Abstract
Background
TK2 is a nuclear gene encoding the mitochondrial matrix protein thymidine kinase 2 (TK2), a critical enzyme in the mitochondrial nucleotide salvage pathway. Deficiency of TK2 activity causes mitochondrial DNA (mtDNA) depletion, which in humans manifests predominantly as a mitochondrial myopathy with onset typically in infancy and childhood. We previously showed that oral treatment of the Tk2 H126N knock-in mouse model (Tk2−/−) with the TK2 substrates, deoxycytidine (dCtd) and thymidine (dThd), delayed disease onset and prolonged median survival by 3-fold. Nevertheless, dCtd + dThd treated Tk2−/− mice showed mtDNA depletion in brain as early as postnatal day 13 and in virtually all other tissues at age 29 days.
Methods
To enhance mechanistic understanding and efficacy of dCtd + dThd therapy, we studied the bioavailability of dCtd and dThd in various tissues as well as levels of the cytosolic enzymes, TK1 and dCK that convert the deoxynucleosides into dCMP and dTMP.
Findings
Parenteral treatment relative to oral treatment produced higher levels of dCtd and dThd and improved mtDNA levels in liver and heart, but did not ameliorate molecular defects in brain or prolong survival. Down-regulation of TK1 correlated with temporal- and tissue-specificity of response to dCtd + dThd. Finally, we observed in human infant and adult muscle expression of TK1 and dCK, which account for the long-term efficacy to dCtd + dThd therapy in TK2 deficient patients.
Interpretations
These data indicate that the cytosolic pyrimidine salvage pathway enzymes TK1 and dCK are critical for therapeutic efficacy of deoxynucleoside therapy for Tk2 deficiency.
Fund
National Institutes of Health P01HD32062.
Keywords: Thymidine kinase 2, TK2, mtDNA depletion syndrome, Nucleoside, Deoxycytidine, Thymidine
Research in context.
Evidence before this study
Thymidine kinase 2 (TK2) deficiency is a devastating disorder that typically manifests in infancy or early childhood as a myopathy. We previously showed that treatment with deoxypyrimidine monophosphate or pyrimidine deoxynucleoside delays disease onset and prolongs survival of the Tk2 knock-in mouse. To improve efficacy of deoxynucleoside treatment in the TK2 mouse, we have investigated the molecular mechanism of this therapy. To gather further evidence, we performed searches in PubMed, including, but not limited, to the terms: “deoxynucleoside transporter”, “deoxynucleoside and blood brain barrier”, “TK2 deficiency”, “Mitochondrial DNA depletion” and “mitochondrial myopathy”.
Added value of this study
In this paper, together with Blazquez et al., we describe the mechanisms responsible for the incomplete response of the Tk2 knock-in mice to deoxynucleoside treatment. We showed that levels of the cytosolic kinases Tk1 and Dck in target tissues are critical in dictating response to deoxycytidine (dCtd) and deoxythymidine (dThd) therapy in Tk2 deficiency. In addition, we showed that, in the presence of these kinases, improved delivery of the drugs produced higher levels of dCtd and dThd and improved response in multiple tissues, but not in brain.
Implications of all the available evidence
TK2 deficiency is a progressive and often fatal disease. Under a compassionate use protocol, dCtd and dThd have shown evidence of efficacy in TK2 deficient patients [38] leading to a phase 2 clinical trial (NCT03845712). Because long-term efficacy has not been established, it is critical to better understand all the factors contributing to the response to deoxynucleoside therapy for TK2 deficiency. Furthermore, patients affected with other disorders due to unbalanced mitochondrial nucleotide pools may benefit from analogous treatments based upon experience gained with the dCtd and dThd for TK2 deficiency.
Alt-text: Unlabelled Box
1. Introduction
In dividing cells, pyrimidine nucleotides for DNA replication are synthesized by both de novo and cell cycle-dependent cytosolic salvage pathways (Fig. 1) [1]. The de novo pathway involves conversion of ribonucleotide diphosphates (rNDP) to deoxynucleoside diphosphates (dNDP) via ribonucleotide reductase whereas salvage pathways serially phosphorylate deoxnucleoside to generate deoxynucleoside triphosphate (dNTPs). In contrast to replicating cells, quiescent cells downregulate the cytosolic pathways and instead, rely on the mitochondrial salvage pathway for replication and maintenance of mitochondrial DNA (mtDNA). Thymidine kinase 2 (TK2), which is essential for mitochondrial pyrimidine salvage, phosphorylates deoxycytidine (dCtd) and thymidine (deoxythymidine [dThd]) to generate deoxycytidine monophosphate (dCMP) and deoxythymidine monophosphate (dTMP), which are subsequently phosphorylated to dCTP and dTTP and incorporated into mtDNA during replication. Mutations in the nuclear gene TK2, impairing TK2 activity cause dNTP pool imbalances that ultimately lead to mtDNA depletion and multiple deletions as well as deficiencies in oxidative phosphorylation (OxPhos) enzyme activities and levels. TK2 deficiency is inherited as an autosomal recessive disorder and manifests predominantly as a mitochondrial myopathy during infancy or childhood leading to early death [2,3], although there are cases of adult-onset myopathy [4]. In fact, recent reports are expanding the phenotypic spectrum of TK2 deficiency, which spans three major clinical presentations: 1) Infantile-onset myopathy (~40% of cases) characterized by severe mtDNA depletion in muscle and early mortality (median survival of 20 months); 2) Childhood-onset (1–12 years-old) myopathy (~40% of cases) also with severe muscle mtDNA depletion but longer survival (>9 years) compared to the infantile-onset presentation; and 3) Late-onset myopathy (~20% of cases) with mild weakness at onset and slow progression, multiple mtDNA deletions, reduced mtDNA copy number, or both in muscle and a median survival of 50 years [5]. A recent review indicates that the late-onset form may have a worse prognosis than initially reported due to the high risk of early and progressive respiratory insufficiency [6].
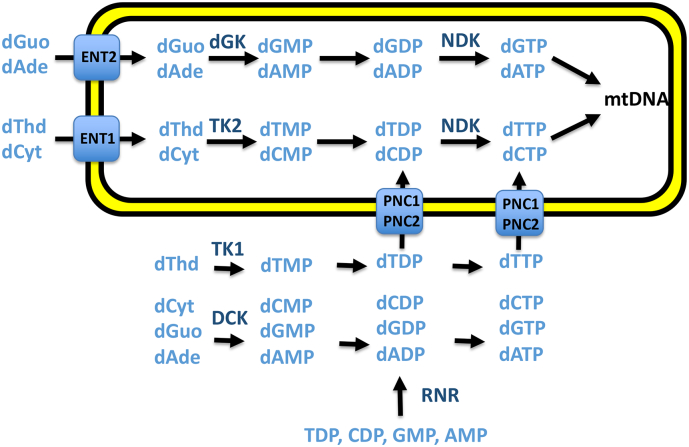
Fig. 1.
Deoxynucleotide salvage and de novo synthesis pathways. In the deoxynucleotide salvage pathways, thymidine (dThd) is phosphorylated by thymidine kinase 1 (TK1) in cytosol and by thymidine kinase 2 (TK2) in mitochondria. In contrast, deoxycytidine (dCtd) is phosphorylated by deoxycytidine kinase (DCK) in cytosol and by TK2 in mitochondria. The purine nucleosides are phosphorylated by DCK in cytosol and by deoxyguanoside kinase (DGK) in mitochondria. In the de novo pathway, ribonucleoside diphosphates are converted to deoxynucleoside diphosphates by ribonucleotide reductase (RNR). Equilibrative nucleoside transporter 1 (ENT1) and 2 (ENT2) transport pyrimidine and purine nucleosides across mitochondrial membranes. Purine and pyrimidine nucleotide carrier 1 (PNC1) and 2 (PNC2) transport deoxynucleoside diphosphates and deoxynucleoside triphosphates across mitochondrial membranes.
We previously generated a TK2 H126N knock-in mouse model (Tk2−/−) which recapitulates molecular and biochemical features of TK2 deficiency patients [7]. Although brain and spinal cord are the most affected tissues in the mouse model, patients manifest predominantly a myopathy. This mouse model has been useful for developing therapies for TK2 deficient patients as well as to better understand the mechanisms of the disease and mechanisms of action of therapies [8,9]. In previous studies, we showed that oral treatment of the Tk2 H126N knock-in mouse (Tk2−/−) with dCtd + dThd (each at 520 mg/kg/day) delayed disease onset and prolonged the median lifespan of the animals by 3-fold [8]. We also demonstrated efficacy of dTMP + dCMP therapy in Tk2−/− mice; however, rapid catabolism of these deoxynucleotides to dCtd + dThd indicated that deoxynucleosides are the active therapeutic agents [9]. Nevertheless, with both therapies we observed mtDNA depletion in brain as early as at postnatal day 13 (P13) as well as in other tissues at P29. In contrast, small intestine showed a robust therapeutic response to this treatment, with correction of mtDNA depletion at P29. The fact that the treatment was orally administered, which presumably lead to a higher bioavailability of dCtd and dThd in intestine relative to other tissues, likely accounted for the strong response. Furthermore, we previously observed that thymidine phosphorylase (TP) activity, which catabolizes dThd, increases dramatically from P13 to P29 in small intestine [9]. This high TP activity may partially account for the diminishing response to treatment in mice during this period, as the bioavailability of the nucleosides decreases in other tissues. In this study, we have observed that parenteral dCtd + dThd administration was more efficient than oral treatment at raising levels of nucleosides in blood and liver as well as rescuing levels of mtDNA in liver and heart. Nevertheless, parenteral treatment did not improve the therapeutic response to dCtd + dThd in brain, even when bioavailability was further increased using Focused Ultrasound (FUS)-induced Blood Brain Barrier (BBB) opening. In fact, we observed that TK1 protein levels in brain, and not nucleoside levels, were the limiting factor of the therapeutic response in Tk2−/− mouse brain. Thus, both extrinsic factors (treatment route and nucleoside bioavailability) and intrinsic factors (catabolic and metabolic enzyme levels) contribute to the effectiveness of dCtd + dThd therapy in TK2 deficiency.
