Abstract
Introduction:
Exposure to biomass fuel (BMF) from traditional cookstoves inflicts an enormous burden of morbidities in women across the developing world. This study aims to assess the lung function and its association with the indoor air pollutants generated using BMF.
Materials and Methods:
This cross-sectional study including 310 women was conducted in a rural village of India. Households were divided into two groups based on the cooking fuel, the BMF group and the liquefied petroleum gas (LPG) group. Information on respiratory symptoms and socioeconomic status was obtained using a standard questionnaire. Indoor air concentration for PM10 and PM2.5 was measured during cooking hours. Pulmonary function tests (PFTs) were conducted for the women inhabitants.
Results:
On comparing the two groups, the concentration of PM10 (890.26 ± 59.59 vs. 148.66 ± 31.97) μg/m3 and PM2.5 (728.90 ± 50.20 vs. 99.76 ± 41.80) μg/m3 (P < 0.01) were higher in the group using BMF. The respiratory symptoms such as wheezing, dyspnea, chronic cough, and nocturnal cough, were significantly more common in the group using BMF. A significant difference was seen in the lung function indices between the two groups. A significant negative correlation of respiratory indices with duration of exposure and the particulate matter (PM) values suggested a greater decline on lung function among women exposed to increased concentrations of PM. On comparing participants with normal and abnormal PFT, it was seen that the use of BMF (odds ratio [OR] 8.01; 95% confidence interval [CI] 4.80, 13.36, P < 0.001) and the duration of exposure to BMF (OR 1.16; 95% CI 1.13, 1.20., P < 0.001) increased the odds of having an abnormal PFT.
Conclusions:
This study shows a high prevalence of respiratory symptoms and an abnormal pulmonary function in women exposed to BMF.
KEY WORDS: Biomass fuel, indoor air pollution (IAP), lung function, particulate matter
INTRODUCTION
Cooking is an indispensable part of our daily activities. Various agents used for combustion include electricity, liquefied petroleum gas (LPG), and biomass fuel (BMF) (dung cake, crop residues, wood, charcoal, and coal). Cleaner fuels such as electricity are available for cooking in the developed world due to their better infrastructure and high per capita income, whereas majority of the developing world uses LPG and biomass agents for cooking.[1,2,3] Nearly half (41%) of the world's population uses BMFs as the combustion agent for cooking, as they are cheap and easily accessible.[4,5]
Combustion of BMFs produces toxic compounds including particulate matter (PM), carbon monoxide, sulfur dioxide, nitrogen oxides, benzene, and formaldehyde. PM can be defined as the portion of air pollution formed by small particles and liquid droplets comprising organic chemicals, metals, soil, and dust particles.[6] It is categorized depending on the size; particles ≤10 μg in diameter are designated PM10 and those ≤2.5 μg in diameter as PM2.5.[7] The ill effects of these substances can be intensified if they are found in high concentration in the indoor air because of poor ventilation in the cooking area.[4] Indoor air pollution has been identified by the WHO as the second largest cause of morbidity; second only to unsafe drinking water and sanitation.[8] Biomass combustion has been associated largely with respiratory morbidity, and few studies have also found association with middle ear infections, perinatal disease, cancers of the nasopharynx and larynx, cataract, blindness, and eclampsia.[2,9] Recent studies also found a higher risk of hypertension and cardiovascular diseases in pregnant women exposed to BMF smoke.[10]
Although studies have suggested that indoor air pollution due to biomass combustion can have an adverse impact leading to respiratory symptom and diseases,[11,12] most studies are only questionnaire based and have not routinely measured the levels of indoor air pollution and performed spirometry. This study was conducted to evaluate the exposure to indoor air pollutants (PM10, PM2.5) occurring due to the use of BMFs among women in rural India and assess its impact on the respiratory symptoms and the lung function indices.
MATERIALS AND METHODS
A population-based cross-sectional study was conducted in a rural village of Western Uttar Pradesh to assess the indoor air pollutants caused using BMF and assess its impact on the lung function. The ethical approval was obtained from the Institutional Ethics Committee of Vardhaman Mahavir Medical College and Safdarjung Hospital (VMMC and SJH), New Delhi, and a written informed consent was obtained from all the participants.
Sampling frame
Western Uttar Pradesh has villages where many households use traditional chulhas burning dung cake, wood, and biomass, and some have now access to LPG under various government schemes.
Households were first sequentially visited to identify the primary cooking fuel used and were categorized as biomass cooking and LPG cooking houses. From the two categories, using a stratified random sampling technique, houses were randomly selected using a computer-based random sequence generator by an independent person of the department, not involved in the study, to form two study groups: (1) biomass group and (2) LPG group.
From each of the household selected, all the women above the age of 18 and who were residing in the hose for more than 6 months were selected. Participants were excluded if they had a previous history of any respiratory ailment such as chronic obstructive pulmonary disease or Asthma. Participants were also excluded if a spirometry was contraindicated (that is, patients with a recent history of thoracic, abdominal, and eye surgery; a recent myocardial infarction and patients with aneurysms).
