
ALT and INR were measured as part of routine clinical care in the Biochemistry Laboratory at the Royal Infirmary of Edinburgh. Enzyme-linked immunosorbent assay (ELISA) was used to measure K18 – (Peviva M65 ELISA (classic)) and ccK18 (M30 Apoptosense ELISA, Bioaxxess, Tewkesbury, UK). miR-122 was measured by reverse transcription polymerase chain reaction (RT-PCR) as previously described [15]. The concentration of miR-122 was expressed as the DCt using spiked-in C. elegans miR-39 as an external normaliser and quantified as copy number per μL by generating a standard curve [15]. GLDH was measured by its oxogluterate reduction activity (Alpha Laboratories Ltd., Eastleigh, UK). Biomarkers were measured in the Centre for Cardiovascular Science at the University of Edinburgh with the researcher blinded to the treatment allocation.
2.8. Statistical analysis
A detailed statistical analysis plan was finalised before locking of the trial database. This is detailed in the published protocol [14]. This initial exploration of safety and tolerability of calmangafodipir did not apply formal hypothesis tests; instead, 95% confidence intervals are presented where appropriate to indicate plausible effect sizes. The primary outcome analysis reported the number and percentage of patients experiencing an AE/SAE by randomised group and overall. Clinical observations and haematology and clinical biochemistry parameters were summarised by measurement time point and change from baseline. The ECG results were summarised by treatment group and overall at 2.5, 10 and 20 h. Conventional and exploratory biomarkers were summarised descriptively by treatment group and overall at baseline, 10 h and 20 h. Change from baseline was also summarised. Biomarkers were analysed by treatment group and overall at each time point using the mean and 95% confidence interval. Biomarkers were compared between each calmangafodipir dose and the combined NAC alone group using the difference in means and its 95% confidence interval. Where required, measures were log-transformed and reported by geometric means and their ratio.
2.9. Role of the funder
Two investigators in this study were employed by PledPharma AB (study funder). Their input was in study design (DH) and monitoring (MB). The funder of the study had no role in data collection, data analysis, data interpretation, or writing of the paper. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The corresponding author wrote the paper.
3. Results
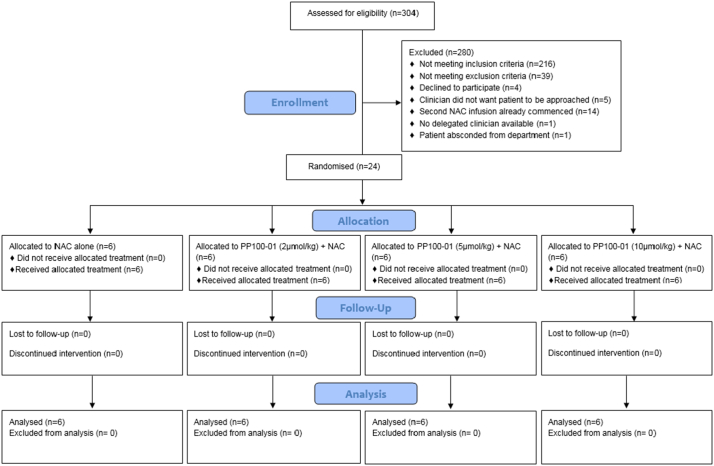
From 5th June 2017 to 10th May 2018 304 patients presenting with acute poisoning were screened in the Emergency Department of the Royal Infirmary of Edinburgh (Fig. 1). Patients screened had toxicology presentations that included, but were not exclusively, paracetamol overdose. The main reasons for not being included were failure to meet inclusion criteria (n = 216, predominately because patient was not a paracetamol overdose that needed treatment) or due to meeting an exclusion criteria (n = 39). Twenty-four patients were randomised and all 24 received the full dosing of their allocated treatment. The 90 day follow up of the final participant was completed on 8th August 2018 which was the protocol-defined end-of-trial date. No patients were lost to follow up.
Fig. 1.
CONSORT diagram for the POP Trial.
The baseline demographics of the 4 treatment groups are presented in Table 1. By chance, the NAC alone group reported ingestion of less paracetamol (normalised to body weight) than the calmangafodipir treated groups and had a lower median presentation paracetamol concentration. By chance, the NAC alone group presented to hospital, and started NAC treatment, an average of about 3 h later after overdose than the calmangafodipir treated groups. In each treatment group the following number of patients started treatment with NAC > 8 h after a single overdose: NAC alone: 3/6; NAC + calmangafodipir (2 μmol/kg): 2/6; NAC + calmangafodipir (5 μmol/kg): 2/6; NAC + calmangafodipir (10 μmol/kg): 1/6. Supplementary Fig. 1 represents graphically a post-hoc analysis of the relation between the paracetamol concentration and time from overdose for the single acute overdoses in this trial. The majority of the study participants had co-ingested other agents (Supplementary Table 1).
Table 1.
