Abstract
Prior studies have suggested that a substantial number of eligible heart failure (HF) patients fail to receive β‐blocker therapy, or receive it at a suboptimal dose. The aim of this study is to assess the benefit of a predefined management program designed for β‐blocker up‐titration, evaluating the synergistic effect of cardiac resynchronization therapy (CRT) and β‐blockers in a HF population. The Resynchronization Therapy and β‐Blocker Titration (RESTORE) study is a prospective, case‐control, multicenter cohort study designed to test the hypothesis that a β‐blocker up‐titration strategy based on a predefined management program maximizes the beneficial effect of CRT, increasing the number of patients reaching the target dose of β‐blockers and improving their clinical outcome. All study patients receive an implantable defibrillator for CRT delivery in accordance with current guidelines. Enrollments started in December 2011 and are scheduled to end in December 2014. Approximately 250 consecutive patients will be prospectively enrolled in 6 Italian centers and followed up for 24 months after implantation. The primary endpoint is to demonstrate that CRT may allow titration of β‐blockers until the optimal dose, or at least to the effective dose, in patients with HF. This study might provide important information about the benefit of a predefined management program for β‐blocker up‐titration in patients receiving CRT. Moreover, assessment of health‐care utilization and the consumption of resources will allow estimating the potential utility of remote monitoring by means of an automated telemedicine system in facilitating the titration of β‐blockers in comparison with a standard in‐hospital approach.
Introduction
β‐Blockers are one of the cornerstones of the pharmacological treatment of heart failure (HF).1 Randomized controlled trials have shown that β‐blockers reduce hospital admissions and improve survival and quality of life.2, 3, 4, 5, 6 However, in clinical practice, we often fail to reach the target dose of β‐blockers because of their relatively low tolerability and the insufficiency of health‐care management systems.7, 8, 9, 10 In patients with symptomatic HF despite optimized pharmacological therapy, and with intraventricular conduction delay (prolonged QRS complex duration), cardiac resynchronization therapy (CRT) is effective in improving clinical conditions and quality of life11, 12 and reducing hospitalizations for HF13, 14 and overall mortality.14, 15 Considerable evidence suggests that, in CRT patients, there is a greater possibility of increasing the dose of β‐blockers.16, 17, 18, 19 Moreover, in these patients, the use of larger doses of β‐blockers maximizes the clinical benefits of CRT, suggesting a possible synergistic effect of the 2 therapies.19, 20 However, large surveys describing current clinical practice have shown that, even in HF patients receiving CRT, β‐blockers are frequently under‐prescribed and/or used at suboptimal doses.16, 17 For this reason, the implementation of specific strategies to facilitate β‐blocker up‐titration in CRT patients is warranted. The aim of the Resynchronization Therapy and β‐Blocker Titration (RESTORE) study is to evaluate the effectiveness of a predefined management program designed for β‐blocker up‐titration in patients receiving CRT.
Methods
The RESTORE study is a prospective, case‐control, multicenter cohort study designed to test the hypothesis that a β‐blocker up‐titration strategy based on a predefined management program maximizes the beneficial effect of CRT in terms of both the number of patients reaching the target dose of β‐blockers and their clinical response to the therapy. The study flowchart is depicted in the Figure 1. This is an independent, nonsponsored study and was approved by the institutional review boards of the 6 participating Italian centers. No extramural funding will be used to support this work. The trial will be conducted in accordance with the Declaration of Helsinki, and all subjects will provide written consent. This trial is registered with ClinicalTrials.gov as NCT02173028. The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the article.
Figure 1.
