ABSTRACT
Background
Optimal medical therapy (OMT) for patients with chronic heart failure and a reduced ejection fraction (HF‐REF) includes angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, β‐blockers, and mineralocorticoid receptor antagonists, plus a diuretic.
Hypothesis
We hypothesized that OMT is less often prescribed in HF‐REF patients (≤35%) with New York Heart Association (NYHA) class II symptoms compared with those with NYHA class III/IV symptoms.
Methods
This was a cross‐sectional, observational, multicenter survey of hospital‐based cardiologists, office‐based cardiologists, and general practitioners in Germany.
Results
Out of a total of 384 patients enrolled, 144 had REF ≤35%. Patients with REF had NYHA class II symptoms in 39.6% (n = 57) and NYHA class III/IV symptoms in 60.4% (n = 87). The REF/NYHA class II group had a higher proportion of males than the REF/NYHA class III/IV group. For angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and β‐blockers, prescription rates were high and comparable between groups. However, prescription rates for mineralocorticoid receptor antagonists were lower compared with other guideline‐recommended treatments. Multivariate analyses indicated that OMT prescription was reduced for older patients and increased for patients cared for by an office‐based cardiologist.
Conclusions
Given the high proportion of patients with reduced left ventricular systolic function but only minor symptoms, HF‐REF appears to be underdiagnosed, and a higher proportion of patients than are currently recognized could potentially be candidates for OMT.
Introduction
Heart failure (HF) with reduced left ventricular ejection fraction (HF‐REF) may become clinically apparent with moderate to severe symptoms (classified as New York Heart Association [NYHA] class III or IV) or rather mild symptoms (NYHA class I or II). Because of the lack of overt disease in patients with mild symptoms, it is reasonable to expect that HF may frequently remain undiagnosed and often undertreated.
To date, the major clinical trials for the treatment of HF have primarily included patients with REF (left ventricular ejection fraction [LVEF] ≤35%).1 There is broad evidence to support the use of 3 classes of neurohumoral antagonists: angiotensin‐converting enzyme inhibitors (ACEIs) and/or angiotensin receptor blockers (ARBs), β‐blockers, and mineralocorticoid receptor antagonists (MRAs), in patients with HF‐REF and NYHA class III/IV symptoms.2 More recent evidence suggests that neurohumoral antagonists are also beneficial for patients with HF‐REF and NYHA class II symptoms.3, 4, 5, 6, 7, 8, 9 This has been incorporated into the 2012 version of the European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure, which state that optimal medical therapy (OMT) for patients with HF‐REF and a NYHA class ≥ II should include all 3 classes of neurohumoral antagonists (ie, ACEI/ARB, β‐blocker, and MRA) plus a diuretic.10
The Registry in Germany Focusing on Level‐Specific and Evidence‐Based Decision Finding in the Treatment of Heart Failure (REFLECT‐HF) survey was a cross‐sectional, observational, multicenter survey of treatment patterns of hospital‐based cardiologists (HBCs), office‐based cardiologists (OBCs), and general practitioners (GPs). The aim of the present analysis was to assess the use of OMT in patients with REF but mild symptoms.
Methods
The REFLECT‐HF survey was conducted in 10 regional clusters across Germany, in which either an HBC (n = 5) or an OBC (n = 5) served as a main center, with 5 satellites per center that were either OBCs (when the center was an HBC) or GPs (when the center was an OBC) in the respective area. In the end, a total of 384 patients were included at 5 HBCs, 26 OBCs, and 18 GPs. Physicians received a compensation of €100 per patient for the complete documentation of their patients.
The recruitment target was consecutive patients (age ≥18 years) with a documented history of chronic HF, with 20 patients for each HBC, 10 for each OBC, and 5 for each GP. Patients were required to have a NYHA class of ≥ II, and/or a LVEF <50%. Patients who were unable to complete the questionnaires because of psychiatric reasons, dementia, or other neurological diseases were excluded.
Information was collected on each participating physician, on patient demographics, the diagnosis of heart failure (NYHA class, LVEF), medical history, device and pharmacological treatments (drug classes were recorded, but not specific compounds or dose levels), quality of life (using the Minnesota Living With Heart Failure [MLHF] questionnaire), hospitalization‐related parameters, electrocardiography (rhythm, branch blocks, heart rate, and QRS interval), and laboratory values.
The study protocol was approved by the International Ethics Committee in Freiburg, Germany, on September 26, 2011. All patients provided written informed consent.
Statistical Analysis
Quantitative data (eg, patient age) in the tables and figures are presented by using either mean ± SD or 95% confidence intervals, and qualitative data (eg, patient sex) are expressed as respective proportions. Group differences were evaluated by applying χ2 tests for qualitative and Wilcoxon tests for quantitative data; P values for the latter were derived from the 2‐sided test situation.
