Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease. We investigated whether serum inflammatory markers, C-reactive protein (CRP), leptin, and nutritional status (assessed by measurement of serum levels of prealbumin and anthropometry) correlated with COPD severity.
Materials and Methods:
One-hundred and two COPD patients (mean age 56.94 ± 10.95 years) were recruited and classified into severity categories based on the GOLD guidelines. Serum concentrations of CRP, prealbumin, and leptin were measured. Anthropometry included body mass index (BMI), mid-upper arm circumference (MUAC), and sum of four skinfold thicknesses (triceps, biceps, suprailiac, and subscapular).
Results:
Twenty-one patients had moderate, 44 had severe, and 37 had very severe COPD. Levels of CRP (mg/dl) (mean ± standard error [SE]) in moderate, severe, and very severe COPD were 0.60 ± 0.096, 2.16 ± 0.39, and 4.15 ± 0.463, respectively. Levels of prealbumin (mg/dl) (mean ± SE) in moderate, severe, and very severe COPD were 15.7 3 ± 0.92, 10.95 ± 0.85, and 11.15 ± 0.79 mg/dl, respectively. Levels of leptin (ng/ml) (mean ± SE) in moderate, severe, and very severe COPD were 13.81 ± 3.88, 8.45 ± 2.25, and 4.40 ± 1.06, respectively. BMI values in the three groups were 23.44 ± 1.16 kg/m2, 20.33 ± 0.62 kg/m2, and 18.86 ± 0.52 kg/m2, respectively. Sum of four skinfold thickness and MUAC was significantly reduced in very severe group as compared to moderate and severe group. Very severe COPD patients had a significantly lower leptin, BMI, and 6-min walk test. Serum CRP was significantly higher in very severe COPD.
Conclusion:
Patients with increasing severity of COPD had a significantly greater serum inflammatory marker level and poorer nutritional status.
KEY WORDS: Chronic obstructive pulmonary disease, C-reactive protein, inflammation, leptin, nutrition, prealbumin
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death globally, as well as in India. According to the latest global burden of disease study (GBD, 1990–2016), COPD accounted for 8.7% of total deaths and 4.8% of disease-adjusted life years in India.[1] Furthermore, COPD prevalence continues to increase, from 3.3% in 1990 to 4.2% in 2016 (GBD 2018).[2] COPD is a systemic inflammatory disease with several extrapulmonary effects. Ongoing inflammation may lead to a more rapid progression of the disease and increased systemic effects. Inflammatory biomarkers, such as C-reactive protein (CRP), prealbumin, and leptin among others have attracted much interest as potential biomarkers for monitoring disease progression and predicting mortality. Dahl et al. demonstrated that circulating plasma CRP levels are increased in COPD patients and increasing levels are associated with increased mortality.[3,4] High levels of circulating CRP have been linked to a higher risk of cardiovascular disease,[5] hospitalization, and death in COPD.[6] Small increases in serum CRP levels are associated with both disease activity and future risk of hospitalization and death from COPD.[3,6,7] Systemic inflammation in stable COPD has been associated with reduced lung function, diminished muscle strength, reduced exercise capacity, and 6-min walking distances (6MWD).[8,9] Leptin, a protein synthesized by adipose tissue and encoded by the ob gene, plays an important role in body weight regulation. Inappropriately increased leptin levels are thought to induce excessive metabolic effects underlying anorexia and loss of body weight.[10,11] Circulating leptin levels are reported to correlate with the body mass index (BMI) in humans.[12,13] Takabatake et al.[14] reported that serum leptin levels were significantly lower in COPD patients than healthy controls. Malnutrition is another major problem in COPD patients and has received increasing attention in recent years. Poor nutritional status in COPD patients has been related to adverse effects that may contribute to complications and increased mortality.[15] Among the various indices of nutritional status in COPD patients, serum prealbumin has been reported to be a highly sensitive and powerful prognostic marker.[16]
Whether a link exists between systemic inflammation and nutrition in COPD patients is unclear. Although the level of systemic inflammation and nutritional status are independently associated with mortality and severity of COPD, the interrelationship between the two is not well defined. Our study was aimed to assess the serum inflammatory markers levels and nutritional status in COPD patients. We investigated whether inflammatory markers, serum CRP and leptin as well as nutritional status (prealbumin and anthropometry) correlate with the severity of airway obstruction in stable COPD patients.
