ABSTRACT
Background
The impact of health insurance carrier and socioeconomic status (SES) on the adherence to appropriate use criteria (AUC) for radionuclide myocardial perfusion imaging (MPI) is unknown.
Hypothesis
Health insurance carrier's prior authorization and patient's SES impact adherence to AUC for MPI in a fee‐for‐service setting.
Methods
We conducted a prospective cohort study of 1511 consecutive patients who underwent outpatient MPI in a multi‐site, office‐based, fee‐for‐service setting. The patients were stratified according to the 2009 AUC into appropriate/uncertain appropriateness and inappropriate use groups. Insurance status was categorized as Medicare (does not require prior authorization) vs commercial (requires prior authorization). Socioeconomic status was determined by the median household income in the ZIP code of residence.
Results
The proportion of patients with Medicare was 33% vs 67% with commercial insurance. The rate of inappropriate use was higher among patients with commercial insurance vs Medicare (55% vs 24%; P < 0.001); this difference was not significant after adjusting for confounders known to impact AUC determination (odds ratio: 1.06, 95% confidence interval: 0.62‐1.82, P = 0.82). The mean annual household income in the residential areas of patients with inappropriate use as compared to those with appropriate/uncertain use was $72 000 ± 21 000 vs $68 000 ± 20 000, respectively (P < 0.001). After adjusting for covariates known to impact AUC determination, SES (top vs bottom quartile income area) was not independently predictive of inappropriate MPI use (odds ratio: 0.9, 95% confidence interval: 0.53‐1.52, P = 0.69).
Conclusions
Insurance carriers prior authorization and SES do not seem to play a significant role in determining physicians adherence to AUC for MPI.
Introduction
Until 2006, there had been an exponential increase in the use of myocardial perfusion imaging (MPI) with single‐photon emission computed tomography (SPECT).1, 2, 3 The expansion in MPI use led third‐party payers to implement prior authorization processes and drastic reimbursement cuts.1, 4 Professional cardiology and imaging societies addressed the escalation in use by developing appropriate use criteria (AUC) to guide physicians in optimal MPI use.4, 5, 6, 7 Although much of the increase in use was likely due to the added diagnostic and prognostic value of MPI,8, 9, 10 financial incentive and self‐referral practices may have contributed to this escalation in MPI utilization.1 Private insurance carriers enforced prior authorization requirements using radiology benefit managers (RBM) to deter MPI testing, whereas the Centers for Medicare & Medicaid Services (CMS) did not implement such measures. In other domains of medical care, factors such as socioeconomic status (SES) and health insurance carrier have been shown to impact access to care, clinical decision‐making, and the type of testing patients receive.11, 12, 13 We hypothesized that SES and prior authorization impact adherence to appropriate use of MPI in community‐based, fee‐for‐service, in‐office testing.
Methods
We investigated the study question in a cross‐sectional analysis of a prospective cohort of 1511 consecutive patients who had undergone outpatient SPECT‐MPI in 11 practices of 22 practitioners (2 cardiologists and 20 primary‐care physicians) in Chicago area between August 2007 and May 2010.14 The detailed methods used in the assembly of the cohort were published elsewhere.14 The MPI studies were performed in the referring physician's office. The revenue from the technical fees of testing was captured by the referring physicians in a fee‐for‐service model pursuant to the in‐office testing exemption of the Stark Law. All studies were performed after the initiation of the prior authorization process implemented by major commercial carriers.
Clinical Data
Detailed patient demographics, referral diagnosis, risk factors, and cardiovascular history were prospectively tabulated. The referring physicians' clinical notes (paper or electronic) preceding MPI were reviewed to determine symptoms and rationale for testing. Framingham 10‐year coronary heart disease (CHD) risk estimates were calculated.15 Chest pain syndromes were classified as typical angina, atypical angina, and nonanginal chest pain on the basis of location, exacerbation with exercise, and resolution with rest or nitroglycerin.16 If clinical notes did not describe the chest pain, it was considered nonanginal. Dyspnea was classified as “nonanginal chest pain.” Subsequently, the pretest probability of obstructive coronary artery disease (CAD) was determined according to Diamond and Forrester tables.16 These methods were used in a similar landmark study.17
Stress Myocardial Perfusion Imaging
Patients underwent 1‐day, rest/stress, technetium‐99 m sestamibi protocol conforming to published guidelines.18 One of 3 stress modalities was chosen as clinically appropriate: exercise Bruce protocol, standard 6‐minute adenosine infusion, or adenosine‐stress with low‐level exercise.19, 20 Electrocardiograms (ECG) were considered uninterpretable for stress‐induced ischemia if they depicted a left bundle branch block, paced ventricular rhythm, left ventricular hypertrophy with secondary ST‐segment abnormalities, Wolff‐Parkinson‐White pattern, or baseline ST‐segment depression ≥0.5 mm. All MPI studies were acquired using a dual‐head dedicated cardiac SPECT camera.
