ABSTRACT
Despite beneficial effects on morbidity and mortality after acute myocardial infarction (AMI), concerns remain about the safety of statin therapy, particularly their potential effects on cognitive and physical function, in elderly individuals. Among statin‐naive AMI patients age ≥65 years in a multicenter US registry, we examined the association between statin prescription at discharge and change in cognition (via Modified Telephone Interview for Cognitive Status [TICS‐M]) assessed at 1 and 6 months after AMI. Short Form‐12 Physical Component score, hand grip, walk time, and chair‐rise tests were used to assess physical function. We conducted noninferiority testing to evaluate the hypothesis that the mean change in cognitive function was no worse among patients recently started on statins compared with those who were not. Among 317 elderly AMI patients, 262 patients (83%) were prescribed a statin at discharge and 55 were not. After matching for propensity to be discharged on statin after AMI, the effect of statin treatment on change in TICS‐M from 1 to 6 months (estimated difference, 0.11 points; 95% confidence interval: −2.11 to 2.32, P = 0.92) showed noninferiority (inferiority threshold 3 points). There were no significant differences in any physical function measure. Among statin‐naive elderly individuals recovering from AMI, initiation of statin therapy was not associated with detectable changes in short‐term cognitive or physical function. These findings support the general safety of statin therapy for secondary prevention in this population.
Introduction
As part of a multifaceted approach to risk reduction, statins are a class Ia American College of Cardiology/American Heart Association (ACC/AHA) recommendation for secondary prevention after acute myocardial infarction (AMI).1 This recommendation extends to all ages, including those age >65 years, although older individuals are commonly undertreated.2, 3 Multiple reasons may prompt clinicians to not treat this population, including a relatively lower number of older individuals enrolled in clinical trials, confusion regarding the relative strength of total cholesterol as a therapeutic target,4 polypharmacy, and concern for side effects, specifically cognitive function5 or other health‐related quality‐of‐life domains.
More than 60 heterogeneous reports of statin‐associated memory loss, occurring mostly within a few months of statin initiation or dose increases, have been reported with simvastatin, pravastatin, or atorvastatin.6 Potential confounding factors, including medical comorbidities, neurologic conditions, and other medication therapies, varied widely. The nature of the memory loss was based almost completely on patient report, with no objective measures being reported. The reversibility of these impairments was also variable. Therefore, it is difficult to draw any firm conclusions from this case series.
Though systematic reviews of small trials designed specifically to address cognition do not support adverse effects on cognition,7, 8 the findings cannot necessarily be extrapolated to the elderly, as they were underrepresented in these trials. Other health‐related quality‐of‐life domains, such as physical function, are similarly void,9 particularly in the post‐AMI setting. Large clinical trials10, 11, 12, 13 designed primarily to address the effect of statins on cardiovascular disease in the elderly have also not demonstrated an increased risk of detriments to cognition or physical function; however, the outcomes studied were exploratory and the trials were likely underpowered to measure these outcomes.11, 13 Additionally, patients with adverse reactions to statins may be screened out during the run‐in phase of relevant clinical trials.10, 11
To address the paucity of high‐quality data assessing cognitive and physical side effects from starting therapy in the elderly, particularly among the high‐risk group recovering from an AMI, we used the Translation Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH) study, a prospective multicenter, post‐AMI registry to address the question of whether statin therapy impacted cognition and physical function following AMI in patients age >65 years.
Methods
Study Protocol
The design and methods of the TRIUMPH study have been previously described.14 In brief, 4340 patients were enrolled in the TRIUMPH study from April 11, 2005, to December 31, 2008. Eligible patients had elevated cardiac biomarkers and additional supporting evidence of an AMI (electrocardiographic ST‐segment changes or prolonged ischemic signs/symptoms). Trained data collectors performed detailed baseline chart abstractions to document patients' medical history, the processes of inpatient care, laboratory results, and treatments. Prior to discharge, patients underwent a standardized interview by research staff to document their sociodemographic and clinical data. They were then contacted for follow‐up interviews at 1 and 6 months after AMI to reassess health status and interval events. In addition, consenting patients had an additional in‐home geriatric assessment at 1 and 6 months after AMI. TRIUMPH excluded patients with dementia. All patients provided written informed consent approved by the participating institution, and each participating center provided institutional review board approval.