Simple and multiple logistic regression models were also performed. For models accounting for multiple variables, stepwise selection was applied based on the P values of the parameter estimates: Only such variables remained in the final model where respective P values of the Wald statistic were <0.1. Variables with a large amount of missing values were not included into the model‐selection process to reach a high number of evaluable patients for the model calculation. Other possible reasons for variables not being included were lack of variability or collinearity issues. Results of the logistic regression models are presented in terms of estimated odds ratio and corresponding 95% confidence intervals. All statistical analyses were carried out using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patient Characteristics
The REFLECT‐HF survey included a total of 384 patients documented at 48 physicians between January 16 and August 30, 2012. This analysis includes data from 364 patients with HF who displayed NYHA class II–IV symptoms with LVEF <50%.
Out of 364 patients considered valid for this analysis, 144 had both a REF (defined as LVEF ≤35%) and either NYHA class II (n = 57; 39.6%) or NYHA class III/IV (n = 87; 60.4%). REF/NYHA class II patients were less often females (8.8% vs 28.7% with REF/NYHA class III/IV; P = 0.0039; Table 1), which had a similar trend in analyses disregarding the LVEF and only considering the NYHA class (24.3% vs 33.3%; P = 0.0561).
Table 1.
Patient Characteristics
| All Patients With LVEF ≤35% by NYHA Class | All Patients by NYHA Class | |||||
|---|---|---|---|---|---|---|
| NYHA II + LVEF ≤35%, n = 57 | NYHA III/IV + LVEF ≤35%, n = 87 | P Value | NYHA II, n = 202 | NYHA III/IV, n = 162 | P Value | |
| Age, y | 64.2 ± 12.8 | 66.5 ± 11.7 | 0.3588 | 67.9 ± 12.3 | 69.7 ± 11.0 | 0.3120 |
| Female sex | 8.8 | 28.7 | 0.0039 | 24.3 | 33.3 | 0.0561 |
| BMI, kg/m2 | 28.4 ± 4.5 | 28.5 ± 5.7 | 0.7116 | 28.8 ± 4.3 | 28.9 ± 5.7 | 0.4331 |
| Cr, mg/dL | 1.3 ± 0.5 | 1.3 ± 0.4 | 0.7760 | 1.3 ± 0.6 | 1.3 ± 0.4 | 0.6216 |
| eGFR, mL/min/1.73 m2 | 67.5 ± 29.5 | 64.8 ± 22.6 | 0.9738 | 64.8 ± 25.1 | 61.1 ± 22.3 | 0.3449 |
| <30 | 6.3 | 6.3 | 1.0000 | 6.3 | 5.9 | 0.9347 |
| K+, mmol/L | 4.3 ± 0.3 | 4.4 ± 0.5 | 0.6233 | 4.4 ± 0.5 | 4.4 ± 0.5 | 0.7375 |
| >5.5 | 0.0 | 2.9 | 0.5022 | 0.0 | 1.5 | 0.3556 |
| NT‐proBNP, pg/mL | 629.2 ± 665.4 | 976.0 ± 856.2 | 0.3977 | 554.7 ± 532.6 | 872.9 ± 772.1 | 0.1040 |
| ≥125 | 20.0 | 14.3 | 0.7503 | 26.3 | 14.3 | 0.3037 |
| Heart rate, bpma | 75.8 ± 16.2 | 73.7 ± 9.5 | 0.9917 | 72.0 ± 11.9 | 72.1 ± 11.3 | 0.7031 |
| Rhythmb | 0.3386 | 0.0930 | ||||
| Sinus rhythm | 76.2 | 69.2 | 76.8 | 65.6 | ||
| AF | 23.8 | 26.2 | 21.8 | 30.4 | ||
| Other | 0.0 | 4.6 | 1.4 | 4.0 | ||
| LBBB | 34.9 | 32.8 | 0.8244 | 30.4 | 28.7 | 0.7680 |
| QRS >130 msec | 42.9 | 32.8 | 0.3272 | 31.0 | 31.7 | 0.9110 |
| Ischemic etiology of HF | 56.1 | 60.9 | 0.5685 | 57.9 | 60.5 | 0.6198 |
| Prior MI | 45.6 | 42.5 | 0.7151 | 38.1 | 38.3 | 0.9762 |
| Hypertensive heart disease | 22.8 | 26.4 | 0.6228 | 32.2 | 26.5 | 0.2422 |
| MLHF summary score | 27.2 ± 20.4 | 42.5 ± 19.3 | <0.0001 | 28.5 ± 18.4 | 42.4 ± 19.0 | <0.0001 |
| Physical dimension | 13.3 ± 9.2 | 20.9 ± 8.0 | <0.0001 | 14.3 ± 8.9 | 21.0 ± 8.3 | <0.0001 |
| Emotional dimension | 4.2 ± 5.0 | 7.4 ± 6.0 | 0.0004 | 4.7 ± 5.0 | 7.3 ± 5.9 | <0.0001 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; Cr, creatinine; eGFR, estimated glomerular filtration rate; HF, heart failure; K+, potassium; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MLHF, Minnesota Living With Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; NYHA, New York Heart Association; QRS, QRS interval; SD, standard deviation.