MATERIALS AND METHODS
One-hundred and two patients with stable COPD were recruited from the Outpatient Clinic at All India Institute of Medical Sciences over a period of 3 years (2008–2011). Diagnosis and Classification of COPD severity was based on the Global Initiative for Chronic Obstructive Lung Disease recommendation.[17] All patients should have been clinically stable for at least 3 months prior to enrolment. Patients with a clinical diagnosis of asthma and other established alternative diagnoses like bronchiectasis, pneumonia, tuberculosis, or other inflammatory diseases such as malignancy, connective tissue disorders, arthritis, inflammatory bowel diseases, liver cirrhosis, thyroid disease, and end-stage renal failure or those who had recently undergone a surgical procedure were excluded. Written informed consent was obtained from all the participants and participants who refused consent were excluded. The study was approved by the Institute Ethics Committee. All participants were informed in detail regarding the proposed investigations.
Baseline evaluation included a detailed pro forma containing demographic characteristics, history, quantification of smoking, and severity of disease based on spirometric postbronchodilator forced expiratory volume (FEV1). Smoking index was calculated (number of bidi/cigarettes per day × number of years of smoking).[18] Baseline investigations including (hemogram, biochemistry, Borg scale, and 6-minute walk test) and spirometry were performed in all the patients. The reversibility of airway obstruction was assessed according to GOLD definitions.[17] Assessment of serum inflammatory markers (serum CRP and leptin) and nutritional status (serum prealbumin and anthropometry) were performed in all the patients. Anthropometry included calculation of BMI, mid-upper arm circumference (MUAC), and sum of four skinfold thicknesses (biceps, triceps, subscapular, and suprailiac). Height was measured to the nearest cm and weight to the nearest 100 g. Skinfold thickness were measured using Holtain calipers (Crymych UK). Six-minute walk test was performed as per the ATS guidelines (2007).[19] Smoking status was classified[18] into three categories: active smokers, nonsmokers, and reformed smokers (not smoking tobacco ≥12 months).
Determination of C-reactive protein, prealbumin, and leptin
Venous blood sample (5 ml) was drawn after an overnight 12 h fast. The blood sample was centrifuged at 3000 rpm for 15 min; sera were separated and stored at −80°C until assayed. Serum CRP (Agappe, Kerela) and leptin (DBC, Diagnostics Biochem Canada Inc.,) levels in samples were estimated by the latex enhanced immunoturbidimetry (LEIT) and enzyme-linked immunosorbent assay (ELISA) method, respectively. Assessment of serum prealbumin (Spinreact, Spain) was performed by quantitative turbidimetric method. All measurements were performed according to the instructions as specified in the manual by the manufacturer.
Spirometry
Spirometry was performed using a dry rolling seal electronic spirometer (Morgan UK S232). The results were reported in absolute volumes as well as percent predicted. The highest value of at least three measurements of FEV1 and forced vital capacity was used.
Statistical analysis
Statistical analysis was carried out using Stata 12.0 (Stata Corp LP, Texas, USA). Data were presented as number (%) or mean ± standard deviation/median (min–max) as appropriate. Categorical variable was compared using Chi-square test. Continuous variables were tested for normality using Shapiro–Wilk test. Variables which did not follow normal distributions were compared using Kruskal–Wallis test followed by post hoc analysis-rank sum test adjusted for Bonferroni correction. Correlation was calculated using Spearman's rank correlation. Analysis of covariance was used to compare the mean values among the groups adjusting for confounder (BMI). P < 0.05 was considered statistically significant.
RESULTS
One-hundred and two patients with stable COPD were recruited [Figure 1]. The mean age was 56.94 ± 10.95 years. The details of baseline characteristics are summarized in Table 1. There were 88 male and 14 female. Of the 102 patients, 21 had moderate (FEV1 64.14% ± 10.71% predicted), 44 severe (FEV1 39.72% ± 5.72% predicted), and 37 very severe (FEV1 23.13% ± 4.25% predicted) COPD. Median (min–max) level of smoking index of patients was 450.0 (20.0–2250.0). There were 22 (21.6%) patients who were never smoked, risk factors for COPD other than smoking were biomass fuel exposure (n = 10), possible environmental or occupational exposure (n = 9), passive smoking (n = 2), and previous tuberculosis (n = 1), while 45 (44.12%) were current smokers. Twenty-nine (28.4%) patients were reformed smokers. Twenty (19.60%) patients had comorbid conditions such as hypertension and diabetes mellitus. Sixty-six (64.70%) patients had no past history of hospitalization. 60% of the patients were using inhaled corticosteroids. Mean BMI of the patients was 20.44 ± 4.40 kg/m2. Mean BMI of COPD patients in moderate, severe, and very severe COPD was 23.4 ± 1.16 kg/m2, 20.3 ± 0.62 kg/m2, and 18.9 ± 0.52 kg/m2, respectively. With increasing severity of COPD, a significant decline in mean BMI was observed (P = 0.028) [Table 1]. Six MWD in moderate, severe, and very severe COPD patients was 360.0 (240.0–660.0) m, 330.0 (120.0–600.0), and 295.0 (150.0–660.0), m respectively. A significant decline was found with severity of COPD (P = 0.036). The details of baseline characteristics are summarized in Table 1.
