ABSTRACT
Background
Existing data on the risk of ischemic stroke in hyperthyroidism‐related atrial fibrillation (AF) and the impact of long‐term anticoagulation in these patients, particularly those with self‐limiting AF, remain inconclusive.
Hypothesis
Risk of stroke in hyperthyroidism‐related AF is the same as nonhyperthyroid counterparts.
Methods
This was a single‐center observational study of 9727 Chinese patients with nonvalvular AF from July 1997 to December 2011. Patients with AF diagnosed concomitantly with hyperthyroidism were identified. Primary and secondary endpoints were defined as hospitalization with ischemic stroke and intracranial hemorrhage in the first 2 years. Patient characteristics, duration of AF, and choice of antithrombotic therapy were recorded. Self‐limiting AF was defined as <7 days' duration.
Results
Out of 9727 patients, 642 (6.6%) had concomitant hyperthyroidism and AF at diagnosis. For stroke prevention, 136 and 243 patients (21.1% and 37.9%) were prescribed warfarin and aspirin, respectively, whereas the remaining patients (41.0%) received no therapy. Ischemic stroke occurred in 50 patients (7.8%), and no patient developed hemorrhagic stroke. Patients with CHA2DS2‐VASc of 0 did not develop stroke. Warfarin effectively reduced the incidence of stroke compared with aspirin or no therapy in patients with CHA2DS2‐VASc ≥1 and non–self‐limiting AF, but not in those with self‐limiting AF or CHA2DS2‐VASc of 0. Presence of hyperthyroidism did not confer additional risk of ischemic stroke compared with nonhyperthyroid AF.
Conclusions
Patients with hyperthyroidism‐related AF are at high risk of stroke (3.9% per year). Warfarin confers stroke prevention in patients with CHA2DS2‐VASc ≥1 and non–self‐limiting AF. Overall stroke risk was lower in hyperthyroid non–self‐limiting AF patients compared with nonhyperthyroid counterparts.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice and confers a 5‐fold higher risk of ischemic stroke.1, 2 It has been reported that 13% of patients with new‐onset AF have biochemical evidence of hyperthyroidism3; 5% to 15% of patients with hyperthyroidism present with AF.4, 5, 6, 7 Although current international guidelines based on well‐validated risk scores for ischemic stroke and bleeding recommend long‐term oral anticoagulation for AF patients with CHA2DS2‐VASc score (congestive heart failure, hypertension, age ≥75 years, age = 65–74 years, diabetes, previous stroke, vascular disease, sex category) ≥1, there is much less consensus on the need for anticoagulation in patients with hyperthyroidism‐related AF. First, despite the strong link between hyperthyroidism and AF, and between AF and ischemic stroke, published data concerning the risk of ischemic stroke among patients with hyperthyroidism‐related AF remain conflicting.8, 9, 10 In most observational reports, contemporary ischemic stroke risk‐stratification schemes such as CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes, previous stroke) and CHA2DS2‐VASc scores have seldom been utilized.11, 12, 13, 14 Second, hyperthyroidism is often regarded as a reversible cause of AF10; AF recurrence following normalization of thyroid status is considered less likely in contrast to nonthyroid AF,15, 16, 17, 18 thus long‐term anticoagulation may be deemed unnecessary. Nonetheless, randomized controlled trials that target specifically patients with hyperthyroidism‐related AF are lacking. In reality, such a study is not ethically possible, given the well‐documented benefit of anticoagulation in AF; thus, a real‐world registry offers a good alternative. The objective of this study was to determine the clinical benefit of warfarin therapy in patients with hyperthyroidism‐related AF with regard to the risk of ischemic stroke using a recently established real‐world cohort of AF patients.19, 20, 21
Methods
Patients
Between July 1997 and December 2011, a total of 9727 Chinese patients with nonvalvular AF at Queen Mary Hospital, Hong Kong, were identified from the computer‐based clinical management system.19, 20, 21 Among these patients, those with AF diagnosed concurrently with hyperthyroidism (within 1 month) were identified. The final analysis included 642 patients with concurrent hyperthyroidism and AF who were categorized according to prescribed antithrombotic therapy.