Assessment
Indoor air quality in terms of PM1, PM2.5, and PM10 was determined at every 5-min interval for a continuous 9-h period in the household which consented for air quality assessment using the GRIMM aerosol spectrometer (model number 1.108, Germany). The instrument was placed at a height of 2 m in the cooking area to measure the direct exposure; the 9 h average was taken which included the morning cooking time.
The age, gender, body mass index, the socioeconomic status, the cooking details, the smoking status (including exposure to environmental tobacco smoke), and the presence of respiratory symptoms were recorded for each woman participating in the study. This was done using an interviewer-administered questionnaire designed for the study.[13] The questionnaire was translated into the local language (Hindi) and back-translated into English by an independent translator, and a pilot study was conducted to identify issues of logistics and understanding. Chronic cough was defined as cough on most days for 3 consecutive months or more during the year for the past 2 years or more. Chronic sputum was defined as sputum on most days for 3 consecutive months or more during the year for the past 2 years or more. Dyspnea was defined as breathlessness when walking, which required the subject to stop or slow down for breathing while walking on the level (corresponding to grade 2 dyspnea by the mMRC scale). Wheezing referred to the occurrence of wheezing/whistling sounds in breathing during exertion on most days or nights. The cooking details recorded included the type of fuel used (current and in the past), the average hours of cooking per day. The biomass exposure index was calculated by multiplying the average hours spent on cooking per day with the number of years of cooking.[14,15]
When recording the smoking history, participants that had never smoked and had no exposure to environmental tobacco smoke were classified as “ never smoker,” while participants who did not smoke but had a household member who smoked in the house were classified as having environmental tobacco smoke exposure. The participants who had quit smoking for more than an year were categorized as reformed smoker and participants who continued to smoke were classified as current smokers.
Pulmonary function assessment was performed using a Pony FX portable flow spirometer that was calibrated daily with a 3 L syringe. Spirometry was performed in accordance with the American Thoracic Society/European Respiratory Society guidelines[16] with participants in a standing position with a nose clip applied and was asked to take tidal breaths before a deep breath. Participants were then asked to take a deep breath with mouth placed tightly around the mouthpiece and then were asked to perform full expiration. Spirometric measurements included forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and FEV1/FVC (expressed as a percentage). The quality of the spirometry data was assessed by one of the authors. The participants were classified as having a normal spirometry, pure obstructive defect, a “possible” restrictive defect, and a mixed ventilator defect. The bronchodilator testing was not performed.
Sample size calculation
In the previous studies, the prevalence of abnormal spirometry in rural women has been reported to be 46% of the patients exposed to BMF and 28% among those not exposed.[17] The required sample size was estimated to be 113 in each group for a two-sided significance level of 95% and power of 80%.
Statistical analysis
All of the data obtained thereby were recorded systematically and analyzed using standardized statistical methods. Categorical variables were compared using the Chi-square test or Fisher exact test, and numerical variables using the t-test for independent samples or the Wilcoxon rank-sum test. Statistical significance was set at 5% (corresponding to a P < 0.05). The Pearson correlation coefficient and the regression coefficient was estimated between the respiratory indices and number of years cooking using biomass, the biomass index, PM1, PM2.5, and PM10 values. The participants with a normal and an abnormal pulmonary function test (PFT) were also compared and logistic regression analysis was used to identify variables that predicted an abnormal PFT.
RESULTS
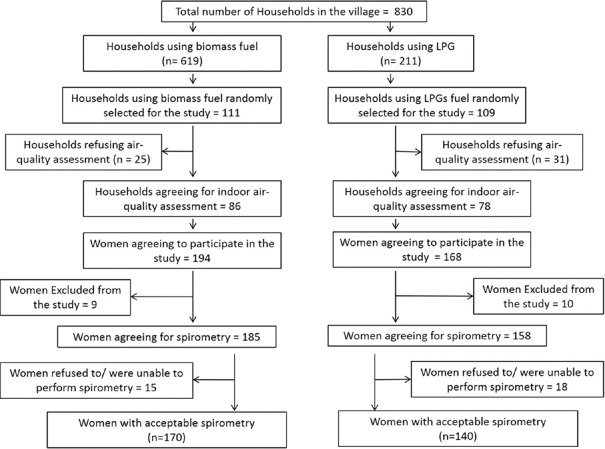
A total of 220 households were visited, out of which 164 agreed for indoor air quality assessment. Most of the houses studied had a separate room for cooking, although often not adequately ventilated. Three hundred and sixty-two women agreed to take part in the questionnaire survey. Of these, 19 were excluded due to the exclusion criteria and 33 refused to/were unable to perform spirometry. There were 310 women who were able to perform acceptable spirometry; 170 in the BMF group and 140 in the LPG group [Figure 1].
Figure 1.