Figure 1.
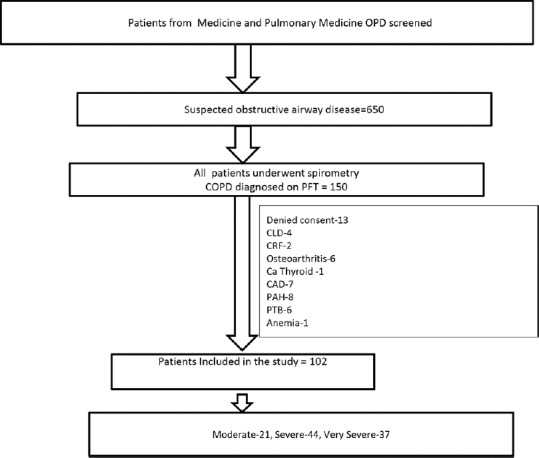
Flow of participants through the study
Table 1.
Baseline characteristics of the study participants: Comparison of various parameters of patients with severity of chronic obstructive pulmonary disease
| Moderate (n=21) | Severe (n=44) | Very severe (n=37) | P | |
|---|---|---|---|---|
| Age (years) | 58.57±12.20 | 58.45±10.90 | 54.22±10.85 | 0.284 |
| Sex/male (%) | 16 (76.2) | 38 (86.4) | 34 (91.9) | 0.166 |
| BMI (kg/m2) | 23.44±1.16 | 20.33±0.62 | 18.86±0.52 | 0.028 |
| 6-min walk test (m) | 360 (240-660) | 330 (120-600) | 295 (150-660) | 0.036 |
| Smoking index | 464.33 (0-3000) | 488.16 (0-2000) | 400.00 (0-3000) | 0.418 |
| FEV1 (percentage predicted) | 64.14±10.71 | 39.72±5.72 | 23.13±4.25 | <0.001 |
| FVC (percentage predicted) | 72.4±18.0 | 56.97±10.1 | 42.43±11.5 | <0.001 |
BMI: Body mass index, FEV: Forced expiratory volume, FVC: Forced vital capacity. Data presented as Mean±SD and Median (Min-Max)
Levels of serum CRP was elevated in all the three groups, but patients with very severe COPD exhibited highest levels. Levels of CRP increased significantly with the severity of COPD [Table 2]. The difference in mean (SE) CRP levels was statistically significant (P < 0.001) among the severity levels. The mean of CRP was higher in severe group than moderate group (P < 0.001) and mean CRP was highest in very severe group than severe with P < 0.001 [Table 2]. Mean (± SE) level of serum prealbumin was lower in patients with severe COPD. In our study, 23 (22.55%) patients had prealbumin level between 10 and 15.0 mg/dl indicate mild malnutrition. Nine (8.82%) patients had moderate (prealbumin 5–10 mg/dl) and 10 (9.80%) patients were (prealbumin level <5 mg/dl) severely low level. Sixty patients (58.82%) had no risk of malnutrition, i.e., prealbumin level was in the normal range (20–40 mg/dl). The difference in mean (± SE) prealbumin levels was statistically significant (P = 0.002) among the severity levels [Table 2]. Mean (± SE) levels of serum leptin were significantly lower in patients with very severe COPD. The difference found in the levels of leptin among the three groups was statistically significant (P = 0.053) [Table 2]. It was found that after adjusting for BMI, the serum level of CRP and prealbumin was statistically significantly (P < 0.001) different among the three groups, but leptin was not statistically significant [Table 2]. There was no significant difference in serum CRP (Z score = 0.30, P = 0.76) and leptin (z = 1.07, P = 0.28) with respect to use of inhaled corticosteroids.
Table 2.
