ABSTRACT
Background
The prevalence and contemporary trends of pre–heart transplantation (HT) coagulopathy and associated clinical outcomes have not been studied from a national database.
Hypothesis
Pre‐HT coagulopathy is associated with increased in‐hospital mortality.
Methods
Among 2454 adult HT recipients from the 2003 to 2010 Nationwide Inpatient Sample databases, 707 (29%) had pre‐HT coagulopathy (defined as a comorbidity variable, based on International Classification of Diseases, Ninthe Revision, Clinical Modification and Diagnosis Related Group codes). We used propensity scores for coagulopathy to assemble a matched cohort of 664 pairs of patients with and without coagulopathy balanced in 54 baseline characteristics.
Results
The prevalence of pre‐HT coagulopathy increased from 17% in 2003 to 44% in 2010 (P for trend <0.001). In‐hospital mortality occurred in 8.6% and 4.7% of matched HT recipients with and without coagulopathy, respectively (hazard ratio: 1.81; 95% confidence interval [CI]: 1.17‐2.80; P = 0.008). Coagulopathy was not significantly associated with post‐HT graft complications (odds ratio [OR]: 1.20; 95% CI: 0.95‐1.52; P = 0.131) but was associated with increased blood transfusions (OR: 1.92; 95% CI, 1.54–2.41; P < 0.001). Coagulopathy and no‐coagulopathy groups had no difference in median length of stay (22 days in each group, P = 0.746), but median total hospital charges were higher among patients with coagulopathy compared to those without (US$425 643 vs US$389 656; P = 0.008).
Conclusions
In this national study of HT recipients, pretransplant coagulopathy was common, increased over time, and was not significantly associated with post‐HT graft complications or increased hospital stay. However, it was associated with increased bleeding risk, in‐hospital mortality, and total hospital charges. These findings may have implications for the selection of patients for HT.
Introduction
Heart transplantation (HT) remains the gold standard treatment for advanced heart failure (HF) patients.1, 2, 3 However, there is a disproportionately high demand–supply ratio in the United States, making appropriate recipient selection crucial to derive maximum benefit from transplantation.4 Impaired coagulation profile and thrombocytopenia are commonly seen in hospitalized patients with advanced HF.5 Potential etiologies include coagulopathy due to hepatic congestion from right HF,6, 7 thrombocytopenia due to increased sequestration of platelets in congested spleen,8, 9 coagulopathic complications from the use of mechanical circulatory support (MCS) devices,10, 11, 12, 13, 14, 15 use of anticoagulant or antiplatelet medications,15, 16 and consumptive coagulopathy from cardiogenic shock.17 Baseline coagulopathy has been shown to increase risk of bleeding, thrombotic events, and even early mortality among patients supported with left ventricular assist devices (LVAD).10 In addition, post‐procedure coagulopathy has been shown to be associated with both early and late morbidity in patients undergoing HT and other cardiac surgeries.18, 19, 20, 21 However, the literature is limited regarding the implication of pre‐HT coagulopathy and its association with in‐hospital survival among HT recipients. In our retrospective study of the Nationwide Inpatient Sample (NIS) databases from 2003 to 2010, we report the prevalence and trends of pre‐HT coagulopathy and its association with in‐hospital outcomes among HT recipients.
Methods
Data Source
The NIS, maintained by the Agency for Healthcare Research and Quality (AHRQ) as a part of the Healthcare Cost and Utilization Project, is the largest publicly available all‐payer inpatient care database in the United States.22 It contains discharge‐level data from approximately 8 million hospital stays per year from nearly 1000 hospitals, representing a 20% stratified sample of all US community hospitals. A discharge weight is included for each patient discharge record to represent the relative proportion of the total US inpatient hospital population for each record, allowing us to obtain national estimates.23 Therefore, the cohort represented in this study is broadly representative of advanced HF patients undergoing HT within the United States.
Patient Population
Patients undergoing HTs were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) procedure code 37.51. Between 2003 and 2010, ∼64 million hospital records were included in the NIS, of which 2808 discharge records were of patients who underwent HT procedures. Of the 2808 patients, 17 without data on coagulopathy, 317 patients under 18 years of age, 9 patients with history of previous HTs, and 11 patients with another organ transplantation during same hospitalization were excluded, leading to a final sample size of 2454 discharge records. Details on the assembly of the study population are given in the Supplemental Figure 1 in the online version of this article. Patients were further stratified by the presence of coagulopathy as a comorbidity at baseline.
Figure 1.
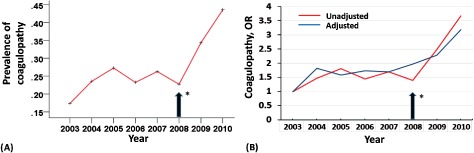
Trends in prevalence of pre–heart transplant (HT) coagulopathy among heart transplant recipients. (A) Prevalence of pre‐HT coagulopathy was calculated as the number of patients with coagulopathy divided by the number of HT recipients each year; P trend <0.001. (B) Trends in pre‐HT coagulopathy presented as unadjusted and adjusted odds ratio (OR) for each year related to 2003 (reference year).
