ABSTRACT
Background
Right‐heart dysfunction is associated with poor prognosis in heart failure with preserved left ventricular ejection fraction (HFpEF). It remains unclear whether sleep‐disordered breathing (SDB) treatment using positive airway pressure (PAP) improves right‐heart and pulmonary function and exercise capacity and reduces mortality rates of HFpEF patients.
Hypothesis
PAP may improve right‐heart and pulmonary function, exercise capacity and prognosis in HFpEF patients with SDB.
Methods
One hundred nine consecutive patients with HFpEF (left ventricular ejection fraction >50%) and moderate to severe SDB (apnea‐hypopnea index ≥15/h) treated with medications were divided into 2 groups: 31 patients with PAP (PAP group) and 78 patients without PAP (non‐PAP group). Right ventricular fractional area change (RV‐FAC), tricuspid valve regurgitation pressure gradient (TR‐PG), tricuspid valve E/E′, forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC), percentage of vital capacity, and peak VO2 were determined before and 6 months later, and all‐cause mortality was followed up for 916 days.
Results
All parameters improved in the PAP group (RV‐FAC, 36.0% –46.5%; TR‐PG, 31.1 mm Hg–22.4 mm Hg; tricuspid valve E/E′, 7.8–5.1; FEV1/FVC, 83.9%–89.8%; percentage of vital capacity, 83.5%–89.9%; and peak VO2, 16.6 mL/kg/min–19.6 mL/kg/min; P <0.05, respectively) but not in the non‐PAP group. Importantly, all‐cause mortality was significantly lower in the PAP group than in the non‐PAP group (0% vs 12.8%; log‐rank P = 0.014).
Conclusions
Positive airway pressure improves right‐heart and pulmonary function and exercise capacity and may reduce all‐cause mortality in patients with HFpEF and SDB.
Introduction
Chronic heart failure (CHF) is a major public‐health problem in many countries. Heart failure with preserved ejection fraction (HFpEF) has a poor prognosis similar to that of heart failure with reduced ejection fraction (HFrEF).1 Heart failure with preserved ejection fraction is not caused by a single pathophysiological factor but is in fact a complex, integrated, multisystem dysfunction of the left and right ventricles, diastolic and systolic function, atrial reserve, heart rate (HR), and microcirculation2; has several comorbidities; and is associated with not only cardiac but also noncardiac mortality.2, 3 Hence, effective pharmacotherapy for HFpEF is still unclear,1 and management of comorbidities may have an overall greater prognostic impact in HFpEF.3
Sleep‐disordered breathing (SDB)—either obstructive sleep apnea (OSA) or Cheyne‐Stokes respiration with central sleep apnea (CSR‐CSA)—is observed in patients with CHF regardless of rEF or pEF4 and has adverse prognostic impacts in CHF.5 Sleep‐disordered breathing is thought to cause cardiac biventricular systolic and diastolic dysfunction, left ventricular (LV) hypertrophy,6 coronary‐artery ischemia,7 direct sympathetic stimulation, renal damage, and arterial stiffness8 and is associated with obesity, arterial hypertension,8 diabetes mellitus, and atrial fibrillation, all of which cause HFpEF. In addition, OSA causes excessive negative intrathoracic pressure and pulmonary‐artery vasoconstriction, which in turn increases venous return, right‐heart volume and pressure overload, pulmonary hypertension, and transmural pressure and changes LV relaxation properties and LV hypertrophy.9 On the other hand, right‐heart dysfunction10, 11 and impaired exercise capacity1 exist and are associated with poor prognosis in CHF patients.
Bitter et al reported that adaptive servo‐ventilation (ASV) improved LV diastolic function and exercise capacity of HFpEF patients with CSR‐CSA.12 We have previously reported that ASV reduced rehospitalization and/or cardiac mortality of HFpEF patients with SDB with favorable effects such as improvement of LV diastolic function and arterial stiffness.13 In addition, effective SDB treatment using positive airway pressure (PAP), including not only ASV but also continuous positive airway pressure (CPAP), improves LV and right ventricular (RV) systolic function14, 15 and prognosis of HFrEF patients.5, 16 Furthermore, CPAP reduces cardiovascular events and mortality in OSA patients.17 Therefore, we examined whether SDB treatment using PAP improves right‐heart function, pulmonary function, and exercise capacity and reduces mortality rates of HFpEF patients with SDB.
