Abstract
The gastrointestinal mucosa is critical for maintaining the integrity and functions of the gut. Disruption of this barrier is a hallmark and a risk factor for many intestinal and chronic inflammatory diseases. Inflammatory bowel disease (IBD) and HIV infection are characterized by microbial translocation and systemic inflammation. Despite the clinical overlaps between HIV and IBD, significant differences exist such as the severity of gut damage and mechanisms of immune cell homeostasis. Studies have supported the role of metabolic activation of immune cells in promoting chronic inflammation in HIV and IBD. This inflammatory response persists in HIV+ persons even after long-term virologic suppression by antiretroviral therapy (ART). Here, we review gut dysfunction and microbiota changes during HIV infection and IBD, and discuss how this may induce metabolic reprogramming of monocytes, macrophages and T cells to impact disease outcomes. Drawing from parallels with IBD, we highlight how factors such as lipopolysaccharides, residual viral replication, and extracellular vesicles activate biochemical pathways that regulate immunometabolic processes essential for HIV persistence and non-AIDS metabolic comorbidities. This review highlights new mechanisms and support for the use of immunometabolic-based therapeutics towards HIV remission/cure, and treatment of metabolic diseases.
Keywords: Inflammatory bowel disease; Inflammation; HIV cure; Macrophage metabolism; Immunometabolism, microbiome
1. Introduction
Approximately 37 million people are currently living with HIV infection globally, with about 21.7 million receiving antiretroviral treatment (ART) [1]. The use of ART has greatly reduced the incidence of AIDS-related morbidity and mortality with most HIV-infected individuals having nearly normal life expectancy [2]. However, ART does not eradicate HIV [3]. People living with HIV, even after suppressive ART, experience high incidence of non-AIDS associated comorbidities, including cardiovascular disease (CVD), frailty, and osteoporosis, liver and kidney disease, and non-AIDS-associated cancers [2,[4], [5], [6], [7]]. Chronic immune activation and inflammation have been identified as the most common risk factor underlying these co-morbidities [[8], [9], [10]]. Chronic inflammation during suppressive ART is multi-factorial, with microbial translocation through the gut barrier being a significant contributor [7].
The gastrointestinal (GI) tract is the major site of HIV replication and persistence of the HIV reservoir [11,12], with early events following HIV infection resulting in rapid loss of GI mucosal integrity. These alterations lead to an increase in GI permeability and translocation of microbial products from the gut lumen across the damaged mucosa into the circulation leading to chronic systemic inflammation [7]. The significance of gut microbiota and gut integrity in HIV pathogenesis is underpinned by clinical trials showing that daily probiotic supplementation to ART-naïve HIV+ persons, decreased immune cell activation, lowered levels of serum inflammatory markers [13], and reduced microbial translocation [14]. The benefits were recapitulated in ART-suppressed HIV+ persons [15], highlighting the potential beneficial effects of some probiotic species in modulating GI disorders and impacting HIV disease progression. However, a lack of understanding of the underlying molecular and biochemical pathways mediating chronic immune activation and inflammation in HIV+ persons precludes the discovery of novel and more specific therapeutics to eradicate gut-resident HIV-reservoir cells, and prevent non-AIDS associated comorbidities.
The causes for the “leaky gut syndrome” are multifactorial and in this review, we will showcase how lessons learnt from HIV and inflammatory bowel diseases (IBD) can enable greater understanding of etiology and mechanisms of gut dysfunction in each condition. We will also highlight how IBD itself is a potentially serious non-modifiable risk factor for the development of non-AIDS co-morbidities.
1.1. Microbial dysbiosis “the Achilles heel” of “leaky gut” associated syndromes
The complex intestinal ecosystem is comprised of trillions of bacteria performing crucial homeostatic functions [16]. Several lines of evidence have implicated alterations in the intestinal microbiota (dysbiosis) to infectious diseases and metabolic disorders such as HIV infection, obesity, and CVD, elegantly reviewed by Godfrey and colleagues [7]. In the context of HIV, disease progression is strongly associated with changes in the enteric microbiota and systemic abnormalities, a concept described as a “two-way street” [17]. This vicious pathological cycle exacerbates HIV-associated immune activation and inflammation [18]. Based on observations regarding the beneficial effects of restoring gut microbial homeostasis and immune functions in metabolic disorders and HIV, deciphering the precise molecular mechanisms is paramount.
The composition of gut bacteria varies significantly between HIV+ ART-suppressed, and HIV uninfected persons [19]. However, there is no consensus about specific bacterial diversity at genus or species level [19]. In clinical studies, several factors could act as confounders, affecting the reliability of microbiome data including: sampling differences such as mucosal versus luminal, lack of standardization in sample collection and analysis, and biological effect of diet, medications and geographic location [19]. Additionally, ART regimens, MSM (men who have sex with men) versus heterosexual males [20], level of immune activation and CD4 T cell recovery status on ART [21], as well as the use of Truvada (emtricitabine, tenofovir disoproxil fumarate) as HIV pre-exposure prophylaxis (PreP) in HIV-negative persons [22] have profound effects on the gut microbiome. Notwithstanding, an increase in members of the genus Prevotella in HIV+ versus HIV-negative healthy controls has been reported [18,21,23]. At the biochemical level, enrichment of Lactobacillales in HIV+ persons may result in catabolism of tryptophan to indole-3-aldehyde by way of the tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase (IDO) [24]. This could create a vicious cycle linking dysbiosis with activation of the kynurenine/IDO pathway and pro-inflammatory cytokine production. It could further lead to a loss of Th17 cells from the gut mucosa, further compromising the integrity of the GI tract [25]. A similar increase in Proteobacteria [26] and Actinetobacteria and a decrease in Firmicutes has been reported in IBD, as well as an increased expression of IDO in intestinal biopsies [27]. Interestingly, microbial dysbiosis did not promote disease progression in simian immunodeficiency virus (SIV)-infected macaques [28], highlighting the importance of being aware of model-specific outcomes when trying to understand the mechanism of diseases in humans.
Immune cells themselves also regulate tryptophan biogenesis. In this regard, LPS-conditioned dendritic cells induce IDO isoforms that preferentially induce NF-κB inflammatory pathway, which may contribute to an immunosuppressive gut environment [29,30]. Similar immunosuppressive and tolerogenic response has been described in macrophages over-expressing IDO via Interleukin-32 (IL-32) and Toll-like receptor 9 (TLR9) stimulation [31,32].
1.2. Short chain fatty acid: pathways to targeted therapies
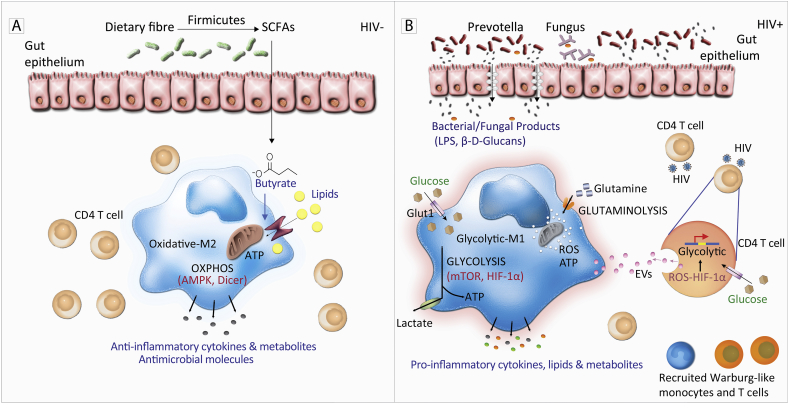
Dysbiosis is characterized by an increase in the number of pathogenic microbes relative to the useful commensal microflora [33]. These changes contribute to diminished levels of anti-inflammatory short chain fatty acids (SCFAs) such as butyrate. Such SCFAs are produced by fermentation of dietary fiber by commensal bacteria contributing to improving gut homeostasis and barrier integrity. SCFAs can restore gut barrier function [34], and may explain some of the protective effects of probiotics and prebiotics in several inflammatory conditions including HIV [[35], [36], [37]]. Seminal work by Arpaia et al. has demonstrated the role of microbial metabolites in restoring intestinal anti-inflammatory regulatory T cells (Treg cells) populations in mouse models [38]. Butyrate promoted gut-associated anti-inflammatory Tregs, while propionate led to their de novo production through histone deacetylase (HDAC) inhibition [38]. In IBD, SCFAs are significantly reduced and supplementation with Lactobacillus strains improved intestinal inflammation and gut barrier function [39]. Similarly, HIV+ persons on ART with increased gut Lactobacillales showed reduced microbial translocation and increased CD4 T cells [40]. It remains to be established how exactly gut microbiota and the associated metabolites mechanistically influence the intestinal barrier and the consequent inflammation in distinctive diseases including IBD and HIV. However, SCFAs like butyrate, can be transported into immune cells to fuel mitochondrial metabolism and ATP synthesis, in order to maintain an oxidative anti-inflammatory state. In the case of probiotic supplementation, butyrate may dampen inflammation by suppressing glycolysis in immune cells (discussed below), while promoting synthesis of antimicrobial molecules by macrophages (Fig. 1A), as previously reviewed [7,41].