Study Design
This was an observational study, and the study protocol was approved by the local institutional review board. Demographic data, cardiovascular risk factors, and medications were recorded at baseline. The primary and secondary endpoints were hospital admission with stroke and intracranial hemorrhage (ICH) during the first 2 years of follow‐up, respectively. Data were retrieved from the medical records and discharge summaries from the territory‐wide information network of all public hospitals in Hong Kong. The index date was defined as the date of the first occurrence of AF. For the registration of outcome during follow‐up, a blanking period of 14 days following the index date was applied, as the occurrence of an ischemic stroke or ICH within the first few days of the diagnosis of AF was most likely related to initial presentation of AF rather than a new event.22
Definitions
The diagnosis of hyperthyroidism was established in the presence of a serum free T4 level >23 pmol/L and concomitant suppressed TSH level <0.03 pmol/L. Self‐limiting AF was defined as the occurrence of AF at the time of hyperthyroidism, with duration <7 days and spontaneous conversion back to sinus rhythm. Hypertension was defined as resting systolic or diastolic blood pressure ≥140/90 mm Hg on 2 occasions or prescription of antihypertensive drugs. Diabetes mellitus was defined as a plasma fasting glucose ≥7.0 mmol/L or prescription of antidiabetic medication. Heart failure was defined according to the Framingham Heart Study. Smoking status was recorded as smoker (past and current) or nonsmoker. Ischemic stroke was defined as a neurological deficit of sudden onset that persisted for >24 hours corresponding to a vascular territory in the absence of primary hemorrhage or other cause (trauma, infection, vasculitis) and confirmed by computed tomography scan or magnetic resonance imaging of the brain.23, 24, 25 Intracranial hemorrhage was diagnosed in the presence of new‐onset neurological symptoms with radiological confirmation and classified as intracerebral hemorrhage, subarachnoid hemorrhage, or subdural hemorrhage.26
Statistical Analysis
Continuous and discrete variables are expressed as mean ± SD and percentages, respectively. Statistical comparisons of the baseline clinical characteristics were performed using the Student t test, 1‐way ANOVA or Fisher exact test as appropriate. Kaplan‐Meier survival analyses with the log‐rank test were carried out. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by univariate and multivariate Cox proportional hazard regression models. Multivariate analyses were performed with an enter regression model in which each variable with a P value of ≤0.1 (based on univariate analysis) was entered into the model. Calculations were performed using statistical software package SPSS version 19.0 (IBM Corp., Armonk, NY). A P value <0.05 was considered to be statistically significant.
Results
Out of 9727 patients with AF, 642 patients (6.6%) had concomitant hyperthyroidism and AF. Table 1 summarizes their clinical characteristics. The mean age was 71.9 ± 14.6 years, and 67.8% were female. The mean CHA2DS2‐VASc and HAS‐BLED (hypertension, abnormal kidney/liver function, stroke, bleeding, labile international normalized ratio, elderly [age ≥65 years], drugs/alcohol) score was 3.3 ± 1.9 and 1.8 ± 1.0, respectively. Three hundred eighteen patients (49.5%) presented with self‐limiting AF.
Table 1.