Study Measures
Our goal was to describe changes in cognitive and physical function in the elderly following AMI. Patients age ≥65 years were assessed with the Modified Telephone Interview for Cognitive Status (TICS‐M), a 13‐item questionnaire modeled after the Mini‐Mental State Examination (correlation r = 0.94)15 that provides an assessment of global cognitive function, at 1 and 6 months. The TICS‐M is a valid and reliable instrument, with higher scores (range, 0–39) representing better cognitive status.16 Scores <23 indicate mild cognitive impairment. Raw scores were adjusted based on level of education achievement (5 points added for <8 years of education, 2 points added for ≥8–10 years of education, no adjustment for 11–12 years of education, and 2 years subtracted for ≥13 years of education). The minimum clinically important difference on the scale is unknown; for this analysis it was estimated as 3 points using Cohen's effect size of 0.5 × the SD of 1‐month scores (6 points) to represent a moderate change.17
Mobility, strength, balance, endurance, and physical activity were assessed using 15‐foot walk speed,18 chair stands,19 and hand grip strength.20 Physical health status was assessed using the Short Form‐12 Physical Component Score (SF‐12 PCS),21 with higher scores indicating better health status (score of 50 normalized to the mean health status of the US population with 10 points representing 1 SD from that mean). Per prior work, we defined the minimum clinically important difference on this scale as 2.5 points.22 Patients were also asked at 1‐ and 6‐month follow‐up to self‐report memory difficulties, exhaustion, and problems with medications, including side effects. Adherence to medications was ascertained by phone interview.
Statistical Analysis
The primary analysis compared change in cognition (via mean change in TICS‐M) from 1 to 6 months by statin exposure. Patients taking statins at the time of admission for AMI were excluded so that the analyses focused upon those without prior statin exposure that may have already modified their mental or physical function. Baseline and follow‐up characteristics were compared between those discharged on statins vs not, using t tests for continuous variables and χ2 test for categorical variables.
To account for the selection bias of those prescribed a statin at discharge vs those not prescribed a statin, a propensity score was derived to assess the probability of statin prescription. Using a nonparsimonious multivariable logistic regression model, statin exposure was modeled as the dependent variable with covariates including baseline cognitive and physical function, age, sex, race, body mass index, self‐rated health status, socioeconomic status, marriage status, insurance status, diabetes mellitus, peripheral vascular disease, hypertension, prior cerebrovascular accident, prior MI, alcohol abuse, chronic kidney disease, type of AMI, physical function, depression, type of revascularization, and Global Registry of Acute Coronary Events (GRACE) score. Statin users and non–statin users were matched 1:1 using optimal matching on the logit of the propensity score. The caliper width was 0.2 × the pooled SD of the logit propensity scores for the groups.23 Balance of baseline characteristics between the groups before and after propensity matching was examined using absolute standardized differences, where the standardized difference was defined as the mean difference as a percentage of the pooled standard deviation in 2 groups (standard difference of <10% is considered well‐balanced).24 Sex and baseline finances were slightly unbalanced (standardized differences of 22 and 14) and were adjusted for in the final model (see Supporting Information, Table 1, in the online version of this article). Of those included in the propensity score match, 16% of patients were missing ≥1 variable, with the highest missing variable as “history of depression” at 7% (see Supporting Information, Table 2, in the online version of this article).
Table 1.