Flow diagram for study participants
On comparing the baseline characteristics of the two groups [Table 1], it was found that the women living in households that cooked with LPG were younger than those living in households that cooked with BMF (P < 0.001). Sixty-one (35.9%) and 89 (63.6%) participants in the BMF group, and the LPG group were in the age group of 18–40 years, respectively. The socioeconomic status was also significantly different; the middle socioeconomic group women used LPG for cooking, while the lower socioeconomic group continued to use biomass as the cooking fuel. This could be attributed to the infrastructure development of the country, whereby cleaner fuel has been made available to the rural communities under various government schemes. Majority of the females in the study had been exposed to environment tobacco smoke, but the smoking status was proportionally equal among the two groups.
Table 1.
Baseline characteristics
| Biomass (n=170) (%) | Nonbiomass (LPG group) (n=140) (%) | P | |
|---|---|---|---|
| Age (years), mean±SD | 42.52±10.22 | 37.36±10.78 | <0.001 |
| BMI (kg/m2), mean±SD | 23.94±3.02 | 22.74±1.89 | 0.003 |
| Types of family | |||
| Nuclear family | 86 (50.58) | 66 (47.14) | 0.569 |
| Joint family | 84 (49.41) | 74 (52.86) | |
| Duration of exposure to fuel (years) | 27.58±12.68 | 3.24±5.03 | <0.001 |
| Biomass fuel index | 63.79±36.55 | 6.47±10.07 | <0.001 |
| Smoking status | |||
| Never smoker | 64 (37.64) | 57 (40.71) | 0.910 |
| Environmental tobacco smoke exposure | 75 (44.12) | 60 (42.85) | |
| Smoking left for >1 year | 13 (7.64) | 11 (7.85) | |
| Currently smoking | 18 (10.59) | 12 (8.57) | |
| Socioeconomic status | |||
| Upper | 0 (0.0) | 0 (0.0) | <0.001 |
| Upper-middle | 8 (4.70) | 37 (26.43) | |
| Lower-middle | 141 (82.94) | 102 (72.86) | |
| Upper-lower | 21 (12.35) | 1 (0.71) | |
| Lower | 0 (0.0) | 0 (0.0) | |
| Comorbidities | |||
| Diabetes mellitus | 17 (10.00) | 10 (7.14) | 0.423 |
| Hypertension | 12 (7.06) | 9 (6.43) | 0.999 |
| Coronary artery disease | 5 (2.94) | 4 (2.86) | 0.999 |
| Stroke | 2 (1.18) | 1 (0.71) | 0.999 |
| Air quality assessment | |||
| PM10 (μg/m3), mean±SD | 876.48±62.21 | 163.44±40.32 | <0.001 |
| PM2.5 (μg/m3), mean±SD | 736.45±53.39 | 101.65±38.17 | <0.001 |
| PM1 (μg/m3), mean±SD | 657.65±50.75 | 81.19±34.66 | <0.001 |
*Indian rupees (1 US$ ≈ 70INR). SD=Standard deviation, PM=Particulate matter, LPG=Liquefied petroleum gas, BMI=Body mass index
The 9-h average value of PM2.5 was found to be 736.45 ± 53.39 μg/m3 in the houses cooking using BMF as opposed to 101.65 ± 38.17 μg/m3 in the houses using LPG (P < 0.001). Average peak concentration of PM2.5 reached as high as 1810.7 μg/m3 at the time of cooking, indicating a much higher level of direct exposure during the cooking hours [Figure 2]. Average PM10 concentration was 736.48 ± 53.39 μg/m3 in houses using biomass compared to 163.66 ± 31.97 μg/m3 in the houses cooking using LPG [Figure 3]. It was also observed the pollutant levels returned to precooking levels after almost 3 h in the BMF group, while in the LPG group, it took about 1 h.
Figure 2.
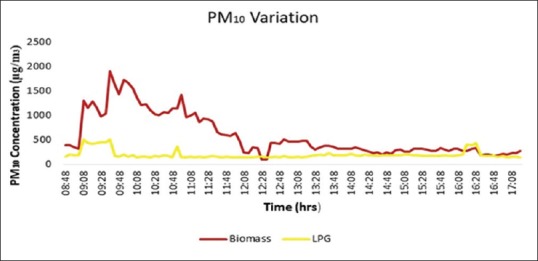
Nine-hour average variation of PM10 including morning cooking hours
Figure 3.
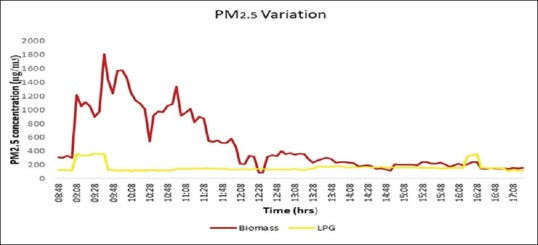
Nine-hour average variation of PM2.5 including morning cooking hours
When we assessed the respiratory symptoms in the two groups [Table 2], dyspnea, wheezing, nocturnal cough, and chronic bronchitis were significantly higher in biomass-exposed group. Fifty-eight patients (34.12%) in the BMF group used inhaled therapies compared to four (2.86%) in the LPG group (P ≤ 0.001). On comparing the spirometry indices between the groups [Table 3], it was seen that the participants from the BMF group showed a lower lung function. The spirometry was classified as being normal, pure obstructive, “possibly” restrictive, and mixed [Table 4]. While only 28.82% patients in the BMF group had a normal PFT, 76.43% in the LPG group were normal. The severe-to-very severe obstruction was significantly higher in women cooking on traditional stoves as opposed to moderately severe obstruction seen in women cooking on LPG.