Baseline Characteristics
| All, N = 642 | No Therapy, n = 263 | Aspirin, n = 243 | Warfarin, n = 136 | P Value | |
|---|---|---|---|---|---|
| Mean age, y | 71.9 ± 14.6 | 71.8 ± 14.7 | 74.5 ± 13.3 | 67.8 ± 15.5 | <0.01a |
| Female sex | 435 (67.8) | 179 (68.1) | 171 (70.4) | 85 (62.5) | 0.29 |
| Type of AF | 0.56 | ||||
| Paroxysmal | 318 (49.5) | 126 (47.9) | 127 (52.3) | 65 (47.8) | |
| Nonparoxysmal | 324 (50.5) | 137 (52.1) | 116 (47.7) | 71 (52.2) | |
| HT | 289 (45.0) | 91 (34.6) | 132 (54.3) | 66 (48.5) | <0.01a |
| DM | 126 (19.6) | 44 (16.7) | 58 (23.9) | 24 (17.6) | 0.11 |
| Smoker | 192 (29.9) | 79 (30.0) | 75 (30.9) | 38 (27.9) | 0.84 |
| Dialysis | 19 (3.0) | 7 (2.7) | 9 (3.7) | 3 (2.2) | 0.66 |
| HF | 147 (22.9) | 50 (19.0) | 68 (28.0) | 29 (21.3) | 0.05 |
| CAD | 83 (12.9) | 20 (7.6) | 41 (16.9) | 22 (16.2) | <0.01a |
| PAD | 10 (1.6) | 1 (0.4) | 7 (1.1) | 2 (0.3) | 0.08 |
| Prior stroke/TIA | 103 (16.0) | 18 (6.8) | 54 (22.2) | 31 (22.8) | <0.01a |
| Prior ICH | 9 (1.4) | 3 (1.1) | 4 (1.6) | 2 (1.5) | 0.89 |
| Mean CHA2DS2‐VASc | 3.3 ± 1.9 | 2.8 ± 1.7 | 3.8 ± 1.9 | 3.2 ± 2.0 | <0.01a |
| CHA2DS2‐VASc | <0.01a | ||||
| 0 | 45 (7.0) | 22 (8.4) | 9 (3.7) | 14 (10.3) | |
| 1 | 85 (14.2) | 45 (18.7) | 24 (10.3) | 16 (13.1) | |
| 2 | 91 (15.2) | 47 (19.5) | 24 (10.3) | 20 (16.4) | |
| 3 | 134 (22.4) | 56 (23.2) | 49 (20.9) | 29 (23.8) | |
| 4 | 100 (16.8) | 44 (18.3) | 38 (16.2) | 18 (14.8) | |
| 5 | 111 (18.6) | 35 (14.5) | 54 (23.1) | 22 (18.0) | |
| 6 | 53 (8.9) | 31 (13.2) | 31 (132.) | 11 (9.0) | |
| 7 | 17 (2.8) | 11 (4.7) | 11 (4.7) | 5 (4.1) | |
| 8 | 4 (0.7) | 2 (0.9) | 2 (0.9) | 1 (0.8) | |
| 9 | 2 (0.3) | 1 (0.4) | 1 (0.4) | 0 (0) | |
| Mean HAS‐BLED | 1.8 ± 1.0 | 1.6 ± 0.9 | 2.0 ± 1.0 | 1.7 ± 1.1 | <0.01a |
| HAS‐BLED | <0.01a | ||||
| 0 | 65 (10.1) | 30 (11.4) | 15 (6.2) | 20 (14.7) | |
| 1 | 180 (28.0) | 89 (33.8) | 56 (23.0) | 35 (25.7) | |
| 2 | 261 (40.7) | 106 (40.3) | 102 (42.0) | 53 (39.0) | |
| 3–6 | 136 (21.2) | 38 (14.4) | 70 (28.8) | 28 (20.6) |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, age = 65–74 years, diabetes, previous stroke, vascular disease, sex category; DM, diabetes mellitus; HAS‐BLED, hypertension, abnormal kidney/liver function, stroke, bleeding, labile international normalized ratio, elderly (age ≥65 years), drugs/alcohol; HF, heart failure; HT, hypertension; ICH, intracranial hemorrhage; PAD, peripheral arterial disease; SD, standard deviation; TIA, transient ischemic attack.
Data are expressed as mean ± SD or n (%).
P < 0.05.