Baseline Characteristics of Study Population by Statin Exposure
| Characteristic | Discharged on Statin? | P Value | |
|---|---|---|---|
| Yes, n = 262 | No, n = 55 | ||
| Age, y | 73 ± 7 | 75 ± 7 | 0.14 |
| CKDa | 78 (30) | 25 (46) | 0.02 |
| BMI, kg/m2 | 27.9 ± 5.4 | 28.0 ± 6.4 | 0.97 |
| Male sex | 148 (57) | 28 (51) | 0.45 |
| Caucasian | 214 (82) | 39 (71) | 0.07 |
| LDL‐C at baseline, mg/dL | 99 ± 32 | 100 ± 37 | 0.80 |
| LDL‐C at 1 month, mg/dL | 75 ± 25 | 95 ± 38 | −0.001 |
| History of depression | 21 (9) | 6 (11) | 0.60 |
| Medication or counseling for depression | 12 (7) | 5 (10) | 0.37 |
| DM | 62 (24) | 20 (36) | 0.05 |
| PVD | 12 (5) | 7 (13) | 0.03 |
| Hypertension | 178 (68) | 44 (80) | 0.08 |
| Prior CVA | 11 (4) | 3 (6) | 0.72 |
| Prior MI | 30 (12) | 9 (16) | 0.31 |
| History of alcohol abuse | 8 (3) | 3 (6) | 0.41 |
| In‐hospital PCI | 190 (73) | 26 (47) | −0.001 |
| In‐hospital CABG | 26 (10) | 8 (15) | 0.31 |
| Married | 149 (57) | 32 (58) | 0.86 |
| Less than high school education | 123 (47) | 24 (44) | 0.65 |
| Has insurance coverage for meds, baseline | 211 (84) | 49 (89) | 0.32 |
| Finances at the end of the month, baseline | 0.10 | ||
| Some money left over | 159 (62) | 29 (53) | |
| Just enough to make ends meet | 85 (33) | 19 (35) | |
| Not enough to make ends meet | 13 (5) | 7 (13) | |
| Avoid care due to cost, baseline | 30 (12) | 6 (11) | 0.93 |
| GRACE 6‐month mortality risk score | 123 ± 22 | 133 ± 22 | 0.002 |
Abbreviations: BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SD, standard deviation.
Data are expressed as n (%) or mean ± SD for continuous variables.
Defined as eGFR <60 mL/min/1.73 m2 at the time of discharge.
A noninferiority analysis was then used to evaluate if the mean change in cognition was no worse among statin‐naive patients recently started on statins as compared with those who were not, using a prespecified noninferiority margin of 3 points (0.5 × SD of 1‐month scores). With the available sample size (n = 317, allocation ratio 4.8 statin treatment to no treatment) we had >85% power to demonstrate noninferiority for our primary outcome, using a threshold of 3 points on the TICS‐M scale.
To examine any bias due to missing follow‐up TICS‐M scores (n = 392, 55%), we performed a sensitivity analysis to assess the potential impact of missing data on our findings. For this analysis, we created a logistic model for the probability of missing follow‐up TICS‐M scores. We then inversely weighted the observed findings by the propensity to be missing follow‐up scores so that the greatest weight was given to those most likely to be like the patients with missing follow‐up data.25
Missing covariate data were imputed by sequential regression imputation incorporating all baseline and outcome variables using IVEware (Imputation and Variance Estimation Software; University of Michigan Survey Research Center, Institute for Social Research, Ann Arbor, MI). All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2‐sided P value of <0.05.
Results
Study Population
Among the 1333 patients in the TRIUMPH cohort who were age ≥65 years, 709 (53%) were statin‐naive (Figure 1). Cognitive scores were not significantly different for those excluded due to statin use at the time of AMI relative to those included in this analysis (22 ± 5 at 1 month, P = 0.437; and 22 ± 7 at 6 months, P = 0.174). We excluded 392 patients who were missing TICS‐M scores at 1 or 6 months, yielding a total of 317 patients included in this analysis. Patients excluded due to missing scores were less likely to be Caucasian (69% of excluded vs 80% in study, P ≤ 0.001), less likely to have at least a high school education (61% excluded vs 46% in study, P ≤ 0.001), had lower socioeconomic status (52% with money left over at end of the month of those excluded vs 60% in study, P = 0.01), and had more comorbidities (see Supporting Information, Table 3, in the online version of this article).