Methods
Subjects and Study Protocol
This was a prospective observational study that enrolled 109 consecutive patients with HFpEF and SDB who were referred for overnight polysomnography at Fukushima Medical University between 2009 and 2012. Patients included in this study were those having (1) symptomatic CHF, which was previously defined based on the Framingham criteria18 and New York Heart Association (NYHA) class ≥II at enrollment; (2) left ventricular ejection fraction (LVEF) ≥50%; (3) a strict regime of pharmacotherapy treatment; (4) a stable clinical status defined as receiving medical therapy with no worsening of CHF for ≥3 months prior to study enrollment; and (5) moderate to severe SDB (defined as apnea‐hypopnea index [AHI] ≥15/h). Exclusion criteria were: (1) age <20 or >80 years; (2) ongoing treatment of SDB; (3) severe valvular disease, pericardial disease, and cardiomyopathy; (4) pacemaker implantation; (5) end‐stage renal disease and/or on dialysis; and (6) documented cancer. We offered PAP therapy to all 109 HFpEF patients with moderate to severe SDB who met the above inclusion criteria, and 52 patients agreed to use PAP. After 1 attempt, 18 patients declined to continue the therapy for the following reasons: economic reasons (n = 7), mask intolerance (n = 5), subjective intolerance to positive airway pressure (n = 4), and no specific reason (n = 2). Thus, 34 out of 52 patients desired to use PAP continuously. After a few days of follow‐up, 3 additional patients stopped PAP use due to subjective intolerance to positive airway pressure (n = 2) and mouth drying (n = 1). Consequently, 31 patients were in the PAP group, treated with usual medications plus PAP (CPAP, n = 16; ASV, n = 15); and the remaining 78 patients were in the non‐PAP group, treated with usual medical care alone. Written informed consent was obtained from all study subjects. The study protocol was approved by the ethical committee of Fukushima Medical University. The investigation conforms with the principles outlined in the Declaration of Helsinki.
We evaluated NYHA functional class; blood pressure (BP); HR; blood laboratory values including plasma B‐type natriuretic peptide (BNP), estimated glomerular filtration rate (eGFR), and C‐reactive protein; echocardiographic parameters; pulmonary function testing results; and cardiopulmonary exercise testing data at baseline and 6 months later in each group. Patients were followed up for cardiac death and all‐cause mortality. Cardiac death included death due to ventricular fibrillation or worsening heart failure. Noncardiac death included death due to respiratory failure, renal failure, infection, sepsis, stroke, or digestive hemorrhage. Survival time was calculated from the date of hospitalization until the date of death or last follow‐up. Status and dates of death were obtained from the patients' medical records. If these data were unavailable, status was ascertained by a telephone call to the patient's referring hospital physician. The survey was performed blindly to the analyses of this study. The primary outcome of our study was evaluation of changes in right‐heart function, and the secondary outcome was cardiac and all‐cause mortality.
Polysomnography and Sleep‐Disordered Breathing Treatment
All subjects underwent polysomnography with the use of standard techniques and scoring criteria.19 Briefly, the polysomnography was performed using a computerized system (Alice 5; Philips Respironics, Murrysville, PA) that monitored the electroencephalogram, electrooculogram, submental electromyogram, electrocardiogram, thoracoabdominal motion, oronasal airflow by thermistor and nasal pressure transducer, and arterial oxyhemoglobin saturation (SPO2) by pulse oximetry. Apnea‐hypopnea index ≥15/h was defined as moderate to severe SDB. The major polysomnographic parameters investigated were AHI, obstructive apnea index, mixed apnea index, central apnea index, hypopnea index, arousal index, 3% oxidative desaturation index, lowest pulse oxyhemoglobin saturation (lowest SPO2), mean pulse oxyhemoglobin saturation (mean SPO2), and slow‐wave sleep. All recordings were scored manually by an experienced polysomnographer blinded to the cardiac assessment. After the diagnostic night, another titration was scheduled for each patient. Chronic heart failure patients have not only simple SDB but also complicated and altered SDB, including OSA and CSR‐CSA.9, 20 Hence, it is difficult to completely separate patients with OSA and CSR‐CSA. We basically chose the following PAP devices: CPAP for OSA‐dominant SDB (REMstar Auto; Philips Respironics, Murrysville, PA) and ASV for CSR‐CSA–dominant SDB (VPAP Adapt SV; ResMed, Sydney, Australia). In consideration of severity and medical history of heart failure and cost of PAP therapy, the attending physician selected the PAP device individually for each patient. The aim was to reduce AHI to <5/h before hospital discharge using a minimum required PAP support.
Echocardiography
Echocardiography was performed blindly by an experienced echocardiographer using the standard techniques. Echocardiographic parameters investigated included interventricular septum thickness, LV dimension, posterior wall thickness, relative wall thickness, LVEF, left atrial (LA) volume, the ratio of early transmitral flow velocity to mitral annular velocity (mitral valve E/E′), mitral regurgitation score, inferior vena cava diameter, tricuspid valve regurgitation score, tricuspid valve regurgitation pressure gradient (TR‐PG), RV fractional area change (RV‐FAC),21 Doppler‐derived tricuspid lateral annular systolic velocity (tricuspid valve S′), and ratio of the peak transtricuspid velocity during early diastole to the peak tricuspid valve annular velocity during early diastole (tricuspid valve E/E′).21 The severity of mitral regurgitation and tricuspid regurgitation was graded using a 4‐point scoring system as follows: 0 = none, 1 = mild, 2 = moderate, and 3 = severe (severe regurgitation was excluded in our study subjects). All recordings were performed on ultrasound systems (ACUSON Sequoia; Siemens Medical Solutions USA, Inc., Mountain View, CA).
