ABSTRACT
Sinus of Valsalva aneurysm (SOVA), a congenital or acquired cardiac defect that is present in roughly 0.09% of the general population, often presents as an incidental finding during cardiac imaging. Although an echocardiogram is the standard imaging technique for such findings, cardiac computed tomography angiography (CCTA) has been increasingly utilized. If SOVA is diagnosed, CCTA is also a useful test for patients who are at low to intermediate risk for coronary artery disease (CAD) prior to surgical repair. CCTA can accurately rule out CAD, obviating the need for invasive angiography in most cases, which may be more risky in SOVA patients because their coronaries may be more difficult to engage and their aortic root may be more prone to injury. Although surgery has previously been the treatment of choice, transcatheter techniques have added to the spectrum of nonsurgical alternatives for repair. We report here 4 incidental SOVA cases and review the current literature.
Background
Definition
A sinus of Valsalva aneurysm (SOVA) is an enlargement of the aortic root area between the aortic valve annulus and the sinotubular ridge.1 In a normal heart, the left and right sinus each contain their respective coronary artery ostia, whereas the posterior sinus is a noncoronary sinus. Sinuses function to permit aortic valve opening during systole without the occlusion of coronary artery ostia. Sinus diameter varies by gender. The upper limit of normal for men is 4.0 cm and 3.6 cm for women, with slight variations when adjusted for body surface area.2 The anatomic positioning of each sinus within the heart is a major determinant of clinical outcome in the case of SOVA formation and/or rupture. The right sinus lies adjacent to the interventricular septum and the right ventricular parietal bands. The left sinus is proximal to the anterior left ventricular free wall as well as the anterior mitral leaflet. The noncoronary (posterior) sinus rests above the interventricular septum, a portion of the anterior mitral leaflet, and forms a complex with the transverse sinus.
Causes
SOVAs can be either acquired or congenital. Although there is debate regarding whether congenital or acquired subtypes are more frequent, SOVA is a consequence of weakness of the elastic lamina at the junction of the aortic media and the annulus fibrosis. In congenital SOVA, the pathology is frequently associated with Marfan's syndrome, Ehlers‐Danlos syndrome, or other connective tissue disorders.3, 4 Patients with a biscuspid aortic valve (BAV), which is the most common congenital heart defect, with a prevalence estimated to be between 0.5% and 2%, may also be more likely to develop SOVA. A retrospective review of 86 cases of SOVA at a single institution noted that 9% of patients had concurrent BAVs.5 Further notable associated cardiac lesions included ventricular septal defects (31%) and aortic regurgitation (44%).5 Embryologically, the SOVA forms first as a blind diverticulum secondary to pressure forces on the aortic root.6 Thus, congenital defects potentiating these pressure forces can lead to development of a SOVA.
Acquired SOVAs are similarly associated with connective tissue pathologies. Infectious etiologies are well established mechanisms for elastic tissue weakening. Syphilis, bacterial endocarditis, and tuberculosis have each been linked to SOVA formation. Chronic changes of atherosclerosis and cystic medial necrosis can weaken the intimal layer of vasculature and lead to SOVA. Chest trauma, vasculitic diseases, and iatrogenic injury during aortic valve replacement have all been reported as causes of acquired SOVA.1, 6, 7, 8
Epidemiology
Although the true prevalence of SOVAs is unknown, the estimated rate is approximately 0.09% of the general population.9 SOVAs comprise 0.1% to 3.5% of all congenital cardiac defects.6 SOVAs usually affect the right coronary sinus, followed by the noncoronary sinus, and finally the left coronary sinus. Typically, men are more affected (4:1), and there is a higher reported incidence in Asian groups.6 If ruptured SOVAs remain untreated, the prognosis is poor, with a 1‐year life expectancy.10
Clinical Consequences
The anatomic setting of a SOVA usually predicts the clinical outcome of aneurysm rupture. Rupture of the right and noncoronary sinuses typically results in communication between the aorta and the right ventricular outflow tract or the aorta and the right atrium. Left SOVA rupture is clinically less significant, with resultant communication to the left atrium and left ventricular outflow tract.11
Ruptures typically occur between 20 and 40 years of age, with notable outliers in infancy or late adulthood.12 The speed at which a rupture occurs is a major determinant of clinical outcome, in addition to size and location. The right ventricle is the most common location of rupture, followed by the right atrium. Symptoms can include substernal chest pain, abdominal pain, and mild to severe dyspnea. In many cases, patients may experience symptoms of acute heart failure, cardiac tamponade, hemodynamic compromise, and even sudden cardiac death.6 Rupture into the interventricular septum has also been reported with resultant left ventricular outflow tract obstruction.13
Nonruptured SOVAs can present with a variety of cardiac pathologies aside from ischemic disease. However, most nonruptured SOVAs are asymptomatic. In few cases, dyspnea, palpitations, or angina may be present. In other cases, the initial findings may be arrhythmia, atrial fibrillation, or complete heart block.6 Large SOVAs can serve as a nidus for thrombus formation. Major coronary arteries have been obstructed by aneurysm with thrombus formation, leading to ischemic cardiac disease.14 These patients often present with symptoms mimicking acute coronary syndromes. Both ruptured and nonruptured SOVAs can be complicated by aortic regurgitation, occurring in up to 30% to 50% of patients.6 For this reason, aortic valve replacement is usually required in addition to operative repair of the SOVA.