Levels of C-reactive protein, prealbumin, and leptin in various chronic obstructive pulmonary disease severity categories adjusted for body mass index
| Variables | Overall (n=102) | Mean±SE | P | P moderate with severe | Moderate with very severe | Severe with very severe | ||
|---|---|---|---|---|---|---|---|---|
| Moderate | Severe | Very severe | ||||||
| CRP (mg/dl) | ||||||||
| Unadjusted | 3.10±0.38 | 0.60±0.096 | 2.16±0.39 | 4.15±0.463 | <0.001 | 0.001 | 0.001 | 0.001 |
| Adjusted | 0.37±0.55 | 2.17±0.36 | 4.274±0.40 | <0.001 | ||||
| Prealbumin (mg/dl) | ||||||||
| Unadjusted | 12.01±0.53 | 15.73±0.92 | 10.95±0.85 | 11.15±0.79 | 0.002 | 0.001 | 0.001 | 0.67 |
| Adjusted | 16.07±1.17 | 10.93±0.76 | 10.935±0.76 | <0.001 | ||||
| Leptin (ng/mL) | ||||||||
| Unadjusted | 8.08±1.34 | 13.81±3.88 | 8.45±2.25 | 4.40±1.06 | 0.008 | 0.053 | 0.001 | 0.18 |
| Adjusted | 8.600±2.587 | 8.640±1.694 | 7.126±1.897 | 0.823 | ||||
Ref range: CRP: (0-0.3 mg/dl), prealbumin: (20.0-40.0 mg/dl), Leptin: (3.8-7.4 ng/ml). CRP: C-reactive protein, SE: Standard error
The findings of anthropometric parameters are presented in Table 3. Mean values of MUAC in moderate, severe, and very severe COPD patients were 25.93 ± 3.46, 24.22 ± 3.43, and 22.69 ± 2.59 cm, respectively. Median (Min-max) level of sum of four skinfold thickness (biceps, triceps, subscapular, and suprailiac) in moderate-severe and very severe COPD were 55.0 (23.0–133.0), 39.30 (3.0–165.0), and 27.0 (2.0–97.0), respectively (P = 0.006).
Table 3.
Anthropometric parameters in various chronic obstructive pulmonary disease severity categories
| Variables | Overall (n=102) | Moderate | Severe | Very severe | P | P moderate with severe | Moderate with very severe | Severe with very severe |
|---|---|---|---|---|---|---|---|---|
| MUAC (cm) | 24.01±3.35 | 25.93±3.46 | 24.22±3.43 | 22.69±2.59 | 0.001 | 0.13 | 0.00 | 0.09 |
| Sum of four skinfold thickness (mm) | 44.57 (2.0-16.05) | 55.00 (23.0-133.0) | 39.30 (3.0-165.0) | 27.00 (2.0-97.0) | 0.006 | 0.03 | 0.00 | 1.00 |
| W/H ratio | 0.90±0.07 | 0.92±0.09 | 0.91±0.07 | 0.90±0.06 | 0.627 | 1.00 | 1.00 | 1.00 |
SE: Standard error, MUAC: Mid-upper arm circumference, W/H: Waist to hip. Data presented as Mean ± SD and Median (Min-Max)
DISCUSSION
We studied serum inflammatory markers and nutritional status in stable patients of COPD and correlation between inflammatory and nutritional markers with severity of disease. The results of the current study indicate that serum CRP level was significantly higher in patients with COPD and CRP levels increased with the severity of COPD, and there was a significant difference in the level of serum CRP and severity of disease. The study shows that increased systemic inflammation is present in COPD patients, and CRP is an important biomarker in reflecting severity of disease.
The findings are in coherent with the previous studies conducted by Yende et al.[20] that the level of CRP increases with severity of COPD as compared to normal individuals. Meta-analysis conducted by Gan et al.[7] observed that there is an increase in the level of CRP in COPD patients as compared to control population, indicating a persistent systemic inflammation in patients with COPD. de Torres et al.[6] demonstrated that serum CRP level was significantly raised by severity of COPD. Conversely, Pinto-Plata et al.[21] showed that there was no significant difference between the severity of disease and serum CRP levels. In patients with mild-to-moderate COPD, Man et al.[22] have reported a positive trend between quintiles of CRP and increased cardiovascular, cerebrovascular, and cancer deaths, but not deaths from respiratory disease. In addition, it has been reported by van Durme et al.[23] that raised serum levels of CRP are associated with an increased risk of developing COPD in a population-based sample of smokers. de Torres et al.[6] reported that CRP is elevated in clinically stable COPD patients who are actively smoking.