Patient demographics. Patients were allocated to 4 treatment groups as described in the study protocol.
| NAC alone |
NAC + 2 μmol/kg calmangafodipir |
NAC + 5 μmol/kg calmangafodipir |
NAC + 10 μmol/kg calmangafodipir |
||
|---|---|---|---|---|---|
| (N = 6) | (N = 6) | (N = 6) | (N = 6) | ||
| Age (years) at randomisation | Mean (SD) | 32.2 (12.5) | 42.5 (13.1) | 42.7 (12.7) | 22.7 (3.3) |
| Median | 30.0 | 41.0 | 42.5 | 22.0 | |
| Minimum, Maximum | 19, 52 | 27, 66 | 26, 59 | 19, 28 | |
| Sex | Male | 4 (67%) | 2 (33%) | 2 (33%) | 3 (50%) |
| Female | 2 (33%) | 4 (67%) | 4 (67%) | 3 (50%) | |
| Time from ingestion of paracetamol to hospital presentation (hours) | Mean (SD) | 8.8 (6.2) | 6.0 (6.2) | 5.8 (7.2) | 4.9 (5.2) |
| Median | 7.6 | 2.6 | 2.1 | 2.1 | |
| Minimum, Maximum | 1.9, 16.6 | 1.4, 15.1 | 1.3, 19.5 | 1.4, 14.6 | |
| Type of overdose | Acute, ≤8 h to NAC | 2 (33%) | 4 (67%) | 4 (67%) | 4 (67%) |
| Acute, >8 h to NAC | 3 (50%) | 2 (33%) | 2 (33%) | 1 (17%) | |
| Staggered intentional | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) | |
| Supra-therapeutic | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Presentation paracetamol concentration (mg/L) | Mean (SD) | 76 (81) | 127 (90) | 74 (44) | 127 (47) |
| Median | 58 | 114 | 88 | 132 | |
| Minimum, Maximum | 8, 228 | 16, 272 | 8, 117 | 76, 201 | |
| Total paracetamol ingested (mg/kg) | Mean (SD) | 185 (156) | 235 (77) | 229 (72) | 397 (476) |
| Median | 116 | 222 | 244 | 227 | |
| Minimum, Maximum | 28, 418 | 154, 340 | 142, 303 | 88, 1357 | |
| Time from ingestion of paracetamol to start of NAC treatment (hours) | Mean (SD) | 12.1 (5.2) | 9.8 (5.7) | 10.2 (6.9) | 8.6 (4.1) |
| Median | 10.3 | 6.5 | 7.3 | 6.7 | |
| Minimum, Maximum | 7.7, 20.3 | 5.5, 17.9 | 6.0, 23.8 | 5.3, 16.0 | |
| Time from ingestion of paracetamol to start of calmangafodipir (hours) | Mean (SD) | N/A | 12.6 (6.8) | 10.8 (4.1) | 11.8 (5.4) |
| Median | N/A | 9.5 | 9.0 | 8.9 | |
| Minimum, Maximum | N/A | 8.5, 26.1 | 7.6, 18.0 | 7.6, 26.1 | |
| Any other drugs ingested | Yes | 5 (83%) | 4 (67%) | 5 (83%) | 5 (83%) |
| No | 1 (17%) | 2 (33%) | 1 (17%) | 1 (17%) | |
| Serum creatinine (μmol/L) | Mean (SD) | 74.7 (11.0) | 67.5 (13.3) | 67.3 (17.2) | 69.5 (13.1) |
| Median | 73.5 | 63.5 | 66.0 | 68.5 | |
| Minimum, Maximum | 59, 92 | 57, 94 | 50, 98 | 53, 86 | |
All randomised patients were analysed for the safety and tolerability primary outcomes (Table 2). During the 7 days after randomisation 23 out of 24 patients had at least one AE. Eleven patients had at least one SAE within the 90 day follow up period; 5 patients had at least one SAE within 7 days of randomisation. These SAEs were spread across the 4 treatment groups. Supplementary Table 2 presents the nature of the AEs and SAEs. There were no AEs or SAEs judged to be probably or definitely related to calmangafodipir. Seven patients experienced AEs judged definitely related to NAC. There were no anaphylactoid reactions to NAC. One death, that occurred 32 days after the start of NAC and calmangafodipir (5 μmol/kg) treatment, was judged to be unrelated to either NAC or calmangafodipir. There was one SUSAR reported for calmangafodipir in the 10 μmol/kg cohort. The SUSAR was hypokalaemia needing potassium replacement therapy that prolonged the patient's hospital stay by 7 h. This was judged as probably related to NAC and possibly related to calmangafodipir. Secondary outcomes demonstrated no safety concerns for calmangafodipir in combination with NAC (Supplementary Table 2).
Table 2.