Concerning the use of anticoagulation among the 642 patients, 263 patients (41.0%) received no aspirin or warfarin (no therapy), 243 patients (37.9%) were prescribed aspirin (80–160 mg daily), and 136 patients (21.1%) received warfarin. Patients prescribed no therapy had the lowest CHA2DS2‐VASc score, followed by those on warfarin and patients on aspirin (2.8 ± 1.7, vs 3.2 ± 2.0, vs 3.8 ± 1.9; P < 0.01). Patients prescribed no therapy also had the lowest HAS‐BLED score compared with patients on warfarin or aspirin (1.6 ± 0.9, vs 1.7 ± 1.1, vs 2.0 ± 1.0; P < 0.01).
Ischemic Stroke
Within the first 2 years of the diagnosis of hyperthyroidism and AF, 50 patients developed an ischemic stroke (7.8%) with an annual incidence of 3.9% per year. None of the patients with CHA2DS2‐VASc score of 0 developed ischemic stroke during the period, irrespective of antithrombotic strategy. To further identify factors that could predict ischemic stroke, patients were categorized as those who developed ischemic stroke and those who did not.1, 2 Table 2 summarizes factors predictive of ischemic stroke together with the corresponding HRs based on Cox proportional hazard model and 95% CIs. Of note, only increasing age >75 years (HR: 6.02, 95% CI: 1.42–25.63, P = 0.02) and renal failure requiring dialysis (HR: 4.44, 95% CI: 1.73–11.40, P < 0.01) were associated with an increasing risk of ischemic stroke in both univariate and multivariate analyses. The use of warfarin therapy was independently associated with the 67% lower risk of ischemic stroke compared with patients with no therapy (HR: 0.33, 95% CI: 0.12–0.91, P = 0.03). Figure 1A depicts the Kaplan‐Meier analysis of ischemic stroke among patients on warfarin, aspirin, and no therapy (log‐rank: 7.23, P = 0.03). To provide useful information to facilitate the clinical decision for antithrombotic treatment in patients with concomitant hyperthyroidism and AF, Kaplan‐Meier analyses were performed with patients with CHA2DS2‐VASc ≥1 and either non–self‐limiting or self‐limiting AF (Figure 1B and 1C). In patients with non–self‐limiting AF at the time of hyperthyroidism diagnosis, warfarin therapy was associated with a reduced risk of ischemic stroke (log rank: 6.23, P = 0.04; Figure 1B). Nonetheless, among patients with self‐limiting AF at presentation of hyperthyroidism, Kaplan‐Meier analysis failed to show any difference in risk for ischemic stroke, regardless of therapy or no therapy (Figure 1C).
Table 2.
Associations Between Baseline Factors and Ischemic Stroke
| No. of Ischemic Strokes | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Age | |||||
| <65 years | 2 | Ref | Ref | ||
| 65–75 years | 13 | 4.92 (1.11–27.80) | 0.04a | 3.73 (0.93–16.76) | 0.09 |
| >75 years | 35 | 8.13 (1.96–33.83) | <0.01a | 6.02 (1.42–25.63) | 0.02a |
| Female sex | 38 | 1.51 (0.79–2.88) | 0.22 | ||
| Nonparoxysmal AF | 27 | 1.25 (0.71–2.17) | 0.44 | ||
| HT | 30 | 1.79 (1.02–3.16) | 0.04a | 1.35 (0.75–2.41) | 0.32 |
| DM | 14 | 1.58 (0.85–2.93) | 0.15 | ||
| Smoker | 15 | 1.04 (0.57–1.90) | 0.91 | ||
| Renal failure on dialysis | 5 | 4.18 (1.66–10.53) | <0.01a | 4.44 (1.73–11.40) | <0.01a |
| HF | 10 | 0.93 (0.46–1.85) | 0.83 | ||
| CAD | 11 | 1.85 (0.95–3.60) | 0.07 | 1.47 (0.72–2.94) | 0.28 |
| PAD | 0 | 0.05 (0.00–1401) | 0.56 | ||
| Prior ischemic stroke | 13 | 1.87 (1.00–3.53) | 0.05 | 1.81 (0.94–3.46) | 0.08 |
| Prior ICH | 1 | 1.37 (0.19–9.91) | 0.76 | ||
| Antithrombotic therapy | |||||
| No therapy | 19 | Ref | Ref | ||
| Aspirin | 26 | 1.27 (0.70–2.29) | 0.56 | 0.99 (0.54–1.81) | 0.96 |
| Warfarin | 5 | 0.36 (0.14–0.98) | 0.04a | 0.33 (0.12–0.91) | 0.03a |
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; HF, heart failure; HR, hazard ratio; HT, hypertension; ICH, intracranial hemorrhage; PAD, peripheral arterial disease; Ref, reference.