2. Materials and methods
2.1. Mice
Generation and characterization of Tk2 H126N knock-in mice were previously reported [7]. All experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the Columbia University Medical Center, and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed and bred according to international standard conditions, with a 12-h light, 12-h dark cycle.
2.2. Treatment administration and experimental plan
To assess the pharmacokinetics of oral, intraperitoneal (IP), and intravenous (IV, via tail vein) treatment, 6-month-old Tk2+ mice received one dose of deoxycytidine (dCtd) and deoxythymidine (dThd) (Hongene Biotechnology, Inc.) at a concentration of 520 mg/kg in 100 μl of either autoclaved water for oral gavage or phosphate buffered saline (PBS) 1× for IP or IV injection. Blood for high performance liquid chromatography (HPLC) measurements were collected from the retro-orbital sinus before treatment and up to two additional samples for each mouse covering the following time-points: T = 0, 5 min, 15 min, 30 min, 45 min, 60 min, 2 h, 6 h, and 24 h. At least two mice were euthanized at each time-point; blood, brain, and liver were collected for HPLC measurements.
To assess the therapeutic efficacy of IP dCtd + dThd therapy or IP dThd therapy, doses of 520 mg/kg of each nucleoside were prepared in 50 μl of autoclaved PBS 1× and administered daily to Tk2 H126N knockin mice (Tk2−/−) and age-matched controls (Tk2+) starting at postnatal day 4. At age 21 days, mice were separated from their mothers and treatment was continued with doses prepared in 100 μl of PBS 1×.Mutant and control Tk2+ mice were weighed and observed daily. Mice were either euthanized at postnatal day 13 or 29 for molecular and biochemical characterization, or continued under treatment and followed for assessment of survival. Organs (brain, liver, heart, kidney, small intestine, and quadriceps muscle) were removed and frozen in the liquid phase of isopentane, pre-cooled near its freezing point (−160 °C) with dry ice. All the experiments were performed with at least 3 mice per group. Both heterozygous and homozygous Tk2 wild-type mice were considered as control group (Tk2+) since no clinical and biochemical differences were observed previously [7,10].
To assess safety and efficacy of nucleoside delivery to the brain with FUS-induced BBB opening, untreated Tk2+ mice received pulsed FUS beam targeted transcranially to the left hemisphere. Mice were either euthanized at postnatal day 29 for safety assessment (brain hematoxylin and eosin staining) or after 15 min, 1 h, 2 h, 6 h, or 24 h of treatment for nucleoside concentrations measurement in each brain hemisphere as well as in liver (measured by HPLC).
To assess the therapeutic efficacy of FUS-induced BBB opening, Tk2−/− mice were treated with 520 mg/kg of dCtd + dThd administered in 50 μl of autoclaved PBS 1× by daily IP injection, starting at postnatal day 4. At age 21 days, mice were separated from their mother and received two pulses of FUS in combination with lipid microbubbles (described below). mtDNA copy number was determined.
2.3. Phenotype assessment
Body weight was assessed daily, because we previously observed that plateauing of weight at P10 was the first sign of disease and followed by head tremor [7]. To define the degree of safety and efficacy of each therapy, we compared survival time, age-at-onset of disease, type and severity of abnormal signs, side-effects, and proportion of treatment termination due to adverse events in treated and untreated Tk2−/− mice. Behavior, survival time, and body weights of the mice were assessed daily beginning at postnatal day 4.
2.4. dNTP pool by polymerase extension assay
Mitochondrial levels of dATP, dGTP, dCTP, and dTTP were measured using a radioactivity assay as previously described [11,12] with one modification; reactions were spotted in Zeta-Probe® GT Genomic Tested Blotting Membranes (Biorad, Hercules, CA, USA) instead of Whatman DE81 discs (GE Healthcare Life Sciences, UK).
2.5. Nucleosides measurements by HPLC
Deoxythymidine (dThd), deoxycytidine (dCtd) deoxyuridine (dU), uracil (U), and thymine (Thy) levels were assessed by a gradient-elution HPLC method as described [13,14], with minor modifications. Briefly, deproteinized samples were injected into an Alliance HPLC system (Waters Corporation, Milford, MA) with an Alltima C18NUC reversed-phase column (Grace AllTech Corporation, Columbia, MD) at a constant flow rate of 1.5 ml/min (except where indicated) using four buffers: eluent A (20 mM potassium phosphate, pH 5.6), eluent B (water) and eluent C (methanol). Samples were eluted over 60 min with a gradient as follows: 0–5 min, 100% eluent A; 5–25 min, 100–71% eluent A, 29% eluent B; 25–26 min, 0–100% eluent C; 26–30 min, 100% eluent C; 30–31 min, 0–100% eluent B; 31–35 min, 100% eluent B (1.5–2 ml/min); 35–45 min, 100% eluent B (2 ml/min); 45–46 min, 100% eluent B (2–1.5 ml/min); 46–47 min, 0–100% eluent C; 47–50 min, 100% eluent C; 50–51 min, 0–100% eluent A; and 51–60 min, 100% eluent A.
Absorbance of the elutes were monitored at 267 nm and dThd and dU peaks were quantified by comparing their peak areas with a calibration curve obtained with standards in aqueous solution. For definitive identification of dThd, dU, U, and T peaks for each sample, we used a second aliquot treated with an excess of purified E. coli TP (Sigma) to specifically catabolize dThd to T and dU to U. The lower detection limit of this method is 0·05 mmol/l for all nucleosides. Results were expressed as nmol/mg of protein.
2.6. RT-qPCR: mitochondrial DNA quantification
mtDNA levels were assessed as previously described [10] by real-time PCR using the ddCt method in a Step One Plus Real Time PCR System (Applied Biosystems). For murine COX I (mtDNA), we used the following primers and probes:
Forward: 5′-TGCTAGCCGCAGGCATTACT-3′
Reverse: 5′-CGGGATCAAAGAAAGTTGTGTTT-3
MGB-FAM detection probe: 5′-TACTACTAACAGACCGCAACC-3′
For mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH, nuclear DNA [nDNA]) we used the GAPDH Endogenous Control assay (catalog number 4352339E, Applied Biosystems, Invitrogen, Foster City, CA).. mtDNA values were normalized to nDNA values and expressed as a percentage relative to wild-type (100%).
2.7. Human muscle samples
Frozen human muscle specimens were obtained as diagnostic leftovers from one infant and three adult non-mitochondrial myopathy patients. Informed consent was obtained from each patient. Muscle samples (20–40 mg) were homogenized using 0·9–2·0 mm Stainless steel beads and the Bullet Blender Storm (Next Advance Inc.) in CPT medium (10% W/V) containing 50 mM Tris HCl, 100 mM NaCl, 1 mM EDTA, 0·5% SDS and pH adjusted to 7·4. After homogenization, samples were centrifuged at 2500 rpm for 20 min and supernatants were used for immunoblotting.
2.8. Immunoblotting
To assess protein levels of the mitochondrial respiratory chain complexes, thirty micrograms of whole mouse brain extracts were obtained using the same protocol as for human muscle samples and electrophoresed in 10–20% Tris-Glycine Gel (Novex™ WedgeWell™, ThermoFisher Scientific Invitrogen, Waltham, MA) and transferred to Immun-Blot™ PVDF membranes (Bio-Rad, Hercules, CA). Membranes were probed with MitoProfile® Total OXPHOS Rodent WB Antibody Cocktail of antibodies (MitoScience LLC Cat# MS604, RRID:AB_2629281) against CI subunit Ndufb8, CII-30 kDa, CIII-Core protein 2, CIV subunit I, and CV alpha subunit. For assessment of mouse TK1 and dCK protein levels, 20 μg of brain, heart, liver, intestine, and muscle were electrophoresed and membranes were probed with rabbit anti-TK1 (SAB2107332, Sigma-Aldrich, St Louis, MO) and rabbit anti-dCK (Sigma-Aldrich Cat# SAB2100535, RRID:AB_10599413). For assessment of TK1 protein levels in human samples, 20 μg of tissue were electrophoresed and membranes were probed with rabbit anti-TK1 (Abcam Cat# ab76495, RRID:AB_1524488). In all membranes, levels of vinculin were assessed as loading control (Abcam Cat# ab18058, RRID:AB_444215). Protein–antibody interaction was detected with either anti-mouse (Sigma-Aldrich Cat# A0168, RRID:AB_257867) or anti-rabbit (Sigma-Aldrich Cat# A2074, RRID:AB_257972) peroxidase-conjugated mouse IgG antibody, using Amersham™ ECL Plus western blotting detection system (GE Healthcare Life Sciences, UK). Quantification of proteins were carried out using NIH ImageJ 1.37 V software. Average grey value was calculated within selected areas as the sum of the grey values of all the pixels in the selection divided by the number of pixels.
2.9. Mitochondrial OxPhos enzyme activities by spectrophotometer analysis
Mitochondrial OxPhos enzymes analysis was performed in cerebrum tissue as previously described [15,16].