BMI is a simple, accurate, and reproducible calculation based on height and weight. It is taken as one of the markers for assessing nutritional status. Many studies are available in the literature to correlate the relationship between serum CRP levels and BMI in patients with COPD. We found in our study that BMI was inversely correlated with CRP in severe COPD. However, the correlation was not statistically significant. This result suggests that loss of body mass may be caused through systemic inflammation. Conversely, the previous studies conducted by Broekhuizen et al.,[24] Eid et al.,[25] and Pinto-Plata[21] that have shown CRP and BMI correlated in patients with COPD. However, our study does not support the findings of Kolsum et al.,[26] in which BMI was not related to CRP. In the study by Landbo et al.[27] and Schols et al.,[10] reduced BMI has been shown to be an independent risk factor for mortality in COPD and to be correlated with disease severity. CRP elevation was statistically significant even after the adjustment with confounding factor BMI. Malnutrition is the noticeable factor in COPD and needs special attention. The markers of malnutrition were strongly associated with poor quality of life.[28] In our study, the median levels of leptin decreased with the severity of COPD. It has been found that there was a significant difference in the level of serum leptin with severity of disease. It was observed that leptin though significant in univariate analysis but after adjusting with confounding factor BMI, it was not significant. The study showed that serum leptin is strongly and significantly associated with BMI in patients with moderate-to-very severe COPD. We observed that BMI and sum of four skinfold thickness decrease with the severity of COPD. Similar results have been reported by Yang et al.[29] indicating that leptin levels were significantly correlated with BMI and ideal body weight in the patients with stable COPD, but the correlation was not present in the patients with acute exacerbation of COPD. This finding was in accordance with the previous study conducted by Considine et al. and Dyck et al.[13,30] demonstrating the correlation between serum leptin concentrations, percentage body fat, and BMI. Takabatake et al.[14] recently reported that serum leptin levels were significantly lower in COPD patients than healthy controls. In addition, it was seen that the sum of four skinfold thickness and MUAC was correlated significantly with leptin in patients with moderate and very severe COPD. Prealbumin is a negative acute phase reactant that tends to decrease during inflammation. Due to their short half-lives, prealbumin can be used to monitor chronic malnutrition and can easily detect acute changes in a short time.[31] The current study indicates that level of prealbumin decreases with the severity of the disease. Another finding of the study was that the level of prealbumin was significant even after the adjustment with confounding factor BMI. This study showed that prealbumin was inversely correlated with CRP in moderate and severe COPD. It has been found that there is evidence of Inflammation with significant increase in CRP and decrease in prealbumin levels. This study showed that prealbumin was inversely correlated with BMI in patients with moderate-to-very severe COPD. Devoto et al.[32] and McWhirter and Pennington[33] reported that, even in stable COPD, prealbumin serves as a significant indicator of the severity of the disease and nutritional status. The study of McWhirter and Pennington[33] and Devoto et al.[32] revealed an association of serum prealbumin with the severity of COPD. In another study, Gocmen et al.[34] found that serum prealbumin levels of the patients with severe COPD were significantly lower than the patients with mild COPD. Laaban et al.[35] found significant differences in prealbumin levels between one group of COPD patients who required ventilator support due to acute respiratory insufficiency and another group who did not require ventilation. The present study has some limitations. First, this study is a cross-sectional study and age- and gender-matched normal healthy controls were not studied. Diabetes was not included as an exclusion criterion, and it may be a possible confounding factor.