Pulmonary Function Testing and Cardiopulmonary Exercise Testing
Pulmonary function testing was performed using a spirometer (CHESTAC‐8900; Chest, Tokyo, Japan) when patients were in a stable phase. Forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) and percentage of vital capacity were determined.22
Subjects underwent incremental, symptom‐limited exercise testing using an upright cycle ergometer with a ramp protocol (Strength Ergo 8; Fukuda Denshi Co. Ltd., Tokyo, Japan). Breath‐by‐breath oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were measured during exercise using an AE‐300S respiratory monitor (Minato Medical Science, Osaka, Japan). Peak VO2 was measured as an average of the last 30 seconds of exercise. Ventilatory response to exercise (expressed as a VE/VCO2 slope) was calculated as the regression slope relating VE to CO2 from the start of exercise until the respiratory compensation point (the time at which ventilation is stimulated by CO2 output and end‐tidal CO2 tension begins to decrease).23 Ventilatory anaerobic threshold was calculated with the V‐slope method.
Statistical Analysis
Normally distributed data are presented as mean ± SD, non–normally distributed data are presented as median (interquartile range), and categorical variables are expressed as numbers and percentages. The baseline characteristics of the 2 groups were compared using the independent Student t test for normally distributed data and the Mann‐Whitney U test for non–normally distributed data for continuous variables, whereas the χ2 test was used for categorical variables. We used the analysis of variance (ANOVA) followed by the Tukey post‐hoc test. Within‐group and between‐group comparisons of measurements were carried out using paired and unpaired t tests for normally distributed data and the Mann‐Whitney U test and the Wilcoxon signed‐rank test for non–normally distributed data. Correlations between change in RV function and peak VO2 were assessed using Pearson correlation analysis. The event‐free rate was analyzed by the Kaplan‐Meier method and compared by the log‐rank test. Cox proportional hazard analysis was used to examine factors related to cardiac death and all‐cause mortality. To prepare for potential confounding, we considered the following clinical factors known to affect the risk of cardiac or all‐cause mortality in HF patients: age, sex, NYHA functional class III or IV, body mass index, systolic BP, HR, presence of ischemic etiology, hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation, chronic kidney disease, anemia, BNP, AHI, and use of β‐blockers, renin‐angiotensin aldosterone inhibitors, diuretics, and PAP therapy. Parameters with statistical significance in the univariate analysis (P < 0.10) were included in the multivariate analysis. A value of P < 0.05 was considered significant for all comparisons. All analyses were performed using SPSS version 21.0 (IBM, Armonk, NY).
Results
Clinical characteristics of the study subjects are shown in Table 1. Body mass index was significantly higher, and prevalence of anemia and diuretic usage was lower, in the PAP group than in the non‐PAP group. There were no significant differences between both groups with respect to age, sex, NYHA class, other comorbidities, and medications.
Table 1.
Comparisons of Clinical Characteristics Between NIPPVPAP and Non‐PAP Groups
| PAP, n = 31 | Non‐PAP, n = 78 | P Value | |
|---|---|---|---|
| Age, y | 65.7 ± 11.7 | 68.5 ± 14.2 | 0.324 |
| Male sex | 23 (74.2) | 46 (59.0) | 0.137 |
| NYHA functional class (I/II/III/IV) | 0/20/11/0 | 0/47/28/3 | 0.533 |
| Epworth Sleepiness Scale | 7.1 ± 4.4 | 5.2 ± 3.7 | 0.085 |
| BMI, kg/m2 | 26.0 ± 4.3 | 23.7 ± 3.9 | 0.010 |
| Ischemic etiology | 5 (16.1) | 9 (11.5) | 0.518 |
| Hypertension | 26 (83.9) | 62 (79.5) | 0.601 |
| DM | 10 (32.3) | 27 (34.6) | 0.815 |
| Dyslipidemia | 26 (83.9) | 62 (79.5) | 0.601 |
| AF | 12 (38.7) | 36 (46.2) | 0.480 |
| CKD | 15 (48.4) | 49 (62.8) | 0.167 |
| Anemia | 8 (25.8) | 39 (50.0) | 0.021 |
| COPD | 8 (25.8) | 22 (28.2) | 0.800 |
| NIPPVPAP | |||
| CPAP/ASV | 16/15 | 0/0 | — |
| Medications | |||
| β‐Blocker | 27 (87.1) | 58 (74.4) | 0.148 |
| RAS inhibitor | 29 (93.5) | 63 (80.8) | 0.097 |
| Diuretic | 10 (32.3) | 42 (53.8) | 0.042 |
| Aldosterone antagonist | 13 (41.9) | 35 (44.9) | 0.781 |
| Statin | 12 (38.7) | 19 (24.4) | 0.134 |
Abbreviations: AF, atrial fibrillation; ASV, adaptive servo ventilation; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; DM, diabetes mellitus; NYHA, New York Heart Association; PAP, positive airway pressure; RAS, renin‐angiotensin‐aldosterone; SD, standard deviation.
Data are presented as mean ± SD or n (%).
Polysomnographic data at baseline and titration night are shown in Table 2. Apnea‐hypopnea index, obstructive apnea index, mixed apnea index, central apnea index, hypopnea index, arousal index, 3% oxidative desaturation index, lowest SPO2, mean SPO2, and slow‐wave sleep were significantly improved in the PAP group (all 31 patients). During the 6 months, the mean usage was 5.9 ± 1.3 h/night with PAP.
Table 2.