Table 2.
Respiratory symptoms in the two groups
| Biomass (n=170) (%) | Nonbiomass (LPG group) (n=140) (%) | P | |
|---|---|---|---|
| Wheezing on exertion | 126 (74.12) | 45 (32.14) | <0.001 |
| Dyspnea mMRC scale ≥Grade 2 (breathlessness) | 55 (32.35) | 11 (7.85) | 0.002 |
| Cough and phlegm | |||
| Chronic cough | 128 (75.29) | 89 (63.57) | <0.001 |
| Chronic sputum production | 109 (64.12) | 61 (43.57) | 0.025 |
| Nocturnal cough | 113 (66.47) | 44 (31.43) | <0.001 |
| Use of inhaled therapies (inhaled bronchodilators/steroids) | 58 (34.12) | 4 (2.86) | <0.001 |
LPG=Liquefied petroleum gas
Table 3.
Respiratory indices
| PFT parameters | Biomass | Nonbiomass (LPG group) | P (comparing the biomass fuel group with the LPG group) | ||||
|---|---|---|---|---|---|---|---|
| Age 18-40 years (n=61) | >40 years (n=109) | Total group (n=170) | Age 18-40 years (n=89) | >40 years (n=51) | Total group (n=140) | ||
| FVC (%) | 86.59±14.20 | 73.22±16.07* | 78.02±16.67 | 94.57±6.84 | 82.73±14.13* | 90.26±11.58 | <0.001 |
| FEV1 (%) | 77.62±20.73 | 43.89±16.93* | 55.99±24.48 | 91.27±12.23 | 71.88±21.71* | 84.21±18.76 | <0.001 |
| FEV1/FVC (%) | 82.28±8.16 | 58.95±14.48* | 67.32±16.84 | 84.58±4.85 | 79.51±9.63* | 82.74±7.37 | <0.001 |
*Significantly different compared to the 18-40 years age group. LPG=Liquefied petroleum gas, FVC=Forced vital capacity, FEV1=Forced expiratory volume in 1 s, PFT=Pulmonary function tests
Table 4.
Pulmonary function test results - lung function changes in asymptomatic and symptomatic patient
| PFT result | Biomass fuel group | Nonbiomass fuel group (LPG group) | P | ||||
|---|---|---|---|---|---|---|---|
| Asymptomatic (n=21) | Symptomatic (n=149) | Total (n=170) | Asymptomatic (n=39) | Symptomatic (n=101) | Total (n=140) | ||
| Normal spirometry (%) | 21 | 28 | 49 (28.82) | 39 | 68 | 107 (76.43) | <0.001 |
| Pure obstructive defect (%) | 0 | 46 | 46 (27.06) | 0 | 8 | 8 (5.71) | |
| Mild obstruction | 0 | 0 | 0 | 0 | 0 | 0 | |
| Moderate obstruction | 0 | 0 | 0 | 0 | 2 | 2 | |
| Moderately severe obstruction | 0 | 6 | 6 | 0 | 6 | 6 | |
| Severe obstruction | 0 | 32 | 32 | 0 | 0 | 0 | |
| Very severe obstruction | 0 | 8 | 8 | 0 | 0 | 0 | |
| Possible restrictive defect (%) | 0 | 36 | 36 (21.18) | 0 | 22 | 22 (15.71) | |
| Mild | 0 | 1 | 1 | 0 | 2 | 2 | |
| Moderate | 0 | 5 | 5 | 0 | 8 | 8 | |
| Moderately severe | 0 | 29 | 29 | 0 | 10 | 10 | |
| Severe | 0 | 1 | 1 | 0 | 2 | 2 | |
| Mixed defect (%) | 0 | 39 | 39 (22.94) | 0 | 3 | 3 (2.14) | |
Symptomatic - defined as having either breathlessness, wheezing, or chronic cough. PFT=Pulmonary function tests, LPG=Liquefied petroleum gas
There was a statistically significant negative correlation between the number of years spent on cooking with biomass and the PM concentrations with the various spirometric indices [Table 5], suggesting a higher decline in lung function with increase in the years of exposure and exposure to higher concentrations of PM.
Table 5.