Fig. 1.
Model showing potential inflammatory and immunometabolic consequences of gut barrier dysfunction in HIV infection. In HIV-negative persons the presence of Firmicutes in the gut enhances fermentation of dietary fiber to short chain fatty acids which have anti-inflammatory functions as well as promoting break down of glucose and fatty acid via oxidative metabolism (B). Damaged epithelial cells facilitate translocation of bacterial and fungal products across the intestinal lumen through the epithelial cells and into the blood stream. ROS and the inflammatory environment may cause low levels of HIV transcription and release of pro-glycolytic/inflammatory extracellular vesicles (EVs) by metabolically active CD4 T cells (B). The activation of monocytes/macrophages may increase the demand of glucose facilitated by increased Glucose Transporter 1 (Glut1) expression. High glycolytic metabolism by recruited monocytes and pro-inflammatory M1-like macrophages results in increased production of cytokines such as IL-6 and TNF which contribute to the chronic inflammation observed in HIV+ people. Figures designed using image stocks from nice-consultants.com.
1.3. Monocyte driven inflammation underlies HIV pathology and associated age-related comorbidities
During HIV infection, there is persistent low-level inflammation and immune activation that leads to disease progression and the risk of serious non-AIDS comorbidities [42]. Monocyte activation is associated with all-cause risk of mortality in HIV+ persons on ART. Several works have shown that HIV infection is associated with monocytes and macrophages exhibiting Warburg-like features [43]. This Warburg effect is characterized by increased glycolysis and lactate production in the presence of physiological concentrations of oxygen (aerobic glycolysis). It also corresponds to a situation where glycolytic enzymes and substrates regulate immune cell activation and inflammatory states, beyond their metabolic roles [44]. This may also represent the metabolic basis of immunosenescence and immune cell aging [45]. It could also contribute to the development of non-AIDS co-morbidities such as CVD, HIV-associated neurocognitive disorder (HAND), osteoporosis and frailty [6,[46], [47], [48], [49], [50], [51]]. This immune activation and inflammatory state is characterized by increased circulating levels of IL-6, soluble CD14 (sCD14) and sCD163 even after suppressive ART [10].
1.4. Microbial translocation in health and disease: Insights into gastrointestinal and systemic inflammation
Gut barrier dysfunction increases bacterial extracellular vesicles (EV)-associated LPS and other LPS products in IBD and HIV+ persons [52,53]. This scenario reveals a complex interplay of factors in both IBD and HIV that would potentiate and aggravate the pathogenesis of both diseases. Thus, HIV-mediated immune deficiency could compromise the host's ability to clear LPS in IBD, whereas pre-existing IBD could heighten inflammation and recruitment of HIV target cells, in addition to the other pleiotropic effects of microbial translocation in both settings.
Besides LPS products increased fungal colonization of the gut during HIV infection represents another significant contributor to inflammation in HIV. Plasma levels of the fungal polysaccharide (1 → 3)-β-D-glucan (β-DG) are elevated in long-term ART treated HIV+ persons and correlates with gut damage, bacterial translocation, immune activation, and inflammation [54]. Monocytes exposed to fungal β-glucans undergo metabolic reprogramming, leading to ‘trained immunity’ characterized by an enhanced proinflammatory status upon secondary stimulation [55]. Thus β-DG presents an important immunometabolic regulator in HIV and a potential therapeutic target to limit development of non-AIDS associated comorbidities [54].
1.5. Mechanisms of gut barrier breach
There is evidence that HIV infection breaches the gut barrier in a way similar to IBD. Indeed expression of junctional complex proteins, claudin 1, cadherin, and Zonula occludens protein 1 (ZO-1), in gut was significantly downregulated in gut biopsies from HIV+ persons with incomplete CD4 T cell recovery [56]. In these persons, increased infiltration of CD8 T cells, rapid depletion of CD4 T cells, oxidative stress and release of TNF, IL-2 and IL-4 was associated with GI damage and intestinal permeability through disruption of tight junctions [57,58] and enterocyte apoptosis [59]. It remains unclear how HIV itself may participate in gut barrier dysfunction and immune cell trafficking in inflamed gut submucosa. However, Kanmogne and colleagues reported that in the brain, exposure of epithelial cells to HIV envelope glycoprotein gp120 disrupted the tight junctions and enhanced monocyte transmigration across the blood-brain barrier [60]. The common ontogeny of epithelial cells despite their anatomical location could allow for a possible extrapolation of this phenomenon to mucosal tight junctions during HIV infection.
Whilst HIV and IBD share some similarities, differences exist such as the area of the intestine affected and the degree to which disruption is manifested. For example, Crohn's disease and ulcerative colitis have profound damage and ulceration. In contrast, studies in ART-naive HIV+ persons, with late-stage HIV infection showed only mild-to-moderate enteritis or colitis in the duodenum and jejunum. Further, CD4 T cell loss was more pronounced in the small (duodenum, jejunum, and ileum) versus large (colon) intestine of untreated HIV+ persons [61].
1.6. The role of Th17 cells in HIV infection and IBD
A balance between the effector and regulatory functions of Th17 and Tregs is important in maintaining gut barrier integrity and functions [62]. IL-17, secreted by Th17 cells increases the expression of tight junction proteins claudin-1 and claudin-2 essential for maintaining gut integrity and inhibiting bacterial translocation [63]. Conversely, separate studies showed that Th17 cells are severely depleted and functionally impaired during HIV infection thereby triggering mucosal barrier damage and microbial translocation [64]. This could be due to the fact that a significant proportion of Th17 cells express CCR5, a HIV co-receptor [65].
It should be noted that the depletion of Th17 cells is common in both HIV infection and IBD, although in the latter there is a differential upregulation of cytokine secretion (IL-17, IL-22) [66]. At a glance, Th17 effector functions appear to be more important in regulating HIV-mediated gut barrier dysfunction than in IBD with both diseases acting as independent predisposing or aggravating risk factors. Therefore, exploration of the role of Th17 dynamics in the context of HIV infection, IBD and gastrointestinal repair would yield interesting pathophysiological insights upon which novel therapies may be developed to improve gut functions.