On physical exam, a murmur can be auscultated in patients with large or ruptured SOVAs. The classic finding is a “loud, superficial sawing murmur prolonged continuously over the first and second heart sounds.”9 Electrocardiogram demonstrates left ventricular hypertrophy and nonspecific ST‐T wave changes. Chest x‐ray may illustrate a bulging structure in the right caval shadow.6 Further imaging findings are noted below.
SOVA Imaging
Many imaging modalities can be utilized in the diagnosis of a SOVA, with cardiac computed tomography (CT) being of only recent popularity. Traditionally, transthoracic and transesophageal echocardiogram have been the first‐line imaging techniques. In the case of a ruptured SOVA, echocardiographic evaluation with color Doppler will reveal continuous flow in systole and diastole, because the aorta is a high‐pressure system. It may also demonstrate a flutter of the tricuspid valve as the color jet moves from the aorta to the right chamber of the heart.11 Magnetic resonance imaging, contrast aortography, and CT have been used as supplemental or confirmatory tests. Magnetic resonance imaging used with multiplanar sequencing has allowed for the evaluation of intracardiac shunts in ruptured SOVAs. New cardiac CT imagers are capable of wide‐range rotation and electrocardiography (ECG)‐gated technology to capture images during less than a single cardiac cycle.15 This results in less radiation dose and motion‐free images due to faster acquisition time. Thus, it permits accurate diagnostic imaging of even small SOVAs in a dynamic setting.
Medical Management
Ruptured SOVAs traditionally require surgical intervention. However, many recent reports have documented good clinical outcomes using transcatheter closure devices as surgical alternatives. No randomized trials comparing the 2 approaches have yet been designed. In the setting of a nonruptured SOVA, concurrent factors should first be considered prior to repair. In findings such as outflow tract obstruction, arrhythmia, or infection, intervention is required.
The first transcatheter closure of a SOVA was reported in 1994 by Cullen et al using a Rashkind umbrella.16 Since then, multiple approaches have been explored. Abidin et al report successful SOVA repair with a septal occluder device.17 Tong et al report a series of 13 patients who underwent transcatheter repair of a SOVA using a ductal occluder.18 Given the saccular nature of SOVAs, the ductal occluder was chosen for its well‐conformed shape. Patients were maintained on aspirin and clopidogrel for 6 months after the repair. During a 60‐month follow‐up period, the group remained without any severe complications including device embolization, residual shunt, right ventricular outflow tract obstruction, new aortic regurgitation, or site rupture.18 Zhong et al describe transcatheter closure in a case series of 22 patients using both septal and ductal devices with minimal procedure‐related adverse effects.19
Rittger et al report the use of an Amplatzer vascular plug (AVP II) for SOVA closure in a 52‐year‐old female. The authors note that the adjustable nature of the proximal and distal discs on the device make it ideal for placement in otherwise tortuous anatomy.20 Although not without complications, transcatheter closure techniques are growing in popularity over open surgery. In patients with nonruptured SOVA and no symptoms, the role of further surgical therapy is unknown.