Correlation and regression analysis
| FEV1% | FVC% | FEV1/FVC% | |
|---|---|---|---|
| Duration of exposure to biomass | |||
| Correlation coefficient (r) | −0.810 | −0.620 | −0.769 |
| P | <0.001 | <0.001 | <0.001 |
| Regression coefficient (95% CI) | −1.35 (−1.46-−1.24) | −0.62 (−0.71-−0.54) | −0.75 (−0.83-−0.69) |
| Biomass index | |||
| Correlation coefficient (r) | −0.663 | −0.456 | −0.634 |
| P | <0.001 | <0.001 | <0.001 |
| Regression coefficient (95% CI) | −0.43 (−0.49-−0.40) | −0.18 (−0.22-−0.14) | −0.25 (−0.28-−0.21) |
| PM1 (μg/m3) | |||
| Correlation coefficient (r) | −0.529 | −0.374 | −0.494 |
| P | <0.001 | <0.001 | <0.001 |
| Regression coefficient (95% CI) | −0.048 (−0.056-−0.039) | −0.020 (−0.026-−0.015) | −0.026 (−0.031-−0.021) |
| PM2.5 (μg/m3) | |||
| Correlation coefficient (r) | −0.533 | −0.378 | −0.497 |
| P | <0.001 | <0.001 | <0.001 |
| Regression coefficient (95% CI) | −0.043 (−0.051-−0.036) | −0.019 (−0.024-−0.014) | −0.024 (−0.028-−0.019) |
| PM10 (μg/m3) | |||
| Correlation coefficient (r) | −0.534 | −0.380 | −0.497 |
| P | <0.001 | <0.001 | <0.001 |
| Regression coefficient (95% CI) | −0.039 (−0.046-−0.032) | −0.017 (−0.021-−0.012) | −0.021 (−0.025-−0.017) |
PFT=Pulmonary function tests, LPG=Liquefied petroleum gas, PM=Particulate matter, CI=Confidence interval, FVC=Forced vital capacity, FEV1=Forced expiratory volume in 1 s
On comparing the participants with normal and abnormal spirometry [Table 6], it was seen that the use of BMF (odds ratio [OR] 8.01; 95% confidence interval [CI] 4.80, 13.36. P <0.001) and the duration of exposure to BMF (OR 1.16; 95% CI 1.13, 1.20. P <0.001) increased the odds of having an abnormal PFT; this persisted despite adjusting for the age, BMI, smoking status, and socioeconomic status. It was also seen that the increase in the concentration of PM10 and PM2.5 by 100 μg/m3 increased the risk of having an abnormal PFT by approximately 36% and 39%, respectively.
Table 6.
Factors associated with an abnormal spirometry
| Mean±SD | P | OR (95% CI) | OR (postadjustment*) (95% CI) | ||
|---|---|---|---|---|---|
| Normal spirometry (n=156) | Abnormal spirometry (n=154) | ||||
| Age (years), mean±SD | 33.69±9.01 | 46.78±8.09 | <0.001 | 1.17 (1.13-1.21) | - |
| BMI (kg/m2), mean±SD | 22.12±1.73 | 24.69±2.77 | <0.001 | 1.62 (1.43-1.83) | - |
| Types of family (%) | |||||
| Nuclear family | 81 (51.92) | 71 (46.10) | 0.301 | 1.0 | - |
| Joint family | 75 (48.08) | 83 (53.90) | 1.26 (0.81-1.97) | - | |
| Cooking fuel used (%) | |||||
| LPG used | 107 (68.59) | 33 (21.43) | <0.001 | 1.0 | 1.0 |
| Biomass fuel used | 48 (30.77) | 121 (78.57) | 8.01 (4.80-13.36) | 12.99 (5.78-29.20) | |
| Duration of exposure to fuel (years) | 5.40±7.71 | 27.92±13.45 | <0.001 | 1.16 (1.13-1.20) | 1.16 (1.11-1.21) |
| Biomass index | 13.34±21.09 | 62.79±39.08 | <0.001 | 1.06 (1.04-1.07) | 1.04 (1.03-1.06) |
| Smoking history (%) | |||||
| Never smoker | 74 (47.44) | 47 (30.52) | 0.025 | 1.0 | - |
| Environmental tobacco smoke exposure | 58 (37.18) | 77 (50.0) | 2.09 (1.27-3.45) | ||
| Smoking left for >1 year | 11 (7.05) | 13 (8.44) | 1.86 (0.77-4.50) | ||
| Currently smoking | 13 (8.33) | 17 (11.04) | 2.06 (0.92-4.63) | ||
| Socioeconomic status (%) | |||||
| Upper | 0 (0.0) | 0 (0.0) | <0.001 | - | - |
| Upper-middle | 36 (23.08) | 9 (5.84) | 1.0 | - | |
| Lower-middle | 110 (70.51) | 133 (86.36) | 4.83 (2.23-10.47) | - | |
| Upper-lower | 10 (6.41) | 12 (7.79) | 4.80 (1.58-14.60) | - | |
| Lower | 0 (0.0) | 0 (0.0) | - | - | |
| Air quality assessment | |||||
| PM10 (μg/m3), mean±SD | 387.31±335.03 | 723.78±299.69 | <0.001 | 1.0028 (1.0021-1.0036) | 1.0036 (1.0024-1.0047) |
| PM2.5 (μg/m3), mean±SD | 301.49±299.08 | 599.97±266.33 | <0.001 | 1.0032 (1.0024-1.0040) | 1.0039 (1.0027-1.0053) |
| PM1 (μg/m3), mean±SD | 263.39±271.67 | 532.96±243.12 | <0.001 | 1.0035 (1.0026-1.0043) | 1.0044 (1.0030-1.0058) |
*OR adjusted for the age, BMI, smoking status, and the socioeconomic status. OR=Odds ratio, BMI=Body mass index, SD=Standard deviation, PM=Particulate matter
DISCUSSION
The current population-based cross-sectional study showed the increased prevalence of respiratory symptoms and lower pulmonary function among the women cooking on traditional chulhas using BMF as opposed to those using LPG stoves. It was also seen that women exposed to BMF had higher odd of having an abnormal lung function test. The pollutants generated during the incomplete combustion of the BMFs have been thought to be the culprit.