2.10. Focused ultrasound blood brain barrier opening
The experimental FUS system was previously described [[17], [18], [19]]. Briefly, the targeting procedure utilized a grid system to locate skull sutures [[20], [21], [22]]. The FUS focus was placed 3 mm below the skull taking into account 18% attenuation for acoustic pressure loss through the skull [[20], [21], [22]]. For experiments on safety and nucleoside delivery to the brain, each animal received pulsed FUS beams (pulse length 6·56 ms; duration 2 min) at acoustic pressures of 225, 300 and 450 kPa, targeted transcranially 1·2 mm anterior and 0·3 mm left of the lambda suture, while the right hemisphere served as a control. Hence, each animal served as its own control, thereby reducing the variability caused by physiologic differences among animals. For assessments of mtDNA copy numbers, each animal received two pulsed FUS beams, targeting 1·2 mm anterior and 0·3 and 0·5 mm left to lambda, at acoustic pressures of 300 kPa.
Lipid-coated microbubbles (4–5 μm) were synthesized as previously described [23]. Prior to microbubbles administration, a 30-s sonication using the same acoustic settings described above was applied in order to measure the baseline background signal required for acoustic emission analysis. The injected microbubbles were freshly diluted before each injection. A bolus of 50 μl diluted microbubbles in saline solution (8 × 108 microbubbles/ml) was injected intravenously through the tail vein immediately before sonication, together with a dose of 50 μl of dCtd + dThd, so each animal received 520 mg/kg of dCtd + dThd.
2.11. Statistical methods
Data are expressed as the mean ± standard deviation (SD) of at least 3 experiments per group. For data grouped in columns, Mann-Whitney test was used to compare each group. For survival curves, Mantel-Cox test was used to compare groups. A p-value of <0·05 was considered to be statistically significant.
2.12. Data availability
The raw data that support the findings of this study and protocols used are available from the corresponding author, upon reasonable request.
3. Results
3.1. Parenteral treatment raises levels of nucleosides in blood and liver more effectively than in brain
The highest levels of dCtd and dThd in plasma, were observed after IV administration, followed by IP injection, and oral gavage. For both compounds, the peaks of maximum concentration in plasma were at 5 min post-IV injection and 20 min after IP injection. Levels of dCtd were only slightly elevated after oral gavage (<1% of levels reached with either IV and IP injection). In contrast, dThd peak concentrations in plasma were attained 1 h after oral treatment, and were 2–5% of peak levels reached after either IV or IP injections. Plasma concentrations of dU (peak of 1·3 mM 2 h after IP injection) and Thy (peak of 27 μM 1 h after IP injection) were increased in plasma after IV and IP treatment, indicating robust deoxynucleoside catabolism of dCtd to dU by cytidine deaminase (CDA) and dThd to Thy by TP (Fig. 2). Because peak levels of dU were higher than Thy, activity of CDA appears to be higher than for TP.
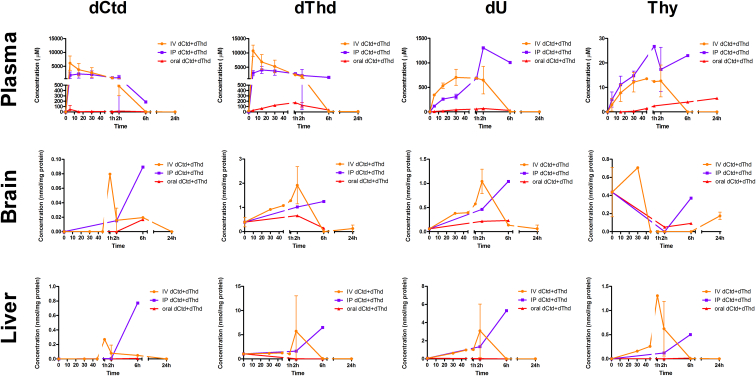
Fig. 2.
Pharmacokinetics of dCtd + dThd treatment. Concentration of dCtd (deoxycytidine), dThd (thymidine), dU (deoxyuridine) and thymine after administration of 520 mg/kg of dCtd + dThd using oral gavage (red line) intra-peritoneal injection (blue line) or intra-venous injection (orange line). Concentrations are either as μM (plasma) or nmol/mg of protein (tissues). Time points < 1 h after treatment are represented in minutes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Levels of dCtd in brain peaked at 1 h after IV injection and at 6 h after IP injection and oral gavage. Maximum levels of dCtd were 4–5 fold higher after parenteral administration compared to the oral treatment (Fig. 2). Levels of dThd in brain were also elevated with peaks at 2 h after administration via all routes. Paradoxically, although IP and IV treatment provided higher concentrations of dThd in brain, cerebral concentrations of dThd after oral gavage was in the same order of magnitude as that reached by parenteral routes indicating that most of the deoxynucleosides in plasma after IV or IP treatment did not reach the brain (Fig. 2). Although similar concentrations of dCtd and dThd in plasma after parenteral treatment (4–10 mM), levels of dThd in brain were 10-fold higher than levels of dCtd (1–2 nmol/mg vs 0·08–0·1 nmol/mg). The higher concentrations of dThd relative to dCtd in the brain is likely due to the higher catabolic activity of CDA relative to TP.