Polysomnographic Data at Baseline and Titration
| PAP, n = 31 | Non‐PAP, n = 78 | P Value | ||
|---|---|---|---|---|
| AHI, /h | Baseline | 39.0 ± 19.4 | 30.2 ± 15.7 | 0.030 |
| Titration | 4.6 ± 2.6a | |||
| Obstructive apnea index, /h | Baseline | 9.4 ± 6.0 | 8.9 ± 4.3 | 0.841 |
| Titration | 1.0 ± 0.6a | |||
| Mixed apnea index, /h | Baseline | 3.1 ± 2.1 | 3.7 ± 2.6 | 0.653 |
| Titration | 0.4 ± 0.2a | |||
| Central apnea index, /h | Baseline | 8.4 ± 4.8 | 6.5 ± 5.9 | 0.476 |
| Titration | 0.8 ± 0.5a | |||
| Hypopnea index, /h | Baseline | 18.1 ± 10.8 | 10.7 ± 6.8 | 0.031 |
| Titration | 2.5 ± 1.2a | |||
| Arousal index, /h | Baseline | 25.0 ± 13.9 | 25.2 ± 14.5 | 0.972 |
| Titration | 10.8 ± 5.2a | |||
| 3% oxidative desaturation index, /h | Baseline | 30.1 ± 19.3 | 28.0 ± 22.0 | 0.779 |
| Titration | 5.5 ± 3.7a | |||
| Lowest SPO2, % | Baseline | 75.1 ± 11.6 | 79.2 ± 9.7 | 0.086 |
| Titration | 88.8 ± 3.6a | |||
| Mean SPO2, % | Baseline | 91.7 ± 9.5 | 94.2 ± 3.2 | 0.042 |
| Titration | 96.4 ± 1.9b | |||
| Slow‐wave sleep, % | Baseline | 1.7 ± 1.3 | 2.9 ± 1.7 | 0.567 |
| Titration | 7.8 ± 5.9a |
Abbreviations: AHI, apnea‐hypopnea index; PAP, positive airway pressure; SPO2, arterial oxyhemoglobin saturation.
P < 0.01 vs baseline.
P < 0.05.
Changes in vital signs and laboratory data from baseline to 6 months later are shown in Table 3. In the PAP group, NYHA class, BP, HR, BNP, and C‐reactive protein were significantly decreased, and eGFR was increased. Decreases of NYHA class, BP, HR, and BNP, and increases of eGFR, were both significantly greater in the PAP group than in the non‐PAP group.
Table 3.
Time Course of NYHA Functional Class, Vital Signs, Laboratory Data, Pulmonary Function Testing, and Cardiopulmonary Function Testing Parameters
| NYHA Functional Class, Vital Signs, and Laboratory Data | PAP, n = 31 | Non‐PAP, n = 78 | P Value | |
|---|---|---|---|---|
| NYHA class | Baseline | 2.4 ± 0.5 | 2.4 ± 0.6 | 0.488 |
| 6 months | 1.6 ± 0.5a | 2.3 ± 0.7 | ||
| Δ | −0.7 ± 0.5 | −0.2 ± 0.7 | <0.001 | |
| SBP, mm Hg | Baseline | 133.0 ± 12.9 | 123.7 ± 17.6 | 0.007 |
| 6 months | 120.7 ± 10.6b | 121.9 ± 19.4 | ||
| Δ | −12.8 ± 11.2 | −1.7 ± 16.9 | 0.001 | |
| DBP, mm Hg | Baseline | 76.7 ± 10.7 | 70.3 ± 13.3 | 0.013 |
| 6 months | 71.9 ± 7.9a | 71.0 ± 13.4 | ||
| Δ | −5.3 ± 12.2 | 0.8 ± 14.2 | 0.038 | |
| HR, bpm | Baseline | 67.7 ± 12.4 | 71.1 ± 15.4 | 0.423 |
| 6 months | 60.7 ± 10.3b | 71.2 ± 13.8 | ||
| Δ | −7.3 ± 9.2 | 0.2 ± 17.9 | 0.032 | |
| BNP, pg/mLc | Baseline | 209.1 (174.8) | 134.2 (113.8) | 0.025 |
| 6 months | 71.6 (106.0)b | 173.1 (209.9)a | ||
| Δ | −105.7 (138.0) | 27.5 (150.1) | <0.001 | |
| eGFR (mL/min/1.73 cm2) | Baseline | 60.0 ± 17.3 | 52.8 ± 22.5 | 0.162 |
| 6 months | 66.2 ± 17.6b | 52.1 ± 22.7 | ||
| Δ | 6.6 ± 6.0 | −0.8 ± 7.8 | <0.001 | |
| CRP (mg/dL)c | Baseline | 0.42 (0.48) | 0.26 (0.36) | 0.016 |
| 6 months | 0.15 (0.38)b | 0.27 (0.41) | ||
| Δ | −0.22 (0.33) | 0.04 (0.29) | <0.001 | |
| Pulmonary Function Testing | PAP, n = 17 | Non‐PAP, n = 42 | P Value | |
| FEV1 /FVC, % | Baseline | 83.9 ± 25.9 | 84.0 ± 24.5 | 0.782 |
| 6 months | 89.8 ± 29.7a | 84.9 ± 28.7 | ||
| Δ | 5.6 ± 11.1 | 1.0 ± 16.2 | 0.288 | |
| % Vital capacity | Baseline | 83.5 ± 22.3 | 85.0 ± 21.3 | 0.663 |
| 6 months | 89.9 ± 24.6b | 82.8 ± 20.2 | ||
| Δ | 6.6 ± 7.7 | −2.1 ± 13.6 | 0.020 | |
| Cardiopulmonary Exercise Testing | PAP, n = 25 | Non‐PAP, n = 46 | P Value | |
| Peak VO2, mL/kg/min | Baseline | 16.6 ± 2.6 | 15.6 ± 4.5 | 0.488 |
| 6 months | 19.6 ± 2.5a | 15.1 ± 3.2 | ||
| Δ | 3.0 ± 4.3 | −0.5 ± 3.1 | 0.035 | |
| VE/VCO2 slope | Baseline | 34.4 ± 5.8 | 34.3 ± 10.2 | 0.972 |
| 6 months | 27.1 ± 3.2a | 31.7 ± 4.2 | ||
| Δ | −7.3 ± 6.3 | −2.6 ± 8.3 | 0.190 |
Abbreviations: BNP, B‐type natriuretic peptide; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate by the Modification of Diet in Renal Disease formula; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HR, heart rate; IQR, interquartile range; NYHA, New York Heart Association; PAP, positive airway pressure; peak VO2, peak oxygen uptake; SBP, systolic blood pressure; VE/VCO2 slope, rate of increase in ventilation per unit increase in carbon dioxide.