Appropriate Use Criteria
Computer‐based logic was used to assign each patient a specific indication and then categorize the studies as appropriate, uncertain, or inappropriate on the basis of the 2009 AUC.5, 14 To study the impact of insurance carrier and SES on inappropriate use, patients with appropriate and uncertain appropriateness scans were combined, thus stratifying the study cohort into 2 groups: Appropriate/Uncertain and Inappropriate. The detailed methods and results of AUC determination were published elsewhere.14
Insurance Carrier and Socioeconomic Status
From billing records, primary insurance carriers were determined and categorized as Medicare (no prior authorization) vs commercial (RBM's prior authorization required). Patients with Medicaid or missing insurance data were excluded. Socioeconomic status was determined using the median annual household income in the patient's ZIP code of residence as reported in 2010 U.S. Census (http://www.census.gov/2010census). Upper vs lower SES was defined on the basis of median annual household income being in the top vs bottom quartile of the study population. Patients with missing ZIP codes were excluded from the analysis. Income values were rounded to the closest $1000.
Statistical Analysis
We based our power calculations on a known 45.5% inappropriate use rate in this cohort,14 33% proportion of Medicare population, and median household income in the Chicago metropolitan area of $57 000. We determined that the sample size would provide >99% power to detect a 20% difference in inappropriate use rate based on insurance carrier and a 5% difference in the mean annual household income based on appropriateness using the χ2 and independent sample Student t tests, respectively, with α = 0.025.
The χ2 test was used to compare dichotomous variables, which were expressed as a number (%). The independent samples Student's t test was used to compare normally distributed continuous variables, which were expressed as mean ± SD. The Mann–Whitney test was used to compare skewed continuous variables, which were expressed as median (interquartile range [IQR]). Multivariate logistic regression analysis models were used to determine whether insurance carrier and SES (exposures) were independently predictive of inappropriate use (outcome), adjusting for confounders known to impact appropriate use based on the 2009 AUC;5 these included: age, sex, CAD risk factors (hypertension [HTN], diabetes mellitus [DM], tobacco use, family history of CAD, and dyslipidemia), known CAD (prior coronary revascularization, prior nonfatal myocardial infarction, or known coronary diameter stenosis ≥50%), inability to exercise, ischemic equivalent symptoms (chest pain or dyspnea), ECG interpretability, and provider specialty (cardiology vs primary care).5, 17, 21 We analyzed insurance and SES in separate logistic regression models due to potential collinearity between insurance carrier and SES and to avoid omitting patients with missing insurance data from the SES analysis. Because the presence of ischemic equivalence symptoms (chest pain or dyspnea) strongly favor appropriate use in the AUC algorithm, we performed multivariate sensitivity analyses in subsets of patients with and without such symptoms. To account for multiple testing for equally weighted aims (insurance carrier and SES), we considered a P value <0.025 to be statistically significant.
The SPSS version 18 software was used for all statistical analysis (SPSS Inc., Chicago, IL). The study was approved by the institutional review board of Rush University Medical Center (Chicago, IL).
Results
Insurance Carrier
Insurance data were available for 1359 (90%) patients; 448 (33%) were insured by Medicare and 909 (67%) had commercial insurance. The most notable insurance carriers are listed in Table 1. Two patients with Medicaid were excluded from the analysis. The baseline characteristics of patients with Medicare vs commercial insurance are compared in Table 2. Notably, patients with Medicare were older and had higher prevalence of known CAD and CAD risk factors, higher likelihood of obstructive CAD, and higher Framingham 10‐year CHD risk. Medicare patients were also more likely to be referred for testing by a cardiologist. Insurance data were missing in 152 (10%) subjects; however, these patients had similar AUC categories distribution and overall clinical profile as those with insurance data (see Supporting Information, Table 1, in the online version of this article).
Table 1.