Primary outcome. Safety and tolerability was the primary outcome as judged by the occurrence of adverse events and serious adverse events. Data are presented for each treatment group as described in the study protocol. The number of events and percentage of group experiencing the event are listed.
| NAC alone |
NAC + 2 μmol/kg calmangafodipir |
NAC + 5 μmol/kg calmangafodipir |
NAC + 10 μmol/kg calmangafodipir |
|
|---|---|---|---|---|
| Event | (N = 6) | (N = 6) | (N = 6) | (N = 6) |
| Any adverse event | 6 (100%) | 6 (100%) | 6 (100%) | 6 (100%) |
| Any serious adverse event | 2 (33%) | 4 (67%) | 2 (33%) | 3 (50%) |
| Adverse event starting after commencement of NAC treatment and within 7 days of consent | 6 (100%) | 5 (83%) | 6 (100%) | 6 (100%) |
| Serious adverse event starting after commencement of NAC treatment and within 7 days of consent | 1 (17%) | 1 (17%) | 1 (17%) | 2 (33%) |
| Adverse event unrelated to NAC | 3 (50%) | 5 (83%) | 3 (50%) | 5 (83%) |
| Adverse event possibly related to NAC | 2 (33%) | 2 (33%) | 2 (33%) | 2 (33%) |
| Adverse event probably related to NAC | 3 (50%) | 2 (33%) | 3 (50%) | 2 (33%) |
| Adverse event definitely related to NAC | 2 (33%) | 3 (50%) | 1 (17%) | 1 (17%) |
| Adverse event unrelated to calmangafodipir | 6 (100%) | 6 (100%) | 5 (83%) | 6 (100%) |
| Adverse event possibly related to calmangafodipir | 0 (0%) | 4 (67%) | 2 (33%) | 2 (33%) |
| Adverse event probably related to calmangafodipir | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Adverse event definitely related to calmangafodipir | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Liver injury was explored with conventional (Table 3) and exploratory biomarkers (Table 4). The confidence intervals for the fold change from baseline to 20 h in ALT or INR included the null value (1) in all treatment groups (Geometric mean of relative change (95%CI): NAC alone: ALT 1.02 (0.62 to 1.69), INR 1.15 (0.98 to 1.35). NAC + calmangafodipir (2 μmol/kg): ALT 0.83 (0.66 to 1.04), INR 1.07 (0.93 to 1.23). NAC + calmangafodipir (5 μmol/kg): ALT 0.87 (0.64 to 1.18), INR 1.10 (1.03 to 1.18). NAC + calmangafodipir (10 μmol/kg): ALT 0.92 (0.57 to 1.49), INR 1.10 (1.00 to 1.21)). In the NAC alone group 2/6 had an increase of 50% or more in ALT from baseline to 20 h. With calmangafodipir 1/18 had this ALT increase (Supplementary Table 3). In the NAC alone group 2/6 patients had an ALT activity > 100 U/L at 20 h after starting NAC, a clinically relevant value used to indicate need for NAC treatment to continue (and a secondary outcome pre-defined in trial protocol). No patients in the calmangafodipir treated groups reached this value (Supplementary Table 3). In total, in the NAC alone group, 3 from 6 patients required additional NAC therapy after the end of the SNAP regimen (1 from each of the following overdose types: <8 h overdose to starting NAC, >8 h and supra-therapeutic). In the calmangafodipir groups, 2 out of 18 patients required extended treatment (1 who took a staggered overdose and another who started NAC treatment < 8 h after overdose). (Table 3). Exploratory biomarkers have greater sensitivity than ALT in the context of paracetamol-induced liver injury [16]. Circulating K18 and ccK18 increased in concentration from baseline to 20 h in all 6 patients treated with NAC alone (Geometric mean of relative change (95%CI): K18 1.85 (1.24 to 2.77); ccK18 2.22 (1.22 to 4.03)) (Table 4). This increase was smaller in the calmangafodipir treated groups; the confidence intervals excluded the null value with the 5 μmol/kg calmangafodipir dose for K18 and ccK18 (ratio of geometric means for the fold change from baseline to 20 h (NAC + calmangafodipir compared to NAC alone) 2 μmol/kg: K18 0.70 (95%CI 0.35 to 1.37), ccK18 0.67 (0.35 to 1.29); 5 μmol/kg; K18 0.48 (0.28 to 0.83), ccK18 0.46 (0.22 to 0.96); 10 μmol/kg K18 0.76 (0.40 to 1.46) ccK18 0.48 (0.19 to 1.27)) (Supplementary Fig. 2).
Table 3.
Secondary outcome. Alanine transaminase activity (ALT) and international normalised ratio (INR) for each of the allocated treatment groups. Data are presented as mean or geometric mean and median as per study protocol. The change from baseline to 20 h after starting NAC is presented as the relative change. A value of 1 indicates no change. SD = standard deviation. GSD = geometric standard deviation. A patients received 12 h of treatment with acetylcysteine (NAC). The number of extra NAC infusions given to each group is listed.