P < 0.05.
P < 0.01 vs baseline.
Data are presented as median (IQR).
Changes in pulmonary function and cardiopulmonary exercise testing are shown in Table 3. In the PAP group, FEV1/FVC, percentage of vital capacity, peak VO2, and VE/VCO2 slope were significantly improved after 6 months. Changes of percentage of vital capacity and peak VO2 were significantly greater in the PAP group than in the non‐PAP group.
Changes in echocardiographic parameters are shown in Table 4. In the PAP group, LV wall thickness, LV dimension, LVEF, LA volume, mitral valve E/E′, mitral regurgitation score, inferior vena cava diameter, TR‐PG, RV‐FAC, tricuspid valve S′, and tricuspid valve E/E′ were significantly improved after 6 months. Relative wall thickness and tricuspid regurgitation score did not change in either group. Changes in LV wall thickness, LV dimension, LVEF, LA volume, mitral valve E/E′, mitral regurgitation score, inferior vena cava diameter, TR‐PG, RV‐FAC, and tricuspid valve E/E′ were greater in the PAP group than in the non‐PAP group. Correlations of changes in peak VO2 and RV function in the PAP group are as follows: TR‐PG, r = −0.338, P = 0.115; RV‐FAC, r = 0.507, P = 0.023; and tricuspid valve E/E′, r = −0.732, P = 0.003). We also analyzed the PAP group separately into the ASV group (n = 15) and CPAP group (n = 16). There were no significant differences in RV function and exercise capacity between the CPAP and ASV groups (see Supporting Table 1 in the online version of this article).
Table 4.
Time Course of Echocardiographic Parameters
| PAP, n = 31 | Non‐PAP, n = 70 | P Value | ||
|---|---|---|---|---|
| IVS thickness, mm | Baseline | 12.6 ± 2.1 | 11.4 ± 2.3 | 0.025 |
| 6 months | 11.8 ± 2.1a | 11.4 ± 2.4 | ||
| Δ | −0.8 ± 1.1 | −0.1 ± 1.1 | 0.001 | |
| LVEDD, mm | Baseline | 50.6 ± 6.9 | 49.7 ± 9.3 | 0.608 |
| 6 months | 48.7 ± 7.3b | 51.6 ± 8.9a | ||
| Δ | −1.6 ± 4.2 | 1.8 ± 5.5 | 0.004 | |
| LVESD, mm | Baseline | 34.0 ± 6.8 | 33.6 ± 8.7 | 0.909 |
| 6 months | 31.1 ± 8.1b | 35.4 ± 8.1b | ||
| Δ | −2.6 ± 6.6 | 1.6 ± 6.1 | 0.003 | |
| Posterior wall thickness, mm | Baseline | 12.0 ± 1.7 | 11.5 ± 1.8 | 0.286 |
| 6 months | 10.9 ± 1.5a | 11.4 ± 1.9 | ||
| Δ | −1.1 ± 1.1 | −0.1 ± 1.3 | <0.001 | |
| Relative wall thickness | Baseline | 0.48 ± 0.09 | 0.48 ± 0.13 | 0.981 |
| 6 months | 0.46 ± 0.10 | 0.46 ± 0.12 | ||
| Δ | −0.02 ± 0.07 | −0.03 ± 0.09 | 0.838 | |
| LVEF, % | Baseline | 58.9 ± 6.8 | 59.5 ± 6.9 | 0.577 |
| 6 months | 61.4 ± 8.4b | 57.5 ± 8.4b | ||
| Δ | 2.8 ± 5.8 | −2.0 ± 8.4 | 0.005 | |
| LA volume, mL | Baseline | 89.4 ± 44.8 | 90.2 ± 74.8 | 0.994 |
| 6 months | 63.9 ± 30.6a | 107.2 ± 87.9a | ||
| Δ | −25.9 ± 30.5 | 16.5 ± 39.7 | <0.001 | |
| Mitral valve E′, cm/s | Baseline | 5.5 ± 2.2 | 7.2 ± 2.9 | 0.006 |
| 6 months | 7.8 ± 3.3a | 6.4 ± 2.2b | ||
| Δ | 2.4 ± 2.7 | −0.8 ± 2.4 | <0.001 | |
| Mitral valve E/E′ | Baseline | 15.2 ± 5.0 | 14.7 ± 7.1 | 0.498 |
| 6 months | 10.7 ± 4.1a | 16.5 ± 9.4b | ||
