ABSTRACT
Background
In patients with acute decompensated heart failure (ADHF), both natriuretic peptides and renal impairment predict adverse outcomes. Our aim was to evaluate the complementary prognosis role of N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and the newly developed Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equations based on cystatin C (CysC) for glomerular filtration rate (GFR) estimation in ADHF patients.
Hypothesis
Renal impairment assessed by CysC‐based CKD‐EPI equations and natriuretic peptides have complementary prognostic value in ADHF patients.
Methods
The study included 613 consecutive patients presenting with ADHF. At admission, plasma levels of NT‐proBNP and CysC were determined. The GFR was estimated using CysC‐based CKD‐EPI equations. The primary endpoint was death from any cause and heart failure readmission.
Results
During the median follow‐up of 365 days (interquartile range, 227–441 days), 323 patients (0.65 %patient‐year) died or were readmitted for heart failure. After multivariate adjustment, estimated GFR <60 mL/min/1.73 m2 and NT‐proBNP >3251 pg/mL were independent predictors of adverse outcomes (P < 0.01). The combination of GFR <60 mL/min/1.73 m2 and NT‐proBNP >3251 pg/mL was associated with the highest risk of adverse outcomes. Furthermore, reclassification analyses demonstrated that use of both NT‐proBNP and CysC‐based CKD‐EPI equations resulted in improving the accuracy for adverse outcomes prediction.
Conclusions
In patients with ADHF, the combination of NT‐proBNP with estimated GFR using CysC‐based CKD‐EPI equations better predicts outcomes than either parameter alone and adds valuable complementary prognosis information to other established risk factors.
Introduction
It is well known that chronic kidney disease (CKD) is common among patients with acute decompensated heart failure (ADHF), and it confers a higher risk for adverse outcomes in these patients1, 2; indeed, important predictive models of mortality in ADHF patients have included CKD alongside other traditional variables predictive of adverse outcome.3, 4 The Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) has proposed 3 new equations to estimate glomerular filtration rate (GFR).5, 6, 7 These equations consider serum creatinine (SCr) concentration, age, sex, and race of the patient (CKD‐EPISCr equation), add cystatin C (CysC) levels (CKD‐EPICysC equation), or combine all of these with SCr (CKD‐EPISCr‐CysC equation). Notably, we have recently demonstrated CysC‐based CKD‐EPI equations to be superior to the Modification of Diet in Renal Disease (MDRD) equation for predicting adverse outcomes in ADHF patients8; however, no data exist on the predictive power of CysC‐based CKD‐EPI equations in combination with natriuretic peptides in this clinical scenario.
Natriuretic peptides are cardiac peptides secreted by the myocardium in response to myocardial stretch, such as that which occurs in the setting of volume overload9; results from natriuretic‐peptide testing are useful for the diagnostic evaluation of ADHF.10, 11 Importantly, natriuretic‐peptide testing not only provides important diagnostic information, but it is also useful for prognosis in the setting of ADHF.12, 13, 14 Given the importance of both CysC‐based CKD‐EPI equations and natriuretic peptides for predicting risk, it is reasonable to expect that the combination of both parameters would provide better clinical risk prediction in this setting, but data are lacking in this regard. Accordingly, in the present study we aim to assess whether the CysC‐based CKD‐EPI equations provide additional prognosis information to admission N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels.
Methods
The local ethics committees approved the study, and informed consent was obtained from each patient at inclusion. The study population consisted of 613 subjects admitted with an established final diagnosis of ADHF (diagnosed clinically using current guidelines)15 from 3 tertiary Spanish hospitals. Blood samples were collected for all patients within 48 hours from admission, processed, and stored at −80 °C until the study analysis. A time window of 48 hours was considered appropriate because hemodynamic status would still reasonably reflect decompensation. Baseline clinical characteristics and hospital events were prospectively recorded. Echocardiography was also performed on all patients before hospital discharge. Left ventricular ejection fraction (LVEF) was measured using the Simpson biplane method. All patients received standard management, as recommended by contemporary guidelines.15 During the entire hospitalization period, clinical‐management decisions about each patient were made by the cardiologist responsible, who was unaware of the patient's CysC concentrations.
We calculated GFR using the CysC‐based CKD‐EPI equations.5, 6, 7 Determination of CysC levels was performed using a BN ProSpec analyzer (Dade Behring GmbH, Liederbach, Germany). The intra‐assay and interassay coefficients of variation for CysC were 2.5% and 2.0%, respectively. NT‐proBNP was measured by electrochemiluminescence immunoassay using a Modular Analytics E170 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The intra‐assay coefficient of variation for NT‐proBNP was 1.8% for 221 pg/mL and 3.1% for 4250 pg/mL.