| NAC alone | NAC + 2 μmol/kg calmangafodipir | NAC + 5 μmol/kg calmangafodipir | NAC + 10 μmol/kg calmangafodipir | |||
|---|---|---|---|---|---|---|
| ALT (U/L) | Baseline | N | 6 | 6 | 6 | 6 |
| Geometric Mean (GSD) | 42.5 (3.6) | 24.6 (2.1) | 29.4 (2.3) | 17.7 (1.5) | ||
| Median | 49.0 | 30.5 | 42.5 | 16.0 | ||
| Minimum, Maximum | 11, 209 | 9, 56 | 7, 67 | 12, 34 | ||
| 10 h | N | 6 | 6 | 6 | 6 | |
| Geometric Mean (GSD) | 41.4 (3.3) | 22.9 (1.8) | 25.3 (2.1) | 15.0 (1.3) | ||
| Median | 48.0 | 28.0 | 32.5 | 14.5 | ||
| Minimum, Maximum | 11, 163 | 10, 44 | 8, 57 | 10, 24 | ||
| 20 h | N | 6 | 6 | 6 | 6 | |
| Geometric Mean (GSD) | 43.3 (3.8) | 20.4 (1.9) | 25.4 (1.8) | 16.4 (1.5) | ||
| Median | 47.5 | 27.0 | 29.0 | 16.5 | ||
| Minimum, Maximum | 9, 226 | 8, 35 | 10, 53 | 8, 27 | ||
| Relative change from baseline to 20 h - ratio | Geometric Mean (GSD) | 1.02 (1.62) | 0.83 (1.25) | 0.87 (1.34) | 0.92 (1.58) | |
| Median | 0.79 | 0.78 | 0.80 | 0.83 | ||
| Minimum, Maximum | 0.70, 2.13 | 0.63, 1.11 | 0.60, 1.43 | 0.65, 2.08 | ||
| INR | Baseline | Missing INR | 0 | 1 | 0 | 0 |
| N | 6 | 5 | 6 | 6 | ||
| Mean (SD) | 1.02 (0.04) | 1.00 (0.12) | 0.98 (0.04) | 1.05 (0.14) | ||
| Median | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Minimum, Maximum | 1.0, 1.1 | 0.9, 1.2 | 0.9, 1.0 | 0.9, 1.3 | ||
| 10 h | N | 6 | 6 | 6 | 6 | |
| Mean (SD) | 1.30 (0.18) | 1.17 (0.20) | 1.20 (0.00) | 1.22 (0.25) | ||
| Median | 1.25 | 1.10 | 1.20 | 1.15 | ||
| Minimum, Maximum | 1.1, 1.6 | 1.0, 1.5 | 1.2, 1.2 | 1.0, 1.7 | ||
| 20 h | N | 6 | 6 | 6 | 6 | |
| Mean (SD) | 1.18 (0.21) | 1.07 (0.19) | 1.08 (0.04) | 1.17 (0.23) | ||
| Median | 1.15 | 1.00 | 1.10 | 1.10 | ||
| Minimum, Maximum | 1.0, 1.5 | 0.9, 1.3 | 1.0, 1.1 | 1.0, 1.6 | ||
| Relative change from baseline to 20 h - ratio | # missing | 0 | 1 | 0 | 0 | |
| N | 6 | 5 | 6 | 6 | ||
| Geometric Mean (GSD) | 1.15 (1.17) | 1.07 (1.12) | 1.10 (1.07) | 1.10 (1.10) | ||
| Median | 1.15 | 1.00 | 1.10 | 1.10 | ||
| Minimum, Maximum | 1.00, 1.36 | 1.00, 1.30 | 1.00, 1.22 | 1.00, 1.23 | ||
| Number of additional NAC infusions after 12 h regimen. | None | 3 (50%) | 5 (83%) | 6 (100%) | 5 (83%) | |
| One | 1 (17%) | 1 (17%) | 0 (0%) | 1 (17%) | ||
| Two | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
Table 4.
Secondary outcome. Keratin-18 (K18), caspase cleaved K18 (ccK18) and microRNA-122 (miR-122) for each of the allocated treatment groups. Data are presented as mean or geometric mean and median as per study protocol. The change from baseline to 20 h after starting NAC is presented as the relative change. A value of 1 indicates no change. SD = standard deviation. GSD = geometric standard deviation. The data for miR-122 are presented as the DCt using spiked-in C. elegans miR-39 as an external normaliser and quantified as copy number per μL. The DCt value is derived as Ct(miR-122)-Ct(miR-39) where Ct refers to the number of PCR cycles required to reach a threshold value for signal detection. Therefore, the lower the DCt value the higher the concentration of miR-122.