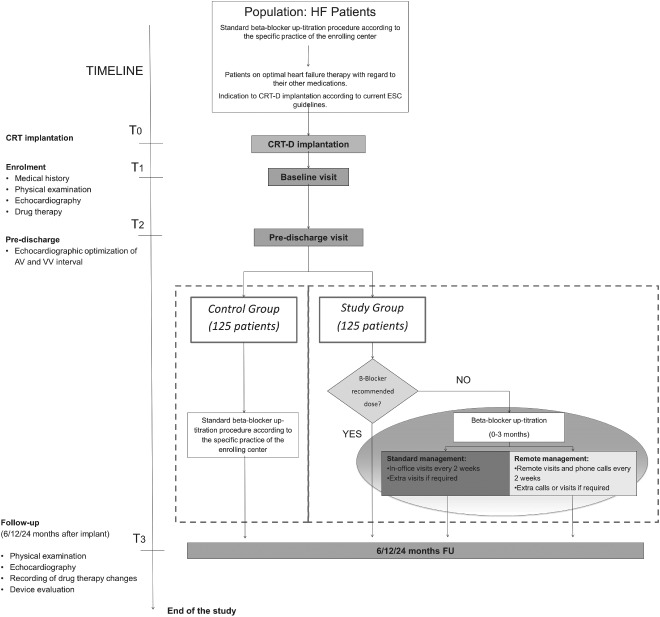
RESTORE study flowchart. Abbreviations: AV, atrioventricular; CRT, cardiac resynchronization therapy; CRT‐D, CRT defibrillator; ESC, European Society of Cardiology; FU, follow‐up; HF, heart failure; RESTORE, Resynchronization Therapy and β‐Blocker Titration; VV, interventricular.
Patient Enrollment and Allocation
Enrollments started in December 2011 and are scheduled to end in December 2014. Approximately 250 consecutive patients will be prospectively enrolled in 6 Italian centers and will be followed up for 24 months after implantation. All patients fulfilling the inclusion/exclusion criteria depicted in Table 1 will be enrolled in the study. The first 125 consecutive patients undergoing CRT defibrillator (CRT‐D) implantation in the study centers will be enrolled as a control group. In the preimplantation and postimplantation period, they will be selected and treated according to the standard practice of the centers. The second group of 125 CRT‐D candidates will be enrolled as the study group. After CRT initiation, they will follow the structured β‐blocker up‐titration program described below.
Table 1.
Patient Selection Inclusion and Exclusion Criteria for Enrollment in the RESTORE Study
| Inclusion criteria: |
|---|
| Patients on optimal HF therapy with regard to their other medications, including diuretics (titrated to the minimum effective dosage), ACEIs, and aldosterone antagonists, with stable daily dosage over the previous month |
| Successful implantation of CRT‐D according to current ESC guidelines21 |
| NYHA functional class II, III, and IV |
| LVEF ≤35% |
| QRS width ≥120 ms with a LBBB morphology |
| Patients with chronic AF will be eligible for the study only if they have undergone AV ablation. |
| Age ≥18 years |
| Exclusion criteria: |
| Life expectancy of <12 months owing to other medical conditions |
| Inability to comply with the protocol and follow‐up requirements |
| Women who are pregnant or not using medically accepted birth control |
| Mechanical tricuspid valve |
| Severe aortic stenosis or other primary valve disease causing cardiomyopathy |
| Participation in other cardiovascular clinical investigations (involving active therapy) |
| Patients on long‐term CRT |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; AV, atrioventricular; CRT, cardiac resynchronization therapy; CRT‐D, CRT defibrillator; ESC, European Society of Cardiology; HF, heart failure; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; RESTORE, Resynchronization Therapy and β‐Blocker Titration.
Procedures
All patients will undergo a baseline screening examination to verify eligibility criteria. Before enrollment, patients should have undergone a β‐blocker up‐titration phase to reach the optimal, or at least the recommended, β‐blocker dose. Reasons for unsuccessful up‐titration, possible intolerance, or any adverse effect will be recorded. The following data are to be collected at the baseline and at scheduled and unscheduled visits: patient's history, medication use, clinical and physical examination results, recording of drug therapy and changes, electrocardiogram, device interrogation data, and echocardiographic data. All patients will undergo 3 evaluations, at 6, 12, and 24 months after device implantation.
Control Group
Each patient will be followed up according to the specific practice of the enrolling center. A standard β‐blocker up‐titration procedure will be adopted and routine clinical assessments will be scheduled during the evaluation period.
Study Group (β‐Blocker Up‐titration Group)
In the study group, all patients not receiving the optimal or recommended dose of β‐blockers (ie, <7.5 mg/d of bisoprolol or <37.5 mg/d of carvedilol) at the time of CRT implantation will undergo an up‐titration procedure, as detailed in the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) and Cardiac Insufficiency Bisoprolol II (CIBIS‐II) trials.2, 5, 22 The maximal tolerated dose will be achieved in the euvolemic state, in accordance with COPERNICUS trial criteria, to determine the optimal medical therapy for each patient. The aim is to reach the maximal β‐blocker dose or at least the stated effective dose (≥7.5 mg/d of bisoprolol or ≥37.5 mg/d of carvedilol). The procedure will last for a maximum of 3 months and end when the patient reaches the recommended dose. The doses will be increased every 2 weeks through phone contact or clinic visit, as depicted in Table 2.