Insurance Carriers (N = 1359)
| Insurance Data Available | N (%) |
|---|---|
| Public | 450 (33.1) |
| Medicare | 448 |
| Medicaid | 2 |
| Commercial | 909 (66.9) |
| BCBS | 577 |
| United HealthCare | 87 |
| Aetna | 48 |
| Cigna | 47 |
| Humana | 23 |
| Great West | 10 |
| Unicare | 8 |
| Miscellaneous | 109 |
Abbreviations: BCBS, Blue Cross and Blue Shield.
Insurance data were missing in 152 subjects.
Table 2.
Baseline Characteristics Based on Insurance Carrier
| Medicare, N = 448 | Commercial, N = 909 | P Value | |
|---|---|---|---|
| Age, y | 72 ± 8 | 53 ± 10 | <0.001 |
| Male sex | 234 (52) | 563 (59) | 0.02 |
| Chest pain or dyspnea | 243 (54) | 518 (57) | 0.34 |
| HTN | 332 (74) | 430 (47) | <0.001 |
| DM | 141 (31) | 170 (19) | <0.001 |
| Dyslipidemia | 248 (55) | 381 (42) | <0.001 |
| Tobacco use | 50 (11) | 118 (13) | 0.34 |
| Family history of CAD | 139 (31) | 342 (38) | 0.02 |
| Framingham 10‐year CHD risk, % | 19 ± 12 | 10 ± 8 | <0.001 |
| Likelihood of obstructive CAD, %a | 23 ± 9 | 16 ± 13 | <0.001 |
| Pharmacologic stress | 200 (45) | 124 (14) | <0.001 |
| Uninterpretable ECGb | 11 (2.5) | 1 (0.1) | <0.001 |
| BMI, kg/m2 | 29 ± 5 | 30 ± 6 | 0.002 |
| Known CAD | 131 (29) | 114 (13) | <0.001 |
| Physician specialty | <0.001 | ||
| Cardiology | 63 (14) | 47 (5) | |
| Primary care | 385 (86) | 862 (95) | |
| AUC classification | <0.001 | ||
| Appropriate | 318 (71) | 389 (43) | |
| Uncertain | 24 (5) | 18 (2) | |
| Inappropriate | 107 (24) | 502 (55) |
Abbreviations: AUC, appropriate use criteria; BMI, body mass index; CAD, coronary artery disease; CHD, coronary heart disease; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; LBBB, left bundle branch block; SD, standard deviation.
Continuous variables are presented as mean ± SD. Dichotomous variables are presented as n (%). There were no missing baseline characteristics data.
Based on Diamond and Forrester tables in patients with chest pain or dyspnea.14
Uninterpretable ECG for myocardial ischemia is defined as LBBB, paced ventricular rhythm, left ventricular hypertrophy with secondary ST‐segment abnormalities, Wolff‐Parkinson‐White pattern, or baseline ST‐segment depression ≥0.5 mm.
As shown in section A of the Figure 1, patients with commercial insurance were more likely to have an inappropriate MPI referral than were those with Medicare (55% vs 24%; P < 0.001). After adjusting for covariates known to impact AUC determination, a multivariate logistic regression analysis (Table 3, model 1) determined that insurance carrier is not independently predictive of inappropriate use (odds ratio [OR]: 1.06, 95% confidence interval [CI]: 0.62‐1.81, P = 0.84). Covariates of age, male sex, known CAD, HTN, DM, dyslipidemia, and inability to undergo exercise stress were independent “protective” predictors of inappropriate MPI use. Provider specialty was not independently predictive of inappropriate use.
Figure 1.
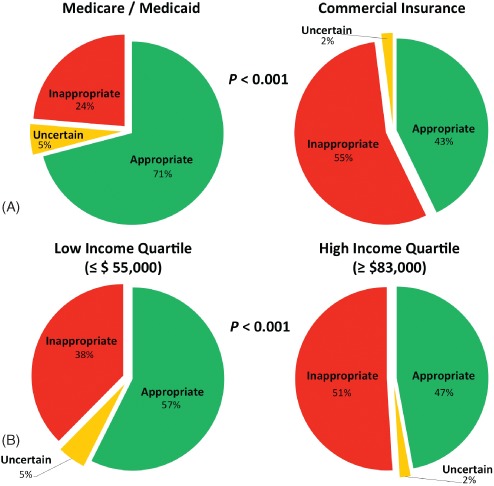
Adherence to appropriate use criteria according to insurance carrier and socioeconomic status.