1.7. Microbial effectors of “Leaky gut” pathogenesis: the role of LPS-mediated signaling on monocyte activation and systemic inflammation
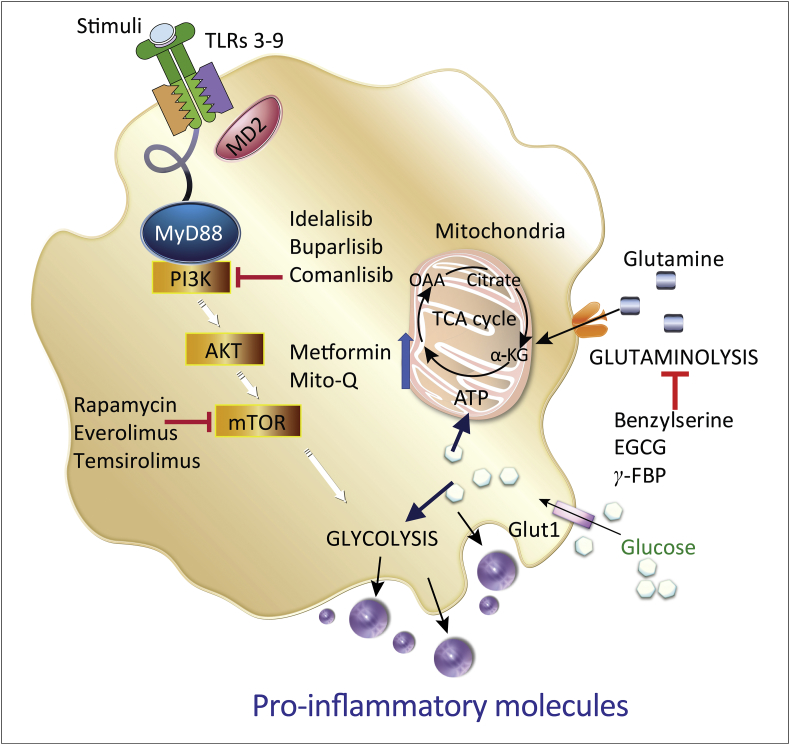
LPS is an integral component of the bacterial structure released upon bacterial lysis and death [67] and can activate monocytes and macrophages by binding to circulating plasma lipopolysaccharide binding protein (LBP), which initiates a metabolic signaling cascade to produce inflammatory molecules (Fig. 2).
Fig. 2.
Key mechanisms of monocyte/macrophage activation and potential therapeutic interventions. Activation of monocytes and macrophages by may be mediated by interactions with TLRs. TLR4 engagement for example induces activation of the PI3K/Akt/mTOR axis and increases glutamine metabolism promoting pro-inflammatory cytokines and toxic nitric oxide production. Also indicated are potential points to therapeutically limit inflammation by repurposing currently available metabolic drugs used to treat cancers. Figures designed using image stocks from nice-consultants.com.
Notably, gut resident macrophages can exhibit an anergic phenotype to inflammatory cues as they do not express CD14 co-receptor for LPS and may have impaired production of inflammatory cytokines. This phenotype is ideal for an environment with constant exposure to microbial flora as previously reviewed [68]. However, during gut barrier dysfunction as observed during HIV infection and IBD, this inflammatory landscape changes considerably due to the recruitment and accumulation of activated blood monocytes in the lamina propria [68] and as observed during HIV infection and IBD [61,69].
In IBD there is increased recruitment of blood monocytes to the gut, which is associated with an upregulation of monocyte-targeted chemokines (MCP-1, CCL2, CCL4) by endothelial cells. This leads to migration of monocytes to the inflamed intestinal tissue amplifying the inflammatory cascade. Furthermore, upregulation of various ligands including P-selectin glycoprotein ligand-1 (PSLG-1), P-selectin, CD34, and VCAM-1 has been observed, and could promote monocyte extravasation into the ileal mucosa [70,71]. Monocyte-derived macrophages are more reactive compared to their gut resident counterparts and have increased secretion of pro-inflammatory cytokines, such as TNF, IL-1 and IL-6, IL-23 [69,72]. Metabolically active monocytes such as those expressing high levels of glucose transporter 1 (Glut1) [73] may be preferentially recruited to the adipose tissues and gut of HIV+ persons creating a vicious inflammatory cycle [7] (Fig. 1 B).
Kamada et al. found a unique highly pro-inflammatory intestinal macrophage subset in Crohn's disease patients [69]. Despite the dogma that intestinal macrophages are devoid of CD14 receptor, this distinctive subset expresses both typical macrophage (CD14, CD33, CD68) and dendritic cell markers (CD205, CD209) [69]. These cells also secrete pro-inflammatory cytokines, such as IL-23, IL-6 and TNF. The increase in IL-23 shifts the balance in favor of IFN-γ secretion from lamina propria mononuclear cells (LPMCs) instead of IL-17, which further heightens abnormal macrophage differentiation and intestinal inflammation in individuals with Crohn's disease [69]. IL-23 has also been implicated as the central regulator for the expansion of Th17 cells expressing CD4 that secrete pro-inflammatory cytokines like IL-17α, IL-22, IFN-γ. The increase in Th17 cells in individuals with IBD is induced by IL-23, which is secreted by lamina propria macrophages [74,75]. In contrast to IBD, HIV is characterized by a pathological loss of Th17 cells and their full function is not achieved even with virologic suppression on ART.
The expression profile and phenotype of intestinal macrophages are similar in HIV infection and IBD, implying that both diseases could have overlapping pathophysiological changes (Table 1).
Table 1.
Comparison of immunopathological changes in HIV and inflammatory bowel disease.
| Marker | HIV | IBD |
|---|---|---|
| Disruption of tight junctions, Villous atrophy | YES [76,77] | YES [78,79] |
| Microbial translocation | ↑LBP, ↑LPS, ↓EndoCAb [[80], [81], [82]] | ↑LBP, ↑EndoCAb, ʘ LPS [83,84] |
| Inflammasome activation | YES [85,86] | YES [87,88] |
| Increased Monocyte/Macrophage infiltration in gut | YES [82,89,90] | YES [72,91,92] |
| Increase in CD38+ HLA-DR+ CD4+ T cells | YES [93,94] | YES [83] |
| Immunometabolic disturbances in T cells | ↑Glut1, ↑HIF-1α, ↑glycolysis in CD4 T cells ↓Mitochondrial respiration in T cells ↑Glycolysis in HIV-infected CD4 cells [[95], [96], [97], [98], [99]] |
No data |
| Immunometabolic disturbances in monocytes/macrophages | ↑Glut1 on monocytes [73] ↑Oxphos and glutaminolysis [100] |
No data |
| Increased secretion of inflammatory cytokines (IL-1, IL-6, TNF. IFN-γ) | YES [76] | Yes [61,69] |
| Th17/Tregs | ↓Th17 cells, ↓IL-17a [64,101] | ↑Th17 cells, IL-17a, IL-21, IL-22 ↓Tregs [[102], [103], [104]] |
| Alteration in the microbiome community | ↑Proteobacteria, Prevotella ↓Firmicutes, Bacteroides [18,105] | ↑Proteobacteria, Actinobacteria ↓Firmicutes [26,106] |
| Probiotics improve gut function | ↓ Microbial translocation, ↑CD4 + cells [40] | Improve gut barrier dysfunction and inflammation [39] |
YES indicates that the change is uniformly observed, ↑, ↓ indicates upregulation or downregulation of a particular factor only, ʘ indicates levels unchanged compared to control population.
In the context of HIV, Cassol and colleagues observed an increased secretion of IL-18, IFN-γ, CCL2, TNF receptor associated factor (TRAF6) and IL-12Rβ1 as well as an increase in CD14 macrophages in colons of HIV+ persons [61]. These data are similar to those from Kamada et al. in persons with Crohn's disease [69]. Similarly, an increased mucosal enrichment of macrophages was observed in the jejunal lamina propria of primary HIV+ patients compared to HIV-uninfected controls. This increased trafficking was associated with elevated levels of integrin β7 on monocytes, a gut-homing molecule that supports monocyte gut infiltration [90].
1.8. Metabolic reprogramming regulates monocyte/macrophage activation and inflammation
1.8.1. Regulators of metabolic reprogramming
All immune cells utilize glucose in order to produce energy to mount an effective immune response against pathogens [[107], [108], [109]]. Glucose is metabolized via two major pathways; oxidative phosphorylation (oxphos), which takes place in the mitochondria to produce maximal amount of ATP, and glycolysis, which occurs in the cytosol and produces less ATP. Aerobic glycolysis produces precursors of protein, lipid, and nucleotide synthesis that are needed by activated and rapidly proliferating cells [110,111].
The key event that marks metabolic reprogramming of LPS-stimulated macrophages is the overexpression of Glut1, the major glucose transporter that supports an increase in glycolytic flux [112]. Freemerman and others have shown that macrophages with increased expression of Glut1 secrete higher levels of TNF, IL-6, and CXCL2 representing a hyperinflammatory state [113]. LPS-mediated signaling in macrophages initiates a metabolic switch from oxphos to glycolysis, similar to the ‘Warburg effect’ [114], which has been shown to be dependent on NAD+ salvage pathway to maintain NAD+ pools [115].