Data are presented as mean ± SD or %.
Only patients with sinus rhythm.
Patients with pacemaker excluded from this analysis.
Patients with REF/NYHA class II also had a lesser score on the MLHF questionnaire (27.2 ± 20.4 vs 42.5 ± 19.3; P < 0.0001), reflecting better quality of life in those with NYHA class II symptoms. The difference in MLHF scores was also observed for NYHA class II vs NYHA class III/IV (P < 0.0001), irrespective of actual LVEF.
As depicted in Figure 1A, the majority of patients seen by HBCs had NYHA class III/IV (70.0% vs 30.0%), whereas OBCs and GPs saw a greater proportion of patients with NYHA class II (60.8% and 59.8%, respectively) compared with NYHA class III/IV (39.2% and 40.2%, respectively; P < 0.0001 for NYHA class II vs III/IV, all 3 physician groups). For the REF/NYHA class II and REF/NYHA class III/IV group, HBCs and GPs primarily saw patients with class III/IV symptoms (83.9% and 66.7%, respectively), whereas OBCs saw a similar proportion of patients with class II (48.4%) and class III/IV (51.6%) symptoms (P = 0.0052 for REF/NYHA class II vs REF/NYHA class III/IV, all 3 physician groups).
Figure 1.
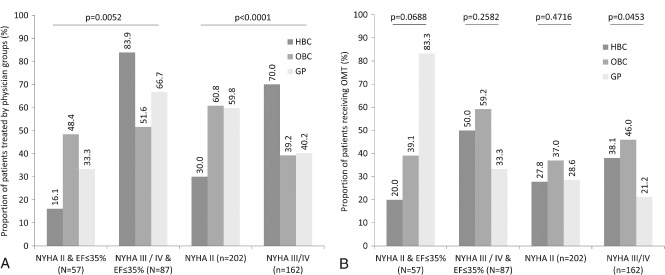
(A) Percentages of patients who were cared for by each type of physician according to NYHA class, with or without consideration of LVEF. (B) Percentages of patients who received OMT by the type of physician, and by NYHA class, LVEF, and NYHA class and LVEF. Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; EF, ejection fraction; GP, general practitioner; HBC, hospital‐based cardiologist; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; OBC, office‐based cardiologist; OMT, optimal medical therapy (treatment with ACEI/ARB, β‐blocker, MRA, and diuretic).
Treatment Patterns
The majority of patients with REF received ACEIs and/or ARBs (94.7% in NYHA class II vs 92.0% in NYHA class III/IV), β‐blockers (93.0% vs 92.0%, respectively), and diuretics (80.7% vs 88.5%, respectively), with no significant differences between the REF/NYHA class II and REF/NYHA class III/IV groups (Table 2). There were, however, nominal differences for the proportion of patients receiving diuretics (80.7% and 88.5%), MRAs (54.4% and 64.4%), anticoagulants (38.6% and 51.7%), and for implantable cardioverter‐defibrillators (ICD; 0.0% and 8.3%). Ivabradine was administered to 0.0% and 10.3% of patients in the REF/NYHA class II and REF/NYHA class III/IV groups, respectively, but this difference only reached borderline significance (P = 0.0524).
Table 2.
Pharmacotherapy/Device Use
| All Patients With LVEF ≤35% by NYHA Class | All Patients by NYHA Class | |||||
|---|---|---|---|---|---|---|
| NYHA II + LVEF ≤35%, n = 57 | NYHA III/IV + LVEF ≤35%, n = 87 | P Value | NYHA II, n = 202 | NYHA III/IV, n = 162 | P Value | |
| % | % | % | % | |||
| ACEIs | 77.2 | 69.0 | 0.2811 | 68.8 | 64.8 | 0.4201 |
| ARBs | 21.1 | 27.6 | 0.3759 | 26.7 | 28.4 | 0.7240 |
| ACEIs and/or ARBs | 94.7 | 92.0 | 0.5206 | 93.1 | 88.9 | 0.1616 |
| β‐Blockers | 93.0 | 92.0 | 0.8203 | 94.1 | 85.2 | 0.0048 |
| Diuretics | 80.7 | 88.5 | 0.1944 | 81.2 | 90.7 | 0.0102 |
| MRAs | 54.4 | 64.4 | 0.2310 | 43.6 | 55.6 | 0.0229 |
| Ivabradine | 0.0 | 10.3 | 0.0524 | 3.9 | 11.0 | 0.0362 |
| Anticoagulants | 38.6 | 51.7 | 0.1225 | 39.6 | 46.9 | 0.1614 |
| CRT | 12.5 | 14.9 | 0.7125 | 9.0 | 8.6 | 0.884 |
| ICD | 0.0 | 8.3 | 0.5035 | 0.0 | 7.7 | 0.2398 |
| ACEI/ARBs, β‐blockers, diuretics, and MRAs combined | 42.1 | 52.9 | 0.2061 | 34.2 | 38.9 | 0.3508 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
Regardless of LVEF, administration of diuretics, MRAs, and ivabradine was more frequent in the NYHA class III/IV group (P = 0.0102, 0.0229, and 0.0362, respectively), whereas β‐blockers were more commonly prescribed in the NYHA class II group (P = 0.0048). The proportion of patients receiving OMT did not differ between either the NYHA class II and the NYHA class III/IV groups or the REF/NYHA class II and REF/NYHA class III/IV groups.