Coagulopathy and Other Covariates Definition
Coagulopathy and other comorbidities were defined based on NIS comorbidity measures assigned using the AHRQ comorbidity software, developed by Elixhauser et al., using ICD‐9‐CM diagnoses and the Diagnosis Related Group (DRG) in effect on the discharge date.24 The AHRQ comorbidity software assigned a variable as a comorbidity by strictly restricting its search to those secondary diagnoses that are not directly related to the principal diagnosis and the DRG assignment of each patient. The software also attempted to eleminiate complications that might have originated during the hospitalization as a result of diagnostic or therapeutic interventions.24 The definition of coagulopathy included the following secondary discharge ICD‐9‐CM diagnoses codes: 286.0‐286.9, 287.1, 287.3‐287.5 and 289.81‐289.82, and excluded the following discharge DRGs: pre‐V25 DRG: 397 and V25‐V29 DRGs: 813.24 The Elixhauser method has been demonstrated to provide effective adjustments for mortality risk among surgical populations.25, 26
Baseline patient characteristics included demographics (age, sex, and race), primary expected payer, elective operative status, median household income for patient's ZIP code (I: $1–%24 999; II: $25 000–$34 999; III: $35 000–$44 999; and IV: ≥$45 000), and clinically relevant comorbidities such as hypertension, diabetes mellitus, tobacco use, dyslipidemia, obesity, atrial fibrillation, alcohol abuse, anemia, rheumatoid arthritis/collagen vascular diseases, chronic pulmonary disease, depression, drug abuse, hypothyroidism, liver disease, malignancy, fluid and electrolyte disorder, paralysis, other neurological disorders, obesity, peripheral vascular disease, psychoses, pulmonary circulation disorders, chronic kidney disease (CKD) etc. (Table 1). Pre‐HT MCS devices (ie, intra‐aortic balloon pump [IABP]), any heart assist device (includes LVAD, right ventricular assist device, total artificial heart) were also included. The ICD‐9‐CM and/or Clinical Classifications Software codes were used to identify these comorbidities. Hospital characteristics such as hospital region (Northeast, Midwest, South, and West), bed size (small, medium, and large) and teaching status (teaching vs nonteaching hospital) were also included. All patients were from urban hospitals.
Table 1.
Baseline Patient Characteristics of Heart Transplant Recipients by Pre‐HT Coagulopathy Before and After Propensity Score Matching
| Variables, N (%) or Mean (±SD) | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| Coagulopathy | P Value | Coagulopathy | P Value | |||
| No, n = 1747 | Yes, n = 707 | No, n = 664 | Yes, n = 664 | |||
| Age, y | 51 (±13) | 52 (±13) | 0.341 | 51 (±13) | 52 (±13) | 0.712 |
| Female | 412 (23.6) | 176 (24.9) | 0.491 | 159 (23.9) | 161 (24.2) | 0.898 |
| Racial distribution | ||||||
| Caucasian | 962 (55.1) | 428 (60.5) | <0.001 | 365 (55.0) | 399 (60.1) | 0.006 |
| African American | 234 (13.4) | 100 (14.1) | 91 (13.7) | 91 (13.9) | ||
| Hispanic | 133 (7.6) | 66 (9.3) | 54 (8.1) | 61 (9.2) | ||
| Other races | 96 (5.5) | 34 (4.8) | 27 (4.1) | 34 (5.1) | ||
| Unknown race | 321 (18.4) | 79 (11.2) | 127 (19.1) | 78 (11.7) | ||
| Elective admission | 467 (26.7) | 222 (31.4) | 0.020 | 217 (32.7) | 204 (30.7) | 0.443 |
| Weekend admission | 339 (19.4) | 144 (20.4) | 0.587 | 123 (18.5) | 137 (20.6) | 0.333 |
| Primary payer status | ||||||
| Medicare | 533 (30.5) | 229 (32.4) | 0.064 | 211 (31.8) | 214 (32.2) | 0.769 |
| Medicaid | 245 (14.0) | 78 (11.0) | 80 (12.0) | 78 (11.7) | ||
| Private | 848 (48.5) | 367 (51.9) | 337 (50.8) | 339 (51.1) | ||
| Median household income | ||||||
| 0–25th percentile | 351 (20.1) | 143 (20.2) | 0.741 | 139 (20.9) | 138 (20.8) | 0.729 |
| 26th–50th percentile (median) | 437 (25.0) | 176 (24.9) | 167 (25.2) | 164 (24.7) | ||
| 51st–75th percentile | 477 (27.3) | 206 (29.1) | 173 (26.1) | 190 (28.6) | ||
| 76th–00th percentile | 482 (27.6) | 182 (25.7) | 185 (27.9) | 172 (25.9) | ||