In liver, maximum levels of dCtd were reached 1 h after IV injection and 6 h after IP injection. Oral gavage slightly raised dCtd levels 6 h after treatment; however, this increase was <10% compared to IP administration and <1% after IV treatment. Maximum levels of dThd were reached 2 h after IV and 6 h after IP injection. Two hours after oral gavage, peak dThd concentration was 0·1–0·5% of levels reached after IP and IV treatment (Fig. 2). As observed in brain, concentrations of dThd in liver were 10-fold higher than dCtd. Peak levels of dCtd and dThd in liver after IP injection were 8·6- and 5·2-fold higher than in brain, while peak levels of dCtd and dThd after IV injection were 3·3- and 3·0-fold higher in liver than in brain, indicating that despite nucleoside transport across the BBB, uptake efficiency is lower for brain than for visceral organs.
3.2. IP dCtd + dThd therapy improves molecular and biochemical features in tissues except brain
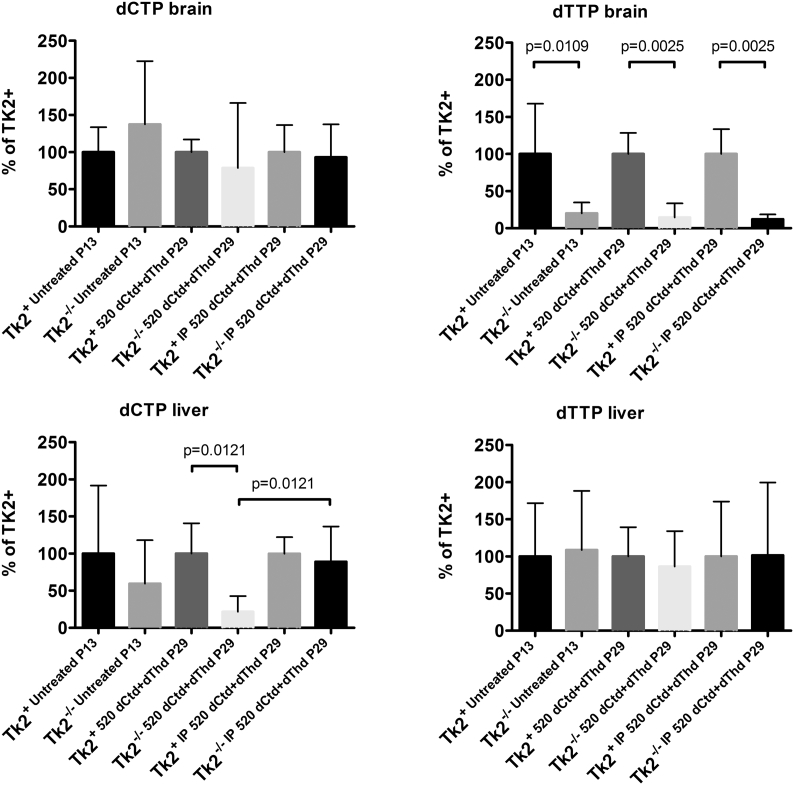
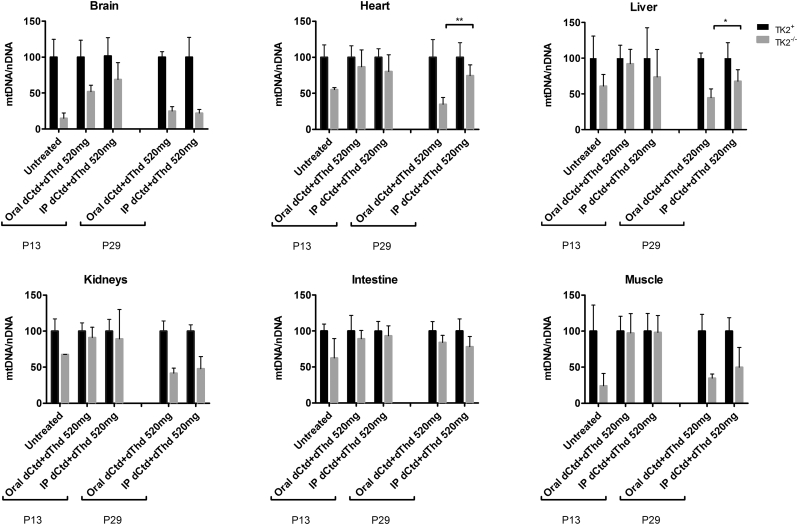
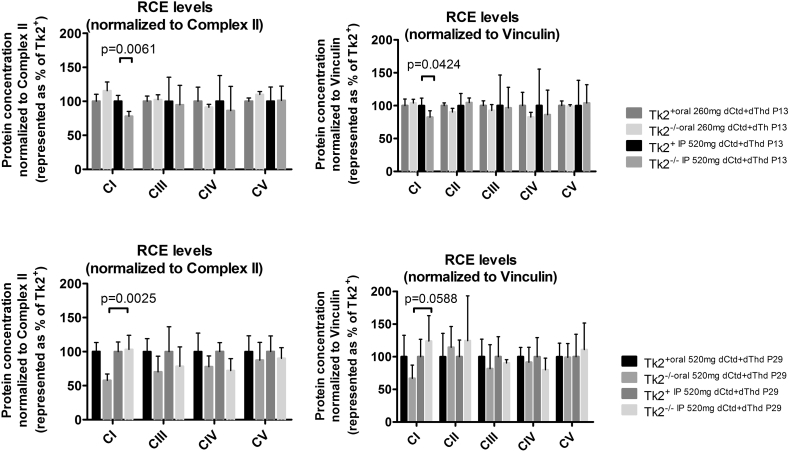
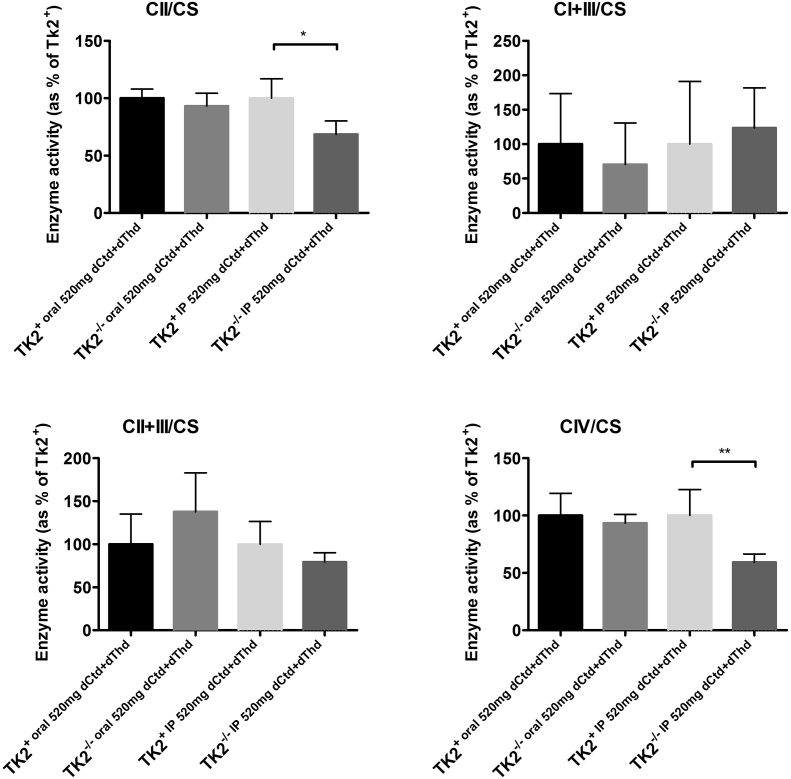
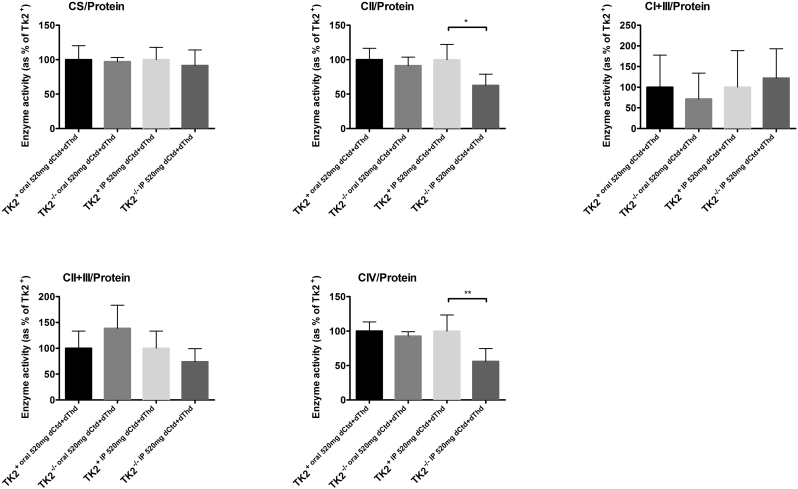
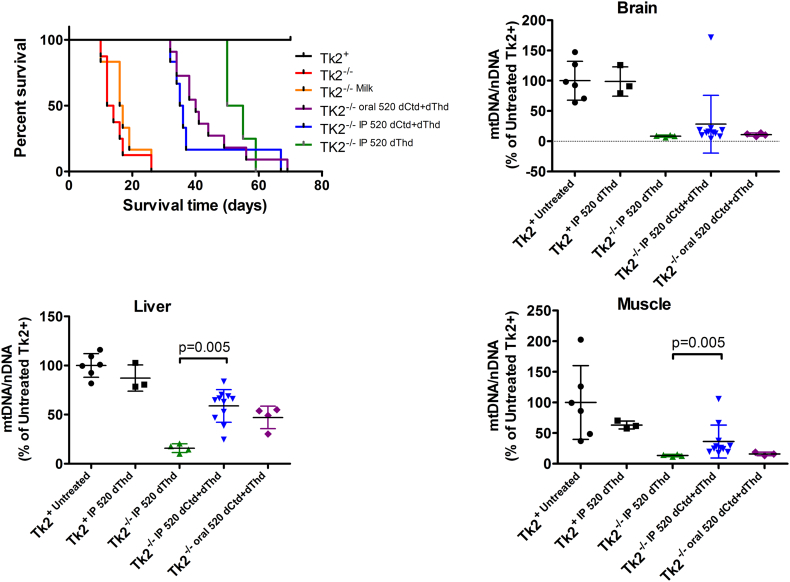
Because parenteral treatment more efficiently raised levels of dCtd and dThd in plasma, liver, and brain relative to oral gavage, we assessed molecular effects of IP treatment of dCtd + dThd (each at 520 mg/kg/day) in the Tk2−/− mice starting at postnatal day 4. Both oral and IP treatment with dCtd + dThd failed to elevate mitochondrial levels of dTTP in the brain at postnatal day 29 compared to treated Tk2+ mice (oral: 14·8 ± 18·7% vs 100 ± 28·5% in Tk2+, p = 0·0025; IP: 12·3 ± 6·3% vs 100 ± 33·6% in Tk2+, p = 0·0025) (Fig. 3). IP treatment only slightly increased levels of mtDNA copy number compared to oral treatment at postnatal day 13 in the brain (68·9 ± 23·5% with IP treatment vs 52·0 ± 9·0% with oral treatment, p > 0·05) but not at postnatal day 29 (22·1 ± 5·1% vs 25·1 ± 6·1%, p > 0·05) (Fig. 4). Despite similar levels of mtDNA copy number in brain, IP treatment was more efficient at rescuing levels of RCE complex I in Tk2−/− mice at postnatal day 29, compared to oral treatment (normalized to complex II: 103·2 ± 20·9% with IP treatment vs 57·7 ± 9·4%, with oral treatment, p = 0·0025; normalized to vinculin: 124·1 ± 38·8% with IP treatment vs 67·0 ± 20·2%, with oral treatment, p = 0·059) (Supplementary Fig. 1). Nevertheless, deficiencies in the activities of both complex II (normalized by CS: 68·4 ± 11·8%, p = 0·036; normalized to protein concentration: 62·6 ± 16·3%, p = 0·036) and complex IV (normalized by CS: 59·4 ± 7·0%, p = 0·009; normalized to protein concentration: 56·1 ± 18·6%, p = 0·009) were observed in Tk2−/− IP 520 dCtd+dThd (Supplementary Fig. 2, Supplementary Fig. 3).
Fig. 3.
Levels of dCTP and dTTP in brain and liver. Values are expressed as percents of levels in Tk2+mice for each treatment. P values < .05 by Mann Whitney tests comparing dNTP levels in Tk2+versus Tk2−/−mice within each treatment are indicated.
Fig. 4.
Levels of Mitochondrial DNA. Values are represented as percents of mtDNA levels in Tk2+ mice within each treatment. P values < 0·05 (*) or <0·01 (**) from Mann Whitney tests between orally- and IP-treated Tk2−/−are indicated.
Supplementary Fig. 1.
Protein levels of the mitochondrial respiratory chain complexes. Brain extracts from mice euthanized either at age 13 days (upper graphs) or 29 days old (lower graphs) were loaded. Values were normalized using either complex II (left) or vinculin (right). P values of Mann Whitney tests are represented as one (p < 0·05), two (p < 0·01), three (p < 0·001) or four (p < 0·0001) stars.
Supplementary Fig. 2.
Mitochondrial OxPhos enzyme activities normalized to CS activity. Values are expressed as percentage of the Tk2+ mice treated with dCtd + dThd via IP injection. P values of Mann Whitney tests are represented as one (p < 0·05), two (p < 0·01) stars.
Supplementary Fig. 3.
Mitochondrial OxPhos enzyme activities at age 29 days normalized to protein concentration. Values are expressed as percentage of the Tk2+ mice treated with dCtd + dThd via IP injection. P values of Mann Whitney tests are represented as one (p < 0·05), two (p < 0·01) stars.
In contrast, IP injection, and not oral gavage, efficiently restored mitochondrial levels of dCTP in liver from Tk2−/− mice at postnatal day 29 (oral: 21·7 ± 21·1% vs 100 ± 40·1% in Tk2+, p = 0·012; IP: 89·0 ± 47·6% vs 100 ± 22·3% in Tk2+, p > 0·05; Tk2−/− oral dCtd+dThdvs Tk2−/− IPdCtd+dThd, p = 0·012) (Fig. 4). Furthermore, mtDNA copy number at postnatal day 29 were higher in Tk2−/− IPdCtd+dThd compared to Tk2−/− oraldCtd+dThd in liver (67·8 ± 16·0% with IP treatment vs 44·9 ± 12·2% with oral treatment, p = 0·030), heart (74·5 ± 14·9% with IP treatment vs 34·8 ± 9·4% with oral treatment, p = 0·0025) and skeletal muscle (49·8 ± 27·6% with IP treatment vs 34·8 ± 5·7% with oral treatment, p > 0·05) (Fig. 4).