Factors Associated With Optimal Medical Therapy
The frequency at which OMT was prescribed was greater for patients with NYHA class III/IV symptoms who saw an OBC, as compared with an HBC or a GP (P = 0.0453). No other significant differences were observed in the frequency of OMT prescription when assessed by physician group (Figure 1B).
For patients with REF and either NYHA class II or class III/IV symptoms, there were no notable differences in the prescription of OMT with respect to physician sex, their working experience, the type of physician (HBC, OBC, or GP), the number of HF patients treated per day, patient sex, or the patient's estimated glomerular filtration rate. On the other hand, in the NYHA class III/IV group, the REF group, as well as the REF/NYHA class II and REF/NYHA class III/IV groups, older patients (> median vs ≤ median) had a reduced likelihood of receiving OMT (Table 3). Similarly, irrespective of LVEF, physicians with a long work experience (> median vs ≤ median) were less likely to prescribe OMT to patients with NYHA class III/IV symptoms, as were GPs vs OBCs.
Table 3.
Univariable Predictors of OMT (% of Patients Receiving ACEI/ARB, β‐Blocker, Diuretic, and MRAs)
| NYHA II + LVEF ≤35%, n = 57, OR (95% CI) | NYHA III/IV + LVEF ≤35%, n = 87, OR (95% CI) | NYHA II, n = 202, OR (95% CI) | NYHA III/IV, n = 162, OR (95% CI) | |
|---|---|---|---|---|
| Physician sex, F vs M | 0.32 (0.03–3.02) | 0.88 (0.26–2.96) | 0.58 (0.27–1.27) | 0.61 (0.25–1.50) |
| Physician working experience > median vs ≤ median | 0.74 (0.25–2.23) | 0.40 (0.16–1.00) | 0.58 (0.32–1.07) | 0.37 (0.19–0.73) |
| GP vs OBC | 7.78 (0.84–72.13) | 0.34 (0.09–1.31) | 0.68 (0.33–1.39) | 0.32 (0.12–0.81) |
| GP vs HBC | 20.00 (0.93–429.90) | 0.50 (0.12–2.08) | 1.04 (0.31–3.46) | 0.44 (0.15–1.24) |
| OBC vs HBC | 2.57 (0.27–24.89) | 1.45 (0.56–3.78) | 1.53 (0.51–4.54) | 1.38 (0.65–2.93) |
| No. of HF patients treated/d > median vs ≤ median | 0.70 (0.24–2.03) | 1.82 (0.72–4.56) | 1.14 (0.63–2.07) | 1.84 (0.93–3.64) |
| Patient sex, F vs M | 0.32 (0.03–3.02) | 0.95 (0.38–2.41) | 0.91 (0.46–1.81) | 1.00 (0.51–1.95) |
| Patient age > median vs ≤ median | 0.28 (0.08–0.93) | 0.31 (0.13–0.75) | 0.65 (0.36–1.18) | 0.29 (0.15–0.56) |
| Patient eGFR <30 vs ≥30 | 0.56 (0.02–17.92) | 1.00 (0.06–17.51) | 1.23 (0.10–14.78) | 0.68 (0.07–6.96) |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; eGFR, estimated glomerular filtration rate; F, female; GP, general practitioner; HBC, hospital‐based cardiologist; HF, heart failure; LVEF, left ventricular ejection fraction; M, male; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; OBC, office‐based cardiologist; OMT, optimal medical therapy; OR, odds ratio.
Factors Associated With Being New York Heart Association Class II Despite Left Ventricular Ejection Fraction ≤35%
Univariate analyses for being NYHA class II with REF were performed using the following 6 variables: age (> median), female sex, body mass index (> median), ischemic origin of HF or prior myocardial infarction, hypertensive heart disease, and heart rate (> median). The only one of these 6 variables that influenced the probability of being NYHA II with REF was female sex, which was a negative correlation. Multivariate analyses demonstrated that female sex was independently associated with a reduced likelihood of being NYHA class II despite REF (Table 4).