| Past medical history | ||||||
| Smoking | 204 (11.7) | 54 (7.6) | 0.003 | 54 (8.1) | 54 (8.1) | 1.000 |
| Drug abuse | 28 (1.6) | 14 (2.0) | 0.514 | 11 (1.7) | 12 (1.8) | 0.833 |
| Diabetes, uncomplicated | 396 (22.7) | 162 (22.9) | 0.895 | 158 (23.8) | 148 (22.3) | 0.515 |
| Diabetes, with chronic complication | 106 (6.1) | 35 (5.0) | 0.282 | 31 (4.7) | 35 (5.3) | 0.614 |
| Hypertension | 670 (38.4) | 270 (38.2) | 0.940 | 255 (38.4) | 250 (37.7) | 0.777 |
| Obesity | 84 (4.8) | 43 (6.1) | 0.197 | 37 (5.6) | 40 (6.0) | 0.725 |
| Peripheral vascular disorders | 64 (3.7) | 33 (4.7) | 0.248 | 30 (4.5) | 31 (4.7) | 0.896 |
| Dyslipidemia | 421 (24.1) | 149 (21.1) | 0.108 | 138 (20.8) | 139 (20.9) | 0.946 |
| Prior myocardial infarction | 237 (13.6) | 95 (13.4) | 0.933 | 86 (13.0) | 91 (13.7) | 0.686 |
| Prior coronary artery bypass grafting | 145 (8.3) | 55 (7.8) | 0.669 | 48 (7.2) | 52 (7.8) | 0.677 |
| Cardiogenic shock | 313 (17.9) | 217 (30.7) | <0.001 | 175 (26.4) | 187 (28.2) | 0.460 |
| Intra‐aortic balloon pump | 185 (10.6) | 105 (14.9) | 0.003 | 86 (13.0) | 92 (13.9) | 0.629 |
| Any heart assist device | 435 (24.9) | 241 (34.1) | <0.001 | 232 (34.9) | 213 (32.1) | 0.269 |
| Swan ganz catheter | 350 (20.0) | 139 (19.7) | 0.834 | 129 (19.4) | 132 (19.9) | 0.836 |
| Pulmonary hypertension | 365 (20.9) | 161 (22.8) | 0.304 | 152 (22.9) | 151 (22.7) | 0.948 |
| Valvular disease | 229 (13.1) | 95 (13.4) | 0.827 | 93 (14.0) | 90 (13.6) | 0.811 |
| Paroxysmal supraventricular tachycardia | 21 (1.2) | 15 (2.1) | 0.086 | 17 (2.6) | 10 (1.5) | 0.173 |
| Atrial fibrillation | 457 (26.2) | 189 (26.7) | 0.770 | 179 (27.0) | 173 (26.1) | 0.709 |
| Atrial flutter | 116 (6.6) | 53 (7.5) | 0.448 | 51 (7.7) | 47 (7.1) | 0.675 |
| Ventricular fibrillation/flutter | 44 (2.5) | 26 (3.7) | 0.118 | 20 (3.0) | 24 (3.6) | 0.540 |
| Ventricular tachycardia | 372 (21.3) | 152 (21.5) | 0.910 | 143 (21.5) | 145 (21.8) | 0.894 |
| Cardiac arrest | 49 (2.8) | 32 (4.5) | 0.031 | 26 (3.9) | 27 (4.1) | 0.889 |
| Diagnostic cardiac catheterization/coronary arteriography | 621 (35.5) | 268 (37.9) | 0.271 | 256 (38.6) | 153 (38.1) | 0.866 |
| Cardiac pacemaker or cardioverter Defibrillator | 682 (39.0) | 316 (44.7) | 0.010 | 293 (44.1) | 291 (43.8) | 0.912 |
| Chronic kidney disease stage lll–V | 362 (20.7) | 180 (25.5) | 0.010 | 163 (24.5) | 163 (24.5) | 1.000 |
| Fluid and electrolyte disorders | 652 (37.3) | 383 (54.2) | <0.001 | 357 (53.8) | 343 (51.7) | 0.442 |
| Deficiency anemias | 388 (22.2) | 198 (28.0) | 0.002 | 176 (26.5) | 182 (27.4) | 0.711 |
| Cancer | 62 (3.5) | 23 (3.3) | 0.717 | 15 (2.3) | 21 (3.2) | 0.311 |
| Chronic blood loss anemia | 25 (1.4) | 16 (2.3) | 0.145 | 13 (2.0) | 14 (2.1) | 0.846 |
| Chronic pulmonary disease | 199 (11.4) | 80 (11.3) | 0.957 | 77 (11.6) | 74 (11.1) | 0.795 |
| Depression | 174 (10.0) | 53 (7.5) | 0.056 | 58 (8.7) | 51 (7.7) | 0.484 |
| Hypothyroidism | 185 (10.6) | 74 (10.5) | 0.929 | 69 (10.4) | 71 (10.7) | 0.858 |
| Liver disease | 34 (1.9) | 20 (2.8) | 0.177 | 19 (2.9) | 18 (2.7) | 0.868 |
| Other neurological disorders | 49 (2.8) | 34 (4.8) | 0.013 | 28 (4.2) | 23 (3.5) | 0.475 |
| Paralysis | 19 (1.1) | 8 (1.1) | 0.925 | |||
| Psychosis | 26 (1.5) | 16 (2.3) | 0.180 | 11 (1.7) | 15 (2.3) | 0.428 |
| Weight loss | 99 (5.7) | 81 (11.5) | <0.001 | 55 (8.3) | 65 (9.8) | 0.338 |
| Hospital bed size | ||||||
| Small | 34 (1.9) | 11 (1.6) | 0.098 | 17 (2.6) | 11 (1.7) | 0.441 |
| Medium | 188 (10.8) | 57 (8.1) | 48 (7.2) | 54 (8.1) | ||
| Large | 1525 (87.3) | 639 (90.4) | 599 (90.2) | 599 (90.2) | ||
| Hospital location by region | <0.001 | |||||
| Midwest | 317 (18.1) | 75 (10.6) | 71 (10.7) | 75 (11.3) | 0.768 | |
| Northeast | 404 (23.1) | 166 (23.5) | 171 (25.8) | 161 (24.2) | ||
| South | 512 (29.3) | 229 (32.4) | 221 (33.3) | 212 (31.9) | ||
| West | 514 (29.4) | 237 (33.5) | 201 (30.3) | 216 (32.5) | ||
| Teaching hospitals | 1671 (95.6) | 675 (95.5) | 0.847 | 629 (94.7) | 633 (95.3) | 0.614 |