Figure 1.
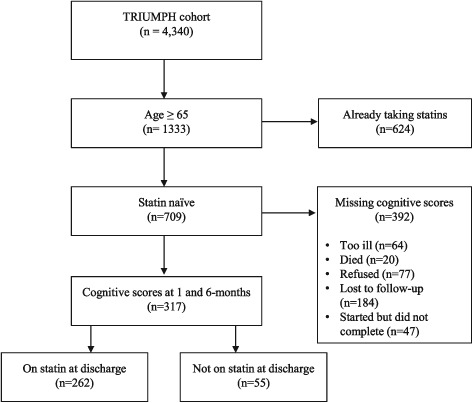
Study flow diagram. Abbreviations: TRIUMPH, Translation Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status.
Table 1 shows baseline characteristics by statin use at discharge. Statin users were less likely to have kidney disease (30% users vs 46% nonusers, P = 0.02) and diabetes mellitus (24% vs 36%, P = 0.05), had lower LDL‐C at 1 month (75 ± 25 mg/dL vs 95 ± 38 mg/dL, P < 0.001), were more likely to receive percutaneous coronary intervention (73% vs 47%, P < 0.001), and had a lower GRACE score (123 ± 22 vs 133 ± 22, P = 0.002). Socioeconomic measures were similar between exposure groups.
Cognition and Physical Function
The mean 1‐month TICS‐M score was 23 ± 6 for those discharged on statin therapy vs 22 ± 6 for nonusers (P = 0.49). Figure 2 demonstrates the distribution of TICS‐M scores at 1 and 6 months by statin use at discharge. Cognitive impairment was common, with 23% of statin users and 30% of nonusers having TICS‐M scores <23 at 1 month post‐AMI. In the unadjusted analysis, all differences in cognitive and physical function from 1 to 6 months after AMI were not statistically different (see Supporting Information, Table 4, in the online version of this article; P < 0.05 for all). In addition, all measures of physical function, including both objective measures (such as grip strength) and self‐reported physical health status, improved with time following AMI for both statin exposure groups, but there were no differences in the degree of improvements between groups (Supporting Information, Table 4).
Figure 2.
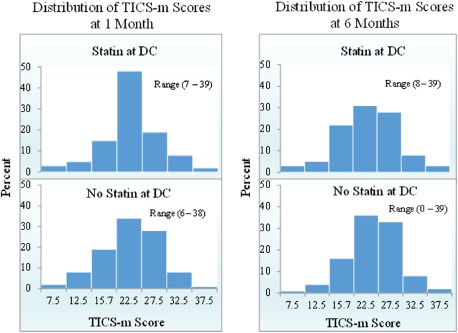
Distribution of TICS‐M scores by statin exposure at 1 and 6 months. Abbreviations: DC, discharge; TICS‐M, Modified Telephone Interview for Cognitive Status.
In our primary analysis, after matching for propensity to be discharged on statin after AMI, the effect of statin treatment on change in TICS‐M from 1 to 6 months was 0.91 points (95% confidence interval: −0.93 to 2.75, P = 0.33). This confidence interval does not include the noninferiority margin of 3 points, which was set a priori.
Mean change in TICS‐M in relation to adherence to statin therapy at 6 months was neither statistically or clinically significant, with a mean change for those adherent of 0.7 ± 5 vs those nonadherent of −0.7 ± 7 (P = 0.11). Nor did the intensity of statin therapy affect cognition (Table 2), with high‐intensity users showing a mean change of −0.1 ± 5 compared with 0.7 ± 5 for moderate‐intensity and −0.4 ± 7 for low intensity, with P = 0.70. Finally, 1‐month self‐reported adverse reactions by statin exposure at discharge (see Supporting Information, Table 5, in the online version of this article) shows that multiple questions related to statin side effects, memory, and physical and overall health were no different between exposure groups.