Like activated monocytes, macrophages of the M1-like pro-inflammatory phenotype have a glycolytic signature, while M2-like macrophages, responsible for producing anti-inflammatory cytokines, rely mostly on oxidative metabolism and beta oxidation of fatty acids to generate ATP [107,116]. Blocking oxidative metabolism has been successful at abrogating the M2-like phenotype and increasing the number of M1-like macrophages in bone marrow derived macrophages and murine models [116,117]. Since the metabolic status of macrophages intricately connects their inflammatory and developmental status, it is conceivable that new macrophage nomenclature may be considered based on their biochemical imprints to better distinguish their functional phenotypes.
Glycolytic influx is also essential for maintaining morphological changes in macrophages that are required for phagocytosis upon LPS stimulation. Venter et al. have shown that a low level of extracellular glucose was required for remodeling of cytoskeleton in M1-like macrophages [118]. These studies suggest that metabolic reprogramming of macrophages is a key event that has both metabolic and non-metabolic consequences on macrophage function.
1.8.2. Metabolic reprogramming in IBD and HIV infection
In a murine model of IBD, a Glut1 conditional knockout in CD4 T cells led to a reduction in disease progression with a less pro-inflammatory cytokine profile. Since Glut1 deletion caused failure of immune cells to increase glucose metabolism, this could have led to abrogation of the inflammatory response [119]. Recent studies reviewed by Venegas et al. have found that metabolism of butyrate, a SCFA, is impaired in inflamed mucosa of IBD patients [120]. Defects in butyrate oxidation could be related to multiple mechanisms, with suggestions that upregulation of Glut1 causes a switch from butyrate to glucose oxidation. This leads to impairment of cellular homeostasis and suppression of the anti-growth action of butyrate. Such a scenario would cause damage to colonocytes and worsen the pathology of IBD by supporting glycolysis and increasing inflammation [121].
The activation of monocytes during HIV infection is accompanied by increased aerobic glycolysis, mediated by increased cell surface expression of Glut1, elevated glucose uptake, and enhanced lactate production even in the presence of sufficient oxygen. High glycolytic metabolism by monocytes is essential for the production of pro-inflammatory cytokines such as IL-6 and TNF [122,123]. Therefore, expression of metabolic regulators such as Glut1 on monocytes may be explored as a potential marker of immune activation and inflammation in people with chronic inflammatory diseases such as IBD and HIV. This is supported by studies where Glut1 is significantly upregulated in both ART-treated and untreated HIV+ persons and is associated with CVD risk [73,124].
1.8.3. LPS reprograms monocyte and macrophage metabolism from oxphos towards glycolysis
Higher levels of L-lactate secretion are observed in activated monocytes in vitro by LPS and IFN-γ. In activated monocytes, the amount of L-lactate secreted due to glycolytic metabolism is higher in the inflammatory intermediate monocyte subset than in other monocyte subpopulations, suggesting that intermediate monocyte inflammatory responses are driven by glycolytic metabolism [122]. Therefore, we propose a model in which gut microbial translocation results in increased level of bacterial products such as LPS in the blood. In turn, LPS induces activation of monocytes and macrophages mediated by increases in Glut1 expression and glycolysis, thus promoting pro-inflammatory cytokine synthesis critical for the development of age-associated comorbidities in HIV+ persons [122,125]. Important in this model is the imbalance of good and bad bacteria such as reduced Firmicutes, essential for the production of anti-inflammatory SCFAs, which helps to maintain gut integrity (Fig. 1A).
Modulation of glucose, lipid and glutamine metabolism are emerging as promising therapeutic approaches to alleviate the impact of inflammatory diseases, such as HIV and IBD [123,126,127]. The increase in metabolism is a characteristic feature of monocytes during a rapid activation period that is regulated at least in part by the PI3K/Akt/mTOR axis and glutamine-metabolizing enzymes such as glutaminase. Therefore, normalization of overactive metabolic activity of monocytes and macrophages by therapeutic targeting of these pathways presents potential to treat existing inflammatory conditions or develop prophylactic treatments for patients at higher risk of leaky gut syndrome and gut inflammation (Fig. 2).
Of note, not all data support this hypothesis. One study found human peripheral blood monocyte-derived macrophages activated with LPS did not undergo metabolic reprogramming towards glycolysis, but instead exhibited an oxidative phenotype. By contrast, mouse bone marrow-derived macrophages challenged with LPS showed increased glycolysis and reduced oxidative phosphorylation. It may be argued that human cells and murine cells have significant biological differences [128]. Regardless, it emphasizes that caution should be taken when interpreting metabolic data from animal models, or different cell types due to intrinsic physiological differences.
1.9. Other factors driving metabolic reprogramming and inflammation in immune cells in HIV+ persons
Besides LPS, other factors may induce monocyte and macrophage activation and metabolic reprogramming. HIV proteins such as viral-protein-r (vpr) produced during residual replication, may induce metabolic switch in HIV-1 infected macrophages from oxphos towards glycolysis through hypoxia-inducible factor 1 (HIF-1α) and peroxisome proliferator-activated receptor gamma (PPARγ)-dependent mechanisms. These metabolic effects may be manifested by pronounced glucose uptake and elevation of critical glycolytic enzymes in infected macrophages [129] and dysregulated systemic lipid metabolism [130]. Metabolic activation of macrophages by HIV may cause accumulation of α-ketoglutarate and glutamine, suggesting a compensatory mechanism to fuel the tricarboxylic acid cycle (TCA) cycle and ATP production via oxphos [108].
Type 1 and II Interferons within the microenvironment may impact activation and glycolysis in macrophages [131,132]. Indeed, IFN-γ through activation of the JAK (Janus tyrosine kinase)-STAT-1 (Signal Transducer and Activator of Transcription 1) induces a robust glycolytic response while reducing oxphos in M1-like pro-inflammatory macrophages [132].
Apart from soluble factors, extracellular vesicles (EVs) have been shown to be potential key players in HIV disease pathogenesis. HIV-infected CD4 T cells release pro-glycolytic EVs, which induce cytokine secretion from bystander lymphocytes and macrophages [97]. Thus the coordinated actions of immune cell metabolic reprogramming and EVs may participate in inflammatory responses that underlie immunometabolic-related sequels in HIV infection [97].
In ART-treated HIV+ persons, the treatment regimen itself may also affect immune cellular metabolism and function due to mitochondrial impairment. In fact CD4 T cells exposed to integrase inhibitors dolutegravir or elvitegravir exhibit reduced functions [96]. Finally, it has been shown that in HIV+ persons opportunistic pathogens, such as cytomegalovirus (CMV), can disrupt epithelial junctions, which significantly impairs gut barrier and potentiate chronic inflammation [133].
1.10. Immunometabolism offers promising opportunities towards HIV remission and cure
Compelling evidence shows that immune cell metabolism underlies mechanisms essential for HIV persistence and inflammation in ART-treated HIV+ persons. A specific plasma metabolic/metabolomic signature is associated with the natural control of HIV infection. Compared to HIV persistent controllers, patients who lost their ability to naturally control HIV without ART have a plasma metabolomic profile enriched with glycolytic intermediates implying disturbances in oxidative metabolism and mitochondrial functions [134]. Systemic lipid dysregulation is also associated with HIV-specific T-cell responses that are important for viral control in untreated HIV infection [134].
Similar immune cellular metabolic changes are associated with CD4 T cell activation, HIV replication and HIV reservoir seeding [97,99,135,136,142]. Indeed, ROS-mediated HIF1-α signaling induces metabolic reprogramming and residual HIV replication in HIV-infected CD4 T cells. Further, extracellular vesicle release from metabolically-stressed CD4 T cells may release their cargo to uninfected recipient macrophages and T cells to induce a glycolytic phenotype that increases HIV infectivity [97,99]. Such immune cell metabolic dysregulation is also linked to T cell exhaustion and reduced functionality [137].