Outcome Measures
All outcomes of interest were established prior to data collection. The primary outcome of interest for this study was all‐cause in‐hospital mortality, defined as “died” during hospitalization encounter in the NIS database. Secondary outcomes of interest were hospital length of stay among survivors, total charges during the hospitalization, post‐HT complications of transplanted heart (includes acute HT rejection), and blood transfusion. Discharge records were used to identify all outcomes of interest. Post‐HT complications of a transplanted heart was defined using discharge diagnosis ICD‐9‐CM code 996.83 (complications of transplanted organ–heart, including transplant failure or rejection).
Assembly of a Balanced Cohort
We used propensity scores for baseline coagulopathy to assemble a cohort in which the 2 comparison groups were balanced on all measured baseline characteristics.27, 28, 29, 30, 31 We estimated propensity scores for each of the 2454 patients using a nonparsimonious multivariable logistic regression model.32 In the model, the presence of coagulopathy was the dependent variable, and 54 baseline characteristics (Table 1) were used as covariates. We used a greedy matching protocol to match 664 or 94% of the 707 patients with coagulopathy with 664 patients without coagulopathy, but had the same propensity score for the presence of coagulopathy. Thus, the final sample size at the matched cohort was 1328.
Statistical Analysis
For descriptive analyses, we used Pearson χ2 and Wilcoxon rank sum tests for the prematch data, and McNemar test and paired sample t test for postmatch comparisons, as appropriate. Cox proportional hazard and Kaplan‐Meier survival analyses were used to determine the association of pre‐HT coagulopathy and in‐hospital mortality in the postmatched cohort (N = 1328). Log‐minus‐log survival plots were used to check proportional hazards assumptions. We conducted subgroup analyses to determine the homogeneity of association between coagulopathy and mortality. Finally, we examined the association of pre‐HT coagulopathy with mortality in the pre‐match cohort using (1) multivariable Cox regression models adjusting for all 54 baseline characteristics used in the propensity model and (2) propensity scores. Univariate and multivariate logistic regression analyses were conducted to determine the association between pre‐HT coagulopathy and other outcomes among pre‐ and postmatched cohorts. All statistical tests were 2‐tailed, and 95% confidence intervals (CIs) were constructed.
Data analysis was performed using IBM SPSS Statistics 20.0 (IBM Corp., Armonk, NY). Categorical variables were expressed as a percentage of the group of origin and continuous variables reported as mean ± standard deviation. Hazard ratio (HR) with a 95% CI were used to report the results of Cox regression models. Odds ratio (OR) with a 95% CI were used to report the results of logistic regression models. Reported probability values were 2‐tailed and were considered statistically significant if P < 0.05.
Results
Trends in Prevalence of Prior Coagulopathy
From 2003 to 2010 NIS databases, 2454 first‐time adult HT recipients were indentified. Pre‐HT coagulopathy was common among these patients (n = 707), representing 29% of the study population. Prevalence of pre‐HT coagulopathy increased from 17% in 2003 to 44% in 2010 (unadjusted OR comparing prevalence in 2010–2003, 3.68; 95% CI: 2.64‐5.15, P < 0.001; Figure 1). When adjusted for demographics, patient comorbidities, and hospital characteristics, prevalence ratios of pre‐HT coagulopathy comparing prevalence rate of 2010 to 2003 attenuated but still remained strongly significant (adjusted OR: 3.19; 95% CI: 2.04‐4.98, P < 0.001; Figure 1). Disorders identified as coagulopathy were diverse, ranging from defibrination syndromes to coagulation defects and thrombocytopenias (see Supplemental Table 1 in the online version of this article).