Table 2.
Structured β‐Blockers Up‐titration Program and Recommended Dosage for the Up‐titration Procedure
| β‐Blocker | First Dose | Increment (mg/d) | Target Dose (mg/d) |
|---|---|---|---|
| Bisoprolol | 1.25 | 2.5, 3.75, 5.0, 7.5, 10 | 10 optimal, 7.5 recommended |
| Carvedilol | 3.125 | 3.125 × 2, 6.25 × 2, 12.5 × 2, 25 × 2 | 50 optimal, 37.5 recommended |
A fractionated dose increase, equivalent to the first dose, may also be prescribed at the investigator's discretion. Compensation, heart rate, blood pressure (BP), and quality of life will be assessed at least every 2 weeks or every time the investigator considers appropriate, to determine whether the patient can tolerate a higher β‐blocker dose. Intolerance for both cardiovascular reasons (hypotension or bradycardia) and noncardiovascular reasons (other contraindications) will be considered. Hemodynamic intolerance will be defined as worsening HF, as indicated by symptomatic hypotension (systolic BP ≤95 mm Hg) and either increased fluid retention (defined as appearance of peripheral edema and/or pulmonary congestion or worsening of preexisting peripheral edema and/or pulmonary congestion) and/or heart rate <60 bpm on ≥2 consecutive clinical assessments performed at 2‐week intervals.
Study Hypotheses
Assuming that CRT enables β‐blocker administration to be initiated in previously intolerant patients and allows further up‐titration of the dose in patients on doses lower than the target doses, the RESTORE study will consider the following hypotheses: (1) the use of a predefined management program for β‐blocker up‐titration in patients receiving CRT is more effective than the usual management in increasing the number of patients who reach the target dose; (2) after CRT implantation, the use of an intensive β‐blocker up‐titration strategy based on a predefined management program maximizes the beneficial effect of CRT in terms of the rate of responders; and (3) a management strategy for β‐blocker up‐titration through remote follow‐up and the use of specific systems for the remote measuring of BP and body weight, as recommended by HF management guidelines, yield similar clinical benefits to those achieved through a strategy of standard in‐office visits, while significantly reducing health‐care utilization and the consumption of resources.
Primary and Secondary Endpoints
The primary endpoint of the study is the proportion of patients that are on treatment with an optimal dose of β‐blockers or with an effective dose after 6 months of CRT. In the study group, the proportion is expected to increase by 20% over control.
Four secondary endpoints will be evaluated. Specifically:
The echocardiographic response, defined as a decrease of ≥15% in left ventricular (LV) end‐systolic volume from the baseline to the 6‐month follow‐up visit. It is hypothesized that this response will be better in the study group than in the control group. Change in LV end‐systolic volume has been shown to be an independent predictor of prognosis.23, 24
The efficacy of remote monitoring by means of an automated telemedicine system in facilitating β‐blocker titration, as compared with the standard approach consisting of regular in‐office visits. The study hypothesis is that both management strategies will enable a comparable proportion of patients to be treated with an optimal dose of β‐blockers, but that remote monitoring will significantly reduce health‐care utilization and subsequent expenditure.
-
The evaluation of clinical outcome after CRT, as a function of optimal or suboptimal β‐blocker therapy. Patient outcome will be measured at 6 and 12 months in terms of:
Degree of LV reverse remodeling.
Change in New York Heart Association functional class.
Ventricular arrhythmias treated by the implantable cardioverter‐defibrillator.
Adverse cardiovascular events (cardiovascular hospital admission, HF hospital admission, mechanical circulatory support, transplantation) and all‐cause mortality.
The efficacy of a structured management program of β‐blocker up‐titration, as compared with the standard approach. We expect an improvement of clinical outcome in the study group with respect to the control group, with consequent reduction of health‐care utilization and related costs.
Additional Analyses
Additional evaluations will be performed, specifically the rate of adverse effects of β‐blockers (eg, hypotension, bradycardia, worsening HF) occurring after CRT initiation, and the ability of automated telemedicine systems to allow remote monitoring and management of such episodes.