Table 4.
Multivariable Predictors of Being NYHA II Despite LVEF ≤35% (Stepwise Multivariable Regression Analysis)
| No. | Simple OR (95% CI) | Multiple OR (95% CI) | |
|---|---|---|---|
| Age > median | 144 | 0.83 (0.42–1.64) | — |
| Female sex | 144 | 0.24 (0.09–0.67) | 0.24 (0.09–0.67)a |
| BMI > median | 144 | 0.80 (0.40–1.56) | — |
| Ischemic origin of HF or prior MI | 144 | 0.80 (0.40–1.58) | — |
| Hypertensive heart disease | 144 | 0.82 (0.38–1.80) | — |
| Heart rate > median | 144 | 1.14 (0.58–2.22) | — |
Abbreviations: CI, confidence interval; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; OR, odds ratio.
Final multiple model is equivalent to simple model.
Discussion
The present subanalysis extends the initial findings of the REFLECT‐HF survey11 by investigating the differences in treatment patterns for patients with reduced LVEF (defined as ≤35%) and either NYHA class II or NYHA class III/IV symptoms. Because patients defined as having NYHA class II heart function have only mild symptoms, both the diagnosis and treatment of these patients is potentially more challenging than for those with overt disease. Based on recent evidence from clinical trials, the 2012 version of the ESC guidelines for the treatment of HF included new recommendations, in particular on the use of all 3 classes of neurohumoral antagonists for patients with REF and NYHA class II–IV symptoms.10
Patient Characteristics
The present subanalysis of the REFLECT‐HF study demonstrates that patient demographics vary according to the degree of left ventricular dysfunction and NYHA class. The NYHA class II group comprised a higher proportion of males than females, and the mean age tended to be lower than that of patients with NYHA class III/IV symptoms. Accordingly, compared with the REF/NYHA class III/IV group, the REF/NYHA class II group was characterized by a significantly higher proportion of males and a slightly lower mean age. This sex‐specific difference was emphasized by the results of multivariate modeling for predictors of being NYHA class II and having REF—the only factor that was identified was female sex, and this was a negative correlation. Thus, the characteristics of patients in this study were consistent with the published literature, which suggests that HF with preserved LVEF (≥50%) is more commonly observed in females and older patients.10, 12, 13 Of note in this survey, approximately 40% of all patients with REF had mild, NYHA class II symptoms.
Guideline Adherence
For the 3 classes of well‐established drugs for the treatment of heart failure (ACEIs/ARBs, β‐blockers, and diuretics), rates of administration were high, with no significant differences between the REF/NYHA class II and REF/NYHA class III/IV groups. These figures are in line with the recommendations of both the 2012 version of the ESC guidelines10 for the treatment of heart failure, as well as regional guidelines, such as the German Society of General Practitioners guidelines.14
In contrast, for MRAs, the prescription rate was much lower, at 54% and 64% for the REF/NYHA class II and REF/NYHA class III/IV groups, respectively. When considering older versions of the ESC guidelines,15 as well as regional guidelines (which had not been updated at the time the REFLECT‐HF survey was conducted), a lower rate of prescription of MRAs relative to the other 3 drug classes may be expected. Prior to the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF) clinical trial, MRAs were only indicated for patients with NYHA class III/IV symptoms. However, the 2012 ESC guidelines were revised to reflect the findings related to the use of MRAs in patients with NYHA class II symptoms.10, 16 Even accounting for the use of the older ESC guidelines or regional guidelines, MRAs appear to be underprescribed, with up to 35% of patients in the REF/NYHA class III/IV group being eligible for, but not receiving, this drug class. Furthermore, the 2012 ESC guidelines recommend that the combination of an ACEI/ARB, a β‐blocker, and an MRA should be initiated as soon as possible following the diagnosis of HF‐REF with NYHA class II symptoms.10, 17 Thus, a considerable subset of patients in the REF/NYHA class II group would be eligible for, but did not receive, an MRA. In the present subanalysis, the probability of receiving all 3 classes of neurohumoral antagonists was highest for NYHA class III/IV patients who were treated by an OBC. Data from the primary REFLECT‐HF analysis, which included an evaluation of the rate of prescription of each drug class according to physician type, indicate that MRAs were more frequently prescribed by OBCs than HBCs and GPs. Interestingly, the REFLECT‐HF analysis also demonstrated that the proportion of patients who were eligible for an MRA but did not receive treatment was greater for those who saw an HBC or an OBC than for those who saw a GP.11