Baseline Patient Characteristics
Before matching, patients with pre‐HT coagulopathy were older, more likely to be white, admitted electively, and had Medicare or private insurance as their primary payer status (Table 1). Patients with pre‐HT coagulopathy were also more likely to have deficiency anemia or chronic blood loss anemia, pulmonary hypertension, cardiogenic shock, cardiac arrest, and higher chance of having MCS. Liver disease, CKD stage lll or higher, and fluid and electrolyte disorders were also more prevalent among patients with pre‐HT coagulopathy. On the other hand, patients with pre‐HT coagulopathy were less likely to have dyslipidemia, history of smoking or alcohol abuse, and less likely to present in a small‐ or medium‐sized hospital in the Midwest region. These and other baseline imbalances were balanced after matching (Table 1). Matched patients (N = 1328) had a mean age of 52 (±12) years, 25% were women, and 28% were nonwhite. Absolute standardized differences for all 54 baseline characteristics between the 2 treatment groups were <10% (mostly <5%), suggesting substantial bias reduction.
Coagulopathy and In‐hospital Mortality
Among the 2454 prematch sample, the primary endpoint of in‐hospital mortality occurred in 9.1% (64/707) and 3.4% (60/1747) of HT recipients with and without coagulopathy, respectively (unadjusted HR when patients with coagulopathy were compared with those without, 2.33; 95% CI: 1.64‐3.31; P < 0.001). Multivariable‐adjusted and propensity‐adjusted HRs for in‐hospital mortality associated with coagulopathy were 2.33 (95% CI: 1.58‐3.45; P < 0.001) and 2.27 (95% CI: 1.57‐3.29; P < 0.001), respectively. Among the 1328 matched sample, all‐cause in‐hospital mortality occurred in 8.6% and 4.6% of HT recipients with and without pre‐HT coagulopathy, respectively (HR: 1.81; 95% CI: 1.17‐2.80; P = 0.008; Table 2). The Kaplan‐Meier survival analyses among the propensity‐matched cohort of HT recipients with and without coagulopathy are displayed in Figure 2. Among the matched sample, the association between pre‐HT coagulopathy and in‐hospital mortality was homogeneous across various subgroups of patients (Figure 3).
Table 2.
Association of Coagulopathy With All‐Cause In‐hospital Mortality, Post–Heart Transplantation Graft Complications, and Blood Transfusion Among Heart Transplant Recipients Before and After Propensity Matching
| All‐Cause Mortality | Events (%) | Absolute Risk Differencea | Hazard Ratiob (95% CI) | P Value | |
|---|---|---|---|---|---|
| Coagulopathy | |||||
| No | Yes | ||||
| Before matching, n = 2454 | n = 1747 | n = 707 | |||
| Unadjusted | 60 (3.4%) | 64 (9.1%) | 5.7% | 2.33 (1.64‐3.31) | <0.001 |
| Multivariable adjusted c | 2.33 (1.58‐3.45) | <0.001 | |||
| Propensity adjusted d | 2.27 (1.57–3.29) | <0.001 | |||
| After matching , n = 1328 | n = 664 | n = 664 | |||
| Propensity‐matched | 31 (4.7%) | 57 (8.6%) | +3.9% | 1.81 (1.17‐2.80) | 0.008 |
| Events (%) | Absolute Risk Differencea | Odds Ratioe (95% CI) | P Value | ||
|---|---|---|---|---|---|
| Coagulopathy | |||||
| No | Yes | ||||
| Post‐HT graft complications | |||||
| Before matching, n = 2454 | n = 1747 | n = 707 | |||
| Unadjusted | 465 (27%) | 225 (32%) | +5% | 1.29 (1.06‐1.56) | 0.009 |
| Multivariable adjustedc | 1.25 (1.02‐1.53) | 0.033 | |||
| Propensity adjustedd | 1.22 (1.00‐1.48) | 0.055 | |||
| After matching, n = 1328 | n = 664 | n = 664 | |||
| Propensity matched | 180 (27%) | 205 (31%) | +4% | 1.20 (0.95‐1.52) | 0.131 |
| Blood transfusion | |||||
| Before matching, n = 2454 | n = 1747 | n = 707 | |||
| Unadjusted | 501 (29%) | 314 (44%) | +15% | 1.99 (1.66‐2.38) | <0.001 |
| Multivariable adjustedc | 2.01 (1.64‐2.45) | <0.001 | |||
| Propensity adjustedd | 1.89 (1.56‐2.28) | <0.001 | |||
| After matching, n = 1328 | n = 664 | n = 664 | |||
| Propensity matched | 200 (30%) | 301 (45%) | +15% | 1.92 (1.54‐2.41) | <0.001 |
Abbreviations: CI, confidence interval; HT, heart transplantation.
Absolute risk difference was calculated by subtracting the percentage of events in the no‐coagulopathy group from that of the coagulopathy group (before values were rounded).
Hazard ratios comparing patients with and without coagulopathy.
Adjusted for 54 baseline characteristics.
Adjusted for propensity score.
Odds ratios comparing patients with and without coagulopathy.
Figure 2.
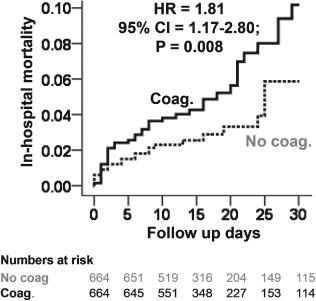
Kaplan‐Meier plots for in‐hospital mortality in the propensity‐matched cohort of heart transplant recipients with and without coagulopathy Abbreviations: CI, confidence interval; Coag., coagulopathy; HR, hazard ratio.