| Δ | −4.8 ± 3.7 | 1.8 ± 6.9 | <0.001 | |
| Mitral regurgitation score | Baseline | 1.1 ± 0.7 | 0.8 ± 0.8 | 0.130 |
| 6 months | 0.5 ± 0.7a | 0.7 ± 0.8 | ||
| Δ | −0.6 ± 0.7 | −0.1 ± 0.8 | 0.010 | |
| Inferior vena cava diameter, mm | Baseline | 16.7 ± 5.9 | 16.8 ± 5.4 | 0.889 |
| 6 months | 13.0 ± 3.5a | 17.1 ± 5.1 | ||
| Δ | −3.7 ± 4.7 | 0.3 ± 4.1 | <0.001 | |
| Tricuspid regurgitation score | Baseline | 0.9 ± 0.7 | 1.0 ± 0.8 | 0.658 |
| 6 months | 0.6 ± 0.6 | 0.9 ± 0.9 | ||
| Δ | −0.4 ± 0.8 | −0.0 ± 0.8 | 0.073 | |
| TR‐PG, mm Hg | Baseline | 31.1 ± 8.9 | 30.0 ± 13.5 | 0.571 |
| 6 months | 22.4 ± 7.8a | 32.4 ± 13.6 | ||
| Δ | −8.8 ± 6.3 | 2.3 ± 10.1 | <0.001 | |
| RV‐FAC, % | Baseline | 36.0 ± 12.3 | 42.6 ± 10.5 | 0.028 |
| 6 months | 46.5 ± 11.2a | 38.9 ± 10.9b | ||
| Δ | 10.3 ± 10.1 | −3.4 ± 11.5 | <0.001 | |
| Tricuspid valve S′, cm/s | Baseline | 8.9 ± 2.1 | 8.9 ± 3.9 | 0.872 |
| 6 months | 11.1 ± 2.8b | 8.8 ± 2.8 | ||
| Δ | 2.1 ± 3.4 | −0.1 ± 3.8 | 0.062 | |
| Tricuspid valve E/E′ | Baseline | 7.8 ± 4.5 | 6.3 ± 2.6 | 0.262 |
| 6 months | 5.1 ± 2.2b | 9.0 ± 6.9b | ||
| Δ | −2.8 ± 4.0 | 2.5 ± 6.1 | 0.008 |
Abbreviations: IVS, interventricular septum; LA, left atrial; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic dimension; mitral valve E/E′, a ratio of the peak transmitral velocity during early diastole to the peak mitral valve annular velocity during early diastole; RV‐FAC, right ventricular fractional area change; tricuspid valve E/E′, ratio of the peak transtricuspid velocity during early diastole to the peak tricuspid valve annular velocity during early diastole; tricuspid valve S′, Doppler‐derived tricuspid lateral annular systolic velocity; TR‐PG, tricuspid valve regurgitation pressure gradient.
P < 0.01 vs baseline.
P < 0.05.
During the follow‐up period (mean, 916 days), in the non‐PAP group there were 10 cardiac deaths (7 caused by worsening heart failure and 3 by ventricular fibrillation) and 5 noncardiac deaths (3 resulting from respiratory failure/pneumonia, 1 from renal failure, and 1 from stroke). In contrast, none of the PAP group died in this follow‐up period. As shown in the Figure, Kaplan‐Meier analysis demonstrated that cardiac mortality (P = 0.045) and all‐cause mortality (P = 0.014) were significantly lower in the PAP group than in the non‐PAP group. Cox proportional hazard analysis to determine cardiac death and all‐cause mortality (see Supporting Figure and Tables 2–3 in the online version of this article).
Figure 1.
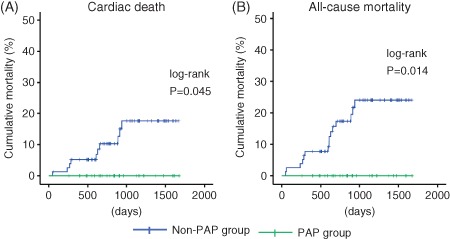
Kaplan‐Meier analysis for (A) cardiac mortality and (B) all‐cause mortality between patients in the PAP and non‐PAP groups. Abbreviations: PAP, positive airway pressure.
Discussion
To the best of our knowledge, the present study is the first to show the possibility that PAP improves right‐heart function, pulmonary function, and exercise capacity in HFpEF patients with SDB. Furthermore, PAP may reduce cardiac and all‐cause mortality of HFpEF patients with SDB.