P < 0.05.
Figure 1.
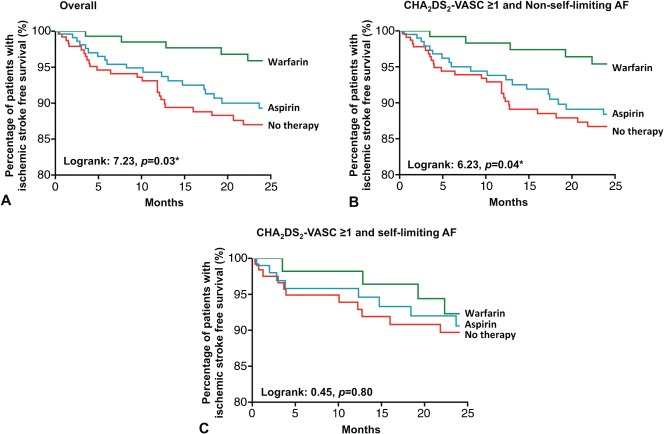
Kaplan‐Meier estimate of percentage of ischemic stroke–free survival in all patients with hyperthyroidism‐related AF (A), in patients with CHA2DS2‐VASc score ≥1 and nonparoxysmal AF (B), and in patients with CHA2DS2‐VASc score ≥1 and paroxysmal AF (C), classified according to antithrombotic therapy. Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, age = 65–74 years, diabetes, previous stroke, vascular disease, sex category.
Intracranial Hemorrhage
No patient developed an ICH during the study period.
Ischemic Stroke Risk in Hyperthyroid and Nonhyperthyroid Atrial Fibrillation
To further compare the ischemic stroke risk between hyperthyroid AF and nonhyperthyroid AF patients, baseline stroke risks for those patients not on any antithrombotic therapy, stratified by different CHA2DS2‐VASc scores, were compared. It was demonstrated that for patients with CHA2DS2‐VASc score of 0, annual ischemic stroke risk in hyperthyroid patients is 0% per year, whereas in those without hyperthyroidism it is 2.56% per year. Similarly, in patients with CHA2DS2‐VASc score of 1–2, annual ischemic stroke risks in patients with and without hyperthyroidism are 3.17% per year and 7.02% per year, respectively. For patients with CHA2DS2‐VASc score of ≥3, hyperthyroid AF patients have an annual stroke risk of 8.61% per year, whereas those without hyperthyroidism have a slightly higher stroke risk of 10.54% per year. Overall annual ischemic stroke risks in AF patients with and without hyperthyroidism are 5.79% per year and 9.12% per year, respectively (Figure 2). In general, hyperthyroid AF patients in this cohort have lower stroke risk compared with nonhyperthyroid counterparts with the same CHA2DS2‐VASc scores.
Figure 2.
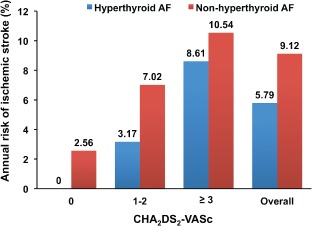
Comparison of annual risk of ischemic stroke in AF patients with and without hyperthyroidism, stratified according to different CHA2DS2‐VASc scores. Abbreviations: CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, age = 65–74 years, diabetes, previous stroke, vascular disease, sex category.