Table 3.
Multivariate Analyses
| ORa | 95% CI | P Value | |
|---|---|---|---|
| Model 1: insurance carrier | |||
| Commercial insurance | 1.06 | 0.62‐1.82 | 0.82 |
| Age, y | 0.87 | 0.84‐0.89 | <0.001 |
| Male sex | 0.03 | 0.02‐0.05 | <0.001 |
| HTN | 0.59 | 0.39‐0.87 | 0.008 |
| DM | 0.31 | 0.19‐0.50 | <0.001 |
| Dyslipidemia | 0.37 | 0.25‐0.55 | <0.001 |
| Tobacco use | 0.70 | 0.41‐1.21 | 0.20 |
| Family history of CAD | 0.81 | 0.55‐1.18 | 0.27 |
| Known CAD | 0.24 | 0.13‐0.42 | <0.001 |
| Inability to exercise | 0.35 | 0.21‐0.59 | <0.001 |
| Chest pain or dyspnea | 0.004 | 0.002‐0.007 | <0.001 |
| Uninterpretable ECGb | 7.55 | 0.50‐114.84 | 0.15 |
| PCP vs cardiologist | 1.30 | 0.64‐2.64 | 0.47 |
| Model 2: SES | |||
| Upper vs lower SESc | 0.90 | 0.53‐1.52 | 0.69 |
| Age, y | 0.86 | 0.84‐0.89 | <0.001 |
| Male sex | 0.03 | 0.01‐0.05 | <0.001 |
| HTN | 0.67 | 0.39‐1.14 | 0.14 |
| DM | 0.16 | 0.08‐0.31 | <0.001 |
| Dyslipidemia | 0.51 | 0.30‐0.87 | 0.01 |
| Tobacco use | 0.74 | 0.34‐1.61 | 0.45 |
| Family history of CAD | 1.21 | 0.72‐2.02 | 0.48 |
| Known CAD | 0.32 | 0.15‐0.67 | 0.003 |
| Pharmacologic stress | 0.27 | 0.13‐0.56 | <0.001 |
| Chest pain or dyspnea | 0.005 | 0.002‐0.01 | <0.001 |
| Interpretable ECGb | 0.95 | 0.08‐10.56 | 0.97 |
| PCP vs cardiologist | 1.64 | 0.66‐4.08 | 0.29 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; LBBB, left bundle branch block; OR, odds ratio; PCP, primary‐care physician; SES, socioeconomic status.
ORs of inappropriate vs appropriate or uncertain appropriateness use.
Uninterpretable ECG for myocardial ischemia is defined as LBBB, paced ventricular rhythm, left ventricular hypertrophy with secondary ST‐segment abnormalities, Wolff‐Parkinson‐White pattern, or baseline ST‐segment depression ≥0.5 mm.
Upper vs lower socioeconomic status is based on the top (≥$83 000) vs bottom (≤$55 000) quartile of median annual household income for the ZIP code of residence.
Subsequently, we performed sensitivity analyses stratifying the cohort based on symptom status (chest pain or dyspnea). Multivariate logistic regression models demonstrated that insurance carrier was not independently predictive of inappropriate use among patients with chest pain or dyspnea (P = 0.94) and those without these symptoms (P = 0.27), after adjusting for all other covariates.
Socioeconomic Status
Annual median household income data for area of residence were available for 1485 (99%) patients who resided in 214 unique ZIP codes. The median household income for the entire cohort was $65 000 (IQR, $55 000–$83 000; min, $28 000; max, $155 000). The mean annual household income in the residential areas of patients with inappropriate use as compared with those with appropriate/uncertain use was $72 000 ± 21 000 vs $68 000 ± 20 000, respectively (Mann–Whitney test P < 0.001). Adjusting for factors known to impact AUC determination, a multivariate logistic regression analysis (see Supporting Information, Table 2, in the online version of this article) demonstrated that household income as a continuous variable was not independently predictive of inappropriate MPI use (OR: 1.0 per $10 000 increment in annual income, 95% CI: 0.92‐1.09, P = 0.99).