Another noteworthy finding of this subanalysis was the lack of administration of ivabradine to patients in the REF/NYHA class II group, despite a high mean heart rate. This is especially pertinent in the context of the finding of a significantly higher heart rate in patients with REF vs those with LVEF >35%. The 2012 version of the ESC guidelines (but not the previous version) advocates the use of ivabradine as an add‐on treatment for patients with an REF and NYHA class II or higher who have a resting heart rate >70 bpm, despite receiving an ACEI/ARB, a β‐blocker, an MRA, and a diuretic. Although rates of administration of both ivabradine and β‐blockers were similar in the REF and the LVEF >35% group, significant differences were observed when comparing the NYHA class II group with the NYHA class III/IV group. Use of β‐blockers was significantly more frequent in the NYHA class II group, whereas use of ivabradine was significantly more frequent in the NYHA class III/IV group. Based on the recommendations of clinical guidelines, it would be expected that β‐blockers are the first‐line drug for the reduction of heart rate. Thus, it appears likely that the combination of β‐blockers and ivabradine was used more frequently in patients with NYHA class III/IV symptoms than in patients with NYHA class II symptoms. Estimates suggest that in real‐life clinical practice, only 30% to 35% of patients attain the therapeutic target dose of β‐blockers, and, in addition, even in patients who receive optimal dose levels, an elevated heart rate is often observed.18 Given that a high heart rate has been identified as an independent predictor of cardiovascular events in patients with HF, increased use of the combination of β‐blockers and ivabradine may confer survival benefits.18, 19, 20, 21
Finally, the use of the nonsurgical devices (ICDs and cardiac resynchronization therapy [CRT]) has also been demonstrated to be beneficial for patients with HF‐REF and mild symptoms. Results from the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) and the Multicenter Automatic Defibrillator Implantation Trial (MADIT)‐CRT indicated that the addition of ICD or CRT to pharmacological treatment improved survival in this patient population.6, 11 Analyses of registries and hospital claims data suggest that only 20% to 50% of eligible patients receive ICD or CRT.22, 23, 24 Our own rates are at the lower end of this estimate, with 14.9% of patients with REF and NYHA group III/IV having a CRT and 8.3% an ICD. Rates were even lower for those with only mild symptoms (12.5% CRT) or in those with LVEF >35%. This may reflect the inclusion of HF patients at office‐based physicians (cardiologists and GPs) rather than in hospitals only, which was the case for the majority of other registries.
Our results are certainly relevant for the German setting, but they need to be put into perspective with European data. The ESC‐HF Long‐Term registry,25, 26 which is a prospective, observational study involving 211 cardiology centers in 21 European and Mediterranean countries, enrolled 7401 patients with chronic HF from May 2011 to April 2013. The median LVEF was 35% (range, 28%–45%), and 74.7% had either NYHA class I or II symptoms. Among the subpopulation of patients with LVEF ≤45% (n = 4792), the rates of prescription of diuretics were 84.3%, ACEIs 70.7%, ARBs 23.5%, β‐blockers 92.7%, MRAs 67.0%, and ivabradine 10.5%. However, data on the subpopulation of patients with REF and NYHA class I or II symptoms were not available at the time of publication,25 making a direct comparison with our data difficult.
Factors Predictive of Receiving Optimal Medical Therapy
The frequency of patients in the present subanalysis who were receiving OMT was highest in the REF/NYHA class III/IV group (53%) and lowest in the LVEF >35% subgroup (30%), with an intermediate value for the REF/NYHA class II group (42%). Because the rate of prescription of the 3 well‐established drug classes for HF was high and relatively uniform across groups, the less‐than‐adequate administration of OMT appears to be primarily associated with the underuse of MRAs. In the case of older patients, physicians may be less willing to prescribe MRAs, possibly because of the presence of risk factors such as reduced renal function. A survey performed in France also indicated that underprescription of OMT was more frequent among older patients, as well as those with renal dysfunction.27 In addition, a recently published study highlighted the requirement for better adherence to guidelines for monitoring creatinine and potassium levels in patients with HF following the initiation of an MRA.28 Improvements in monitoring of renal function in patients receiving MRAs would allow dosage modifications or discontinuation of treatment, potentially resulting in fewer adverse events and this class of drug being associated with a better benefit‐risk profile.
With regard to the increased likelihood of receiving OMT for patients who saw an OBC, this may in part be reflective of differences in the patient population at each type of clinical setting. For example, the REFLECT‐HF analysis indicated that patients cared for by GPs were more often females, and tended to be older, with reduced renal function and a lower NYHA class. The frequency of patients with atrial fibrillation was also higher for GPs. Furthermore, it may be expected that patients who require treatment in a hospital setting have greater morbidity than those who visit OBCs or GPs. In the present subanalysis, differences were also observed in the patient population seen by each physician type, with HBCs caring predominantly for patients with NYHA class III/IV symptoms. The patient population seen by each type of physician may contribute to variations in the implementation of guidelines for 2 reasons: (1) the inherent characteristics of a patient may preclude treatment with a particular agent (ie, patients may have other comorbidities or contraindications that complicate drug prescription); and (2) the relative experience of each type of physician with particular patient populations.