Promising strategies to rewire or re-polarize cells towards their normal metabolic status include use of mTOR inhibitors (e.g rapamycin clinical trialNCT02440789) [123], and AMPK activators (e.g metformin) [138] to regain immune functions [139]. mTOR inhibitors may also suppress reactivation of HIV, a strategy called “lock and block” [140,141] or reduce proliferation of HIV reservoir cells to “starve the reservoir” [123]. Other emerging metabolic targets include Glutaminase (GLS1 and GLS2) that catalyze the conversion of glutamine to glutamate, and glutamate dehydrogenase that converts glutamate to α-ketoglutarate [108,142]. This anaplerotic reaction drives production of mitochondrial ATP as a compensatory energy-producing pathway in glycolytic T cells and macrophages. Indeed, glutamine/glutamate represents a major energy source for surviving HIV-infected macrophages, and inhibiting glutaminase activity with bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) or benzylserine caused death of these latently infected cells [100].
Such interventions may also be adaptable to repolarize pro-inflammatory glycolytic-M1 macrophages towards an anti- oxidative M2 phenotype as promising therapies to treat and prevent metabolic-mediated non-AIDS co-morbidities.
2. Conclusion
Here we bring together evidence to support a model by which increased intestinal permeability and microbial translocation instigate a peripheral pro-glycolytic inflammatory environment in both HIV and IBD. These diseases share several commonalities, of which disruption of the intestinal tight junction, inflammation and immune cellular metabolic reprogramming are hallmarks. However, while these similarities exist, there are unique differences in terms of the genera and species of the microbial population, and mechanisms of immune cell homeostasis.in the gut.
Compromised gut barrier and metabolic remodeling of immune cells may also play a central role in the pathophysiology of obesity, cancer and aging diseases related to, or independent of HIV infection [6,7,43]. Beyond implication for HIV cure [100,125,126] and treating non-AIDS comorbidities [7,73,123], the emergence of immunometabolism provides genuine opportunities for novel biomarker discoveries, and therapeutic approaches to manage inflammatory, and metabolic-mediated non-communicable diseases.
Outstanding questions
There are significant gaps in our knowledge on how fundamental biochemical processes in gut endothelial and immune cells are affected in HIV infection and how this influences reservoir persistence in the gut. To bridge this knowledge gap, it is important to decipher whether gut barrier dysfunction and its inflammatory consequences in HIV infection are the results of perturbed cellular mechanisms or whether altered processes are simply the manifestation of infection. How does pre-existence of IBD or HIV affect the risk of acquisition of the other and clinical progression? A better fundamental understanding of these aspects will improve diagnosis and long-term management of patients.
Search strategy and selection criteria
Content for this review were obtained through PubMed search and Google Scholar using the search terms “HIV leaky gut” “IBD leaky gut” “macrophage metabolism” “monocyte metabolism” “Immunometabolism IBD” “HIV inflammation” “IBD inflammation” “HIV immunometabolism” “Metabolism HIV cure”. To obtain up-to-date scientific evidence we placed preferences for articles published between 2014 and 2019, except where seminal articles were relevant.
Authors' contribution
C.S.P. conceptualized the review, organized, wrote the manuscripts, formulated models, and designed the images. J.A, T.H, R.P, and G.W.M wrote the manuscript. D.S and S.M.C provided critical analysis, provided content and edited the manuscript. M.A-M edited the manuscript and provided critical insights.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
CSP is funded by the Australian Centre for HIV and Hepatitis Virology Research (ACH2) and receives unrestricted funding from Merck Sharp & Dohme. The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program received by the Burnet Institute. The authors also thank nice-consultants.com for expertise assistance on graphic design of figures. C.S.P is grateful to Dr Darren Lockie who provided donations to support his research lab.
References
- 1.Feyissa G.T., Lockwood C., Woldie M., Munn Z. Reducing HIV-related stigma and discrimination in healthcare settings: a systematic review of quantitative evidence. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legarth R.A., Ahlstrom M.G., Kronborg G., Larsen C.S., Pedersen C., Pedersen G. Long-term mortality in HIV-infected individuals 50 years or older: a nationwide, population-based cohort study. J Acquir Immune Defic Syndr. 2016;71(2):213–218. doi: 10.1097/QAI.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 3.Deeks S.G., Lewin S.R., Ross A.L., Ananworanich J., Benkirane M., Cannon P. International AIDS society global scientific strategy: towards an HIV cure 2016. Nat Med. 2016;22(8):839–850. doi: 10.1038/nm.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hearps A.C., Angelovich T.A., Jaworowski A., Mills J., Landay A.L., Crowe S.M. HIV infection and aging of the innate immune system. Sex Health. 2011;8(4):453–464. doi: 10.1071/SH11028. [DOI] [PubMed] [Google Scholar]
- 5.Yeoh H.L., Cheng A., Palmer C., Crowe S.M., Hoy J.F. Frailty in men living with HIV: a cross-sectional comparison of three frailty instruments. Antivir Ther. 2018;23(2):117–127. doi: 10.3851/IMP3185. [DOI] [PubMed] [Google Scholar]
- 6.Yeoh H.L., Cheng A.C., Cherry C.L., Weir J.M., Meikle P.J., Hoy J.F. Immunometabolic and Lipidomic markers associated with the frailty index and quality of life in aging HIV+ men on antiretroviral therapy. EBioMedicine. 2017;22:112–121. doi: 10.1016/j.ebiom.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godfrey C., Bremer A., Alba D., Apovian C., Koethe J.R., Koliwad S. Obesity and fat metabolism in HIV-infected individuals: Immunopathogenic mechanisms and clinical implications. J Infect Dis. 2019;220(3):420–431. doi: 10.1093/infdis/jiz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges A.H., O'Connor J.L., Phillips A.N., Neaton J.D., Grund B., Neuhaus J. Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis. 2016;214(3):408–416. doi: 10.1093/infdis/jiw173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grund B., Baker J.V., Deeks S.G., Wolfson J., Wentworth D., Cozzi-Lepri A. Relevance of Interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anzinger J.J., Butterfield T.R., Angelovich T.A., Crowe S.M., Palmer C.S. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res. 2014;2014:569819. doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoder A.C., Guo K., Dillon S.M., Phang T., Lee E.J., Harper M.S. The transcriptome of HIV-1 infected intestinal CD4+ T cells exposed to enteric bacteria. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong J.K., Yukl S.A. Tissue reservoirs of HIV. Curr Opin HIV AIDS. 2016;11(4):362–370. doi: 10.1097/COH.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gori A., Rizzardini G., Van't Land B., Amor K.B., van Schaik J., Torti C. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the "COPA" pilot randomized trial. Mucosal Immunol. 2011;4(5):554–563. doi: 10.1038/mi.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villar-Garcia J., Hernandez J.J., Guerri-Fernandez R., Gonzalez A., Lerma E., Guelar A. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. 2015;68(3):256–263. doi: 10.1097/QAI.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 15.d'Ettorre G., Ceccarelli G., Giustini N., Serafino S., Calantone N., De Girolamo G. Probiotics reduce inflammation in antiretroviral treated, HIV-infected individuals: results of the "Probio-HIV" clinical trial. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon S.M., Frank D.N., Wilson C.C. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS. 2016;30(18):2737–2751. doi: 10.1097/QAD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon S.M., Lee E.J., Kotter C.V., Austin G.L., Gianella S., Siewe B. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2016;9(1):24–37. doi: 10.1038/mi.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gootenberg D.B., Paer J.M., Luevano J.M., Kwon D.S. HIV-associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis. 2017;30(1):31–43. doi: 10.1097/QCO.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong A.J.S., Shaffer M., Nusbacher N.M., Griesmer C., Fiorillo S., Schneider J.M. An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome. 2018;6(1):198. doi: 10.1186/s40168-018-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu W., Feng Y., Jing F., Han Y., Lyu N., Liu F. Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front Microbiol. 2018;9:1451. doi: 10.3389/fmicb.2018.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dube M.P., Park S.Y., Ross H., Love T.M.T., Morris S.R., Lee H.Y. Daily HIV pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate-emtricitabine reduced Streptococcus and increased Erysipelotrichaceae in rectal microbiota. Sci Rep. 2018;8(1):15212. doi: 10.1038/s41598-018-33524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutlu E.A., Keshavarzian A., Losurdo J., Swanson G., Siewe B., Forsyth C. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2) doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyboh K., Jenabian M.A., Mehraj V., Routy J.P. HIV and the gut microbiota, partners in crime: breaking the vicious cycle to unearth new therapeutic targets. J Immunol Res. 2015;2015:614127. doi: 10.1155/2015/614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vujkovic-Cvijin I., Swainson L.A., Chu S.N., Ortiz A.M., Santee C.A., Petriello A. Gut-resident Lactobacillus abundance associates with IDO1 inhibition and Th17 dynamics in SIV-infected macaques. Cell Rep. 2015;13(8):1589–1597. doi: 10.1016/j.celrep.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagalingam N.A., Lynch S.V. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2012;18(5):968–984. doi: 10.1002/ibd.21866. [DOI] [PubMed] [Google Scholar]