To distinctly separate patients on the basis of socioeconomic status, lower vs upper status was determined on the basis of residing in a ZIP code with median annual household income in the bottom (≤$55 000) vs top (≥$83 000) quartile of the study population. As shown in Table 4, patients in lower SES were older, more obese, had higher prevalence of known CAD and CAD risk factors, and had higher likelihood of obstructive CAD and Framingham 10‐year CHD risk. They were also more likely to be referred for testing by a cardiologist. As shown in section B of the Figure 1, patients in lower SES were less likely to undergo inappropriate MPI testing (38% vs 51%; P < 0.001). Adjusting for factors known to impact AUC determination, a multivariate logistic regression analysis (Table 3, model 2) demonstrated that upper SES was not independently predictive of inappropriate MPI use (OR: 0.9, 95% CI: 0.53‐1.52, P = 0.69).
Table 4.
Baseline Characteristics Based on SES
| Characteristic | Lower SES, n = 383a | Upper SES, n = 359a | P Value |
|---|---|---|---|
| Age, y | 60 ± 13 | 57 ± 12 | 0.002 |
| Male sex | 222 (58) | 204 (57) | 0.75 |
| Chest pain or dyspnea | 226 (59) | 191 (53) | 0.11 |
| HTN | 245 (64) | 167 (47) | <0.001 |
| DM | 138 (36) | 46 (13) | <0.001 |
| Dyslipidemia | 203 (53) | 135 (38) | <0.001 |
| Tobacco use | 50 (13) | 33 (9) | 0.10 |
| Family history of CAD | 107 (28) | 167 (47) | <0.001 |
| Framingham 10‐year CHD risk, % | 15 ± 11 | 11 ± 10 | <0.001 |
| Likelihood of obstructive CAD, %b | 19 ± 12 | 17 ± 9 | 0.02 |
| Pharmacologic stress | 121 (32) | 55 (15) | <0.001 |
| Uninterpretable ECGc | 7 (12) | 6 (1.7) | 0.87 |
| BMI, kg/m2 | 31 ± 6 | 29 ± 6 | <0.001 |
| Known CAD | 86 (22) | 50 (14) | 0.003 |
| Physician specialty | <0.001 | ||
| Cardiology | 57 (15) | 11 (3) | |
| Primary care | 326 (85) | 348 (97) | |
| AUC classification | <0.001 | ||
| Appropriate | 220 (57) | 169 (47) | |
| Uncertain | 19 (5) | 7 (2) | |
| Inappropriate | 144 (38) | 183 (51) |
Abbreviations: AUC, appropriate use criteria; BMI, body mass index; CAD, coronary artery disease; CHD, coronary heart disease; DM, diabetes mellitus; ECG, electrocardiogram; HTN, hypertension; LBBB, left bundle branch block; SD, standard deviation.
Continuous variables are presented as mean ± SD. Dichotomous variables are presented as n (%). There were no missing baseline characteristics data.
Upper vs lower socioeconomic status is based on the top (≥$83 000) vs bottom (≤$55 000) quartile of median annual household income for the ZIP code of residence.
Based on Diamond and Forrester tables in patients with chest pain or dyspnea.14
Uninterpretable ECG for myocardial ischemia is defined as LBBB, paced ventricular rhythm, left ventricular hypertrophy with secondary ST‐segment abnormalities, Wolff‐Parkinson‐White pattern, or baseline ST‐segment depression ≥0.5 mm.
Subsequently, we performed sensitivity analyses stratifying the cohort based on the symptom status. Multivariate logistic regression models demonstrated that SES (upper vs bottom income quartile) was not independently predictive of inappropriate use among patients with chest pain or dyspnea (P = 1.0) and those without these symptoms (P = 0.94), after adjusting for all other confounders.
Discussion
This is the first investigation to study the impact of insurance carrier and SES on the adherence to AUC for SPECT‐MPI. The study is particularly unique in the sense that it investigated patients tested in a community, office‐based, fee‐for‐service setting. Univariate analyses suggested that patients with commercial health insurance or higher household income were more likely to undergo inappropriate MPI than those with Medicare or lower SES, respectively. However, differences in inappropriate MPI use, on the basis of insurance carrier or SES, dissipated once we adjusted for clinical covariates known to impact appropriate use determination. We also demonstrated that physician specialty (cardiology vs primary care) was not independently predictive of adherence to AUC, as differences observed in univariate analysis were shown to be insignificant after adjusting for clinical covariates.