Study Limitations
Although the REFLECT‐HF survey comprised data from the 3 main groups of physicians who treat patients with HF in Germany, and is thus reflective of real‐world clinical practice, the study has some limitations. In particular, because REFLECT‐HF was a cross‐sectional study, data on the time since diagnosis of HF, as well as morbidity and mortality rates, were not obtained. Other limitations of this study have already been discussed in the primary publication of the REFLECT‐HF survey.11 These include a change of guideline recommendations recommending the use of MRAs in patients with NYHA class II HF and REF. Finally, though the findings may apply to the German health care system, they should be validated in different health care systems.
Clinical Implications
Using OMT is associated with an improved prognosis, even in those patients with only moderate, NYHA class II symptoms. The reluctance to intensify treatment, based on a lack of clinical consequences and a fear of treatment‐associated side effects, but improved prognosis, should be overcome by a closer cooperation and exchange of different physician groups caring for the HF patient.
Conclusion
Given the high proportion of patients with a reduced left ventricular systolic function but only minor symptoms, HF‐REF appears to be underdiagnosed, and a higher proportion of patients than are currently recognized could potentially be candidates for OMT.
Acknowledgments
The statistical evaluation by Michael Obermayer (GKM Gesellschaft für Therapieforschung mbH, Munich) is acknowledged.
Steering Committee: Michael Böhm (Homburg/Saar), Carsten Tschöpe (Berlin), Ulrich Tebbe (Detmold), Jost Henner Wirtz (Dinslaken), Jan Lokies (Berlin), Peter Bramlage (Mahlow).
Investigators: Ulrich Tebbe (Detmold), Stefan Blankenberg (Hamburg), Stefan Störk (Würzburg), Bernd‐Dieter Gonska (Karlsruhe), Günter Piske (Berlin), Gunter Stenzel (Riesa), Clemens Bauknecht (Rottweil), Heribert Brück (Erkelenz), Peter Bosiljanoff (Munich), Jens Taggeselle (Markkleeberg), Gunter Seidel (Detmold), Sinisa Miketic (Detmold), Torsten Figura (Rinteln), Susanne Backhaus (Buchholz), Dag‐Alexander Keilhau (Hamburg), Jens Beermann (Wedel), Behrus Subin (Hamburg), Wilhelm Josef Mieseler (Höchberg), Claus Günthert (Höchberg), Michael Dobler (Karlstadt), Heinrich Bechtold (Schrozberg), Matthias Salefsky (Aschaffenburg), Ulrich Staedt (Speyer), Thomas Dieterle (Ettlingen), Sabine Raulin (Karlsruhe), Mustafa Durak (Heidelberg), Petra Lange‐Braun (Berlin), Andreas Förster (Berlin), Rene Oliver (Berlin), Claudia Zemmrich (Berlin), Werner Rieker (Berlin), Heike Stenzel (Riesa), Peter Kindermann (Riesa), Jan Nimetschik (Gröditz), Petra Bauer (Bad Liebenwerda), Andreas Hagenow (Elsterwerda), Ottmar Banning (Waldmössing), Klaus Götz (Bad Dürrheim), Johannes Guhl (Villingen‐Schwenningen), Karl Stuff (Donaueschingen), Helmut Hermanns (Erkelenz), Franz‐Josef Geffers (Erkelenz), Ulrich Arendt (Mönchengladbach), Alexandra Haupt‐Pichler (Munich), Cornelia Groos‐März (Munich), Constanze Schmidt (Borna), Thomas Theuner (Markkleeberg), Astrid Meier (Markkleeberg), Cornelia Schlott (Zwenkau).
All authors were involved in the conception and design of the present survey. Michael Böhm and Peter Bramlage drafted the first version of the manuscript and all authors revised the manuscript for important intellectual content. All authors approved the final manuscript and voted for its submission.
Michael Böhm, Carsten Tschöpe, Ulrich Tebbe, Jost Henner Wirtz, Jan Lokies, and Peter Bramlage have received research funding from a number of pharmaceutical companies interested in heart failure, including the sponsor of the present study, Pfizer Pharma GmbH, Berlin. Peter Bramlage was a paid consultant to Pfizer in connection with the development of this manuscript. Eva Turgonyi, Katharina Lins, and Anke M. Strunz are employees of the sponsor.