- 27.Wolf A.M., Wolf D., Rumpold H., Moschen A.R., Kaser A., Obrist P. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113(1):47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz A.M., Flynn J.K., DiNapoli S.R., Vujkovic-Cvijin I., Starke C.E., Lai S.H. Experimental microbial dysbiosis does not promote disease progression in SIV-infected macaques. Nat Med. 2018;24(9):1313–1316. doi: 10.1038/s41591-018-0132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q., Harden J.L., Anderson C.D., Egilmez N.K. Tolerogenic phenotype of IFN-gamma-induced IDO+ dendritic cells is maintained via an autocrine IDO-kynurenine/AhR-IDO loop. J Immunol. 2016;197(3):962–970. doi: 10.4049/jimmunol.1502615. [DOI] [PubMed] [Google Scholar]
- 30.Salazar F., Awuah D., Negm O.H., Shakib F., Ghaemmaghami A.M. The role of indoleamine 2,3-dioxygenase-aryl hydrocarbon receptor pathway in the TLR4-induced tolerogenic phenotype in human DCs. Sci Rep. 2017;7:43337. doi: 10.1038/srep43337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan H., Dong M., Liu X., Shen Q., He D., Huang X. Multiple myeloma cell-derived IL-32gamma increases the immunosuppressive function of macrophages by promoting indoleamine 2,3-dioxygenase (IDO) expression. Cancer Lett. 2019;446:38–48. doi: 10.1016/j.canlet.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Nicoli F., Paul S., Appay V. Harnessing the induction of CD8(+) T-cell responses through metabolic regulation by pathogen-recognition-receptor triggering in antigen presenting cells. Front Immunol. 2018;9:2372. doi: 10.3389/fimmu.2018.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong J.M., de Souza R., Kendall C.W., Emam A., Jenkins D.J. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Hu G.X., Chen G.R., Xu H., Ge R.S., Lin J. Activation of the AMP activated protein kinase by short-chain fatty acids is the main mechanism underlying the beneficial effect of a high fiber diet on the metabolic syndrome. Med Hypotheses. 2010;74(1):123–126. doi: 10.1016/j.mehy.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 35.D'Angelo C., Reale M., Costantini E. Microbiota and probiotics in health and HIV Infection. Nutrients. 2017;9(6) doi: 10.3390/nu9060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.d'Ettorre G., Rossi G., Scagnolari C., Andreotti M., Giustini N., Serafino S. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis. 2017;5(3):244–260. doi: 10.1002/iid3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim C.J., Walmsley S.L., Raboud J.M., Kovacs C., Coburn B., Rousseau R. Can probiotics reduce inflammation and enhance gut immune health in people living with HIV: study designs for the probiotic Visbiome for inflammation and translocation (PROOV IT) pilot trials. HIV Clin Trials. 2016;17(4):147–157. doi: 10.1080/15284336.2016.1184827. [DOI] [PubMed] [Google Scholar]
- 38.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., De Roos P. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Santiago J., Gianella S., Massanella M., Spina C.A., Karris M.Y., Var S.R. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27(12):1921–1931. doi: 10.1097/qad.0b013e3283611816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caputa G., Castoldi A., Pearce E.J. Metabolic adaptations of tissue-resident immune cells. Nat Immunol. 2019;20(7):793–801. doi: 10.1038/s41590-019-0407-0. [DOI] [PubMed] [Google Scholar]
- 42.Neuhaus J., Jacobs D.R., Jr., Baker J.V., Calmy A., Duprez D., La Rosa A. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aounallah M., Dagenais-Lussier X., El-Far M., Mehraj V., Jenabian M.A., Routy J.P. Current topics in HIV pathogenesis, part 2: inflammation drives a Warburg-like effect on the metabolism of HIV-infected subjects. Cytokine Growth Factor Rev. 2016;28:1–10. doi: 10.1016/j.cytogfr.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Palmer C.S., Henstridge D.C., Yu D., Singh A., Balderson B., Duette G. Emerging role and characterization of Immunometabolism: relevance to HIV pathogenesis, serious non-AIDS events, and a cure. J Immunol. 2016;196(11):4437–4444. doi: 10.4049/jimmunol.1600120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus R.M., Finlay D.K. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem. 2016;291(1):1–10. doi: 10.1074/jbc.R115.693903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anzinger J.J., Butterfield T.R., Angelovich T.A., Crowe S.M., Palmer C.S. Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res. 2014;2014 doi: 10.1155/2014/569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swartz T.H., Dubyak G.R., Chen B.K. Purinergic receptors: key mediators of HIV-1 infection and inflammation. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zungsontiporn N., Tello R.R., Zhang G., Mitchell B.I., Budoff M., Kallianpur K.J. Non-classical monocytes and monocyte chemoattractant Protein-1 (MCP-1) correlate with coronary artery calcium progression in chronically HIV-1 infected adults on stable antiretroviral therapy. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shan Z., Clish C.B., Hua S., Scott J.M., Hanna D.B., Burk R.D. Gut microbial-related choline metabolite trimethylamine-N-oxide is associated with progression of carotid artery atherosclerosis in HIV infection. J Infect Dis. 2018;218(9):1474–1479. doi: 10.1093/infdis/jiy356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukui S.M., Piggott D.A., Erlandson K.M. Inflammation strikes again: frailty and HIV. Curr HIV/AIDS Rep. 2018;15(1):20–29. doi: 10.1007/s11904-018-0372-5. [DOI] [PubMed] [Google Scholar]
- 51.Angelidou K., Hunt P.W., Landay A.L., Wilson C.C., Rodriguez B., Deeks S.G. Changes in inflammation but not in T-cell activation precede non-AIDS-defining events in a case-control study of patients on long-term antiretroviral therapy. J Infect Dis. 2018;218(2):239–248. doi: 10.1093/infdis/jix666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tulkens J., Vergauwen G., Van Deun J., Geeurickx E., Dhondt B., Lippens L. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2018 Dec 5 doi: 10.1136/gutjnl-2018-317726. (pii: gutjnl-2018-317726; Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramendra R., Isnard S., Mehraj V., Chen J., Zhang Y., Finkelman M. Circulating LPS and (1-->3)-beta-D-Glucan: a Folie a Deux contributing to HIV-associated immune activation. Front Immunol. 2019;10:465. doi: 10.3389/fimmu.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehraj V., Ramendra R., Isnard S., Dupuy F.P., Ponte R., Chen J. Circulating (1-->3)-beta-D-Glucan is associated with immune activation during HIV infection. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez-Andres J., Arts R.J.W., Ter Horst R., Gresnigt M.S., Smeekens S.P., Ratter J.M. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog. 2017;13(9) doi: 10.1371/journal.ppat.1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tincati C., Merlini E., Braidotti P., Ancona G., Savi F., Tosi D. Impaired gut junctional complexes feature late-treated individuals with suboptimal CD4+ T-cell recovery upon virologically suppressive combination antiretroviral therapy. AIDS. 2016;30(7):991–1003. doi: 10.1097/QAD.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 57.Buccigrossi V., Laudiero G., Nicastro E., Miele E., Esposito F., Guarino A. The HIV-1 transactivator factor (tat) induces enterocyte apoptosis through a redox-mediated mechanism. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenchley J.M., Douek D.C. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1(1):23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maingat F., Halloran B., Acharjee S., van Marle G., Church D., Gill M.J. Inflammation and epithelial cell injury in AIDS enteropathy: involvement of endoplasmic reticulum stress. FASEB J. 2011;25(7):2211–2220. doi: 10.1096/fj.10-175992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanmogne G.D., Schall K., Leibhart J., Knipe B., Gendelman H.E., Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassol E., Rossouw T., Malfeld S., Mahasha P., Slavik T., Seebregts C. CD14(+) macrophages that accumulate in the colon of African AIDS patients express pro-inflammatory cytokines and are responsive to lipopolysaccharide. BMC Infect Dis. 2015;15:430. doi: 10.1186/s12879-015-1176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo A., Leach S.T., Barres R., Hesson L.B., Grimm M.C., Simar D. The microbiota and epigenetic regulation of T helper 17/regulatory T cells: in search of a balanced immune system. Front Immunol. 2017;8:417. doi: 10.3389/fimmu.2017.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinugasa T., Sakaguchi T., Gu X., Reinecker H.C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118(6):1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim C.J., McKinnon L.R., Kovacs C., Kandel G., Huibner S., Chege D. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J Immunol. 2013;191(5):2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 65.El Hed A., Khaitan A., Kozhaya L., Manel N., Daskalakis D., Borkowsky W. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J Infect Dis. 2010;201(6):843–854. doi: 10.1086/651021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Owaga E., Hsieh R.H., Mugendi B., Masuku S., Shih C.K., Chang J.S. Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. Int J Mol Sci. 2015;16(9):20841–20858. doi: 10.3390/ijms160920841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X., Quinn P.J. Springer Science & Business Media; 2010. Endotoxins: structure, function and recognition. [Google Scholar]
- 68.Smith P.D., Smythies L.E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4(1):31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118(6):2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burgio V.L., Fais S., Boirivant M., Perrone A., Pallone F. Peripheral monocyte and naive T-cell recruitment and activation in Crohn's disease. Gastroenterology. 1995;109(4):1029–1038. doi: 10.1016/0016-5085(95)90560-x. [DOI] [PubMed] [Google Scholar]
- 71.Inoue T., Tsuzuki Y., Matsuzaki K., Matsunaga H., Miyazaki J., Hokari R. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol. 2005;77(3):287–295. doi: 10.1189/jlb.0204104. [DOI] [PubMed] [Google Scholar]
- 72.Zareie M., Singh P.K., Irvine E.J., Sherman P.M., McKay D.M., Perdue M.H. Monocyte/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn's disease. Am J Pathol. 2001;158(3):1101–1109. doi: 10.1016/S0002-9440(10)64057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer C.S., Anzinger J.J., Zhou J., Gouillou M., Landay A., Jaworowski A. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol. 2014;193(11):5595–5603. doi: 10.4049/jimmunol.1303092. [DOI] [PubMed] [Google Scholar]
- 74.Sarra M., Pallone F., Macdonald T.T., Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16(10):1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 75.Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Epple H.J., Schneider T., Troeger H., Kunkel D., Allers K., Moos V. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58(2):220–227. doi: 10.1136/gut.2008.150425. [DOI] [PubMed] [Google Scholar]
- 77.Quigley M.F., Abel K., Zuber B., Miller C.J., Sandberg J.K., Shacklett B.L. Perforin expression in the gastrointestinal mucosa is limited to acute simian immunodeficiency virus infection. J Virol. 2006;80(6):3083–3087. doi: 10.1128/JVI.80.6.3083-3087.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coskun M. Intestinal epithelium in inflammatory bowel disease. Front Med. 2014;1:24. doi: 10.3389/fmed.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edelblum K.L., Turner J.R. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9(6):715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ancuta P., Kamat A., Kunstman K.J., Kim E.Y., Autissier P., Wurcel A. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6) doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brenchley J.M., Price D.A., Schacker T.W., Asher T.E., Silvestri G., Rao S. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 82.Wallet M.A., Rodriguez C.A., Yin L., Saporta S., Chinratanapisit S., Hou W. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 2010;24(9):1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Funderburg N.T., Stubblefield Park S.R., Sung H.C., Hardy G., Clagett B., Ignatz-Hoover J. Circulating CD4(+) and CD8(+) T cells are activated in inflammatory bowel disease and are associated with plasma markers of inflammation. Immunology. 2013;140(1):87–97. doi: 10.1111/imm.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasternak B.A., D'Mello S., Jurickova I.I., Han X., Willson T., Flick L. Lipopolysaccharide exposure is linked to activation of the acute phase response and growth failure in pediatric Crohn's disease and murine colitis. Inflamm Bowel Dis. 2010;16(5):856–869. doi: 10.1002/ibd.21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chattergoon M.A., Latanich R., Quinn J., Winter M.E., Buckheit R.W., 3rd, Blankson J.N. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernandez J.C., Latz E., Urcuqui-Inchima S. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology. 2014;57(1):36–42. doi: 10.1159/000353902. [DOI] [PubMed] [Google Scholar]
- 87.Liu L., Dong Y., Ye M., Jin S., Yang J., Joosse M.E. The pathogenic role of NLRP3 Inflammasome activation in inflammatory bowel diseases of both mice and humans. J Crohns Colitis. 2017;11(6):737–750. doi: 10.1093/ecco-jcc/jjw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang J., Fu S., Sun S., Li Z., Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014;7(5):1139–1150. doi: 10.1038/mi.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allers K., Fehr M., Conrad K., Epple H.J., Schurmann D., Geelhaar-Karsch A. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis. 2014;209(5):739–748. doi: 10.1093/infdis/jit547. [DOI] [PubMed] [Google Scholar]
- 90.Sankaran S., George M.D., Reay E., Guadalupe M., Flamm J., Prindiville T. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82(1):538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reinecker H.C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R.P. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rugtveit J., Brandtzaeg P., Halstensen T.S., Fausa O., Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35(5):669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.d'Ettorre G., Paiardini M., Zaffiri L., Andreotti M., Ceccarelli G., Rizza C. HIV persistence in the gut mucosa of HIV-infected subjects undergoing antiretroviral therapy correlates with immune activation and increased levels of LPS. Curr HIV Res. 2011;9(3):148–153. doi: 10.2174/157016211795945296. [DOI] [PubMed] [Google Scholar]
- 94.Jiang W., Lederman M.M., Hunt P., Sieg S.F., Haley K., Rodriguez B. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palmer C.S., Ostrowski M., Gouillou M., Tsai L., Yu D., Zhou J. Increased glucose metabolic activity is associated with CD4+ T-cell activation and depletion during chronic HIV infection. AIDS. 2014;28(3):297–309. doi: 10.1097/QAD.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korencak M., Byrne M., Richter E., Schultz B.T., Juszczak P., Ake J.A. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duette G., Pereyra Gerber P., Rubione J., Perez P.S., Landay A.L., Crowe S.M. Induction of HIF-1alpha by HIV-1 Infection in CD4(+) T Cells Promotes Viral Replication and Drives Extracellular Vesicle-Mediated Inflammation. MBio. 2018;9(5) doi: 10.1128/mBio.00757-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hegedus A., Kavanagh Williamson M., Huthoff H. HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology. 2014;11:98. doi: 10.1186/s12977-014-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer C.S., Duette G.A., Wagner M.C.E., Henstridge D.C., Saleh S., Pereira C. Metabolically active CD4+ T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett. 2017;591(20):3319–3332. doi: 10.1002/1873-3468.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castellano P., Prevedel L., Valdebenito S., Eugenin E.A. HIV infection and latency induce a unique metabolic signature in human macrophages. Sci Rep. 2019;9(1):3941. doi: 10.1038/s41598-019-39898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raffatellu M., Santos R.L., Verhoeven D.E., George M.D., Wilson R.P., Winter S.E. Simian immunodeficiency virus–induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14(4):421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu X.R., Liu C.Q., Feng B.S., Liu Z.J. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014;20(12):3255–3264. doi: 10.3748/wjg.v20.i12.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geremia A., Biancheri P., Allan P., Corazza G.R., Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13(1):3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 104.Wolk K., Witte E., Hoffmann U., Doecke W.D., Endesfelder S., Asadullah K. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn's disease. J Immunol. 2007;178(9):5973–5981. doi: 10.4049/jimmunol.178.9.5973. [DOI] [PubMed] [Google Scholar]