These findings are significant on multiple levels. First, all MPI studies analyzed in this investigation were performed after major commercial carriers have implemented a RBM prior authorization process, whereas CMS did not impose such measures. Our data suggest that prior authorization measures used were not effective in limiting inappropriate testing, thus questioning the value of this frustrating and time‐consuming process. However, the study does not exclude the potential for even higher inappropriate use rates if prior authorization was not in place. Second, our study indicates that higher reimbursement for SPECT‐MPI by commercial insurance carriers at the time of the study had no impact on appropriate use rates in this office‐based, fee‐for‐service setting. Our data are consistent with others from the Veterans Administration hospitals showing that lack of financial gains did not prevent high inappropriate use rates.22, 23 Third, it has been shown that medical care and access to testing tightly correlates with education level, which is closely tied with income.11, 24 Our study indicates that SES associated with area of residence did not impact patients' selection for testing.
Radiology benefit managers are widely used by private third‐party payers with the primary objective of reducing the utilization of imaging procedures through a prior authorization process that is often based on indiscriminant or poorly thought‐out criteria.1 Morley et al found that prior authorization is a measurable financial burden on physicians.25 Lee et al demonstrated that 28% of the savings incurred in reducing medical imaging volume is shifted to the medical practices.26 Robinson et al concluded that eliminating denial provisions in prior authorization for advanced diagnostic imaging does not result in increased utilization of such imaging.27 Willens et al demonstrated that there is limited agreement between prior authorization determination and AUC for transthoracic echocardiography.28 The aforementioned studies and our investigation suggest that prior authorization may not be effective in reducing the societal cost of advanced imaging caused by inappropriate testing. On the other hand, Saifi et al demonstrated that by implementing self‐directed decision‐support and quality‐improvement software, physicians may be able to dramatically decrease the proportion of inappropriate MPI use.
Our findings highlight the importance of evaluating inappropriate use rates in the context of the patient population being tested. In this study, Medicare patients were more likely to receive appropriate MPI testing simply because they were older and tended to have multiple comorbidities and mobility problems, thus favoring “appropriate” classification by the AUC.5 Once we adjusted for important determinants of appropriateness, disparity in the odds of inappropriate testing dissipated. A similar phenomenon was observed in the analysis of SES, as patients at lower SES, particularly minorities, are generally at greater risk for obesity, DM, and HTN, which elevate their risk,13 thus favoring “appropriate” classification by the AUC.5
Multiple investigators have reported higher inappropriate use rates by primary care physicians than cardiologists.8, 14, 17, 21, 29 In fact, our group had previously reported from this very cohort an inappropriate use rate of 47% by primary care physicians vs 28% by cardiologists (P < 0.001).14 In this report, we demonstrated, by adjusting for significant clinical confounders, the lack of significant specialty‐based differences in inappropriate use. Thus, previously reported specialty‐based differences simply reflect dissimilarity in patient population cared for by respective providers, rather than a vast gap in expertise.30
This cohort has a notably higher inappropriate use rate compared with published studies.31 However, unlike this community‐based study, most published data are derived from university‐based practices with different expertise, population risk profile, and referral biases. Furthermore, publication bias may have eliminated reports from practices with high inappropriate use rate. Therefore, this study was not intended to be a report on current appropriate use rates, but rather to investigate the impact of insurance and socioeconomic factors on appropriate use.
The study provides no knowledge on appropriateness of testing among patients covered by health maintenance organizations, as these carriers do not permit any office‐based testing. No insights can be gained on appropriate use among uninsured or Medicaid patients, who were scarce in our cohort. We speculate that Medicaid coverage was not accepted by the study providers or these patients were not referred for in‐office testing due to below‐cost reimbursement for MPI by the Illinois Medicaid program. Therefore, Medicaid patients were excluded from the analysis because other considerations have played a role in their referral for testing.
Study Limitations
The study is minimally limited by a lack of insurance data in 10% of the study population; however, patients with missing insurance data have a clinical profile similar to the rest of the cohort, thus making them unlikely to have changed the overall results of the study. Another limitation is using median annual household income for residential ZIP codes rather than actual income data; however, the large sample size adjusts for this inaccuracy by averaging the error to more precise mean values. Furthermore, analyzing top vs bottom ZIP code income quartiles effectively separated the patients based on their socioeconomic status. Finally, the majority of patients resided in middle‐class or affluent neighborhoods; therefore, little knowledge could be gained regarding poor patients, who may have greater difficulty affording insurance copayments and deductibles.