Figure 3.
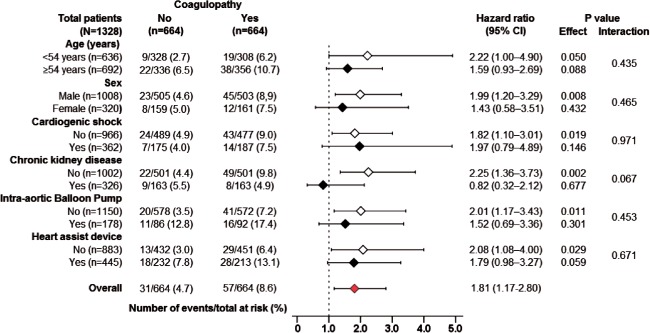
Hazard ratio and 95% confidence interval (CI) for in‐hospital mortality associated with pre–heart transplant coagulopathy in subgroups of matched patients.
Coagulopathy and Secondary Outcomes
Post‐HT graft complications occurred in 31% and 27% of matched coagulopathy and no‐coagulopathy patients, respectively (OR: 1.20; 95% CI: 0.95‐1.52; P = 0.145). Blood transfusion occurred in 45% and 30% of matched patients with and without coagulopathy, respectively (OR: 1.92; 95% CI: 1.54‐2.41; P < 0.001). Matched patients with and without coagulopathy had a median length of stay of 22 days in each group (P = 0.884). Matched patients with and without coagulopathy had a median total hospital charge of US$429 580 and US$402 201, respectively (P < 0.001). Pre‐HT coagulopathy and secondary outcomes among prematch cohort are displayed in the Table 2 (see also Supplemental Table 2 in the online version of this article).
Discussion
The findings from the current analysis demonstrate that, among HT recipients in the United States, the prevalence of pre‐HT coagulopathy was relatively high and significantly increased over the last decade. Coagulopathy was associated with a borderline increase in post‐HT graft complications but independently associated with an increased risk of bleeding (as evidenced by higher blood transfusion rates), total hospital charges, and most importantly, in‐hospital mortality. These findings are important because HT is being increasingly allocated to status 1A and 1B candidates,33 who have more advanced disease and likely to benefit from HT, but also have a higher burden of pre‐HT coagulopathy, and thus at increased risk of post‐transplant early mortality.34 To the best of our knowledge, this is the first report of a significant association between pre‐HT coagulopathy and post‐HT in‐hospital outcomes, based on a propensity‐matched analysis of a large, all‐payer national database.
Pre‐HT coagulopathy increased from 17% in 2003 to 44% in 2010. This increasing trend was strongly driven by an increase in pre‐HT thrombocytopenia from unspecified causes that increased from 4% in 2003 to 34% in 2010. There are several potential explanations for this observed increase in coagulopathy. One explanation is that a slow but steady increase in the proportion of status 1A and 1B patients has been noted over the last few years, but the total number of transplants annually has remained the same.33, 34 Thus, a higher proportion of HT recipients in recent years were from the status 1A or 1B waiting list, who are prone to be coagulopathic, most notably from thrombocytopenia.10, 11, 12, 13 A significant proportion of these patients are critically ill, requiring MCS devices as a bridge to transplant. Continuous flow LVAD (HeartMate ll; Thoratec Corp., Pleasanton, CA) was approved by the FDA in 2008 as a bridge to transplantation and may very well be the reason for an increase in coagulopathy over this time period. Our data also show that 35% to 40% of patients had MCS devices placed during the same hospitalization when they had the HT. Other possibilities include increased detection or documentation of coagulopathy over time during the study period.
The strong unadjusted association of coagulopathy with increased in‐hospital mortality among the prematch cohort was substantially attenuated among the propensity‐matched cohort. This is in part explained by the significant imbalances in various baseline characteristics between patients with and without coagulopathy. For example, patients with pre‐HT coagulopathy were more likely to have fluid and electrolyte imbalances, cardiogenic shock, CKD stage lll or higher, and more patients with pre‐HT coagulopathy had used MCS. All of these characteristics correlate with a sicker group of patients with an increased intrinsic risk of in‐hospital mortality. However, the association remained strong and significant after propensity matching, thus indicating a possible true intrinsic association. Potential mechanistic explanations for an intrinsic association include accelerated activation of procoagulant, anticoagulant, and fibrinolytic factors among transplant recipients with pre‐HT coagulopathy, which may lead to increased post‐transplant complications including bleeding and mortality. Our findings suggest that the higher deaths in those with pre‐HT coagulopathy may be in large part due to a higher incidence of bleeding among those individuals.