3.3. IP dCtd + dThd therapy does not modify disease course of the Tk2−/− mice compared to oral treatment
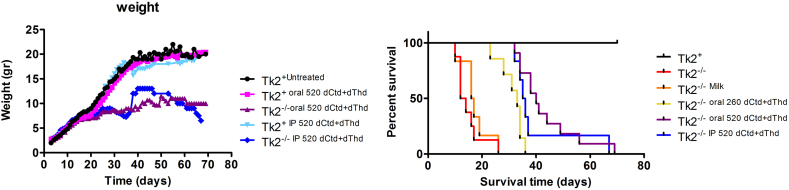
To test whether the rise in mtDNA levels in visceral organs and skeletal muscles of mice given IP treatment had any effect on disease course, we assessed growth rate and survival time. Both Tk2−/− IP dCtd+dThd and Tk2−/− oral dCtd+dThd animals gained weight at the same rate as Tk2+ mice until postnatal day 20, when Tk2−/− growth rate slowed regardless the treatment route (Supplementary Fig. 4). There were no statistically significant differences in survival between IP and oral treatment (Supplementary Fig. 4), although a mild trend towards longer survival was observed in mice with oral treatment (Tk2−/− IP dCtd+dThd, 40·2 ± 13·3 vs Tk2−/− oraldCtd+dThd, 43·2 ± 11·1; p = 0·32). These results demonstrated that IP dCtd + dThd treatment did not delay disease-onset or improve survival time compared to oral therapy despite amelioration of the molecular defects in visceral organs and skeletal muscle. These findings indicate that disease progression in the brain is responsible for both disease-onset and early death. Both Tk2+ IP dCtd+dThd and Tk2+ oraldCtd+dThd showed similar growth rates as untreated Tk2+; furthermore, they appeared normal and showed no signs of side-effects related to the treatment.
Supplementary Fig. 4.
Weight and survival of Tk2−/− mice treated with dCtd + dThd via IP. Weight (left panel) is expressed as the average weight in grams each day. Survival (right panel) is represented as percent survival within each treatment group.
3.4. FUS-induced BBB opening improves delivery of dCtd and dThd into the brain
To assess whether the lack of improvement of Tk2−/− animals with IP 520 mg/kg/day of dCtd + dThd therapy relative to oral therapy was due to poor penetration of dCtd and dThd through the BBB, we first tested whether levels of dCtd and dThd in brain after IV injection of 520 mg could be enhanced using FUS-induced BBB opening in combination with lipid microbubbles compared to IV dCtd + dThd treatment alone. To test the safety of this procedure, two Tk2+ mice at P21 were injected (IV) with monodisperse lipid-coated microbubbles (4–5 μm) and received single pulsed FUS beams at acoustic pressures of 300 and 450 kPa. FUS was placed 0·3 mm left and 1·2 mm anterior to the lambda suture. Mice were euthanized at P29 and hematoxylin and eosin (H&E) staining of whole brain was performed to evaluate tissue damage in the treated (left) hemisphere. Although both mice developed normally after the procedure and no signs of tissue damage were observed in the H&E staining, we detected higher levels of inertial cavitation, which might be harmful, in the animal treated with acoustic pressure of 450 kPa (Supplementary Fig. 5). We then tested the effects of FUS-induced BBB opening on levels of dCtd and dThd in brain. Four Tk2+ mice at P21 were injected (IV) with monodisperse lipid-coated microbubbles (4–5 μm) and one dose of 520 mg/kg of dCtd + dThd. Animals received one pulsed FUS beam at either acoustic pressure of 200 or300 kPa. FUS was placed 0·3 mm left and 1·2 mm anterior to the lambda suture, so within the same animal, we measured dCtd, dThd, dU, and T levels by HPLC in both the treated (left) and untreated (right) hemisphere.
Supplementary Fig. 5.
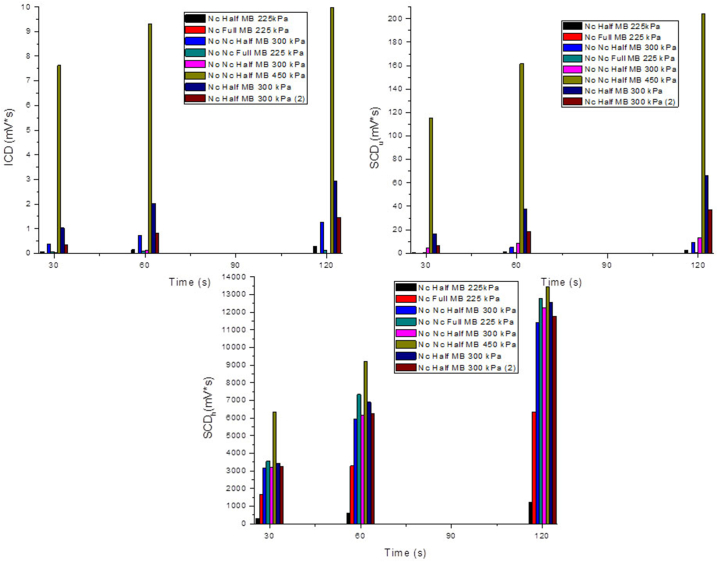
Assessments of FUS-induced BBB disruption. Inertial cavitation dose (ICD) and stable cavitation dose from harmonics (SCDh) and ultraharmonics (SCDu), Different pressures (225, 300 and 450 kPa) were assessed using full or half dose of lipid-coated micro bubbles and with (Nc) or without (No Nc) dCtd + dThd treatment.
We observed that, 1 h after treatment, levels of dThd, dCtd and dU were increased exclusively in the left hemispheres of those mice under FUS procedure using an acoustic pressure of 300 kPa, while right hemispheres of these mice as well as both left and right hemispheres of animals treated with an acoustic pressure of 200 kPa showed levels similar to those animals treated with IV dCtd + dThd alone (Fig. 5). These results indicate that FUS at acoustic pressure of 300 kPa, but not 200 kPa, in combination with lipid microbubbles was efficient opening the BBB and allowed for a higher targeted brain dCtd and dThd delivery. Levels of these compounds in liver were similar between dCtd + dThd + FUS-treated and dCtd + dThd-treated animals (Supplementary Fig. 6), showing that the dCtd + dThd dose received was equivalent in all the animals and that differences in brain are caused by the opening of the BBB with FUS in conjunction with microbubbles.
Fig. 5.
Levels of dCtd and dThd in brain after FUS-induced BBB opening. FUS was performed in two mice using 300 kPa and two additional mice using 200 kPa, while three mice used as controls (“No FUS” controls). Treatment with 520 mg/kg of dCtd + dThd via intravenous injection was followed in all seven mice, and one additional mouse was left without treatment as an “Untreated” control.
Supplementary Fig. 6.
Levels of dCtd and dThd in liver after FUS-induced BBB opening. FUS was performed in two mice using 300 kPa and two additional mice using 200 kPa, while three mice were left as controls (“No FUS” controls). Treatment with 520 mg/kg of dCtd + dThd via intravenous injection was followed in all seven mice, and one additional “Untreated” mouse control.
To assess the pharmacokinetics of dCtd and dThd when administered IV in conjunction with FUS and microbubbles, we measured nucleoside levels in each brain hemispheres of Tk2+ mice at several time points after the treatment. We observed that differences in nucleoside levels in each of the treated and untreated hemispheres were only apparent 1 h after the treatment, which may be explained by the rapid catabolism of dCtd to dU and dThd to T (Supplementary Fig. 7).
Supplementary Fig. 7.
Pharmacokinetics of dCtd + dThd treatment after FUS-treatment. Concentrations of dCtd (deoxycytidine), dThd (thymidine), dU (deoxyuridine) and Thymine after FUS followed by administration of 520 mg/kg of dCtd + dThd using intra-venous injection. Concentrations are expressed as nmol/mg of protein (tissues).
3.5. Increased dCtd and dThd levels in brain does not improve response to dCtd + dThd therapy
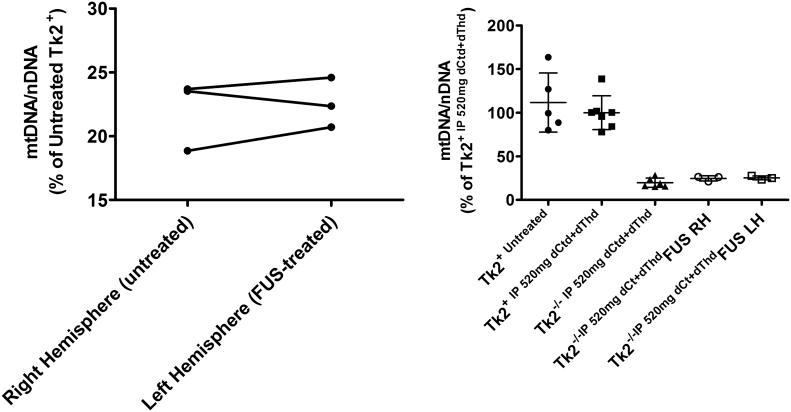
To test whether targeted brain dCtd and dThd delivery by the FUS procedure enhanced parenteral dCtd + dThd treatment, Tk2−/− mice were treated at P21 with FUS in combination with lipid microbubbles and one dose of 520 mg/kg of dCtd + dThd intravenously. Two different FUS beams were placed at 1·2 mm anterior and both 0·3 and 0·5 mm left of the lambda suture. Animals were then maintained until P29 and continued on the IP dCtd + dThd treatment (520 mg/kg/day) until they were euthanized. Brain samples from the area where FUS was performed in the left hemisphere and from the homologous area in the right hemisphere showed no significant differences in the mtDNA levels within each animal (Fig. 6 left panel). Furthermore, mtDNA levels were similar as those found in the Tk2−/− IP dCtd+dThd animals at P29 (Tk2−/− IP 520 mg, 19·6 ± 5·3%; Tk2−/− IP 520 mg+FUS right hemisphere, 24·6 ± 3·06%; Tk2−/− IP 520 mg+FUS left hemisphere, 25·2 ± 2·18%, Fig. 6 right panel) indicating that other factors, rather than lower bioavailability of dCtd and dThd in brain, account for the lack of response in brain to deoxynucleoside treatment.