| NAC alone |
NAC + 2 μmol/kg calmangafodipir |
NAC + 5 μmol/kg calmangafodipir |
NAC + 10 μmol/kg calmangafodipir |
|||
|---|---|---|---|---|---|---|
| (N = 6) | (N = 6) | (N = 6) | (N = 6) | |||
| K18 (U/L) | Baseline (2 h) | Geometric Mean (GSD) | 187 (2.20) | 177 (1.82) | 193 (1.56) | 128 (1.25) |
| Median | 158 | 191 | 200 | 130 | ||
| Minimum, Maximum | 95, 731 | 91, 431 | 102, 353 | 89, 173 | ||
| 10 h | Geometric Mean (GSD) | 182 (1.95) | 152 (1.56) | 170 (1.42) | 111 (1.18) | |
| Median | 172 | 135 | 168 | 110 | ||
| Minimum, Maximum | 91, 472 | 105, 319 | 113, 252 | 89, 143 | ||
| 20 h | Geometric Mean (GSD) | 347 (3.18) | 229 (1.94) | 172 (1.45) | 181 (1.73) | |
| Median | 306 | 212 | 163 | 155 | ||
| Minimum, Maximum | 118, 2606 | 98, 572 | 100, 287 | 103, 507 | ||
| Relative change from baseline to 20 h – ratio | Geometric Mean (GSD) | 1.85 (1.47) | 1.29 (1.89) | 0.89 (1.57) | 1.41 (1.83) | |
| Median | 1.71 | 1.41 | 1.02 | 1.17 | ||
| Minimum, Maximum | 1.24, 3.57 | 0.53, 2.80 | 0.43, 1.45 | 0.74, 4.34 | ||
| ccK18 (U/L) | Baseline (2 h) | Geometric Mean (GSD) | 67 (1.99) | 45 (1.33) | 84 (1.80) | 104 (2.44) |
| Median | 61 | 45 | 71 | 83 | ||
| Minimum, Maximum | 28, 210 | 30, 67 | 52, 244 | 32, 408 | ||
| 10 h | Geometric Mean (GSD) | 72 (2.24) | 53 (1.25) | 56 (1.57) | 78 (2.12) | |
| Median | 60 | 54 | 52 | 74 | ||
| Minimum, Maximum | 34, 281 | 36, 70 | 34, 99 | 33, 279 | ||
| 20 h | Geometric Mean (GSD) | 149 (3.34) | 66 (1.34) | 85 (1.62) | 111 (2.56) | |
| Median | 111 | 69 | 94 | 113 | ||
| Minimum, Maximum | 51, 1451 | 38, 87 | 42, 148 | 38, 530 | ||
| Relative change from baseline to 20 h - ratio | Geometric Mean (GSD) | 2.22 (1.77) | 1.49 (1.55) | 1.02 (1.79) | 1.08 (2.44) | |
| Median | 1.85 | 1.29 | 0.94 | 1.03 | ||
| Minimum, Maximum | 1.48, 6.91 | 0.98, 2.88 | 0.52, 2.68 | 0.38, 3.27 | ||
| miR-122 (DCt) | Baseline (2 h) | Mean (SD) | 5.58 (3.36) | 5.85 (1.50) | 4.43 (3.92) | 8.73 (2.36) |
| Median | 5.17 | 5.89 | 4.47 | 8.71 | ||
| Minimum, Maximum | 1.93, 11.11 | 4.11, 8.18 | −2.06, 8.82 | 5.52, 12.71 | ||
| 10 h | Mean (SD) | 5.41 (3.86) | 6.14 (1.99) | 5.01 (3.36) | 9.00 (1.45) | |
| Median | 4.03 | 6.19 | 6.00 | 9.16 | ||
| Minimum, Maximum | 0.76, 10.93 | 2.94, 8.83 | −1.06, 7.78 | 7.07, 10.73 | ||
| 20 h | Mean (SD) | 4.85 (3.97) | 7.12 (2.26) | 4.49 (2.93) | 8.44 (1.50) | |
| Median | 4.37 | 7.42 | 5.72 | 8.56 | ||
| Minimum, Maximum | −0.04, 11.69 | 4.23, 9.67 | −1.24, 6.58 | 5.94, 10.49 | ||
| miR-122 (copies/mcL) | Baseline (2 h) | Geometric Mean (GSD) | 146,363 (11.7) | 116,749 (2.4) | 194,075 (13.5) | 36,051 (3.9) |
| Median | 143,528 | 93,798 | 298,431 | 28,499 | ||
| Minimum, Maximum | 4871, 2,313,373 | 46,075, 421,291 | 12,405, 11,163,618 | 5892, 360,305 | ||
| 10 h | Geometric Mean (GSD) | 206,205 (13.0) | 109,882 (3.3) | 196,732 (9.4) | 37,066 (2.2) | |
| Median | 351,346 | 97,112 | 125,977 | 31,463 | ||
| Minimum, Maximum | 9042, 6,819,168 | 32,464, 809,183 | 34,866, 10,717,356 | 14,955, 127,755 | ||
| 20 h | Geometric Mean (GSD) | 216,256 (10.8) | 57,664 (3.8) | 202,271 (7.7) | 40,745 (3.2) | |
| Median | 349,165 | 42,716 | 96,912 | 33,750 | ||
| Minimum, Maximum | 4026, 4,488,868 | 11,394, 367,730 | 45,608, 10,150,008 | 7920, 198,075 | ||
| Relative change from baseline to 20 h - ratio | Geometric Mean (GSD) | 1.48 (5.71) | 0.49 (1.98) | 1.04 (8.28) | 1.13 (2.96) | |
| Median | 2.23 | 0.54 | 2.00 | 1.09 | ||
| Minimum, Maximum | 0.07, 10.91 | 0.22, 1.39 | 0.06, 10.55 | 0.30, 3.88 | ||
In comparison to the K18 isoforms, miR-122 had similar mean and median relative changes when NAC + calmangafodipir treatment groups were compared to the NAC alone group (Table 4). miR-122 increased in concentration from baseline to 20 h in patients treated with NAC alone (geometric mean of relative change (95%CI): 1.48 (0.24 to 9.20)). This increase was consistently smaller across calmangafodipir doses (ratio of geometric means for the fold change from baseline to 20 h (NAC + calmangafodipir compared to NAC alone) 2 μmol/kg: 0.33 (0.06 to 1.84); 5 μmol/kg; 0.71 (0.06 to 8.52); 10 μmol/kg 0.76 (0.12 to 4.95)). However, miR-122 had greater variability than K18 isoforms and all confidence intervals included the null value (Supplementary Fig. 2).