Pathophysiology and Treatment of Heart Failure With Preserved Ejection Fraction
Heart failure with preserved ejection fraction has heterogeneity of pathophysiologic mechanisms such as LV diastolic dysfunction, systolic LV‐arterial coupling, myocardial contractile dysfunction, impaired exercise capacity, chronotropic incompetence, LA dysfunction, pulmonary hypertension, volume overload, vascular dysfunction and comorbidities.1 Exercise intolerance,24, 25 impaired pulmonary function,26 pulmonary hypertension,27, 28, 29 right‐heart dysfunction,10, 11, 24 LV hypertrophy,11, 24, 27 LA enlargement,27 and functional mitral regurgitation30 are evident and are associated with poor prognosis in HFpEF patients.
Established pharmacotherapy for HFrEF does not show an improvement in prognosis for HFpEF.1 Nonpharamacological therapy such as exercise training improves peak VO2 in HFpEF patients, for which enhanced skeletal‐muscle perfusion and/or oxygen utilization may be responsible.31
Impacts of Positive Airway Pressure on Cardiac and Pulmonary Function, Exercise Capacity, and Mortality in Heart Failure With Preserved Ejection Fraction
Pulmonary dysfunction exists in HF patients26, 32 and SDB patients.33 In addition, SDB causes excessive negative intrathoracic pressure and pulmonary‐artery vasoconstriction, resulting in right‐heart volume and pressure overload.9 Therapy with CPAP rapidly improves pulmonary congestion and decreases wall stress in decompensated HFpEF patients.34 In addition, CPAP therapy for 2 weeks improves pulmonary function and physical activity in CHF patients, regardless of presence of SDB.35 Furthermore, CPAP therapy for SDB decreases right atrial and LA volume,36 RV diameter,36 pulmonary hypertension,36 and LV hypertrophy36 and improves hypertension37 and cardiac systolic15, 38 and diastolic38 function in OSA patients. Positive airway pressure improves pulmonary congestion including unloading of respiratory muscles, preventing microatelectasis, decreasing dead space, improving alveolar ventilation, decreasing ventricular wall stress, and especially deleting adverse impacts brought on by SDB. Left ventricular ejection fraction improves, and BP and HR decrease by CPAP therapy for CHF patients in meta‐analysis,15 similar to our data. In addition, ASV improves LV diastolic function12, 13 and peak VO2 12 in HFpEF patients. Furthermore, SDB treatment using CPAP for OSA39 and ASV for coexisting CSR‐CSA and OSA40 reduces rehospitalization and cardiac mortality in HFrEF patients.5, 16 Namely, improvements in SDB, pulmonary congestion, right‐heart function, and pulmonary function may be associated with amelioration of exercise capacity. Furthermore, these mechanisms were possibly related to our present results that mortality decreased in the PAP group.
Study Strengths and Limitations
Our study has several strengths and differs from previous studies5, 16 in many ways. For instance, we presented comprehensive right‐heart and left‐heart function, pulmonary function, and exercise capacity brought on by effective PAP therapy in patients with HFpEF and SDB. We demonstrated, although in small numbers of subjects, that PAP might improve cardiac and all‐cause mortality. It is noteworthy that the diagnoses of HFpEF and SDB were accurately made by our experienced cardiologists.
There are some potential limitations. Our study was not a randomized controlled trial but prospective observational study. There might be differences in compliance with standard treatment for heart failure between the 2 groups, namely, the PAP group might have superior compliance than the non‐PAP group. However, in the present study, all patients in both groups visited the outpatient clinic of our hospital every month to continue medications during the follow‐up period. Second, we evaluated RV function using echocardiography, unless using right‐heart catheterization. Finally, because the number of study subjects was few because this study was performed in a single institution, small sample size and a low event rate were not appropriate for Cox proportional analyses. We should pay attention to generalize our results. Hence, further randomized controlled trials with a larger sample size are necessary to establish SDB treatment using PAP as a promising therapy for HFpEF with SDB.
Conclusion
Our data suggest that appropriate SDB management using PAP improves right‐heart and pulmonary function and exercise capacity and consequently may reduce cardiac and all‐cause mortality in patients with HFpEF and SDB.
Supporting information
FigureS1.