Statistical Methods and Data Analysis
Continuous data will be expressed as mean ± SD or median values with interquartile range. Intrapatient differences will be evaluated by means of the Student t test for paired data. The McNemar test will be used to compare intrapatient proportions. A test for proportions will be used to evaluate the predictive role of the structured up‐titration strategy by comparing responders and nonresponders to CRT. Multivariate analysis will be performed to adjust results for any confounding baseline variable. A P value of 0.05 will be considered statistically significant.
The hypothesis underlying the primary objective of the study is that a predefined management program for β‐blocker up‐titration is more effective than the usual management in increasing the number of patients reaching the target dose of β‐blocker. In calculating the sample size, the following assumptions have been made18, 19, 25, 26:
Thirty percent of the patients in each study arm will reach the target β‐blocker dose at the baseline.
Twenty percent absolute difference in the rate of patients receiving the target dose in the study group than in the control group at 6‐month follow‐up.
According to these hypotheses, a sample size of 206 patients (103 per study arm) will have 80% power to detect a difference in proportions of 0.20 (50% in the control group vs 70% in the study group at 6 months). The method of analysis will be the χ2 (McNemar) test of paired proportions with a 2‐sided 0.05 significance level. Assuming a 15% attrition rate, 240 patients will be required for the analysis of primary and secondary endpoints.
Discussion
β‐Blockers are strongly recommended by the current HF guidelines as standard (class I) treatment for all eligible patients with HF caused by symptomatic LV systolic dysfunction.1 This recommendation is the result of several randomized clinical trials, which have demonstrated that β‐blockers improve HF symptoms, slow disease progression, and reduce mortality when combined with conventional therapy.2, 3, 4, 5, 6 Although low doses of β‐blockers may still be beneficial in comparison with placebo, a greater benefit can be achieved when maximal doses are used.27, 28 Hence, β‐blockers should be used at the recommended doses shown to be effective in placebo‐controlled trials.1
Despite extensive clinical‐trial evidence and strong recommendations in guidelines, prior studies have suggested that a substantial number of eligible patients fail to receive β‐blocker therapy, or receive it at a suboptimal dose. Indeed, several clinical trials have reported 43% to 75% rates of HF patients treated with target doses of β‐blockers.29, 30, 31, 32 The data obtained from community‐based surveys are even worse, indicating rates of 37% to 86%, only 6% to 18% of whom receive the target dose.7, 8, 9, 10 β‐Blockers are under‐prescribed or used at suboptimal doses for many reasons, including both their relatively low tolerability and the insufficiency of health‐care management systems. The high incidence of β‐blocker intolerance is due both to their traditional, non–HF‐specific side effects (eg, excessive bradycardia, atrioventricular block, bronchial asthma) and to their acute negative inotropic effects, which may cause hemodynamic deterioration and worsening HF.33, 34, 35, 36
To optimize β‐blocker titration, thereby increasing the number of patients taking the target dose, several specific strategies have been proposed, including nurse‐managed titration protocols37, 38, 39 (with remote follow‐up or in‐office visits) and a remote‐monitoring titration approach through automated telemedicine systems.38, 40, 41, 42 Considerable evidence has shown that these strategies for the close monitoring of any medication‐related adverse events and patient compliance lead to an increase in utilization rates of β‐blockers, a reduction in the time needed to reach the target doses, and an increase in the number of patients receiving target doses, with consequent improvement in patient outcomes.37, 40
Heart failure patients are often candidates for CRT; however, CRT should be considered only when pharmacologic therapy has been optimized.1 Nevertheless, in clinical practice, patients on CRT often do not receive β‐blocker therapy, despite the absence of documented contraindications, or receive it at suboptimal doses,16, 17 and this condition seems to have a negative impact on patient prognosis.17 A modest increase in the use and dose of β‐blockers has been observed after CRT implantation.16, 18, 19 This observation was confirmed by the data from the Cardiac Resynchronization in Combination With β‐Blocker Treatment in Advanced Chronic Heart Failure (CARIBE‐HF) prospective observational study, which suggested that CRT may be effective in enabling the achievement and maintenance of target doses of β‐blockers, probably by preventing excessive bradycardia and improving hemodynamic stability.20 Interestingly, the patients in that study underwent further up‐titration of β‐blockers after CRT implantation and displayed a greater New York Heart Association class reduction and a greater increase in LV ejection fraction than control patients on long‐term follow‐up, thus suggesting a synergistic effect of CRT and β‐blockers. These results should encourage the development of new and more effective strategies for achieving target doses of β‐blockers in patients receiving CRT in the setting of outpatient clinical practices.