Fig. 6.
mtDNA levels in FUS-treated mice. Left panel: mtDNA levels in right (untreated) and left (treated) hemisphere from Tk2−/−. Lines connect values from each mouse brain. Values are expressed as percents of mtDNA level in Tk2+ mice. Right panel: Values are expressed as percents of of mtDNA level in Tk2+ mice treated with 520 mg/kg/day of dCtd + dThd via IP injection. RH and LH denotes right hemisphere and left hemisphere, respectively.
3.6. Down-regulation of TK1 during growth determines failure to response to dCtd + dThd therapy
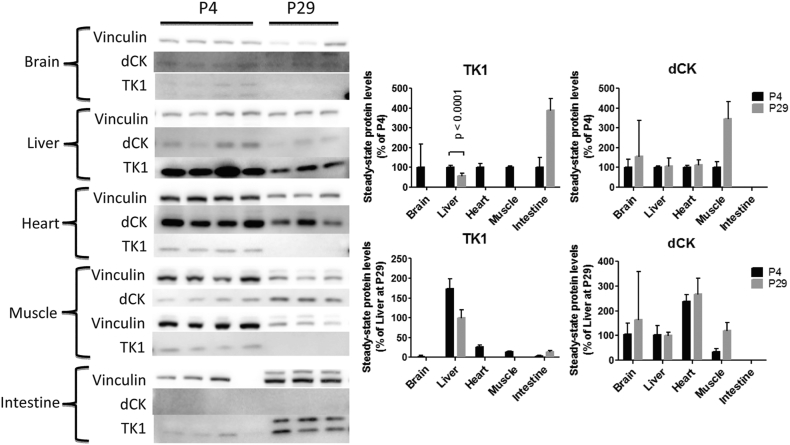
To unravel the intrinsic factors underlying lack of response to dCtd + dThd therapy in brain of Tk2−/− mice, we measured levels of the cytosolic enzymes dCK and TK1 from Tk2+ mice at P4 and P29 and normalized to levels in liver at P29 for comparisons. The highest levels of dCK were found in heart (238·6 ± 26·3% at P4 and 267·6 ± 63·8% at P29) followed by brain (105·7 ± 43·8% at P4 and 163·7 ± 194·7% at P29) liver (103·6 ± 37·5% at P4 and 100·0 ± 13·1% at P29) and muscle (35·1 ± 10·4% at P4 and 121·6 ± 29·9% at P29). Intestinal levels of dCK were below the detection limit. We did not find a significant down-regulation of dCK at P29 with respect to P4 (Fig. 7). In fact, levels of dCK at P29 were higher in brain (154·9 ± 184·2% of levels at P4) and muscle (346·2 ± 85·2% of levels at P4) and similar in heart (112·1 ± 26·7% of levels at P4) and liver (107·9 ± 40·6% of levels at P4).
Fig. 7.
TK1 and dCK levels in mice. Right panel: Immunoblots showing bands corresponding to vinculin (loading control, 130 KDa), TK1 (26 KDa) and dCK (30 KDa). Four first lanes correspond to tissues from mice euthanized at age 4 days and three last lanes correspond to tissues from mice euthanized at age 29 days. Blots have been cropped to improve visualization. Negative controls and molecular size markers were loaded on each immunoblot. Left panel: Quantification of immunoblots from right panel. Vinculin was used as loading control to normalize values from TK1 and dCK bands within each lane. Values are expressed either as percentage of the average value at age 4 days within each tissue or as percentage of the average in liver at age 29 days.
The highest levels of TK1 were found in liver (174·3 ± 24·1% at P4 and 100·0 ± 19·9% at P29). TK1 was poorly expressed at P4 in brain (2·0 ± 2·4%), heart (26·3 ± 5·3%) and muscle (14·0 ± 1·1%), and it was below the detection level in all three tissues at P29 (Fig. 7). In contrast, intestinal TK1 activity increased from P4 (3·8 ± 1·9%) to P29 (14·7 ± 2·3%), indicating that response to dCtd + dThd therapy at older ages is highly dependent on the expression of TK1 in target tissues.
These results suggest that the down-regulation of TK1 in most tissues, as mice continue to develop, prevents these tissues from maintaining responds to the dCtd + dThd therapy; regardless of the dose or bioavailability of each nucleoside.
3.7. Treatment with dThd alone reveals that both dCtd and dThd are necessary for the therapeutic response
To assess the therapeutic properties of dCtd, we treated our Tk2−/− mice with 520 mg/kg/day of dThd using IP injection from postnatal day 4. Although showing a similar survival (no statistical differences compared to dCtd + dThd treatment via IP) mtDNA copy number at postnatal day 29 in liver was dramatically reduced compared to the combined dCtd + dThd treatment via IP (15·8 ± 4·5% vs 58·8 ± 16·6% in dThd and dCtd + dThd treated Tk2−/− mice respectively, p = 0·005) (Fig. 8). mtDNA levels in skeletal muscle were also reduced when treated with dThd compared to dCtd + dThd (13·5 ± 1·7% vs 36·3 ± 26·7% in dThd and dCtd + dThd treated Tk2−/− mice respectively, p = 0·005). Interestingly, mtDNA copy number in brain at P29 was similar in both groups of mice, which likely accounts for the similar survival rate observed. These results are consistent with our previous observation that high dCtd levels using IP treatment effectively rescue mitochondrial dCTP levels in liver and indicate that dCtd is critical to achieve a therapeutic response at least in liver.
Fig. 8.
Survival and mtDNA levels after dThd treatment. Upper left graph represents survival, represented as percent survival within each treatment group, of Tk2−/− mice. Remaining graphs represent mtDNA levels in brain, liver, and muscle. Values as expressed as percents of mtDNA in untreated Tk2+ mice. P values < .05 from Mann Whitney tests between dCtd + dThd- and dThd-treated Tk2−/−mice are indicated.
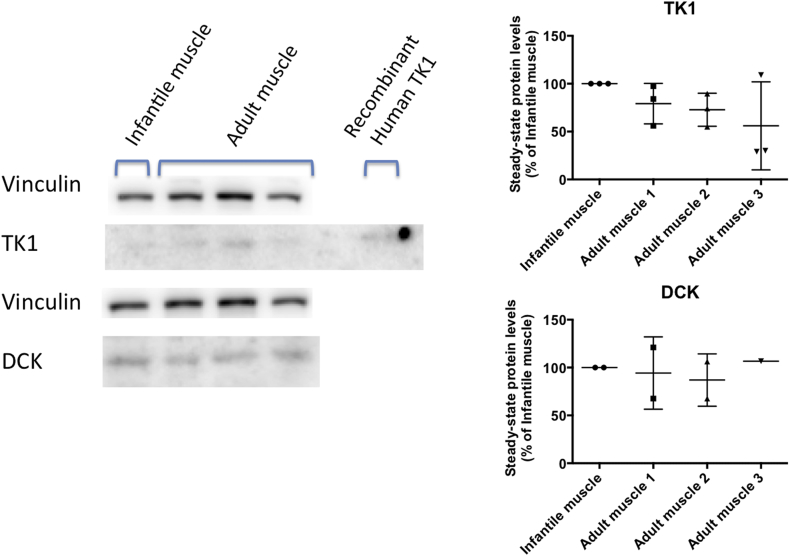
3.8. dCK and TK1 protein levels in human muscle
To assess whether human muscle also manifests down-regulation of dCK, TK1, or both during development, we measured levels of both enzymes in muscle samples from one infant and three adults. Levels of dCK were similar between the infantile sample and the three adult muscle samples (Fig. 9). In contrast, levels of TK1 were lower in the adult muscle samples compared to the infant sample (Fig. 9). Nevertheless, levels of TK1 in the adult muscle samples were still detectable, unlike mice samples, and ranged between 56% and 79% of levels in the infant muscle sample (Fig. 9). This result suggests that down-regulation of TK1 is milder in human muscle respect to mouse muscle and therefore human muscle is more likely to respond to dCtd + dThd therapy than mouse muscle.
Fig. 9.
TK1 and dCK levels in human. Left panel: Representative immunoblots showing bands corresponding to TK1, dCK, and vinculin. The far left lane is loaded with muscle protein extract from an infant subject, three adjacent lanes are loaded with muscle protein extract from three adult subjects and the far right lane is loaded with human recombinant TK1 (Novoprotein Catalog # CI64 10 μg) as positive control. Right panel: Quantitation of TK1 (upper graph) and dCK (lower graph). Vinculin was used as loading control to normalize values from TK1 and dCK bands within each lane. Values are expressed as percents of levels in the infantile subject sample.
4. Discussion
Mitochondrial Depletion Syndrome (MDS) is a group of heterogeneous disorders characterized by reductions in mtDNA copy number causing secondary deficiencies of the OxPhos complexes in affected tissues. TK2 deficiency is a devastating disorder and one of the most common forms of MDS. It predominantly affects infants and children and manifests as a progressive skeletal myopathy leading to early death, although several reports have widened the phenotypic spectrum with three different disease presentations: infantile-onset rapidly progressive myopathy, childhood-onset progressive myopathy, and late-onset slowly progressive myopathy [5].