The study findings are generalizable to the majority of SPECT‐MPI studies performed in the United States in similar clinical settings (eg, for‐profit, community, or office‐based testing). However, they may not be applicable to other clinical establishments, such as academic medical centers or accountable care organizations. Furthermore, the results are generalizable to the majority of patients in the United States, but not to those not represented in this investigation, such as uninsured or Medicaid patients.
Conclusion
Implementation of a prior authorization process by insurance carriers does not seem to significantly impact appropriate selection for SPECT‐MPI. Socioeconomic status does not seem to significantly influence physicians' adherence to AUC for SPECT‐MPI.
Supporting information
TableS1. Baseline Characteristics of Patients with vs. without Insurance Data
TableS2. Multivariate Analysis: Household Income as continuous variable
An abstract from the study was presented in poster format at the 2014 American College of Cardiology Annual Scientific Session, March 31, 2014, Washington, DC.
Dr. Rami Doukky had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Author contributions as follows: Study concept, design, obtaining funding, supervision, statistical analysis, and interpretation of data: Rami Doukky, MD. Administrative, technical, or material support: Nathan Frogge, MBA; Kathleen Hayes‐Brown, MD; Noreen T. Nazir, MD; Fareed M. Collado, MD. Data management and processing: Nathan Frogge, MBA; Kathleen Hayes, MD. Manuscript drafting: Rami Doukky, MD; Noreen T. Nazir, MD. Manuscript editing: Kathleen Hayes‐Brown, MD; Nathan Frogge, MBA. Critical review: Kim A. Williams, MD. Critical revision and final approval of the manuscript: Rami Doukky, MD.
The study was funded by an investigator‐initiated grant from Astellas Pharma US. Grant fiduciary: Rush University Medical Center; principal investigator: Rami Doukky, MD. The funding source had no input in the study design, execution, data analysis and interpretation, or manuscript preparation and approval. Rami Doukky has served on the advisory board of Astellas Pharma and received investigator‐initiated research‐grant support from Astellas Pharma.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Hendel RC. Utilization management of cardiovascular imaging pre‐certification and appropriateness. JACC Cardiovasc Imaging. 2008;1:241–248. [DOI] [PubMed] [Google Scholar]
- 2. Arlington Medical Resources Inc . Myocardial Perfusion Monthly Monitor, November 2012. [Google Scholar]
- 3. AMR/Arlington Medical Resources Inc . Myocardial Study Market Guide, January‐June 2012.
- 4. Hendel RC. The revolution and evolution of appropriateness in cardiac imaging. J Nucl Cardiol. 2008;15:494–496. [DOI] [PubMed] [Google Scholar]
- 5. Hendel RC, Berman DS, Di Carli MF et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Endorsed by the American College of Emergency Physicians. J Am Coll Cardiol. 2009;53:2201–2229. [DOI] [PubMed] [Google Scholar]
- 6. Singh M, Babayan Z, Harjai KJ, et al. Utilization patterns of single‐photon emission cardiac tomography myocardial perfusion imaging studies in a rural tertiary care setting. Clin Cardiol. 2014;37:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gertz ZM, O'Donnell W, Raina A, et al. Application of appropriate use criteria to cardiac stress testing in the hospital setting: limitations of the criteria and areas for improved practice. Clin Cardiol. 2014; doi: 10.1002/clc.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doukky R, Frogge N, Balakrishnan G, et al. The prognostic value of cardiac SPECT performed at the primary care physician's office. J Nucl Cardiol. 2013;20:519–528. [DOI] [PubMed] [Google Scholar]
- 9. Shaw LJ, Hachamovitch R, Berman DS, et al; Economics of Noninvasive Diagnosis (END) Multicenter Study Group . The economic consequences of available diagnostic and prognostic strategies for the evaluation of stable angina patients: an observational assessment of the value of precatheterization ischemia. J Am Coll Cardiol. 1999;33:661–669. [DOI] [PubMed] [Google Scholar]
- 10. Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction [published correction appears in Circulation. 1998;98:190]. Circulation. 1998;97:535–543. [DOI] [PubMed] [Google Scholar]