CONCLUSION
Patients with very severe COPD had significantly decreased serum levels of leptin, BMI, and MUAC as compared to those with and moderate and severe disease. CRP levels tend to increase as COPD progresses and conversely nutritional status worsens. This may be associated with a poor prognosis. Treatment in severe COPD should also be directed toward inflammation and nutritional status, as this may improve prognosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.India State_Level Disease Burden Initiative Collaborators. Nations within a nation: Variations in epidemiological transition across the states of India, 1990_2016 in the global burden of disease study. Lancet. 2017;390:2437–60. doi: 10.1016/S0140-6736(17)32804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.India State_Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The global burden of disease study 1990_2016. Lancet Glob Health. 2018;6:e1363–74. doi: 10.1016/S2214-109X(18)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Man SF. Systemic inflammation and mortality in chronic obstructive pulmonary disease. Can J Physiol Pharmacol. 2007;85:141–7. doi: 10.1139/y06-093. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases. The potential role of systemic inflammation in chronic obstructive pulmonary disease? Circulation. 2003;107:1514–9. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 6.de Torres JP, Cordoba-Lanus E, López-Aguilar C, Muros de Fuentes M, Montejo de Garcini A, Aguirre-Jaime A, et al. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J. 2006;27:902–7. doi: 10.1183/09031936.06.00109605. [DOI] [PubMed] [Google Scholar]
- 7.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax. 2004;59:574–80. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Rio F, Miravitlles M, Soriano JB, Muñoz L, Duran-Tauleria E, Sánchez G, et al. Systemic inflammation in chronic obstructive pulmonary disease: A population-based study. Respir Res. 2010;11:63. doi: 10.1186/1465-9921-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffery Mador M, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Res. 2001;2:216–24. doi: 10.1186/rr60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1220–6. doi: 10.1164/ajrccm.160.4.9811033. [DOI] [PubMed] [Google Scholar]
- 11.Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1239–45. doi: 10.1164/ajrccm.162.4.9912016. [DOI] [PubMed] [Google Scholar]
- 12.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma and RNA in obese and weight -reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 13.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 14.Takabatake N, Nakamura H, Abe S, Hino T, Saito H, Yuki H, et al. Circulating leptin in subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1215–9. doi: 10.1164/ajrccm.159.4.9806134. [DOI] [PubMed] [Google Scholar]
- 15.Ezzell L, Jensen GL. Malnutrition in chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;72:1415–6. doi: 10.1093/ajcn/72.6.1415. [DOI] [PubMed] [Google Scholar]
- 16.Matkovic Z, Cvetko D, Rahelic D, Esquinas C, Zarak M, Miravitlles M, et al. Nutritional status of patients with chronic obstructive pulmonary disease in relation to their physical performance. COPD. 2017;14:626–34. doi: 10.1080/15412555.2017.1386643. [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 18.Jindal SK, Malik SK. Smoking Index-A measure to quantify cumulative smoking exposure. Lung India. 1988;6:195–6. [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Yende S, Waterer GW, Tolley EA, Newman AB, Bauer DC, Taaffe DR, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61:10–6. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–8. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–53. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Durme YM, Verhamme KM, Aarnoudse AJ, Van Pottelberge GR, Hofman A, Witteman JC, et al. C-reactive protein levels, haplotypes, and the risk of incident chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:375–82. doi: 10.1164/rccm.200810-1540OC. [DOI] [PubMed] [Google Scholar]
- 24.Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax. 2006;61:17–22. doi: 10.1136/thx.2005.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–8. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 26.Kolsum U, Roy K, Starkey C, Borrill Z, Truman N, Vestbo J, et al. The repeatability of interleukin-6, tumor necrosis factor-alpha, and C-reactive protein in COPD patients over one year. Int J Chron Obstruct Pulmon Dis. 2009;4:149–56. doi: 10.2147/copd.s5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 28.Chumlea WC, Dwyer J, Bergen C, Burkart J, Paranandi L, Frydrych A, et al. Nutritional status assessed from anthropometric measures in the HEMO study. J Ren Nutr. 2003;13:31–8. doi: 10.1053/jren.2003.50003. [DOI] [PubMed] [Google Scholar]
- 29.Yang YM, Sun TY, Liu XM. The role of serum leptin and tumor necrosis factor-alpha in malnutrition of male chronic obstructive pulmonary disease patients. Chin Med J (Engl) 2006;119:628–33. [PubMed] [Google Scholar]
- 30.Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 31.Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med. 2002;40:1281–91. doi: 10.1515/CCLM.2002.222. [DOI] [PubMed] [Google Scholar]
- 32.Devoto G, Gallo F, Marchello C, Racchi O, Garbarini R, Bonassi S, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52:2281–5. doi: 10.1373/clinchem.2006.080366. [DOI] [PubMed] [Google Scholar]
- 33.McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945–8. doi: 10.1136/bmj.308.6934.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gocmen H, Ediger D, Uzaslan E, Doganay S, Guney NA, Ege E, et al. The relationships of serum prealbumin levels with parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. Eurasian J Med. 2010;42:105–10. doi: 10.5152/eajm.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laaban JP, Kouchakji B, Dore MF, Elizabeth OF, Rochemaure J, David P. Nutrition status of subjects with chronic obstructive pulmonary disease and acute respiratory failure. Chest. 1993;103:1362–8. doi: 10.1378/chest.103.5.1362. [DOI] [PubMed] [Google Scholar]