In our study, average 9-h PM2.5 level observed in the households using BMF was 728.90 ± 50.20 μg/m3, while it was 101.65 ± 38.17 μg/m3 in the LPG households; in both groups, it was above the recommended WHO standard of 25 μg/m3 24-h mean. These high concentrations are due to the pollutants being generated during burning of the fuel, and also their persistence because of poor ventilation in the kitchen. In a study conducted in rural households of South India by Balakrishnan et al.,[4] the levels of 24-h average exposure to PM was reported to be 231 and 82 μg/m3 in households using biomass and LPG, respectively.[4] Similar such studies have demonstrated high PM2.5 and PM10 levels in the household using BMFs, but they have not evaluated the association between the indoor PM concentration and the lung function of those exposed to it.[18,19]
In the current study, the presence of respiratory symptoms was high in both the groups (e.g. chronic cough was reported in 75.29% in the BMF group and 63.57% in the LPG group). This could be explained by the high prevalence of exposure to environmental tobacco smoke in the study population. Furthermore, both groups were exposed to pollutants above the recommended WHO air quality guidelines. On comparing the two groups, we observed a higher frequency of respiratory symptoms in participants using BMF. Similar results have been reported by prior studies.[20,21] A meta-analysis estimating the burden of disease due to BMF exposure had found chronic bronchitis was nearly two times more common among women exposed to BMF for cooking (OR = 2.37, 95% CI: 1.59, 3.54).[22] Kumar et al.[1] also showed the correlation between use of BMF and increased respiratory symptoms among women of rural Indian village.
When the pulmonary function of the participants was assessed, a significant decline in the respiratory indices was seen in the group using BMF. Similar findings of decreased PFT were reported by Kurmi et al.,[23] in a study conducted on rural Nepalese population. In this study, the authors have reported a positive correlation between the use of BMF and lower lung function across all the age groups (OR = 2.10, 95% CI 1.47, 2.99). In a similar study conducted by Rinne et al.[2] demonstrated decreased pulmonary function among children living in homes that cook with BMF when compared with children living in homes that cook with LPG only.
A decline in the spirometric parameters occurs with age and is accelerated in patients with pulmonary disease.[24,25] In the current study, we observed a lower lung function in participants exposed to BMF. Exposure to air pollutants causes constriction of the airway smooth muscles and irritation of the mucous glands, leading to wheezing, and overproduction of sputum. Biomass smoke contains a combination of gases and PM that are known to weaken host defenses.[22] The inhalation of these hazardous chemicals also produces changes in the respiratory tract, causing lung inflammation and activation of alveolar epithelial cells leading to excessive mucous secretion, and finally resulting in airway remodeling.[6,7]
The ill effects of exposure to BMF also include extrapulmonary manifestations. A long-term prospective cohort study conducted by Pope et al.[26] in the American population showed increased cardiovascular morbidity and lung cancer incidence with increasing levels of fine particulate air pollution. A nationwide prospective cohort study from China has reported that solid fuel use for cooking and heating was associated with higher risks of cardiovascular and all-cause mortality.[27] A similar nationwide population-based study from Bangladesh had shown that household air pollution from cooking had an adverse effect on pregnancy and birth outcomes.[28]
The use of BMF has been identified as risk factor for multiple pulmonary and extrapulmonary diseases. According to the WHO, approximately 3.8 million deaths are caused by household air pollution globally.[29] India has disproportionately high mortality and disease burden due to air pollution. This burden is generally highest in the poorer states of India.[30] The poorest section of the society is the most affected by this due to their inability to access alternative cleaner fuels; for them, the cheap and easily available BMF is often the only fuel available. In our study also, women cooking on biomass belonged to lower socioeconomic strata. The problem is further compounded by poor ventilation in places where the food is cooked; leading to exposure to these pollutants for a longer duration. Our study showed elevated levels of PM10 and PM2.5 during the cooking hours with high levels persisting even after cooking. The worst affected are often women, due to the constant exposure to high levels of indoor air pollutants.[31,32,33]
India has a very high burden of chronic respiratory diseases. The increasing contribution of these diseases to the overall disease burden and the high rate of health loss from them, highlights the need for focused policy interventions.[34] There is a need for interventions to alleviate the ill effects of indoor air pollution. The use of cleaner fuel also needs to be encouraged. Pilishvili et al.[35] showed reduced indoor air pollution in rural Kenyan households with a better stove. Similarly, improved biomass stove intervention applied in Mexico revealed improvement in respiratory function of women to be comparable to that of smoking cessation.[36] A systematic review conducted by Thomas et al.[37] also revealed that improved stove intervention in low- and middle-income countries could reduce the exposure to household air pollution. It is also important to ensure adequate ventilation in places where cooking is done.