- 105.Dillon S.M., Lee E.J., Kotter C.V., Austin G.L., Dong Z., Hecht D.K. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong M., Li X., Wegener Parfrey L., Roth B., Ippoliti A., Wei B. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Millet P., Vachharajani V., McPhail L., Yoza B., McCall C.E. GAPDH binding to TNF-α mRNA contributes to posttranscriptional repression in monocytes: a novel mechanism of communication between inflammation and metabolism. J Immunol. 2016;196(6):2541–2551. doi: 10.4049/jimmunol.1501345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shehata H.M., Murphy A.J., Lee M.K.S., Gardiner C.M., Crowe S.M., Sanjabi S. Sugar or fat?-metabolic requirements for immunity to viral infections. Front Immunol. 2017;8:1311. doi: 10.3389/fimmu.2017.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Palmer C.S., Ostrowski M., Balderson B., Christian N., Crowe S.M. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol. 2015;6:1. doi: 10.3389/fimmu.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haas R., Cucchi D., Smith J., Pucino V., Macdougall C.E., Mauro C. Intermediates of metabolism: from bystanders to Signalling molecules. Trends Biochem Sci. 2016;41(5):460–471. doi: 10.1016/j.tibs.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 111.O'Neill L.A., Kishton R.J., Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16(9):553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukuzumi M., Shinomiya H., Shimizu Y., Ohishi K., Utsumi S. Endotoxin-induced enhancement of glucose influx into murine peritoneal macrophages via GLUT1. Infect Immun. 1996;64(1):108–112. doi: 10.1128/iai.64.1.108-112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freemerman A.J., Johnson A.R., Sacks G.N., Milner J.J., Kirk E.L., Troester M.A. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelly B., O'Neill L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cameron A.M., Castoldi A., Sanin D.E., Flachsmann L.J., Field C.S., Puleston D.J. Inflammatory macrophage dependence on NAD(+) salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat Immunol. 2019;20(4):420–432. doi: 10.1038/s41590-019-0336-y. [DOI] [PubMed] [Google Scholar]
- 116.Galván-Peña S., O'Neill L.A. M1/M2 Macrophages: the Arginine Fork in the road to health and disease. vol. 5(420) 2015. Metabolic reprograming in macrophage polarization; p. 275. [Google Scholar]
- 117.Vats D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Venter G., Oerlemans F.T., Wijers M., Willemse M., Fransen J.A., Wieringa B. Glucose controls morphodynamics of LPS-stimulated macrophages. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gerriets V., Macintyre A., Nichols A., Rathmell J. Glucose metabolism in CD4+ T cell subsets modulates inflammatory disease and autoimmunity (P4126) J Immunol. 2013;190(Supplement 1) [191.9] [Google Scholar]
- 120.Parada Venegas D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thibault R., Blachier F., Darcy-Vrillon B., de Coppet P., Bourreille A., Segain J.P. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16(4):684–695. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- 122.Palmer C.S., Anzinger J.J., Zhou J., Gouillou M., Landay A., Jaworowski A. Glucose transporter 1–expressing Proinflammatory monocytes are elevated in combination antiretroviral therapy–treated and untreated HIV+ subjects. J Immunol. 2014;193(11):5595–5603. doi: 10.4049/jimmunol.1303092. [DOI] [PubMed] [Google Scholar]
- 123.Palmer C.S., Palchaudhuri R., Albargy H., Abdel-Mohsen M., Crowe S.M. Exploiting immune cell metabolic machinery for functional HIV cure and the prevention of inflammaging. F1000Res. 2018;7:125. doi: 10.12688/f1000research.11881.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Butterfield T.R., Hanna D.B., Kaplan R.C., Kizer J.R., Durkin H.G., Young M.A. Increased glucose transporter-1 expression on intermediate monocytes from HIV-infected women with subclinical cardiovascular disease. AIDS. 2017;31(2):199–205. doi: 10.1097/QAD.0000000000001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Palmer C.S., Cherry C.L., Sada-Ovalle I., Singh A., Crowe S.M. Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis. EBioMedicine. 2016;6:31–41. doi: 10.1016/j.ebiom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Planas D., Routy J.P., Ancuta P. New Th17-specific therapeutic strategies for HIV remission. Curr Opin HIV AIDS. 2019;14(2):85–92. doi: 10.1097/COH.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 127.Belizario J.E., Faintuch J., Garay-Malpartida M. Gut microbiome Dysbiosis and Immunometabolism: new Frontiers for treatment of metabolic diseases. Mediators Inflamm. 2018;2018:2037838. doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vijayan V., Pradhan P., Braud L., Fuchs H.R., Gueler F., Motterlini R. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide - a divergent role for glycolysis. Redox Biol. 2019;22:101147. doi: 10.1016/j.redox.2019.101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Datta P.K., Deshmane S., Khalili K., Merali S., Gordon J.C., Fecchio C. Glutamate metabolism in HIV-1 infected macrophages: role of HIV-1 Vpr. Cell Cycle. 2016;15(17):2288–2298. doi: 10.1080/15384101.2016.1190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Low H., Cheng L., Di Yacovo M.S., Churchill M.J., Meikle P., Bukrinsky M. Lipid metabolism in patients infected with Nef-deficient HIV-1 strain. Atherosclerosis. 2016;244:22–28. doi: 10.1016/j.atherosclerosis.2015.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ahmed D., Cassol E. Role of cellular metabolism in regulating type I interferon responses: implications for tumour immunology and treatment. Cancer Lett. 2017;409:20–29. doi: 10.1016/j.canlet.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 132.Wang F., Zhang S., Jeon R., Vuckovic I., Jiang X., Lerman A. Interferon gamma induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine. 2018;30:303–316. doi: 10.1016/j.ebiom.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maidji E., Somsouk M., Rivera J.M., Hunt P.W., Stoddart C.A. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tarancon-Diez L., Rodriguez-Gallego E., Rull A., Peraire J., Vilades C., Portilla I. Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection. EBioMedicine. 2019;42:86–96. doi: 10.1016/j.ebiom.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ricciardi S., Manfrini N., Alfieri R., Calamita P., Crosti M.C., Gallo S. The translational machinery of Human CD4(+) T Cells Is Poised for activation and controls the switch from quiescence to Metabolic Remodeling. Cell Metab. 2018;28(6):895–906. doi: 10.1016/j.cmet.2018.08.009. [e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Valle-Casuso J.C., Angin M., Volant S., Passaes C., Monceaux V., Mikhailova A. Cellular metabolism is a major determinant of HIV-1 Reservoir Seeding in CD4(+) T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2019;29(3):611–626. doi: 10.1016/j.cmet.2018.11.015. [e5] [DOI] [PubMed] [Google Scholar]
- 137.Pallett L.J., Schmidt N., Schurich A. T cell metabolism in chronic viral infection. Clin Exp Immunol. 2019 doi: 10.1111/cei.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Routy J.P., Isnard S., Mehraj V., Ostrowski M., Chomont N., Ancuta P. Effect of metformin on the size of the HIV reservoir in non-diabetic ART-treated individuals: single-arm non-randomised lilac pilot study protocol. BMJ Open. 2019;9(4) doi: 10.1136/bmjopen-2018-028444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O'Sullivan D., Sanin D.E., Pearce E.J., Pearce E.L. Metabolic interventions in the immune response to cancer. Nat Rev Immunol. 2019;19(5):324–335. doi: 10.1038/s41577-019-0140-9. [DOI] [PubMed] [Google Scholar]
- 140.Kessing C.F., Nixon C.C., Li C., Tsai P., Takata H., Mousseau G. In vivo suppression of HIV rebound by Didehydro-Cortistatin a, a "block-and-lock" strategy for HIV-1 treatment. Cell Rep. 2017;21(3):600–611. doi: 10.1016/j.celrep.2017.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Besnard E., Hakre S., Kampmann M., Lim H.W., Hosmane N.N., Martin A. The mTOR complex controls HIV latency. Cell Host Microbe. 2016;20(6):785–797. doi: 10.1016/j.chom.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Clerc I E., Abba Moussa D S., Vahlas Z M., Tardito S H.W., Oburoglu L N.N., Hope TJ A. Entry of glucose- and glutamine-derived carbons into the citric acid cycle supports early steps of HIV-1 infection in CD4 T cells. Nat. Metab. 2019;1(7):717–730. doi: 10.1038/s42255-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]