Discussion
To the best of our knowledge, this is the largest real‐world cohort of patients with concomitant hyperthyroidism and AF in whom the clinical benefit of warfarin therapy for stroke prevention has been studied. First, we showed that the risk of ischemic stroke among patients with hyperthyroidism‐related AF was substantial, except in those with CHA2DS2‐VASc score of 0, whose ischemic stroke risk was truly low (0% per year) and similar to those with nonthyroid AF. Second, warfarin therapy was associated with a reduced risk of ischemic stroke compared with aspirin and no therapy. Nonetheless, the reduced risk of ischemic stroke attributable to warfarin therapy was evident only in patients with CHA2DS2‐VASc score ≥1 and non–self‐limiting, not self‐limiting, AF.
Hyperthyroidism is a common metabolic disorder that can exacerbate preexisting cardiac disease or cause de novo cardiovascular abnormalities such as AF,27, 28 heart failure,29 and pulmonary hypertension.30, 31 Despite the well‐known association of AF with ischemic stroke, stroke prevention in patients with hyperthyroidism‐related AF is less well established than in those with nonthyroid AF. First, it remains controversial whether hyperthyroidism‐related AF confers a higher ischemic stroke risk than nonthyroid AF.8 The incidence of ischemic stroke among patients with hyperthyroidism‐related AF has been reported to be 8% to 24% in early observational series.11, 12, 13 A recent study with age‐ and sex‐matched cohorts of 480 AF patients has demonstrated a higher incidence of ischemic stroke among patients with hyperthyroidism‐related AF.28 Nevertheless, in the world's largest AF registry, involving 182,678 AF patients, history of thyroid disease per se was not shown to be associated with higher ischemic stroke risk.32 Such a discrepancy is most likely a result of the difference in temporal relationship between hyperthyroidism and AF in these studies. In our present study, it was shown that by comparing patients with or without hyperthyroidism, those with hyperthyroidism‐related AF were not at higher risk of ischemic stroke than their nonhyperthyroid counterparts. Of note, when stratified into different CHA2DS2‐VASc scores, hyperthyroidism‐related AF was consistently at a slightly lower risk of stroke. Such finding differs from our previous study, which showed higher incidence of ischemic stroke among hyperthyroid AF patients.28 Possible explanations include the relatively small number of patients in the previous study and failure to segregate patients on no antithrombotic therapy, aspirin, or warfarin. With a larger number of hyperthyroid AF patients (642) included in the current study, the baseline ischemic stroke risk of hyperthyroidism‐related AF becomes more representative.
Randomized controlled trials have demonstrated the benefits of long‐term warfarin therapy over placebo in reducing AF‐related ischemic stroke.33 Atrial fibrillation in the presence of hyperthyroidism is often regarded as reversible,10 and recurrence following normalization of thyroid status is considered less likely in contrast to nonthyroid AF.15, 16, 17, 18 Long‐term anticoagulation may thus be deemed unnecessary. However, no randomized controlled trial targeting specifically patients with hyperthyroidism‐related AF to evaluate the efficacy and safety of long‐term anticoagulation therapy has been performed. Such a trial would be difficult and not feasible given the well‐documented benefit of anticoagulation therapy in AF. Consequentially, a large real‐world registry like this study may provide a good alternative. In the present study, it is evident that among patients with hyperthyroidism related‐AF and CHA2DS2‐VASc score of 0, the risk of ischemic stroke is virtually zero, irrespective of the type of AF (self‐limiting or not), thus the use of anticoagulation therapy is deemed inappropriate. Nevertheless, among patients with CHA2DS2‐VASc score ≥1, warfarin therapy was associated with a reduced ischemic stroke risk only in those with non–self‐limiting AF, not those with self‐limiting AF. This concurs with the clinical observation that, unlike nonthyroid AF, hyperthyroidism‐related AF is associated with a lower risk of recurrence following normalization of hyperthyroidism and thus also the risk of ischemic stroke. The risk of ischemic stroke in patients with self‐limiting AF, therefore, remains low.