Strengths
An adequate sample size along with a population-based random sampling allows the result to accurately reflect the ill effects of BMF use on the respiratory function of rural women. In the study, we have monitored the indoor air quality by estimating PM10 and PM2.5 that are released from incomplete combustion of biomass in the traditional stoves, permitting us to accurately assess the indoor air pollution generated due to the burning of BMF and persisting in the ambient air due to inadequate ventilation. Another strength of the study was that the participants had answered a questionnaire as well as performed a spirometry. This allows for a precise evaluation of the impact that indoor air pollution had on the pulmonary function of the participants.
Limitations
Although our study measured the concentration of PM10 and PM2.5, other pollutants such as volatile organic compounds and gases such as carbon monoxide were not measured. Furthermore, a detailed PFT including diffusion capacity and body plethysmography could not be performed to further categorize the participants with an abnormal PFT. Although the adverse effects of indoor air pollution include long-term extrapulmonary manifestations, the current study did not study attempt to study them. We suggest that in future, studies focusing on interventions to mitigate the ill effects of BMF and their long-term impacts need to be conducted. Such interventions can include engineering methods to reduce exposure to PM, provision of ventilation, and shifting to cleaner cooking fuels.
CONCLUSIONS
The current study shows that household using BMF have higher levels of PM and have more respiratory complaints and reduced lung function. The use of BMFs increases the probability of a reduced lung function. It is important to use safer alternative fuels and/or develop techniques to mitigate the ill effects of indoor air pollution.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kumar R, Singh K, Nagar S, Kumar M, Mehto UK, Rai G, et al. Pollutant levels at cooking place and their association with respiratory symptoms in women in a rural area of Delhi-NCR. Indian J Chest Dis Allied Sci. 2015;57:225–31. [PubMed] [Google Scholar]
- 2.Rinne ST, Rodas EJ, Bender BS, Rinne ML, Simpson JM, Galer-Unti R, et al. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural ecuador. Respir Med. 2006;100:1208–15. doi: 10.1016/j.rmed.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rumchev K, Zhao Y, Spickett J. Relationship of pulmonary function among women and children to indoor air pollution from biomass use in rural ecuador. Respir Med. 2006;100:1208–15. doi: 10.1016/j.rmed.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balakrishnan K, Sankar S, Parikh J, Padmavathi R, Srividya K, Venugopal V, et al. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of Southern India. Environ Health Perspect. 2002;110:1069–75. doi: 10.1289/ehp.021101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devakumar D, Qureshi Z, Mannell J, Baruwal M, Sharma N, Rehfuess E, et al. Women's ideas about the health effects of household air pollution, developed through focus group discussions and artwork in Southern Nepal. Int J Environ Res Public Health. 2018;15 doi: 10.3390/ijerph15020248. pii: E248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8:166–75. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulin L, Hansel N. Particulate air pollution and impaired lung function. F1000Res. 2016;5 doi: 10.12688/f1000research.7108.1. pii: F1000 Faculty Rev-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilbert JJ. The world health report 2002 – Reducing risks, promoting healthy life. Educ Health (Abingdon) 2003;16:230. doi: 10.1080/1357628031000116808. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal S, Yamamoto S. Effect of indoor air pollution from biomass and solid fuel combustion on symptoms of preeclampsia/eclampsia in Indian women. Indoor Air. 2015;25:341–52. doi: 10.1111/ina.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander D, Northcross A, Wilson N, Dutta A, Pandya R, Ibigbami T, et al. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am J Respir Crit Care Med. 2017;195:1629–39. doi: 10.1164/rccm.201606-1177OC. [DOI] [PubMed] [Google Scholar]
- 11.Kurmi OP, Semple S, Devereux GS, Gaihre S, Lam KB, Sadhra S, et al. The effect of exposure to biomass smoke on respiratory symptoms in adult rural and urban Nepalese populations. Environ Health. 2014;13:92. doi: 10.1186/1476-069X-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asante KP, Kinney P, Zandoh C, Vliet EV, Nettey E, Abokyi L, et al. Childhood respiratory morbidity and cooking practices among households in a predominantly rural area of Ghana. Afr J Infect Dis. 2016;10:102–10. doi: 10.21010/ajid.v10i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhabra P, Sharma G, Kannan AT. Prevalence of respiratory disease and associated factors in an urban area of Delhi. Indian J Community Med. 2008;33:229–32. doi: 10.4103/0970-0218.43227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behera D, Jindal SK. Respiratory symptoms in Indian women using domestic cooking fuels. Chest. 1991;100:385–8. doi: 10.1378/chest.100.2.385. [DOI] [PubMed] [Google Scholar]