Surgical Management
Surgical intervention is recommended for a ruptured SOVA and/or a SOVA with associated intracardiac abnormalities such as ventricular septal defect or significant aortic valve regurgitation. An unruptured but symptomatic or enlarging SOVA should also be considered for surgical repair. Although specific guidelines for repair of a SOVA are yet to be established, it is generally accepted to follow repair guidelines for aortic root aneurysm. According to the 2010 American Guidelines on Thoracic Aortic Disease, surgical repair should be considered in those with aneurysms >5.5 cm, >5 cm in those with bicuspid valves, >4.5 cm in the setting of connective tissue disease, or a growth rate of >0.5 cm/year.21
Since the first successful surgical repair of a SOVA by Lillehei in 1957, different surgical approaches and repair techniques have been described. All surgical repairs are done with cardiopulmonary bypass and cardioplegic arrest. There are 3 main operative approaches: (1) through the aortic root via an aortotomy, (2) through the cardiac chamber in which the aneurysm has ruptured, or (3) a dual approach through both an aortotomy and an incision into the involved cardiac chamber. The choice of approach is determined by the presence of aortic valvular pathology such as aortic regurgitation, the size of the SOVA, the presence of concomitant cardiac anomaly such as a ventricular septal defect, and the cardiac chamber involved. There are2 main closure techniques: primary closure and patch closure. Primary closure has been routinely used for the repair of small SOVAs. Patch closure is preferred for repair of larger SOVAs, as primary closure in those cases may distort the aortic sinus resulting in aortic valve incompetence, or may cause excessive tissue tension in the repair site resulting in delayed recurrent rupture.
With an operative mortality rate of 1.9% to 3.6% and actual survival rates of close to 90% at 15 years,22, 23, 24 surgical repair of SOVAs can be performed with acceptably low mortality and good long‐term outcome. Early surgical intervention should therefore be considered prior to worsening symptoms and the development of complications.
Case Presentations
Case 1
A 76‐year‐old male with a past medical history of hypertension and hyperlipidemia was found to have asymptomatic atrial fibrillation on a routine electrocardiogram at his physician's office. The patient was recommended cardioversion and underwent transesophageal echocardiography prior to the procedure to evaluate for left atrial appendage (LAA) thrombus. Although an LAA thrombus was absent, an incidental finding of a 5.3‐cm right coronary cusp (RCC) aneurysm was noted. The patient did have trace aortic insufficiency along with mild mitral and tricuspid insufficiency. Of note, he had diffuse intimal thickening, consistent with grade 2 atheromatous disease in the descending aorta. He underwent CT imaging with a nongated scan, and definitive findings of a SOVA was not noted. The patient was further followed up with an ECG‐gated, 640‐slice cardiac CT at our institution, which confirmed the presence of the 5.3‐mm right SOVA (Figure 1). He underwent successful repair of the SOVA with a Dacron graft patch closure and preservation of the aortic root. Aortic valve sparing surgery has been shown to have excellent outcomes and prevent the need for long‐term anticoagulation and other complicating factors.25
Figure 1.
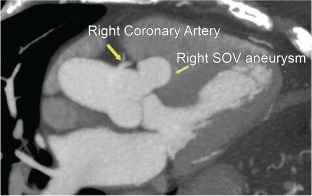
Right coronary cusp sinus of Valsalva (SOV) aneurysm.
Case 2
A 54‐year‐old female with past medical history of hyperlipidemia, emphysema, and a strong family history of coronary artery disease presented to an outside cardiologist with complaints of palpitations. She was sent for routine exercise stress echocardiogram, which demonstrated a normal exercise response on the electrocardiogram but an incidental dilated proximal aorta ranging from 4.5 to 4.7 cm. She was referred for a dedicated transthoracic echocardiogram, which showed moderate aortic root dilation to 4.6 cm and aneurysmal dilatation of the sinuses of Valsalva. Finally, cardiac computed tomography angiography (CCTA) was performed, which confirmed dilation of all 3 sinuses, with dimensions measuring 4.1 cm at the RCC, 4.3 cm at the left coronary cusp (LCC), and 5.6 cm at the noncoronary cusp (NCC) (Figure 2). As valve‐sparing surgery has been noted to demonstrate excellent outcomes,25 she was offered a valve‐sparing aortic root replacement and is currently pending surgical intervention.
Figure 2.
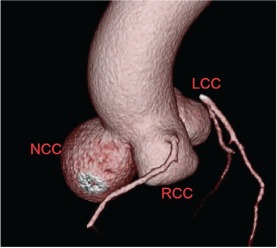
Noncoronary cusp sinus of Valsalva aneurysm (all 3 coronary cusps dilated). Abbreviations: LCC, left coronary cusp; NCC, noncoronary cusp; RCC, right coronary cusp.