We previously showed that oral treatment with dCtd and dThd, the substrates for TK2, delayed disease onset and prolonged the lifespan of animals by 3-fold [8]. Although the treatment prevented mtDNA depletion in most tissues at postnatal day 13, mtDNA copy number was reduced in multiple tissues at day 29 and was strikingly low in near-terminal mice. Both normal levels of mtDNA copy number in small intestine, even in near-terminal animals, and previous data have revealed up regulation of TP activity in small intestine from postnatal day 13 to postnatal day 29 [9], indicating that bioavailability of dCtd and dThd in target tissues is critical for this therapy. Furthermore, catabolism of dThd to T by TP could account for the lack of response to the oral treatment at later ages.
To test this hypothesis, we initially studied the pharmacokinetics of dCtd + dThd therapy in adult mice using different routes of administration to avoid TP catabolism in the small intestine. In fact, parenteral routes of administration dramatically increased levels of dCtd and dThd in both plasma and liver, while levels in brain were only slightly higher than those reached with the oral treatment. Uptake of nucleosides in the BBB depends highly on the equilibrative nucleoside transporters (ENT)-1 and ENT-2 [24] and functional characterization of ENT-1 has revealed that this transporter has a Vmax value below the mM range, therefore uptake of dCtd and dThd by ENT-1 is likely saturated at the concentrations reached in plasma by either IP or IV treatment [25,26].
Consistent with this observation, IP treatment of dCtd + dThd rescued mitochondrial dCTP levels in liver and resulted in higher mtDNA copy number in liver, heart, and muscle at P29, compared to the oral treatment. However, levels of both mitochondrial dTTP and mtDNA in brain as well as disease onset and survival were comparable with IP and oral treatment. These results supported our hypothesis that higher bioavailability (levels) of dCtd and dThd in visceral tissues, is critical for optimal response to this therapy. We further tested whether, as in visceral organs, a higher bioavailability of dCtd and dThd in brain improves the response of this tissue to the therapy. FUS-induced BBB opening has been shown to be a safe and efficient way to transiently disrupt the BBB [17,19,27]. Using FUS in conjunction with lipid microbubbles during IV dCtd + dThd treatment, we efficiently increased levels of both dCtd and dThd in brain after treatment. In fact, levels of dCtd and dT one hour after the treatment with FUS were in the same range as those found in liver. Nevertheless, the higher delivery of nucleosides into the brain was not sufficient to rescue mtDNA copy number in the targeted area, indicating that other factors are responsible for the lack of response of the brain to the dCtd + dThd therapy.
The cytosolic enzymes dCK and TK1 enzymes, which phosphorylate dCtd to dCMP and dThd to dTMP, may partially compensate for Tk2 deficiency and are postulated to serve as the initial step in the mechanism of action of dCtd + dThd therapy. dCMP and dTMP need to be transported from cytosol to the mitochondria through the pyrimidine nucleotide carrier PNC1 [28], thus bypassing Tk2 activity. We previously described that down-regulation of cytosolic TK1 activity in mice, when the pyrimidine salvage pathway is forced to rely exclusively on mitochondrial Tk2, determines the onset and tissue specificity of Tk2 deficiency [10]. However, based upon our new immunoblot data, we infer that dCK and TK1 activities in target tissues play critical roles in the time- and tissue-specific response to dCtd + dThd treatment in Tk2−/− mice. Regulation of Tk1 and dCK differ, as Tk1 is reported to be cell-cycle dependent and is down-regulated in post-mitotic tissues [29], whereas dCK is not cell cycle dependent and its expression can be up-regulated by decreased levels of dCTP [30]. Our data show an up-regulation of dCK levels in brain and muscle from P4 to P29 and a severe down-regulation of TK1 at P29 to non-detectable levels, which likely explains the poor response to therapy in animal at this age. At P4, the lowest levels of TK1 are found in brain, which may explain why this is the first tissue to manifest poor response to dCtd + dThd therapy, even after increasing the bioavailability of dCtd and dThd in brain with the parenteral treatment and FUS-induced breakdown of the BBB. Higher expression of TK1 in heart and muscle at P4 delays the onset in these organs with respect to brain and predisposes them to better responses when higher levels of dCtd and dThd are achieved with parenteral treatment. Furthermore, up-regulation of TK1 in intestine from P4 to P29 likely accounts for the striking response of this tissue to dCtd + dThd therapy.
Our observation that TK1 levels correlates with tissue response to dCtd + dThd therapy in addition to the fact that oral administration of dThd alone (and not with dCtd alone) has been shown to be as efficacious as oral dCtd + dThd therapy at extending lifespans of the Tk2 KO mouse [31] indicate that dThd is the primary therapeutic component of this treatment; however, our study also reveals that both, dCtd and dThd, are necessary for optimal therapeutic response in some tissues. While down-regulation of TK1 in brain leads to reduced mitochondrial dTTP levels and triggers the onset of central nervous system dysfunction, TK1 activity in liver spares this tissue from decreased dTTP. In contrast, decreased dCTP level appears to be the cause of mtDNA depletion in liver despite high activity of dCK in this tissue, because dCtd is rapidly catabolized to dU, thereby reducing the efficiency of this branch of the dCtd + dThd therapy. Increased bioavailability of dCtd using parenteral treatment, in combination with endogenous dCK activity rescued dCTP deficiency in liver mitochondria, thereby preventing mtDNA depletion and improving therapeutic response in liver.
In contrast to our Tk2 H126N knock-in mouse model, which manifests a prominent encephalopathy [7], most patients with TK2 deficiency present with mitochondrial myopathy [2,4,[32], [33], [34], [35], [36]], indicating that skeletal muscle is the major target tissue for dCtd + dThd therapy in humans. Although the number of human muscle samples was low and therefore results should be considered preliminary observations, our study suggests that down-regulation of TK1 in human muscle is not as dramatic as in mice. Thus, this human TK2 deficient myopathy is likely to respond to dCtd + dThd therapy, regardless of age. We further speculate that in TK2 deficient patients with partial residual enzyme activity, levels of TK2 activity in tissues may contribute to treatment response. Furthermore, our data in Tk2 deficient mice further suggest that administration of dThd alone may not be as effective as dCtd + dThd therapy rescuing mtDNA levels in skeletal muscle.
In summary, our study demonstrates the critical roles of TK1 and dCK in the mechanism of action of dCtd + dThd therapy in our Tk2−/− mouse model. Our data support the hypothesis that deoxynucleosides (dCtd and dThd) are phosphorylated in the cytosol by Dck and Tk1, respectively. In the presence of these enzymes, higher bioavailability of deoxynucleoside may enhance the therapy, but in the absence of these enzymes, there is no response to this treatment. After deoxynucleosides phosphorylation, monophosphates are further transported into the mitochondria by the pyrimidine nucleotide carrier PNC1 [37], bypassing the defects in TK2 enzyme activity. We also showed the vital role of both dCtd and dThd bioavailability in the response to the deoxynucleoside substrate enhancement therapy. Although parenteral administration prevented catabolism of the dCtd + dThd in small intestine, increased bioavailability, and thus yielded higher levels of mtDNA copy number in several tissues such as liver and heart, the slight increase of mtDNA in skeletal muscle was not sufficient to justify a change of the administration route in patients currently on this therapy. Furthermore, parenteral treatment in patients may cause side-effects that might compromise the safety of the therapy.
Although the mechanism of action of the dCtd + dThd therapy via TK1 and dCK activity appears straight forward, our study indicates multiple factors modulating response to this treatment. Our assessment of effects of TK1 and dCK levels and nucleoside bioavailability in tissues of deoxynucleoside treated Tk2−/− mice indicate that response to this substrate enhancement therapy is influenced by both anabolic and catabolic enzymes and nucleoside transporters in target tissues. Understanding all these variables will help us not only to optimize the dCtd + dThd treatment for TK2 deficiency, but also to translate and develop novel therapies based on nucleosides supplementation in other MDS disorders in which mitochondrial dNTP metabolism is compromised.
The following are the supplementary data related to this article.
Funding sources
This work was supported by research grants from the NIH (P01 HD32062) (MH). MH is supported by the Arturo Estopinan TK2 Research Fund, Nicholas Nunno Foundation, JDM Fund for Mitochondrial Research, Shuman Mitochondrial Disease Fund, and the Marriott Mitochondrial Disease Clinic Research Fund (MMDCRF) from the J. Willard and Alice S. Marriott Foundation. MH also acknowledges support from NIH grants (R01 HD057543, and R01 HD056103 from NICHD) and the Office of Dietary Supplements, as well as U54 NS078059 from NINDS and NICHD. Funders had no role in study design, data collection, data analysis, data interpretation, drafting of the manuscript or submission for publication.
Author contributions
CLG and MH contributed to the conception and design of the study, CLG, HH, CS, HOA, MJSQ, MJF, ST contributed to the acquisition and analysis of data; CLG, KT, EEK and MH contributed to the interpretation of data. CLG and MH contributed to the drafting of the manuscript and figures. All authors contributed to critically revise the manuscript.
Declaration of Competing Interest
MH is a co-inventor on a patent application for deoxynucleoside therapies for mitochondrial DNA depletion syndrome including TK2 deficiency. Columbia University Irving Medical Center (CUIMC) has licensed the pending patent applications related to the technology to Modis Therapeutics, Inc. (formerly Meves Pharmaceuticals, Inc.) and CUIMC may be eligible to receive payments related to the development and commercialization of the technology. MH is a paid consultant to Modis Therapeutics, Inc. This relationship is de minimus for Columbia University Medical Center (MH). The other authors declare no conflicts of interest.
Acknowledgements
We would like to thank Dr. Marҫal Pastor-Anglada for his input on nucleoside transporters, and Drs. Cora Blázquez, Ramón Martí and Yolanda Cámara for their collaboration and discussion throughout the study and critical revision of the manuscript.