GLDH was measured as pre-defined in our protocol. It was below the published lower limit of quantification (1 U/L) in 16 out of 72 samples. As per our trial protocol, the GLDH analysis is presented in Supplementary Table 4 [17]. There was no difference between groups.
4. Discussion
Paracetamol overdose is a common reason for emergency hospital admission. The only current treatment is NAC, but this antidote loses efficacy when treatment is delayed. In this study we demonstrate that calmangafodipir, in addition to NAC, did not result in any safety issues.
Calmangafodipir prevents cell injury by reducing oxidative stress – a central mechanism responsible for paracetamol-induced hepatocyte necrosis. It has been safely administered to patients with cancer and demonstrated to reduce the incidence of peripheral neuropathy. In this paper, we begin the process of exploring the clinical utility of calmangafodipir in paracetamol overdose. As a starting point, it was important to determine whether there were any safety concerns when combined with NAC, an agent that commonly produces ADRs. Our data demonstrate no increase in AEs or SAEs at 3 ascending doses of calmangafodipir when combined with the SNAP NAC regimen. An advantage of using the SNAP regimen in this trial, rather than the more widely used 21 h NAC regimen, is it produces substantially fewer ADRs. No patients in this trial had an anaphylactoid reaction yet these reactions occur in up to 30% of those treated with the 21 h regimen [9]. The improved safety profile of the SNAP regimen facilitated our analysis of any emergent calmangafodipir toxicity. From this phase 1 study we conclude that there are no safety issues that preclude, or need special consideration in, future clinical trials.
Our pre-defined secondary objective was to explore conventional and exploratory biomarkers of liver injury. More patients in the NAC alone group required additional NAC therapy after completion of the SNAP regimen compared to the NAC + calmangafodipir groups due to more patients having ALT increases. However, this phase 1 study is small and no firm conclusions can be drawn using ALT. In part, this is because early in the disease process - soon after overdose - ALT can remain within the healthy reference interval despite evolving liver injury. The exploration of liver injury in this trial was facilitated by the recent development of a panel of chemically and bio-analytically distinct biomarkers for drug-induced liver injury. A full-length version of K18 is released by necrotic cell death. A shorter, caspase cleaved form of K18 (ccK18) is released following cell apoptosis (programmed cell death). Both forms of K18, when measured in the first serum sample at presentation at the hospital after paracetamol overdose, correlate with peak ALT activity during that hospital stay [16,18]. K18 is more sensitive than ALT – it distinguishes patients with and without acute liver injury at hospital presentation when ALT activity is still in the normal range. K18 is supported for exploratory use in assessing drug-induced liver injury in clinical trials, both by the EMA and the FDA [19,20]. miR-122 is a microRNA biomarker specific for liver injury that is fully conserved (translational) across in vitro models, in vivo models and humans. Similar to K18, miR-122 is an early marker for acute liver injury that predicts a rise in ALT activity following paracetamol overdose. When miR-122 was measured at hospital presentation in patients requiring NAC therapy its concentration correlated significantly with peak hospital stay ALT activity and miR-122 was significantly higher in those patients who developed subsequent acute liver injury [16,18]. This is consistent with miR-122 having enhanced sensitivity and specificity in this context-of-use.
The baseline measurements of the exploratory biomarkers were similar across the 4 treatment groups consistent with successful randomisation of patients with regard to the biomarker concentrations prior to calmangafodipir administration. The variability across subjects was substantially higher for miR-122 than K18, a limitation of miR-122 that has been described in other studies [17]. All 6 patients in the NAC alone group had an increase in both K18 forms from baseline to 20 h. In the calmangafodipir-treated groups there was not an increase in the exploratory biomarkers. We note that GLDH was too low to be reliably quantified in this study. This is consistent with published evidence that demonstrates GLDH has reduced sensitivity when directly compared to K18 and miR-122 in patients with paracetamol overdose [14].
The data suggest that, with development, calmangafodipir may have value as an additional therapy to NAC in patients at increased risk of liver injury after paracetamol overdose. Such patients may include late presenters after overdose (greater than around 8 h) or those with evidence of liver injury at presentation. The exploratory biomarkers used in this study can sensitively identify liver injury before ALT is elevated and rapid clinical assays would represent potential companion diagnostics that could be used to identify patients that may benefit from treatment with calmangafodipir.