The RESTORE study will provide the first prospective, controlled, large‐scale evaluation of the effectiveness of a predefined management program for β‐blocker up‐titration in patients receiving CRT. The impact of this program will be evaluated both in terms of the number of patients reaching and maintaining the target dose of β‐blockers in the medium and long term, and in terms of enhanced clinical and instrumental response to CRT. If the results show that CRT facilitates the optimization of β‐blocker therapy and confirm the synergistic effects of CRT and β‐blockers, the management model validated in this study could be proposed in clinical practice as a standard strategy for those patients who have received CRT without having reached the target dose of β‐blockers.
Acknowledgments
The authors acknowledge Carmine Ciardiello (HT‐MED, Naples, Italy) for his contribution to this study.
M.M. and S.V. are employees of Boston Scientific Italy. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. McMurray JJ, Adamopoulos S, Anker SD, et al; ESC Committee for Practice Guidelines . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 2. The CIBIS‐II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 3. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4. Hjalmarson A, Goldstein S, Fagerberg B, et al; MERIT‐HF Study Group . Effects of controlled‐release metoprolol on total mortality, hospitalizations, and well‐being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT‐HF). JAMA. 2000;283:1295–1302. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. [DOI] [PubMed] [Google Scholar]
- 6. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. [DOI] [PubMed] [Google Scholar]
- 7. Komajda M, Follath F, Swedberg K, et al; Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003;24:464–474. [DOI] [PubMed] [Google Scholar]
- 8. Adams KF Jr, Fonarow GC, Emerman CL, et al; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100 000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 9. Tandon P, McAlister FA, Tsuyuki RT, et al. The use of β‐blockers in a tertiary care heart failure clinic: dosing, tolerance, and outcomes. Arch Intern Med. 2004;164:769–774. [DOI] [PubMed] [Google Scholar]
- 10. Fonarow GC, Yancy CW, Albert NM, et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail. 2008;1:98–106. [DOI] [PubMed] [Google Scholar]
- 11. Linde C, Abraham WT, Gold MR, et al; REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. [DOI] [PubMed] [Google Scholar]
- 12. Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. [DOI] [PubMed] [Google Scholar]
- 13. Moss AJ, Hall WJ, Cannom DS, et al; MADIT‐CRT Trial Investigators. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 14. Tang AS, Wells GA, Talajic M, et al; Resynchronization‐Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac‐resynchronization therapy for mild‐to‐moderate heart failure. N Engl J Med. 2010;363:2385–2395. [DOI] [PubMed] [Google Scholar]
- 15. Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 16. Chen S, Ling Z, Kiuchi MG, et al. The efficacy and safety of cardiac resynchronization therapy combined with implantable cardioverter‐defibrillator for heart failure: a meta‐analysis of 5674 patients. Europace. 2013;15:992–1001. [DOI] [PubMed] [Google Scholar]
- 17. Hauptman PJ, Swindle JP, Masoudi FA, et al. Underutilization of β‐blockers in patients undergoing implantable cardioverter‐defibrillator and cardiac resynchronization procedures. Circ Cardiovasc Qual Outcomes. 2010;3:204–211. [DOI] [PubMed] [Google Scholar]
- 18. Voigt A, Shalaby A, Adelstein E, et al. β‐Blocker utilization and outcomes in patients receiving cardiac resynchronization therapy. Clin Cardiol. 2010;33:E1–E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heywood JT, Fonarow GC, Yancy CW, et al. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail. 2010;3:596–605. [DOI] [PubMed] [Google Scholar]
- 20. Aranda JM Jr, Woo GW, Conti JB, et al. Use of cardiac resynchronization therapy to optimize β‐blocker therapy in patients with heart failure and prolonged QRS duration. Am J Cardiol. 2005;95:889–891. [DOI] [PubMed] [Google Scholar]
- 21. Brignole M, Auricchio A, Baron‐Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on Cardiac Pacing and Resynchronization Therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Bristow MR, Cohn JN, et al; US Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. [DOI] [PubMed] [Google Scholar]