Case 3
A 37‐year‐old male with past medical history of hypertension presented with chest pain. CCTA was done to evaluate coronaries and demonstrated no evidence of coronary atherosclerosis; however, there was aortic root dilation to 5.1 cm, with aneurysmal RCC and NCC and a normal LCC (Figure 3). His aortic valve was trileaflet, and there was no history of connective tissue disease. Given that the patient did not meet any of the 2010 Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease for surgical intervention, as mentioned above, and he is subsequently asymptomatic, he was recommended for 6‐month interval follow‐up with CCTA and transthoracic echocardiography (TTE).
Figure 3.
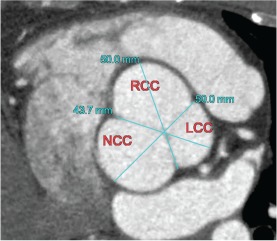
Right and noncoronary cusp sinus of Valsalva aneurysm with a normal left coronary cusp. Abbreviations: LCC, left coronary cusp; NCC, noncoronary cusp; RCC, right coronary cusp.
Case 4
A 50‐year‐old male with no past medical history presented to an outside hospital with left‐sided paresis and was treated with tissue plasminogen activator for a presumed acute cerebrovascular accident (CVA). He reported a preceding 6‐week history of conservatively managed back pain with no associated chest pain, dyspnea, or syncopal episodes. TTE during his hospitalization demonstrated a dilated aorta with echogenic material adjacent to and within the aorta, concerning for dissection with an organized thrombus. The patient was transferred to our institution, where a cardiac CT showed a focal aneurysm, containing thrombus, arising from the under‐surface of the noncoronary sinus of Valsalva. The aortic root at the sinuses of Valsalva was dilated, measuring about 5.4 cm in diameter (Figure 4). Echocardiogram showed a tricuspid aortic valve. As the patient had a focal aneurysm with a normal tricuspid aortic valve with no aortic insufficiency, he underwent successful resection of the thrombus with a pericardial patch repair of the SOVA. When possible, preservation of the native aortic valve is always preferable to valve replacement to avoid the risk of prosthetic valve degeneration, infection, and thrombosis. The patient is currently symptomatically improving and recovering from his CVA.
Figure 4.
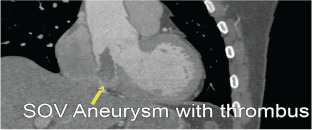
Sinus of Valsalva (SOV) aneurysm with thrombus.
Discussion
SOVAs are rare congenital or acquired cardiac defects that have been increasingly diagnosed as a result of improved imaging techniques. Ruptured or symptomatic nonruptured SOVAs are indications for surgical repair. Over the last 20 years, improved transcatheter techniques have added to the spectrum of nonsurgical alternatives for repair. Given the close association between SOVAs and BAV, it is important for physicians to consider SOVA in the differential when diagnosing BAV. As CCTA in the emergency department setting becomes more popular, we anticipate an increase in incidental findings of cardiac defects, including SOVAs. Ultimately, randomized trial data will be required for optimizing the management of these rare findings.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Bricker AO, Avutu B, Mohammed TL, et al. Valsalva sinus aneurysms: findings at CT and MR imaging. Radiographics. 2010;30:99–110. [DOI] [PubMed] [Google Scholar]
- 2. Troupis JM, Nasis A, Pasricha S, et al. Sinus valsalva aneurysm on cardiac CT angiography: assessment and detection. J Med Imaging Radiat Oncol. 2013;57:444–447. [DOI] [PubMed] [Google Scholar]
- 3. Ott DA. Aneurysm of the sinus of Valsalva. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:165–176. [DOI] [PubMed] [Google Scholar]
- 4. Edwards JE, Burchell HB. Specimen exhibiting the essential lesion in aneurysm of the aortic sinus. Proc Staff Meet Mayo Clin. 1956;31:407–412. [PubMed] [Google Scholar]
- 5. Moustafa S, Mookadam F, Cooper L, et al. Sinus of valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol. 2007;99:1159–1164. [DOI] [PubMed] [Google Scholar]