Table 2.
Change in TICS‐M Score by Intensity of Statin Therapy
| Statin Regimen at Dischargea | P Value | |||||
|---|---|---|---|---|---|---|
| Other, n = 1 | None, n = 54 | Low, n = 62 | Moderate, n = 87 | High/Goal, n = 112 | ||
| Change in TICS‐M from 1 to 6 months, mean ± SD | 3.0 | −0.4 ± 5.5 | −0.4 ± 7.0 | 0.7 ± 5.2 | −0.1 ± 5.2 | 0.70 |
| Relative change in TICS‐M from 1 to 6 months, n (%) | ||||||
| ≥15% increase | 1 (100) | 38 (70) | 47 (76) | 74 (85) | 95 (85) | 0.12 |
| <15% decrease | 0 (0) | 16 (30) | 15 (24) | 13 (15) | 17 (15) | |
Abbreviations: ANOVA, analysis of variance; SD, standard deviation; TICS‐M, Modified Telephone Interview for Cognitive Status.
Comparisons made using 1‐way ANOVA.
Low‐intensity statin defined as simvastatin 10 mg, pravastatin 10 mg–20 mg, lovastatin 20 mg, fluvastatin 20 mg–40 mg; moderate therapy as atorvastatin 10 mg–20 mg, rosuvastatin 10 mg, simvastatin 20 mg–40 mg, pravastatin 40 mg–80 mg, lovastatin 40 mg; high/goal therapy as atorvastatin 80 mg, rosuvastatin 20 mg–40 mg.
Discussion
Among statin‐naive individuals recovering from an AMI, initiation of statin therapy at hospital discharge was not associated with detectable changes in cognition or physical function at 1 or 6 months, nor the change between 1 and 6 months. These findings are consistent with large, post‐AMI clinical trials of elderly patients11, 12 and provide additional insights in a real‐world patient population. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) study was the largest randomized trial of statin therapy in the elderly specifically designed to test cognitive function.12 Cognition was assessed in 5804 subjects at 6 different time points using 4 neuropsychological tests, and the authors found no difference in cognitive decline over a mean follow‐up of 42 months. Our results also match a recent systematic review and meta‐analysis of 14 trials (27 643 patients) that found no evidence of cognitive impairment with statin use but go further in focusing on patients age >65 years.26 As previously noted in this cohort,27 and others,11, 28, 29 cognitive impairment is common in patients age >65 years with coronary artery disease, which tends to be associated with less aggressive medical care and worse 1‐year survival. Although some of this less aggressive care may reflect patient and family preferences, some may represent a concern on the part of clinicians to avoid exacerbating cognitive decline in the elderly through the use of additional medications. The present analysis should reassure providers regarding the lack of adverse cognitive and physical effects of statin therapy in the elderly. With the established mortality and morbidity of statin therapy, these data further support treatment in elderly patients after AMI.
Our findings extend the prior literature assessing the cognitive and physical changes in patients treated with statin therapy. For example, the Cholesterol Reduction In Seniors Program (CRISP) showed that low‐intensity statin therapy (lovastatin doses at 20–40 mg/d) was associated with no detrimental effects to cognition and other health‐related quality‐of‐life measures at 6 months.13 The majority (76%) of statin users in the present analysis used moderate or high‐intensity statin therapy and therefore go further in supporting the safety of the ACC/AHA secondary prevention recommendation for the elderly.