Our results have several important clinical implications. First, although it remains arguable that hyperthyroidism per se confers a higher risk of ischemic stroke, the threshold to initiate anticoagulation therapy (CHA2DS2‐VASc ≥1) as recommended by current guidelines seems appropriate in patients with hyperthyroidism‐related AF.34 Second, transient, self‐limiting AF during the early phase of hyperthyroidism may not impose additional risk of ischemic stroke, as recurrence is less likely following normalization of thyroid status; thus long‐term warfarin therapy may not be necessary.
Study Limitations
Our study had the following limitations. First, it is limited by its cohort‐based and single‐center observational design in primarily hospital‐based patients. Second, despite the fact that patients with concomitant hyperthyroidism and AF treated with warfarin had a substantially reduced risk of ischemic stroke compared with patients on aspirin or no therapy, the decision and selection of antithrombotic strategies was not randomized or controlled. As such, patients prescribed warfarin may have differed from those on aspirin or no therapy as decided by the attending physicians, thus imposing a selection bias in our cohort. In addition, although we carefully ascertained all strokes and ICH by examination of hospitalization records and laboratory and imaging results, patients with a milder form of stroke and/or ICH who were not hospitalized were not included.
Conclusions
The risk of ischemic stroke among patients with hyperthyroidism‐related AF remains substantial. Warfarin appears to be appropriate for stroke prevention in patients with CHA2DS2‐VASc score ≥1 and non–self‐limiting AF.
Gregory Y.H. Lip, MD, has served as a consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Daiichi‐Sankyo, Medtronic, Biotronik, Portola, and Boehringer Ingelheim, and has served on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Medtronic, Daiichi‐Sankyo, and Sanofi Aventis.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Jørgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation: the Copenhagen Stroke Study. Stroke. 1996;27:1765–1769. [DOI] [PubMed] [Google Scholar]
- 2. Sandercock P, Bamford J, Dennis M, et al. Atrial fibrillation and stroke: prevalence in different types of stroke and influence on early and long‐term prognosis (Oxfordshire community stroke project). BMJ. 1992;305:1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 4. Kerr CR, Boone J, Connolly SJ, et al. The Canadian Registry of Atrial Fibrillation: a noninterventional follow‐up of patients after the first diagnosis of atrial fibrillation. Am J Cardiol. 1998;82(8A):82N–85N. [DOI] [PubMed] [Google Scholar]
- 5. Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. [DOI] [PubMed] [Google Scholar]
- 6. Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94–98. [DOI] [PubMed] [Google Scholar]
- 7. Polikar R, Burger AG, Scherrer U, et al. The thyroid and the heart. Circulation. 1993;87:1435–1441. [DOI] [PubMed] [Google Scholar]
- 8. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 suppl):429S–456S. [DOI] [PubMed] [Google Scholar]
- 9. Squizzato A, Gerdes VE, Brandjes DP, et al. Thyroid diseases and cerebrovascular disease. Stroke. 2005;36:2302–2310. [DOI] [PubMed] [Google Scholar]
- 10. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) [published correction appears in J Am Coll Cardiol. 2007;50:562]. J Am Coll Cardiol. 2006;48:854–906. [DOI] [PubMed] [Google Scholar]
- 11. Hurley DM, Hunter AN, Hewett MJ, et al. Atrial fibrillation and arterial embolism in hyperthyroidism. Aust N Z J Med. 1981;11:391–393. [DOI] [PubMed] [Google Scholar]
- 12. Staffurth JS, Gibberd MC, Fui SN. Arterial embolism in thyrotoxicosis with atrial fibrillation. Br Med J. 1977;2:688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuen RW, Gutteridge DH, Thompson PL, et al. Embolism in thyrotoxic atrial fibrillation. Med J Aust. 1979;1:630–631. [DOI] [PubMed] [Google Scholar]