- 6. Feldman DN, Roman MJ. Aneurysms of the sinuses of valsalva. Cardiology. 2006;106:73–81. [DOI] [PubMed] [Google Scholar]
- 7. Nakano T, Okano H, Konishi T, et al. Aneurysm of the left aortic sinus caused by Takayasu's arteritis: compression of the left coronary artery producing coronary insufficiency. J Am Coll Cardiol. 1986;7:696–700. [DOI] [PubMed] [Google Scholar]
- 8. Koh KK, Lee KH, Kim SS, et al. Ruptured aneurysm of the sinus of valsalva in a patient with Behcet's disease. Int J Cardiol. 1994;47:177–‐179. [DOI] [PubMed] [Google Scholar]
- 9. Hope J, ed. A Treatise on the Disease of the Heart and Great Vessels. 3rd ed. Philadelphia PA: Lea and Blanchard, 1839:466–471. [Google Scholar]
- 10. Sawyers JL, Adams JE, Scott HW Jr. A method of surgical repair for ruptured aortic sinus aneurysms with aorticoatrial fistula. South Med J. 1957;50:1075–1078. [DOI] [PubMed] [Google Scholar]
- 11. DeMaria AN, Blanchard DG. Echocardiography In: Fuster V, Walsh RA, Harrington RA, eds. Hurst's the Heart Manual of Cardiology,. 13th ed. New York, NY: McGraw‐Hill; 2011. [Google Scholar]
- 12. Nakamura Y, Aoki M, Hagino I, et al. Case of congenital aneurysm of sinus of valsalva with common arterial trunk. Ann Thorac Surg. 2014;97:710–712. [DOI] [PubMed] [Google Scholar]
- 13. Lee DH, Kang EJ, Park TH, et al. A case of right sinus of valsalva rupture with dissection into interventricular septum causing left ventricular outflow tract obstruction. Korean Circ J. 2013;43:770–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardia‐Rinaldi R, Von Koch L, Howell JP. Aneurysm of the sinus of Valsalva producing obstruction of the left main coronary artery. J Thorac Cardiovasc Surg. 1976;72:123–126. [PubMed] [Google Scholar]
- 15. Toshiba America Medical Systems . Computed tomography: Aquilion ONE family. http://www.medical.toshiba.com/products/ct/aquilion‐one‐family. Accessed June 14, 2014.
- 16. Cullen S, Somerville J, Redington A. Transcatheter closure of a ruptured aneurysm of the sinus of Valsalva. Br Heart J. 1994;71:479–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abidin N, Clarke B, Khattar RS. Percutaenous closure of ruptured sinus of Valsalva aneurysm using an Amplatzer occluder device. Heart. 2005;91:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong S, Zhong L, Liu J, et al. The immediate and follow‐up results of transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with duct occluder. J Invasive Cardiol. 2014;26:55–59. [PubMed] [Google Scholar]
- 19. Zhong L, Tong SF, Zhang Q, et al. Clinical efficacy and safety of transcatheter closure of ruptured sinus of valsalva aneurysm. Catheter Cardiovasc Interv. 2014;84:1184–1189. [DOI] [PubMed] [Google Scholar]
- 20. Rittger H, Gundlach U, Koch A. Transcatheter closure of ruptured Sinus of Valsalva Aneurysm into the right ventricle with an Amplatzer Vascular Plug II [published online ahead of print January 9, 2014]. Catheter Cardiovasc Interv. doi: 10.1002/ccd.25382. [DOI] [PubMed] [Google Scholar]
- 21. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 22. Yan F, Huo Q, Qian J, et al. Surgery for sinus of valsalva aneurysm: 27‐year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16:361–365. [DOI] [PubMed] [Google Scholar]
- 23. Vural KM, Sener E, Tasdemir O, et al. Approach to sinus of Valsalva aneurysms: a review of 53 cases. Eur J Cardiothorac Surg. 2001;20:71–76. [DOI] [PubMed] [Google Scholar]
- 24. Sarikaya S, Adademir T, Elibol A, et al. Surgery for ruptured sinus of valsalva aneurysm: 25 year experience with 55 patients. Eur J Cardioyhoracic Surg. 2013;43:591–596. [DOI] [PubMed] [Google Scholar]
- 25. Forteza A, Centeno J, Bellot R, et al. Aortic valve sparing in 120 patients with aortic root aneurysms. Rev Esp Cardiol (Engl Ed). 2011;64:470–475. [DOI] [PubMed] [Google Scholar]