5. Limitations
None of the patients in this trial developed hepatotoxicity (ALT > 1000 U/L) or acute liver failure. In future studies it will be important to continue to monitor for any safety signals when calmangafodipir is administered to patients with established liver injury and liver failure. Liver injury after overdose is rare if NAC starts within 8 h of overdose. The patients were not stratified at randomisation by their risk of developing liver injury. This, combined with the small patient numbers per treatment group, resulted in the NAC alone group having a higher proportion of patients who started NAC later than 8 h after overdose compared with the NAC and calmangafodipir groups. This difference across treatment groups should be considered when interpreting the effect of calmangafodipir. It should be noted, however, that the change in biomarkers was consistent in the NAC alone group regardless of overdose type. All the NAC alone patients had an increase in K18 and ccK18 from baseline to 20 h including 2 early presenters (<8 h) and 1 supra-therapeutic overdose patient. In 5 out of these 6 patients the concentration of K18 at 20 h was greater than the published upper limit of normal of the healthy reference interval (151 U/L) [17]. With caution, we speculate that this may be consistent with K18 reporting liver injury that is too mild to cause an elevation in ALT. This hypothesis needs to be rigorously studied in subsequent trials that robustly determine whether calmangafodipir reduces paracetamol-induced liver injury biomarkers. These future trials would need to be large in size to demonstrate any impact on patient mortality should that be present. However, it should be feasible to demonstrate an effect on clinically and economically important outcomes such as the development of liver synthetic dysfunction and the length of hospital admission.
In conclusion, calmangafodipir was safe and tolerated in patients treated with NAC for paracetamol overdose and may reduce liver injury biomarkers.
The following are the supplementary data related to this article.
Agents ingested in overdose at the time of paracetamol overdose. Agents are grouped by drug class. Patients grouped by treatment allocation. NSAIDS = non-steroidal anti-inflammatory drugs. SSRIs = selective serotonin reuptake inhibitors.
Secondary safety outcomes. Patients are grouped by treatment allocation (CAM = calmangafodipir). Adverse event details listed per treatment group. Endpoints are described at each measurement time point after starting NAC. The absolute change from baseline to each timepoint is given with mean and standard deviation (SD), median and range. Electrocardiogram (ECG) findings were classed as normal, abnormal (not clinically significant - NCS) or abnormal (clinically significant - CS).
Secondary outcome. Alanine transaminase activity (ALT), international normalised ratio (INR) and paracetamol concentration for each of the allocated treatment groups. Data are presented as the number and percentage of treatment group reaching cut-off values and fold increases that were pre-defined in the study protocol.
Secondary outcome. Glutamate dehydrogenase (GLDH) for each of the allocated treatment groups. Data are presented as geometric mean and median as per study protocol. The change from baseline to 20 h after starting NAC is presented as the relative change. A value of 1 indicates no change. GSD = geometric standard deviation. LLoQ – lower limit of quantification. One sample was not measurable due to lipidaemia. 16 samples were below the LLoQ.
Paracetamol concentration versus time since overdose to blood collection. Sloped lines are an extension of the Rumack-Matthew nomogram corresponding to 100, 150, 200, 300 and 500 mg/L at 4 h post-ingestion (based on a first-order decay of blood paracetamol with a half-life of 4 h). The vertical line at 8 h separates “early” and “late” presenters. N = 6 except NAC alone (N = 5 due to one supra-therapeutic overdose) and NAC and 10umol/kg (N = 5 due to one staggered overdose).
The relative fold change for keratin-18 (K18), caspase cleaved K18 (ccK18) and microRNA-122 (miR-122) from baseline to 20 h after starting acetylcysteine treatment (NAC). Patients are grouped by the treatment allocation. K18 = white symbols. ccK18 = grey symbols. miR-122 = black symbols.
Contributions
The trial was designed by Dr. Dear and Dr. Henriksen. Trial set up and management was provided by ECTU. Patient recruitment and trial delivery was by EMERGE and Edinburgh Royal Infirmary. Sub-study biomarker analysis was by University of Edinburgh.
Declaration of Competing Interest
Dr. Dennis Henriksen and Ms. Marie Bengtson are employed by PledPharma AB. Dr. Dear is a member of the expert advisory group for the EU IMI funded TransBioLine Consortium.
Acknowledgments
Acknowledgments
JWD was supported by an NHS Research Scotland (NRS) Career Research Fellowship through NHS Lothian and acknowledges the contribution of the British Heart Foundation Centre of Research Excellence Award (RE/08/001). Prof Weir was supported in this work by NHS Lothian via ECTU.
Funding
PledPharma AB.