- 23. Packer M. Proposal for a new clinical endpoint to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail. 2001;7:176–182. [DOI] [PubMed] [Google Scholar]
- 24. Yu CM, Fung WH, Lin H, et al. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–688. [DOI] [PubMed] [Google Scholar]
- 25. Maggioni AP, Anker SD, Dahlström U, et al; Heart Failure Association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2013;15:1173–1184. [DOI] [PubMed] [Google Scholar]
- 26. Kelesidis I, Varughese CJ, Hourani P, et al. Effects of β‐adrenergic blockade on left ventricular remodeling among Hispanics and African Americans with chronic heart failure. Clin Cardiol. 2013;36:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grosu A, Senni M, Iacovoni A, et al. Cardiac resynchronization in combination with β‐blocker treatment in advanced chronic heart failure (CARIBE‐HF): the results of the CARIBE‐HF study. Acta Cardiol. 2011;66:573–580. [DOI] [PubMed] [Google Scholar]
- 28. Bristow MR, O'Connell JB, Gilbert EM, et al; Bucindolol Investigators. Dose‐response of chronic β‐blocker treatment in heart failure from either idiopathic dilated or ischemic cardiomyopathy. Circulation. 1994;89:1632–1642. [DOI] [PubMed] [Google Scholar]
- 29. Bristow MR, Gilbert EM, Abraham WT, et al; MOCHA Investigators. Carvedilol produces dose‐related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–2816. [DOI] [PubMed] [Google Scholar]
- 30. Wikstrand J, Hjalmarson A, Waagstein F, et al; MERIT‐HF Study Group . Dose of metoprolol CR/XL and clinical outcomes in patients with heart failure: analysis of the experience in metoprolol CR/XL randomized intervention trial in chronic heart failure (MERIT‐HF). J Am Coll Cardiol. 2002;40:491–498. [DOI] [PubMed] [Google Scholar]
- 31. Simon T, Mary‐Krause M, Funck‐Brentano C, et al. Bisoprolol dose‐response relationship in patients with congestive heart failure: a subgroup analysis in the cardiac insufficiency bisoprolol study (CIBIS‐II). Eur Heart J. 2003;24:552–559. [DOI] [PubMed] [Google Scholar]
- 32. Poole‐Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. [DOI] [PubMed] [Google Scholar]
- 33. Gupta R, Tang WH, Young JB. Patterns of β‐blocker utilization in patients with chronic heart failure: experience from a specialized outpatient heart failure clinic. Am Heart J. 2004;147:79–83. [DOI] [PubMed] [Google Scholar]
- 34. Eichhorn EJ, Bristow MR. Practical guidelines for initiation of β‐adrenergic blockade in patients with chronic heart failure. Am J Cardiol. 1997;79:794–798. [DOI] [PubMed] [Google Scholar]
- 35. Bristow MR. β‐adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. [DOI] [PubMed] [Google Scholar]
- 36. Metra M, Nodari S, D'Aloia A, et al. A rationale for the use of β‐blockers as standard treatment for heart failure. Am Heart J. 2000;139:511–521. [DOI] [PubMed] [Google Scholar]
- 37. Metra M, Giubbini R, Nodari S, et al. Differential effects of β‐blockers in patients with heart failure: a prospective, randomized, double‐blind comparison of the long‐term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–551. [DOI] [PubMed] [Google Scholar]
- 38. Driscoll A, Krum H, Wolfe R, et al; BENCH Study Group . Nurse‐led titration of β‐adrenoreceptor blocking agents in chronic heart failure patients in the community. J Card Fail. 2011;17:224–230. [DOI] [PubMed] [Google Scholar]
- 39. Moyer‐Knox D, Mueller TM, Vuckovic K, et al. Remote titration of carvedilol for heart failure patients by advanced‐practice nurses. J Card Fail. 2004;10:219–224. [DOI] [PubMed] [Google Scholar]
- 40. Gustafsson F, Schou M, Videbaek L, et al; Danish Heart Failure Clinics Network. Treatment with β‐blockers in nurse‐led heart failure clinics: titration efficacy and predictors of failure. Eur J Heart Fail. 2007;9:910–916. [DOI] [PubMed] [Google Scholar]
- 41. Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14:300–305. [DOI] [PubMed] [Google Scholar]
- 42. Spaeder J, Najjar SS, Gerstenblith G, et al. Rapid titration of carvedilol in patients with congestive heart failure: a randomized trial of automated telemedicine versus frequent outpatient clinic visits. Am Heart J. 2006;151:844.e1–844.e10. [DOI] [PubMed] [Google Scholar]