To our knowledge, this is the first post‐AMI analysis of the physical‐function effects of statins in the elderly, assessed objectively at 2 time points. However, our results are consistent with prior studies in other patient populations, particularly in regard to the effects of statins on physical function.30, 31 In the largest study to date on a lower‐risk, non‐AMI population, a secondary analysis of 5777 women age 65 to 79 years from the Women's Health Initiative, statin use was not associated with baseline measures or mean annual change for several performance measures (repeated chair stands, grip strength, 6‐minute walk) with 6 years of follow‐up.32 Using a diverse variety of estimates from self‐reported to objective measures, our results support these prior studies and again showed no significant impact on physical function or health status.
Study Limitations
Our findings should be interpreted in the context of the following potential limitations. The present analysis is not randomized and, thus, subject to all biases associated with observational studies, especially treatment and selection bias. We attempted to address this by using propensity score match pairs for statin treatment bias and a sensitivity analysis (for missing TICS‐M scores) for selection bias. The treatment was not blinded, which may introduce bias on the part of both subjects and providers. This bias tends to exaggerate effect estimates, but we found no difference in baseline, 6‐month, or interval change in scores between treatment groups. Second, cognition via TICS‐M was assessed at 1 month after hospital discharge and may not reflect rapid deterioration in cognitive function. However, the setting of an AMI, where hospitalization and temporary medications can impair cognitive function, is not a stable time period in which to assess cognitive function. Moreover, we found no differences at 1 month between those initiated and not treated with statin therapy, suggesting that there was not a rapid impact of statin therapy on mental functioning. There are differences between those lost to follow‐up and those included in our analysis (Supporting Information, Table 3) that might impact cognition. Patients with cognitive dysfunction at baseline theoretically may be more likely to experience adverse effects from statin therapy. Cognitive adverse effects could influence the likelihood of accepting follow‐up testing and could bias our results to the null. It is also possible that a patient discharged home without a statin would be prescribed a statin between discharge and the 1‐month cognitive testing. We assessed medication use at 1 month and found that 30% of those not discharged on a statin had one initiated prior to the 1‐month assessment. This would tend to bias our results to not finding a difference between groups. Third, it is possible that the TICS‐M, as a measure of global cognitive function, is not sensitive enough to detect the “ill‐defined memory loss” and “confusion” that prompted the 2012 US Food and Drug Administration safety label change for statins. Full neurocognitive testing was impractical in the TRIUMPH study, which showed no moderate changes in cognitive function, but more sensitive assessments may be warranted in future studies.
Conclusion
We found no adverse changes in cognitive or physical function in elderly individuals recovering from an AMI associated with new statin prescription. These findings lend additional support for the general safety of statin therapy in the elderly for secondary prevention.
Supporting information
TableS1: Propensity Score Match
TableS2: Data Missing Rate within Propensity Score Match
TableS3: Characteristics of patients excluded due to missing cognitive data compared to those included in the study
TableS4. Unadjusted Outcomes by Statin Exposure
TableS5: Self‐reported Quality of Life Outcomes by Statin Exposure
Dr. Martin is supported by the Pollin Cardiovascular Prevention Fellowship, a National Institutes of Health training grant (T32HL07024), as well as the Marie‐Josée and Henry R. Kravis endowed fellowship. Dr. Blumenthal is supported by the Kenneth Jay Pollin Professorship in Cardiology.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation 2014;129(25 suppl 2):S46–S48]. Circulation 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. Majumdar SR, Gurwitz JH, Soumerai SB. Undertreatment of hyperlipidemia in the secondary prevention of coronary artery disease. J Gen Intern Med. 1999;14:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maddox TM, Borden WB, Tang F, et al. Implications of the 2013 ACC/AHA cholesterol guidelines for adults in contemporary cardiovascular practice: insights from the NCDR PINNACLE registry. J Am Coll Cardiol. 2014;64:2183–2192. [DOI] [PubMed] [Google Scholar]
- 4. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90 056 participants in 14 randomised trials of statins [published corrections appear in Lancet. 2005;366:1358 and Lancet. 2008;371:2084]. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration . FDA Drug Safety Communication: important safety label changes to cholesterol‐lowering statin drugs. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed August 7, 2012.