- 11. Bernheim SM, Ross JS, Krumholz HM, et al. Influence of patients' socioeconomic status on clinical management decisions: a qualitative study. Ann Fam Med. 2008;6:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenneker MB, Weissman JS, Epstein AM. The association of payer with utilization of cardiac procedures in Massachusetts. JAMA. 1990;264:1255–1260. [PubMed] [Google Scholar]
- 13. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88(4 part 1):1973–1998. [DOI] [PubMed] [Google Scholar]
- 14. Doukky R, Hayes K, Frogge N, et al. The impact of appropriate use on the prognostic value of SPECT myocardial perfusion imaging. Circulation. 2013;128:1634–1643. [DOI] [PubMed] [Google Scholar]
- 15. Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 16. Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary‐artery disease. N Engl J Med. 1979;300:1350–1358. [DOI] [PubMed] [Google Scholar]
- 17. Hendel RC, Cerqueira M, Douglas PS, et al. A multicenter assessment of the use of single‐photon emission computed tomography myocardial perfusion imaging with appropriateness criteria. J Am Coll Cardiol. 2010;55:156–162. [DOI] [PubMed] [Google Scholar]
- 18. Henzlova MJ, Cerqueira MD, Mahmarian JJ, et al. Stress protocols and tracers. J Nucl Cardiol. 2006;13:e80–e90. [DOI] [PubMed] [Google Scholar]
- 19. Doukky R. Pharmacologic stress testing in myocardial perfusion imaging: technical applications In: Mann A, Heller GV, Hendel RC, eds. Nuclear Cardiology: Technical Applications. New York, NY: McGraw‐Hill; 2007:107–124. [Google Scholar]
- 20. Elliott MD, Holly TA, Leonard SM, et al. Impact of an abbreviated adenosine protocol incorporating adjunctive treadmill exercise on adverse effects and image quality in patients undergoing stress myocardial perfusion imaging. J Nucl Cardiol. 2000;7:584–589. [DOI] [PubMed] [Google Scholar]
- 21. Carryer DJ, Askew JW, Hodge D, et al. The impact of ordering provider specialty on appropriateness classification. J Nucl Cardiol. 2012;19:285–290. [DOI] [PubMed] [Google Scholar]
- 22. Nelson KH, Willens HJ, Hendel RC. Utilization of radionuclide myocardial perfusion imaging in two health care systems: assessment with the 2009 ACCF/ASNC/AHA appropriateness use criteria. J Nucl Cardiol. 2012;19:37–42. [DOI] [PubMed] [Google Scholar]
- 23. Winchester DE, Meral R, Ryals S, et al. Appropriate use of myocardial perfusion imaging in a veteran population: profit motives and professional liability concerns. JAMA Intern Med. 2013;173:1381–1383. [DOI] [PubMed] [Google Scholar]
- 24. Alter DA, Naylor CD, Austin P, et al. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–1367. [DOI] [PubMed] [Google Scholar]
- 25. Morley CP, Badolato DJ, Hickner J, et al. The impact of prior authorization requirements on primary care physicians' offices: report of two parallel network studies. J Am Board Fam Med. 2013;26:93–95. [DOI] [PubMed] [Google Scholar]
- 26. Lee DW, Rawson JV, Wade SW. Radiology benefit managers: cost saving or cost shifting? J Am Coll Radiol. 2011;8:393–401. [DOI] [PubMed] [Google Scholar]
- 27. Robinson JD, Hippe DS, Hiatt MD. The effect of a no‐denial policy on imaging utilization. J Am Coll Radiol. 2013;10:501–506. [DOI] [PubMed] [Google Scholar]
- 28. Willens HJ, Hendel RC, Inhaber FR, et al. Appropriateness use criteria for transthoracic echocardiography: relationship with radiology benefit managers preauthorization determination and comparison of the new 2010 criteria to the original 2007 criteria. Am Heart J. 2011;162:772–779. [DOI] [PubMed] [Google Scholar]
- 29. Gupta A, Tsiaras SV, Dunsiger SI, et al. Gender disparity and the appropriateness of myocardial perfusion imaging. J Nucl Cardiol. 2011;18:588–594. [DOI] [PubMed] [Google Scholar]
- 30. Doukky R, Hayes K, Frogge N. Are cardiologists truly better at appropriately selecting patients for stress myocardial perfusion imaging? Int J Cardiol. 2014;176:285–286. [DOI] [PubMed] [Google Scholar]
- 31. Gibbons RJ, Miller TD. Single‐photon emission computed tomography appropriateness: does it matter for patient outcomes? Circulation. 2013;128:1595–1597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1. Baseline Characteristics of Patients with vs. without Insurance Data
TableS2. Multivariate Analysis: Household Income as continuous variable