TableS1. Time Course of echocardiac and Cardiopulmonary Function Test Parameters
TableS2. Cox Proportional Hazard Model of Cardiac Death in HFpEF with SDB
TableS3. Cox Proportional Hazard Model of All‐cause Mortality in HFpEF with SDB
This study was supported in part by a grant‐in‐aid for Scientific Research (No. 21790737) from the Japan Society for the Promotion of Science. Akiomi Yoshihisa and Satoshi Suzuki belong to an endowed department (affiliation with Fukuda Denshi Co., Ltd., Tokyo, Japan).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35:1022–1032. [DOI] [PubMed] [Google Scholar]
- 2. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 3. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bitter T, Faber L, Hering D, et al. Sleep‐disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11:602–608. [DOI] [PubMed] [Google Scholar]
- 5. Damy T, Margarit L, Noroc A, et al. Prognostic impact of sleep‐disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail. 2012;14:1009–1019. [DOI] [PubMed] [Google Scholar]
- 6. Dursunoglu D, Dursunoglu N, Evrengül H, et al. Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur Respir J. 2005;26:283–288. [DOI] [PubMed] [Google Scholar]
- 7. Loo G, Koo CY, Zhang J, et al. Impact of obstructive sleep apnea on cardiovascular outcomes in patients treated with percutaneous coronary intervention: rationale and design of the sleep and stent study. Clin Cardiol. 2014;37:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drager LF, Diegues‐Silva L, Diniz PM, et al. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254. [DOI] [PubMed] [Google Scholar]
- 9. Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57:119–127. [DOI] [PubMed] [Google Scholar]
- 10. Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bitter T, Westerheide N, Faber L, et al. Adaptive servoventilation in diastolic heart failure and Cheyne‐Stokes respiration. Eur Respir J. 2010;36:385–392. [DOI] [PubMed] [Google Scholar]
- 13. Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo‐ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep‐disordered breathing. Eur J Heart Fail. 2013;15:543–550. [DOI] [PubMed] [Google Scholar]
- 14. Kourouklis SP, Vagiakis E, Paraskevaidis IA, et al. Effective sleep apnoea treatment improves cardiac function in patients with chronic heart failure. Int J Cardiol. 2013;168:157–162. [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal S, Nadeem R, Loomba RS, et al. The effects of continuous positive airways pressure therapy on cardiovascular end points in patients with sleep‐disordered breathing and heart failure: a meta‐analysis of randomized controlled trials. Clin Cardiol. 2014;37:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jilek C, Krenn M, Sebah D, et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011;13:68–75. [DOI] [PubMed] [Google Scholar]
- 17. Marin JM, Carrizo SJ, Vicente E, et al. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 18. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 19. Iber C, Ancoli‐Israel S, Chesson AL, Jr, et al, eds. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20. Kasai T, Usui Y, Yoshioka T, et al ; JASV Investigators . Effect of flow‐triggered adaptive servo‐ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne‐Stokes respiration. Circ Heart Fail. 2010;3:140–148. [DOI] [PubMed] [Google Scholar]
- 21. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 22. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [published correction appears in Eur Respir J. 2006;27:242]. Eur Respir J. 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 23. Ponikowski P, Francis DP, Piepoli MF, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103:967–972. [DOI] [PubMed] [Google Scholar]
- 24. Maeder MT, Thompson BR, Htun N, et al. Hemodynamic determinants of the abnormal cardiopulmonary exercise response in heart failure with preserved left ventricular ejection fraction. J Card Fail. 2012;18:702–710. [DOI] [PubMed] [Google Scholar]
- 25. Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andrea R, López‐Giraldo A, Falces C, et al. Lung function abnormalities are highly frequent in patients with heart failure and preserved ejection fraction. Heart Lunc Circ. 2014;23:273–279. [DOI] [PubMed] [Google Scholar]
- 27. Shah AM, Shah SJ, Anand IS, et al; TOPCAT Investigators. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thenappan T, Shah SJ, Gomberg‐Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. [DOI] [PubMed] [Google Scholar]
- 29. Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community‐based study. J Am Coll Cardiol. 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maréchaux S, Neicu DV, Braun S, et al; Lille HFpEF Study Group . Functional mitral regurgitation: a link to pulmonary hypertension in heart failure with preserved ejection fraction. J Card Fail. 2011;17:806–812. [DOI] [PubMed] [Google Scholar]
- 31. Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single‐blind trial. J Am Coll Cardiol. 2013;62:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dimopoulou I, Daganou M, Tsintzas OK, et al. Effects of severity of long‐standing congestive heart failure on pulmonary function. Respir Med. 1998;92:1321–1325. [DOI] [PubMed] [Google Scholar]
- 33. Chaouat A, Weitzenblum E, Krieger J, et al. Prognostic value of lung function and pulmonary haemodynamics in OSA patients treated with CPAP. Eur Respir J. 1999;13:1091–1096. [DOI] [PubMed] [Google Scholar]
- 34. Bellone A, Etteri M, Vettorello M, et al. The effects of continuous positive airway pressure on plasma brain natriuretic peptide concentrations in patients presenting with acute cardiogenic pulmonary edema with preserved left ventricular systolic function. Am J Emerg Med. 2010;28:230–234. [DOI] [PubMed] [Google Scholar]
- 35. Wittmer VL, Simoes GM, Sogame LC, et al. Effects of continuous positive airway pressure on pulmonary function and exercise tolerance in patients with congestive heart failure. Chest. 2006;130:157–163. [DOI] [PubMed] [Google Scholar]
- 36. Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–681. [DOI] [PubMed] [Google Scholar]
- 37. Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–555. [DOI] [PubMed] [Google Scholar]
- 38. Butt M, Dwivedi G, Shantsila A, et al. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail. 2012;5:226–233. [DOI] [PubMed] [Google Scholar]
- 39. Kasai T, Narui K, Dohi T, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133:690–696. [DOI] [PubMed] [Google Scholar]
- 40. Owada T, Yoshihisa A, Yamauchi H, et al. Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep‐disordered breathing. J Card Fail. 2013;19:225–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1.
TableS1. Time Course of echocardiac and Cardiopulmonary Function Test Parameters
TableS2. Cox Proportional Hazard Model of Cardiac Death in HFpEF with SDB
TableS3. Cox Proportional Hazard Model of All‐cause Mortality in HFpEF with SDB