- 15.Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013;137:87–94. [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Revathi M, Kutty TK, Annamalai N. Pulmonary function in rural women exposed to biomass fuel. J Pulm Respir Med. 2012;2:133. [Google Scholar]
- 18.Nayek S, Padhy PK. Approximation of personal exposure to fine particulate matters (PM2.5) during cooking using solid biomass fuels in the kitchens of rural West Bengal, India. Environ Sci Pollut Res Int. 2018;25:15925–33. doi: 10.1007/s11356-018-1831-7. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Du W, Chen Y, Shen G, Su S, Lin N, et al. Household air pollution and personal inhalation exposure to particles (TSP/PM2.5PM1.0PM0.25) in rural Shanxi, North China. Environ Pollut. 2017;231:635–43. doi: 10.1016/j.envpol.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 20.Mbatchou Ngahane BH, Afane Ze E, Chebu C, Mapoure NY, Temfack E, Nganda M, et al. Effects of cooking fuel smoke on respiratory symptoms and lung function in semi-rural women in cameroon. Int J Occup Environ Health. 2015;21:61–5. doi: 10.1179/2049396714Y.0000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majdan M, Svaro M, Bodo J, Taylor M, Muendo RM. Assessment of the biomass related indoor air pollution in kwale district in Kenya using short term monitoring. Afr Health Sci. 2015;15:972–81. doi: 10.4314/ahs.v15i3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sehgal M, Rizwan SA, Krishnan A. Disease burden due to biomass cooking-fuel-related household air pollution among women in India. Glob Health Action. 2014;7:25326. doi: 10.3402/gha.v7.25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurmi OP, Devereux GS, Smith WC, Semple S, Steiner MF, Simkhada P, et al. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013;41:25–30. doi: 10.1183/09031936.00220511. [DOI] [PubMed] [Google Scholar]
- 24.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–60. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Lee J, Park YS, Lee CH, Yoon HI, Lee SM, et al. Age-related annual decline of lung function in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:51–60. doi: 10.2147/COPD.S95028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu K, Qiu G, Chan KH, Lam KH, Kurmi OP, Bennett DA, et al. Association of solid fuel use with risk of cardiovascular and all-cause mortality in rural China. JAMA. 2018;319:1351–61. doi: 10.1001/jama.2018.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MN, B Nurs CZ, Mofizul Islam M, Islam MR, Rahman MM. Household air pollution from cooking and risk of adverse health and birth outcomes in Bangladesh: A nationwide population-based study. Environ Health. 2017;16:57. doi: 10.1186/s12940-017-0272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Accelerate Efforts to Address air Pollution. World Health Organization; 2018. [Last accessed on 2018 Dec 01]. Available from: http://www.searo.who.int/mediacentre/releases/2018/1687/en/ [Google Scholar]
- 30.Balakrishnan K, Dey S, Gupta T, Dhaliwal RS, Brauer M, Cohen AJ, et al. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The global burden of disease study 2017. Lancet Planet Health. 2019;3:e26–39. doi: 10.1016/S2542-5196(18)30261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agrawal S. Effect of indoor air pollution from biomass and solid fuel combustion on prevalence of self-reported asthma among adult men and women in India: Findings from a nationwide large-scale cross-sectional survey. J Asthma. 2012;49:355–65. doi: 10.3109/02770903.2012.663030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KR, Frumkin H, Balakrishnan K, Butler CD, Chafe ZA, Fairlie I, et al. Energy and human health. Annu Rev Public Health. 2013;34:159–88. doi: 10.1146/annurev-publhealth-031912-114404. [DOI] [PubMed] [Google Scholar]
- 33.Mishra V. Effect of indoor air pollution from biomass combustion on prevalence of asthma in the elderly. Environ Health Perspect. 2003;111:71–8. doi: 10.1289/ehp.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.India State-Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: The global burden of disease study 1990-2016. Lancet Glob Health. 2018;6:e1363–74. doi: 10.1016/S2214-109X(18)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilishvili T, Loo JD, Schrag S, Stanistreet D, Christensen B, Yip F, et al. Effectiveness of six improved cookstoves in reducing household air pollution and their acceptability in rural Western Kenya. PLoS One. 2016;11:e0165529. doi: 10.1371/journal.pone.0165529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O, et al. Improved biomass stove intervention in rural Mexico: Impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180:649–56. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 37.Thomas E, Wickramasinghe K, Mendis S, Roberts N, Foster C. Improved stove interventions to reduce household air pollution in low and middle income countries: A descriptive systematic review. BMC Public Health. 2015;15:650. doi: 10.1186/s12889-015-2024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]