- 14. Petersen P, Hansen JM. Stroke in thyrotoxicosis with atrial fibrillation. Stroke. 1988;19:15–18. [DOI] [PubMed] [Google Scholar]
- 15. Shimizu T, Koide S, Noh JY, et al. Hyperthyroidism and the management of atrial fibrillation. Thyroid. 2002;12:489–493. [DOI] [PubMed] [Google Scholar]
- 16. Nakazawa HK, Handa S, Nakamura Y, et al. High maintenance rate of sinus rhythm after cardioversion in post‐thyrotoxic chronic atrial fibrillation. Int J Cardiol. 1987;16:47–55. [DOI] [PubMed] [Google Scholar]
- 17. Nakazawa H, Lythall DA, Noh J, et al. Is there a place for the late cardioversion of atrial fibrillation? A long‐term follow‐up study of patients with post‐thyrotoxic atrial fibrillation. Eur Heart J. 2000;21:327–333. [DOI] [PubMed] [Google Scholar]
- 18. Nakazawa HK, Sakurai K, Hamada N, et al. Management of atrial fibrillation in the post‐thyrotoxic state. Am J Med. 1982;72:903–906. [DOI] [PubMed] [Google Scholar]
- 19. Huang D, Anguo L, Yue WS, et al. Refinement of ischemic stroke risk in patients with atrial fibrillation and CHA2DS2‐VASc score of 1. Pacing Clin Electrophysiol. 2014;37:1442–1447. [DOI] [PubMed] [Google Scholar]
- 20. Siu CW, Lip GY, Lam KF, et al. Risk of stroke and intracranial hemorrhage in 9727 Chinese with atrial fibrillation in Hong Kong. Heart Rhythm. 2014;11:1401–1408. [DOI] [PubMed] [Google Scholar]
- 21. Siu CW, Tse HF. Net clinical benefit of warfarin therapy in elderly Chinese patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:300–306. [DOI] [PubMed] [Google Scholar]
- 22. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish Atrial Fibrillation cohort study. Circulation. 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 23. Ho LY, Siu CW, Yue WS, et al. Safety and efficacy of oral anticoagulation therapy in Chinese patients with concomitant atrial fibrillation and hypertension. J Hum Hypertens. 2011;25:304–310. [DOI] [PubMed] [Google Scholar]
- 24. Siu CW, Jim MH, Ho HH, et al. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007;132:44–49. [DOI] [PubMed] [Google Scholar]
- 25. Siu CW, Jim MH, Lau CP, et al. Low‐molecular‐weight heparin versus unfractionated heparin for thromboprophylaxis in patients with acute atrial fibrillation: a randomized control[led] trial. Acute Card Care. 2011;13:196–198. [DOI] [PubMed] [Google Scholar]
- 26. Chong BH, Chan KH, Pong V, et al. Use of aspirin in Chinese after recovery from primary intracranial hemorrhage. Thromb Haemost. 2012;107:241–247. [DOI] [PubMed] [Google Scholar]
- 27. Siu CW, Jim MH, Zhang X, et al. Comparison of atrial fibrillation recurrence rates after successful electrical cardioversion in patients with hyperthyroidism‐induced versus non–hyperthyroidism‐induced persistent atrial fibrillation. Am J Cardiol. 2009;103:540–543. [DOI] [PubMed] [Google Scholar]
- 28. Siu CW, Pong V, Zhang X, et al. Risk of ischemic stroke after new‐onset atrial fibrillation in patients with hyperthyroidism. Heart Rhythm. 2009;6:169–173. [DOI] [PubMed] [Google Scholar]
- 29. Siu CW, Yeung CY, Lau CP, et al. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siu CW, Zhang XH, Yung C, et al. Hemodynamic changes in hyperthyroidism‐related pulmonary hypertension: a prospective echocardiographic study. J Clin Endocrinol Metab. 2007;92:1736–1742. [DOI] [PubMed] [Google Scholar]
- 31. Wong SM, Tse HF, Siu CW. Pulmonary hypertension and isolated right heart failure complicating amiodarone‐induced hyperthyroidism. Heart Lung Circ. 2012;21:163–165. [DOI] [PubMed] [Google Scholar]
- 32. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
- 33. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 34. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation—developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]