- 6. Wagstaff LR, Mitton MW, Arvik BM, et al. Statin‐associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy. 2003;23:871–880. [DOI] [PubMed] [Google Scholar]
- 7. Swiger KJ, Manalac RJ, Blumenthal RS, et al. Statins and cognition: a systematic review and meta‐analysis of short‐ and long‐term cognitive effects. Mayo Clin Proc. 2013;88:1213–1221. [DOI] [PubMed] [Google Scholar]
- 8. Jukema JW, Cannon CP, de Craen AJ, et al. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol. 2012;60:875–881. [DOI] [PubMed] [Google Scholar]
- 9. Swiger KJ, Manalac RJ, Blaha MJ, et al. Statins, mood, sleep, and physical function: a systematic review. Eur J Clin Pharmacol. 2014;70:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004;5:81–87. [DOI] [PubMed] [Google Scholar]
- 11. Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 12. Shepherd J, Blauw GJ, Murphy MB. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 13. Santanello NC, Barber BL, Applegate WB, et al. Effect of pharmacologic lipid lowering on health‐related quality of life in older persons: results from the Cholesterol Reduction in Seniors Program (CRISP) Pilot Study. J Am Geriatr Soc. 1997;45:8–14. [DOI] [PubMed] [Google Scholar]
- 14. Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 16. Brandt J, Welsh KA, Breitner JC, et al. Hereditary influences on cognitive functioning in older men: a study of 4000 twin pairs. Arch Neurol. 1993;50:599–603. [DOI] [PubMed] [Google Scholar]
- 17. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbraum Associations; 1988. [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 19. Jones CJ, Rikli RE, Beam WC. A 30‐s chair‐stand test as a measure of lower‐body strength in community‐residing older adults. Res Q Exerc Sport. 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 20. Giampaoli S, Ferrucci L, Cecchi F. Hand‐grip strength predicts incident disability in non‐disabled older men. Age Ageing. 1999;28:283–288. [DOI] [PubMed] [Google Scholar]
- 21. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 22. Parker SL, Mendenhall SK, Shau DN, et al. Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same‐level recurrent lumbar stenosis: understanding clinical versus statistical significance. J Neurosurg Spine. 2012;16:471–478. [DOI] [PubMed] [Google Scholar]
- 23. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 24. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. [DOI] [PubMed] [Google Scholar]
- 25. Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. [DOI] [PubMed] [Google Scholar]
- 26. Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta‐analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gharacholou SM, Reid KJ, Arnold SV, et al. Cognitive impairment and outcomes in older adult survivors of acute myocardial infarction: findings from the translational research investigating underlying disparities in acute myocardial infarction patients' health status registry. Am Heart J. 2011;162:860.e1–869.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States [published correction appears in Ann Intern Med. 2009;151:291–292]. Ann Intern Med. 2008;148:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silbert BS, Scott DA, Evered LA, et al. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Anesth Analg. 2007;104:1023–1028. [DOI] [PubMed] [Google Scholar]
- 30. Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55:420–425. [DOI] [PubMed] [Google Scholar]
- 31. Carlsson CM, Papcke‐Benson K, Carnes M, et al. Health‐related quality of life and long‐term therapy with pravastatin and tocopherol (vitamin E) in older adults. Drugs Aging. 2002;19:793–805. [DOI] [PubMed] [Google Scholar]
- 32. Gray SL, Aragaki AK, LaMonte MJ, et al. Statins, angiotensin‐converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc. 2012;60:2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1: Propensity Score Match
TableS2: Data Missing Rate within Propensity Score Match
TableS3: Characteristics of patients excluded due to missing cognitive data compared to those included in the study
TableS4. Unadjusted Outcomes by Statin Exposure
TableS5: Self‐reported Quality of Life Outcomes by Statin Exposure