References
- 1.Wang L. Mitochondrial purine and pyrimidine metabolism and beyond. Nucleosides Nucleotides Nucleic Acids. 2016;35(10−12):578–594. doi: 10.1080/15257770.2015.1125001. [DOI] [PubMed] [Google Scholar]
- 2.Saada A., Shaag A., Mandel H., Nevo Y., Eriksson S., Elpeleg O. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet. 2001;29(3):342–344. doi: 10.1038/ng751. [DOI] [PubMed] [Google Scholar]
- 3.Saada A., Ben-Shalom E., Zyslin R., Miller C., Mandel H., Elpeleg O. Mitochondrial deoxyribonucleoside triphosphate pools in thymidine kinase 2 deficiency. Biochem Biophys Res Commun. 2003;310(3):963–966. doi: 10.1016/j.bbrc.2003.09.104. [DOI] [PubMed] [Google Scholar]
- 4.Behin A., Jardel C., Claeys K.G. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology. 2012;78(9):644–648. doi: 10.1212/WNL.0b013e318248df2b. [DOI] [PubMed] [Google Scholar]
- 5.Garone C., Taylor R.W., Nascimento A. Retrospective natural history of thymidine kinase 2 deficiency. J Med Genet. 2018;55(8):515–521. doi: 10.1136/jmedgenet-2017-105012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez-Gonzalez C., Hernandez-Lain A., Rivas E. Late-onset thymidine kinase 2 deficiency: a review of 18 cases. Orphanet J Rare Dis. 2019;14(1):100. doi: 10.1186/s13023-019-1071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akman H.O., Dorado B., Lopez L.C. Thymidine kinase 2 (H126N) knockin mice show the essential role of balanced deoxynucleotide pools for mitochondrial DNA maintenance. Hum Mol Genet. 2008;17(16):2433–2440. doi: 10.1093/hmg/ddn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Gomez C., Levy R.J., Sanchez-Quintero M.J. Deoxycytidine and deoxythymidine treatment for thymidine kinase 2 deficiency. Ann Neurol. 2017;81(5):641–652. doi: 10.1002/ana.24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garone C., Garcia-Diaz B., Emmanuele V. Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol Med. 2014;6(8):1016–1027. doi: 10.15252/emmm.201404092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorado B., Area E., Akman H.O., Hirano M. Onset and organ specificity of Tk2 deficiency depends on Tk1 down-regulation and transcriptional compensation. Hum Mol Genet. 2011;20(1):155–164. doi: 10.1093/hmg/ddq453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marti R., Dorado B., Hirano M. Measurement of mitochondrial dNTP pools. Methods Mol Biol. 2012;837:135–148. doi: 10.1007/978-1-61779-504-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez L.C., Akman H.O., Garcia-Cazorla A. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet. 2009;18(4):714–722. doi: 10.1093/hmg/ddn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marti R., Lopez L.C., Hirano M. Assessment of thymidine phosphorylase function: measurement of plasma thymidine (and deoxyuridine) and thymidine phosphorylase activity. Methods Mol Biol. 2012;837:121–133. doi: 10.1007/978-1-61779-504-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marti R., Spinazzola A., Tadesse S. Definitive diagnosis of mitochondrial neurogastrointestinal encephalomyopathy by biochemical assays. Clin Chem. 2004;50(1):120–124. doi: 10.1373/clinchem.2003.026179. [DOI] [PubMed] [Google Scholar]
- 15.DiMauro S., Servidei S., Zeviani M. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987;22(4):498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- 16.Birch-Machin M.A., Briggs H.L., Saborido A.A., Bindoff L.A., Turnbull D.M. An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol. 1994;51(1):35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- 17.Tung Y.S., Vlachos F., Feshitan J.A., Borden M.A., Konofagou E.E. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am. 2011;130(5):3059–3067. doi: 10.1121/1.3646905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi J.J., Selert K., Vlachos F., Wong A., Konofagou E.E. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc Natl Acad Sci U S A. 2011;108(40):16539–16544. doi: 10.1073/pnas.1105116108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sierra C., Acosta C., Chen C. Lipid microbubbles as a vehicle for targeted drug delivery using focused ultrasound-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2016;37(4):1236–1250. doi: 10.1177/0271678X16652630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J.J., Pernot M., Small S.A., Konofagou E.E. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med Biol. 2007;33(1):95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Choi J.J., Wang S., Brown T.R., Small S.A., Duff K.E., Konofagou E.E. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer's double transgenic mice using focused ultrasound. Ultrason Imaging. 2008;30(3):189–200. doi: 10.1177/016173460803000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J.J., Pernot M., Brown T.R., Small S.A., Konofagou E.E. Spatio-temporal analysis of molecular delivery through the blood-brain barrier using focused ultrasound. Phys Med Biol. 2007;52(18):5509–5530. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- 23.Feshitan J.A., Chen C.C., Kwan J.J., Borden M.A. Microbubble size isolation by differential centrifugation. J Colloid Interface Sci. 2009;329(2):316–324. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 24.Young J.D., Yao S.Y., Baldwin J.M., Cass C.E., Baldwin S.A. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Aspects Med. 2013;34(2–3):529–547. doi: 10.1016/j.mam.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Rehan S., Jaakola V.P. Expression, purification and functional characterization of human equilibrative nucleoside transporter subtype-1 (hENT1) protein from Sf9 insect cells. Protein Expr Purif. 2015;114:99–107. doi: 10.1016/j.pep.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Huang W., Zeng X., Shi Y., Liu M. Functional characterization of human equilibrative nucleoside transporter 1. Protein Cell. 2017;8(4):284–295. doi: 10.1007/s13238-016-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konofagou E.E., Tung Y.S., Choi J., Deffieux T., Baseri B., Vlachos F. Ultrasound-induced blood-brain barrier opening. Curr Pharm Biotechnol. 2012;13(7):1332–1345. doi: 10.2174/138920112800624364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraro P., Nicolosi L., Bernardi P., Reichard P., Bianchi V. Mitochondrial deoxynucleotide pool sizes in mouse liver and evidence for a transport mechanism for thymidine monophosphate. Proc Natl Acad Sci U S A. 2006;103(49):18586–18591. doi: 10.1073/pnas.0609020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munch-Petersen B. Enzymatic regulation of cytosolic thymidine kinase 1 and mitochondrial thymidine kinase 2: a mini review. Nucleosides Nucleotides Nucleic Acids. 2010;29(4–6):363–369. doi: 10.1080/15257771003729591. [DOI] [PubMed] [Google Scholar]
- 30.Hodzic J., Giovannetti E., Diosdado B., Adema A.D., Peters G.J. Regulation of deoxycytidine kinase expression and sensitivity to gemcitabine by micro-RNA 330 and promoter methylation in cancer cells. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1214–1222. doi: 10.1080/15257770.2011.629271. [DOI] [PubMed] [Google Scholar]
- 31.Blázquez-Bermejo C., Molina-Granada D., Vila-Juliá F. 2019. Age-related metabolic changes limit efficacy of deoxynucleoside-based therapy for TK2 deficiency in mice. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camara Y., Carreno-Gago L., Martin M.A. Severe TK2 enzyme activity deficiency in patients with mild forms of myopathy. Neurology. 2015;84(22):2286–2288. doi: 10.1212/WNL.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 33.Lesko N., Naess K., Wibom R. Two novel mutations in thymidine kinase-2 cause early onset fatal encephalomyopathy and severe mtDNA depletion. Neuromuscul Disord. 2010;20(3):198–203. doi: 10.1016/j.nmd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso M., Salviati L., Sacconi S. Mitochondrial DNA depletion: mutations in thymidine kinase gene with myopathy and SMA. Neurology. 2002;59(8):1197–1202. doi: 10.1212/01.wnl.0000028689.93049.9a. [DOI] [PubMed] [Google Scholar]
- 35.Oskoui M., Davidzon G., Pascual J. Clinical spectrum of mitochondrial DNA depletion due to mutations in the thymidine kinase 2 gene. Arch Neurol. 2006;63(8):1122–1126. doi: 10.1001/archneur.63.8.1122. [DOI] [PubMed] [Google Scholar]
- 36.Tyynismaa H., Sun R., Ahola-Erkkila S. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Hum Mol Genet. 2012;21(1):66–75. doi: 10.1093/hmg/ddr438. [DOI] [PubMed] [Google Scholar]
- 37.Franzolin E., Miazzi C., Frangini M., Palumbo E., Rampazzo C., Bianchi V. The pyrimidine nucleotide carrier PNC1 and mitochondrial trafficking of thymidine phosphates in cultured human cells. Exp Cell Res. 2012;318(17):2226–2236. doi: 10.1016/j.yexcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 38.Domínguez-González Cristina, Miazzi C., Madruga-Garrido Marcos, Mavillard Fabiola, Garone Caterina, Aguirre-Rodríguez Francisco Javier, Alice Donati M., Kleinsteuber Karin, Martí Itxaso, Martín-Hernández Elena, Morealejo-Aycinena Juan P., Munell Francina, Nascimento Andrés, Kalko Susana G., Sardina M. Dolores, Concepcion Alvarez del Vayo, Serrano Olga, Long Yuelin, Tu Yuqi, Levin Bruce, Thompson John L.P., Engelstad Kristen, Uddin Jasim, Torres-Torronteras Javier, Jimenez-Mallebrera Cecilia, Martí Ramon, Paradas Carmen, Hirano Michio. Deoxynucleoside Therapy for Thymidine Kinase 2–Deficient Myopathy. Ann Neurol. 2019;86(2):293–303. doi: 10.1002/ana.25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that support the findings of this study and protocols used are available from the corresponding author, upon reasonable request.