This study was sponsored by Pfizer.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Zhang Y, Kilgore ML, Arora T, et al. Design and rationale of studies of neurohormonal blockade and outcomes in diastolic heart failure using OPTIMIZE‐HF registry linked to Medicare data. Int J Cardiol. 2013;166:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ. CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin‐angiotensin‐aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail. 2011;13:929–936. [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, McMurray JJ, Krum H, et al; EMPHASIS‐HF Study Group . Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 4. Lindley CM, Tully MP, Paramsothy V, et al. Inappropriate medication is a major cause of adverse drug reactions in elderly patients. Age Ageing. 1992;21:294–300. [DOI] [PubMed] [Google Scholar]
- 5. The SOLVD Investigators . Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Hall WJ, Cannom DS, et al; MADIT‐CRT Trial Investigators . Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 7. Tang AS, Wells GA, Talajic M, et al; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators . Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 8. Konstam MA, Neaton JD, Dickstein K, et al; HEAAL Investigators . Effects of high‐dose versus low‐dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double‐blind trial [published correction appears in Lancet. 2009;374:1888]. Lancet. 2009;374:1840–1848. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Bristow MR, Cohn JN, et al; U.S. Carvedilol Heart Failure Study Group . The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2013;34:158]. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 11. Tebbe U, Tschöpe C, Wirtz JH, et al. Registry in Germany focusing on level‐specific and evidence‐based decision finding in the treatment of heart failure: REFLECT‐HF. Clin Res Cardiol. 2014;103:665–673. [DOI] [PubMed] [Google Scholar]
- 12. Quiroz R, Doros G, Shaw P, et al. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am J Cardiol. 2014;113:691–696. [DOI] [PubMed] [Google Scholar]
- 13. von Scheidt W, Zugck C, Pauschinger M, et al. Characteristics, management modalities and outcome in chronic systolic heart failure patients treated in tertiary care centers: results from the Evidence‐based Treatment in Heart Failure (EVITA‐HF) registry. Clin Res Cardiol. 2014;103:1006–1014. [DOI] [PubMed] [Google Scholar]
- 14. Weinbrenner S, Langer T, Scherer M, et al. The German National Disease Management Guideline “Chronic Heart Failure” [article in German]. Dtsch Med Wochenschr. 2012;137:219–227. [DOI] [PubMed] [Google Scholar]
- 15. Dickstein K, Cohen‐Solal A, Filippatos G, et al; ESC Committee for Practice Guidelines . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) [published corrections appear in Eur J Heart Fail. 2009;11:110 and Eur J Heart Fail. 2010;12:416]. Eur J Heart Fail. 2008;10:933–989. [DOI] [PubMed] [Google Scholar]
- 16. Zannad F, McMurray JJ, Drexler H, et al. Rationale and design of the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Eur J Heart Fail. 2010;12:617–622. [DOI] [PubMed] [Google Scholar]
- 17. Mentz RJ, Bakris GL, Waeber B, et al. The past, present and future of renin‐angiotensin aldosterone system inhibition. Int J Cardiol. 2013;167:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Franco A, Sarullo FM, Salerno Y, et al. Beta‐blockers and ivabradine in chronic heart failure: from clinical trials to clinical practice. Am J Cardiovasc Drugs. 2014;14:101–110. [DOI] [PubMed] [Google Scholar]
- 19. Pastor‐Pérez FJ, Manzano‐Fernández S, Goya‐Esteban R, et al. Heart rate control in chronic heart failure: resting versus mean heart rate with prolonged ambulatory ECG recording. Int J Cardiol. 2013;170:e45–e47. [DOI] [PubMed] [Google Scholar]
- 20. Kato N, Kinugawa K, Teruhiko I, et al. Differential impacts of achieved heart rate and achieved dose of β‐blocker on clinical outcomes in heart failure with and without atrial fibrillation. Int J Cardiol. 2014;173:331–333. [DOI] [PubMed] [Google Scholar]
- 21. Ceconi C, Freedman SB, Tardif JC, et al; BEAUTIFUL Echo‐BNP Investigators . Effect of heart rate reduction by ivabradine on left ventricular remodeling in the echocardiographic substudy of BEAUTIFUL. Int J Cardiol. 2011;146:408–414. [DOI] [PubMed] [Google Scholar]
- 22. Fonarow GC, Stough WG, Abraham WT, et al; OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 23. Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence‐based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence‐Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 24. Albert NM, Yancy CW, Liang L, et al. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. [DOI] [PubMed] [Google Scholar]
- 25. Maggioni AP, Anker SD, Dahlström U, et al; Heart Failure Association of the ESC . Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 26. Maggioni AP, Dahlström U, Filippatos G, et al; Heart Failure Association of the European Society of Cardiology . EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail. 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 27. de Groote P, Isnard R, Clerson P, et al. Improvement in the management of chronic heart failure since the publication of the updated guidelines of the European Society of Cardiology. The Impact‐Reco Programme. Eur J Heart Fail. 2009;11:85–91. [DOI] [PubMed] [Google Scholar]
- 28. Allen LA, Shetterly SM, Peterson PN, et al. Guideline concordance of testing for hyperkalemia and kidney dysfunction during initiation of mineralocorticoid receptor antagonist therapy in patients with heart failure. Circ Heart Fail. 2014;7:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