References
- 1.Narayan H., Thomas S.H., Eddleston M., Dear J.W., Sandilands E., Nicholas Bateman D. Disproportionate effect on child admissions of the change in Medicines and Healthcare Products Regulatory Agency guidance for management of paracetamol poisoning: an analysis of hospital admissions for paracetamol overdose in England and Scotland. Br J Clin Pharmacol. 2015;80(6):1458–1463. doi: 10.1111/bcp.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawton K., Bergen H., Simkin S. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ. 2013;346:f403. doi: 10.1136/bmj.f403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee W.M. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40(1):6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A., Jaeschke H. Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology. J Clin Transl Res. 2017;3(Suppl. 1):157–169. doi: 10.18053/jctres.03.2017S1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott L.F., Park J., Ballantyne A., Adriaenssens P., Proudfoot A.T. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;2(8035):432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 6.Keays R., Harrison P.M., Wendon J.A. Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ. 1991;303(6809):1026–1029. doi: 10.1136/bmj.303.6809.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waring W.S., Stephen A.F., Robinson O.D., Dow M.A., Pettie J.M. Lower incidence of anaphylactoid reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose. Clin Toxicol. 2008;46(6):496–500. doi: 10.1080/15563650701864760. [DOI] [PubMed] [Google Scholar]
- 8.NHS Hospital Episode Statistics 2011. http://www.hscic.gov.uk/heshttp://www.hscic.gov.uk/hes
- 9.Bateman D.N., Dear J.W., Thanacoody H.K. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014;383(9918):697–704. doi: 10.1016/S0140-6736(13)62062-0. [DOI] [PubMed] [Google Scholar]
- 10.Pettie J.M., Caparrotta T.M., Hunter R.W. Safety and efficacy of the SNAP 12 hour acetylcysteine regimen for the treatment of paracetamol overdose. EClinicalMedicine. 2019;11:11–17. doi: 10.1016/j.eclinm.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedda S., Laurent A., Conti F. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J Hepatol. 2003;39(5):765–772. doi: 10.1016/s0168-8278(03)00325-8. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson J.O., Ignarro L.J., Lundstrom I., Jynge P., Almen T. Calmangafodipir [Ca4Mn(DPDP)5], mangafodipir (MnDPDP) and MnPLED with special reference to their SOD mimetic and therapeutic properties. Drug Discov Today. 2015;20(4):411–421. doi: 10.1016/j.drudis.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Glimelius B., Manojlovic N., Pfeiffer P. Persistent prevention of oxaliplatin-induced peripheral neuropathy using calmangafodipir (PledOx((R))): a placebo-controlled randomised phase II study (PLIANT) Acta Oncol. 2018;57(3):393–402. doi: 10.1080/0284186X.2017.1398836. [DOI] [PubMed] [Google Scholar]
- 14.POP Trial Investigators Randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with the 12-h regimen of N-acetylcysteine for paracetamol overdose-the PP100-01 for Overdose of Paracetamol (POP) trial: study protocol for a randomised controlled trial. Trials. 2019;20(1):27. doi: 10.1186/s13063-018-3134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oosthuyzen W., Ten Berg P.W.L., Francis B. Sensitivity and specificity of microRNA-122 for liver disease in dogs. J Vet Intern Med. 2018;32(5):1637–1644. doi: 10.1111/jvim.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dear J.W., Clarke J.I., Francis B. Risk stratification after paracetamol overdose using mechanistic biomarkers: results from two prospective cohort studies. Lancet Gastroenterol Hepatol. 2018;3(2):104–113. doi: 10.1016/S2468-1253(17)30266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church R.J., Kullak-Ublick G.A., Aubrecht J. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology. 2019;69(2):760–773. doi: 10.1002/hep.29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoine D.J., Dear J.W., Starkey-Lewis P. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letter of Support for DILI Biomarkers EMA 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/09/WC500213479.pdf
- 20.Letter of Support for DILI Biomarkers FDA 2016. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm434382.htm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Agents ingested in overdose at the time of paracetamol overdose. Agents are grouped by drug class. Patients grouped by treatment allocation. NSAIDS = non-steroidal anti-inflammatory drugs. SSRIs = selective serotonin reuptake inhibitors.
Secondary safety outcomes. Patients are grouped by treatment allocation (CAM = calmangafodipir). Adverse event details listed per treatment group. Endpoints are described at each measurement time point after starting NAC. The absolute change from baseline to each timepoint is given with mean and standard deviation (SD), median and range. Electrocardiogram (ECG) findings were classed as normal, abnormal (not clinically significant - NCS) or abnormal (clinically significant - CS).
Secondary outcome. Alanine transaminase activity (ALT), international normalised ratio (INR) and paracetamol concentration for each of the allocated treatment groups. Data are presented as the number and percentage of treatment group reaching cut-off values and fold increases that were pre-defined in the study protocol.
Secondary outcome. Glutamate dehydrogenase (GLDH) for each of the allocated treatment groups. Data are presented as geometric mean and median as per study protocol. The change from baseline to 20 h after starting NAC is presented as the relative change. A value of 1 indicates no change. GSD = geometric standard deviation. LLoQ – lower limit of quantification. One sample was not measurable due to lipidaemia. 16 samples were below the LLoQ.
Paracetamol concentration versus time since overdose to blood collection. Sloped lines are an extension of the Rumack-Matthew nomogram corresponding to 100, 150, 200, 300 and 500 mg/L at 4 h post-ingestion (based on a first-order decay of blood paracetamol with a half-life of 4 h). The vertical line at 8 h separates “early” and “late” presenters. N = 6 except NAC alone (N = 5 due to one supra-therapeutic overdose) and NAC and 10umol/kg (N = 5 due to one staggered overdose).
The relative fold change for keratin-18 (K18), caspase cleaved K18 (ccK18) and microRNA-122 (miR-122) from baseline to 20 h after starting acetylcysteine treatment (NAC). Patients are grouped by the treatment allocation. K18 = white symbols. ccK18 = grey symbols. miR-122 = black symbols.