The association between coagulopathy and in‐hospital mortality was homogeneous across various subgroups (Figure 3). The association of pre‐HT coagulopathy with mortality was weaker among those with MCS (IABP or heart assist device), compared to those without MCS, though the interaction was not significant. This may indicate that coagulopathy that develops from a mechanical source has a lesser impact on bleeding and mortality. This finding is similar to previous studies that showed that thrombocytopenia secondary to IABP insertion was not associated with major bleeding or in‐hospital mortality, suggesting that thrombocytopenia that develops from a mechanical factor may have less clinical significance than thrombocytopenia that develops in a systemic illness.13, 35, 36 Studies have also shown that pre–cardiac transplant coagulopathy developing after cardiogenic shock is a marker of worse prognosis and increased bleeding and mortality,17 a finding that was used to support institution of MCS before shock‐induced coagulopathy to reduce risk of bleeding and graft failure. However, findings from our subgroup analysis demonstrated a homogenous association of pre‐HT coagulopathy and in‐hospital mortality, both in patients with and in those without cardiogenic shock.
The finding that mortality is increased in those transplant patients who had pre‐HT coagulopathy has important implications. Taking into account that organs are the limiting factor in HT, identifying pre‐HT factors that contribute to poor outcomes is important.34 For example, patients at higher risk for post‐HT mortality due to pre‐HT coagulopathy may also be at higher risk for mortality while on the waiting list for HT. The competing survival benefit from HT in these coagulopathic patients is not established. Thus, future prospective studies should examine how waiting list coagulopathy may alter the expected survival benefit post‐HT across the spectrums of coagulopathic patients. Every individual patient case is unique, and the decision to transplant depends on a multitude of factors, but perhaps coagulopathy is a factor that should be considered.
There are potential limitations to our study. First, the retrospective design of this study renders the possibility for selection bias, although we can expect this to be attenuated by the extremely robust size of the NIS databases from which we based our analyses. Second, there is always a potential for variability as well as unrecognized miscoding among diagnostic, comorbidity, and procedure codes within a large administrative database such as the NIS. In addition, the NIS database is limited to in‐hospital events, allowing us to report only short‐term outcomes. As a result, the true incidence of perioperative mortality and morbidity that follow patient discharge cannot be elucidated and may even be underestimated. The NIS does not include specific laboratory values and lacks transplant‐related variables such as ischemic time, mismatch, hemodynamics, and status of recipient at the time of transplant. It also does not have donor characteristics such as cause of death and risk profiles. Furthermore, it is not possible to determine the cause and circumstances surrounding the coagulopathy such as the inability to conclude whether coagulopathy had resulted from mechanical support or in the setting of shock. In fact, previous studies have shown that the etiology of the coagulopathy can be critical, thus rendering this an interesting direction for future study.
Conclusion
In this national study of HT recipients, the prevalence of pre‐HT coagulopathy was high and has significantly increased over time. Pre‐HT coagulopathy had no association with increased length of hospital stay but was associated with a borderline increase in post‐HT graft complications and an increased risk of bleeding, in‐hospital mortality, and total hospital charges. With increasing prevalence of coagulopathies among the pre‐HT population, pretransplant coagulapathy warrants consideration in selecting candidates who would derive the maximum benefit from HT, and future large‐scale prospective studies are needed to develop optimal management strategies for these higher‐risk HT patients.
Supporting information
FigureS1. Assembly of prematch and postmatch study cohorts.
TableS1. Types of coagulopathy and their frequencies among all heart transplant recipients with coagulopathy
TableS2. Association of coagulopathy with length of stay and total hospital cost among heart transplant recipients before and after propensity matching
Drs Mujib and Khanna have contributed equally to this study.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Johnson MR, Meyer KH, Haft J, et al. Heart transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 pt 2):1035–1046. [DOI] [PubMed] [Google Scholar]
- 2. Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty‐seventh official adult heart transplant report—2010. J Heart Lung Transplant. 2010;29:1089–1103. [DOI] [PubMed] [Google Scholar]
- 3. Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant. 2012;31:1052–1064. [DOI] [PubMed] [Google Scholar]
- 4. Stevenson LW. The urgent priority for transplantation is to trim the waiting list. J Heart Lung Transplant. 2013;32:861–867. [DOI] [PubMed] [Google Scholar]
- 5. Mongirdiene A, Kursvietiene L, Kasauskas A. The coagulation system changes in patients with chronic heart failure. Medicina (Kaunas). 2010;46:642–647. [PubMed] [Google Scholar]
- 6. Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011;20:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein DJ, Seldomridge JA, Chen JM, et al. Use of aprotinin in LVAD recipients reduces blood loss, blood use, and perioperative mortality. Ann Thorac Surg. 1995;59:1063–1067; discussion 1068. [DOI] [PubMed] [Google Scholar]
- 8. Heck J, Keitel K, Wusthoff D, et al. Frequency and pathogenis of thrombocytopenia in cardiac failure [in German]. Dtsch Med Wochenschr. 1976;101:1381–1384. [DOI] [PubMed] [Google Scholar]
- 9. Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62:485–495. [DOI] [PubMed] [Google Scholar]
- 10. Fried J, Levin AP, Mody KM, et al. Prior hematologic conditions carry a high morbidity and mortality in patients supported with continuous‐flow left ventricular assist devices. J Heart Lung Transplant. 2014;33:1119–1125. [DOI] [PubMed] [Google Scholar]
- 11. Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ Heart Fail. 2010;3:675–681. [DOI] [PubMed] [Google Scholar]
- 12. Ravichandran AK, Parker J, Novak E, et al. Hemolysis in left ventricular assist device: a retrospective analysis of outcomes. J Heart Lung Transplant. 2014;33:44–50. [DOI] [PubMed] [Google Scholar]
- 13. Vales L, Kanei Y, Ephrem G, et al. Intra‐aortic balloon pump use and outcomes with current therapies. J Invasive Cardiol. 2011;23:116–119. [PubMed] [Google Scholar]
- 14. Eckman PM, John R. Bleeding and thrombosis in patients with continuous‐flow ventricular assist devices. Circulation. 2012;125:3038–3047. [DOI] [PubMed] [Google Scholar]
- 15. Wadia Y, Etheridge W, Smart F, et al. Pathophysiology of hepatic dysfunction and intrahepatic cholestasis in heart failure and after left ventricular assist device support. J Heart Lung Transplant. 2005;24:361–370. [DOI] [PubMed] [Google Scholar]
- 16. Givertz MM. Cardiology patient pages: ventricular assist devices: important information for patients and families. Circulation. 2011;124:e305–e311. [DOI] [PubMed] [Google Scholar]
- 17. Takayama H, Truby L, Koekort M, et al. Clinical outcome of mechanical circulatory support for refractory cardiogenic shock in the current era. J Heart Lung Transplant. 2013;32:106–111. [DOI] [PubMed] [Google Scholar]
- 18. Davidson S. State of the art—how I manage coagulopathy in cardiac surgery patients. Br J Haematol. 2014;164:779–789. [DOI] [PubMed] [Google Scholar]
- 19. Jimenez JJ, Iribarren JL. Coagulopathy after cardiac surgery. Anesth Analg. 2007;105:1514; author reply 1514–1515. [DOI] [PubMed] [Google Scholar]
- 20. Rozental T, Shore‐Lesserson L. Pharmacologic management of coagulopathy in cardiac surgery: an update. J Cardiothorac Vasc Anesth. 2012;26:669–679. [DOI] [PubMed] [Google Scholar]
- 21. Song HK, Tibayan FA, Kahl EA, et al. Safety and efficacy of prothrombin complex concentrates for the treatment of coagulopathy after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:1036–1040. [DOI] [PubMed] [Google Scholar]
- 22. LaPar DJ, Bhamidipati CM, Mery CM, et al. Primary payer status affects mortality for major surgical operations. Ann Surg. 2010;252:544–550; discussion 550–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrett M, Wilson E, Whalen D. 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Methods Series Report # 2010–03. Online September 9, 2010. U.S. Agency for Healthcare Research and Quality. Available: http://www.hcup-us.ahrq.gov/reports/methods.jsp. Accessed November 1, 2014.
- 24. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 25. Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–360. [DOI] [PubMed] [Google Scholar]
- 26. Stukenborg GJ, Wagner DP, Connors AF. Comparison of the performance of two comorbidity measures, with and without information from prior hospitalizations. Med Care. 2001;39:727–739. [DOI] [PubMed] [Google Scholar]
- 27. Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrica. 1983;70:41–55. [Google Scholar]
- 28. Rubin DB. Using propensity score to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2:169–188. [Google Scholar]
- 29. Austin PC. Primer on statistical interpretation or methods report card on propensity‐score matching in the cardiology literature from 2004 to 2006: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:62–67. [DOI] [PubMed] [Google Scholar]
- 30. Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32:1704–1708. [DOI] [PubMed] [Google Scholar]
- 31. Michels KB, Braunwald E. Estimating treatment effects from observational data: dissonant and resonant notes from the SYMPHONY trials. JAMA. 2002;287:3130–3132. [DOI] [PubMed] [Google Scholar]
- 32. Mujib M, Desai RV, Ahmed MI, et al. Rheumatic heart disease and risk of incident heart failure among community‐dwelling older adults: a prospective cohort study. Ann Med. 2012;44:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mancini DM, Schulze PC. Heart transplant allocation: in desperate need of revision. J Am Coll Cardiol. 2014;63:1179–1181. [DOI] [PubMed] [Google Scholar]
- 34. Singh TP, Milliren CE, Almond CS, et al. Survival benefit from transplantation in patients listed for heart transplantation in the United States. J Am Coll Cardiol. 2014;63:1169–1178. [DOI] [PubMed] [Google Scholar]
- 35. Vonderheide RH, Thadhani R, Kuter DJ. Association of thrombocytopenia with the use of intra‐aortic balloon pumps. Am J Med. 1998;105:27–32. [DOI] [PubMed] [Google Scholar]
- 36. Roy SK, Howard EW, Panza JA, et al. Clinical implications of thrombocytopenia among patients undergoing intra‐aortic balloon pump counterpulsation in the coronary care unit. Clin Cardiol. 2010;33:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1. Assembly of prematch and postmatch study cohorts.
TableS1. Types of coagulopathy and their frequencies among all heart transplant recipients with coagulopathy
TableS2. Association of coagulopathy with length of stay and total hospital cost among heart transplant recipients before and after propensity matching