All patients were clinically followed during a median of 365 days. The primary clinical endpoint for this analysis was defined as the combination of death from any cause and/or heart failure readmission. Death was ascertained from available medical records and death certificates. If hospital records were ambiguous or unavailable, National Death Records were consulted. In patients requiring hospitalization, medical records were carefully reviewed to further characterize the cause of hospitalization.
Continuous variables were tested for a normal distribution by the Kolmogorov‐Smirnov test. Normally distributed data are presented as the mean ± SD and non‐normally distributed data as the median (interquartile range [IQR]). Categorical variables are expressed as percentages. Categorized analyses were performed according to the presence of adverse clinical events during the follow‐up. Differences in baseline characteristics were compared using the Student t test or the Mann–Whitney U test for continuous variables and the χ2 test for categorical variables.
Univariable Cox regression analysis was used for studying the association between adverse clinical events and each CysC‐based CKP‐EPI equations and NT‐proBNP. All other clinical and biochemical characteristics (described in Table 1) were also evaluated in the univariable Cox regression analysis. The independent information retained by CysC‐based CKP‐EPI equations and NT‐proBNP on the 2 endpoints of death from any cause and heart failure readmission and on the combined endpoint was evaluated in multivariable Cox regression analyses adjusted by covariates showing P values <0.10 in univariable analyses, as well as other factors known to be associated with worse outcomes in this clinical scenario: age, sex, heart rate, New York Heart Association function class, history of diabetes mellitus, prior heart failure, chronic obstructive pulmonary disease, LVEF, sodium, hemoglobin, and treatment with β‐blockers. Log‐cumulative hazard plots, time‐dependent covariates, and Schoenfeld residuals were used to evaluate adherence of the Cox proportional hazard assumptions. Interaction between CysC‐based CKP‐EPI equations and NT‐proBNP was assessed using interaction terms in Cox regression models.
Table 1.
Study Population Clinical Characteristics and in Accordance to the Occurrence of the Combined Endpoint
| Variables | Whole Population (N = 613) | Events (n = 323) | No Events (n = 290) | P Value |
|---|---|---|---|---|
| Age, y | 75 ± 10 | 76 ± 9 | 74 ± 10 | <0.001 |
| Male sex | 284 (46) | 157 (49) | 127 (56) | 0.23 |
| SBP, mm Hg | 140 (126–170) | 140 (120–170) | 142 (130–170) | 0.14 |
| Heart rate, bpm | 90 (75–110) | 90 (75–108) | 90 (75–120) | 0.66 |
| CHF | 374 (61) | 235 (73) | 139 (48) | <0.001 |
| LVEF, % | 52 (37–60) | 50 (35–60) | 54 (39–60) | 0.21 |
| LVEF >50% | 430 (70) | 223 (69) | 207 (71) | 0.54 |
| NYHA class III–IV | 276 (45) | 173 (54) | 103 (36) | <0.001 |
| Ischemic cause of HF | 212 (35) | 118 (37) | 94 (32) | 0.46 |
| DM | 312 (49) | 184 (57) | 128 (44) | 0.002 |
| Hypertension | 499 (81) | 265 (82) | 234 (81) | 0.67 |
| Hyperlipidemia | 255 (42) | 128 (40) | 127 (44) | 0.45 |
| PAD | 62 (10) | 39 (12) | 23 (8) | 0.09 |
| AF/flutter | 359 (59) | 201 (62) | 158 (55) | 0.05 |
| Previous stroke | 91 (15) | 40 (12) | 51 (17) | 0.50 |
| COPD | 155 (25) | 92 (29) | 63 (22) | 0.06 |
| Treatment at discharge | ||||
| β‐Blocker | 351 (57) | 169 (52) | 182 (63) | 0.03 |
| ACEI/ARB | 459 (75) | 245 (76) | 214 (74) | 0.64 |
| Antialdosteronic | 204 (33) | 115 (36) | 89 (30.7) | 0.28 |
| Statin | 276 (45) | 148 (46) | 128 (44) | 0.81 |
| Loop diuretic | 530 (86) | 284 (88) | 246 (85) | 0.37 |
| Laboratory parameters | ||||
| Hg (g/dL) | 12.2 ± 2.1 | 12.1 ± 2.1 | 12.8 ± 2.3 | <0.001 |
| SCr (mg/dL) | 1.12 (0.84–1.45) | 1.20 (0.90–1.66) | 1.12 (0.80–1.30) | <0.001 |
| CysC (mg/L) | 1.33 (1.04–1.75) | 1.51 (1.12–1.98) | 1.32 (0.95–1.51) | <0.001 |
| BUN, mg/dL | 51 (35–71) | 59 (41–84) | 44 (32–58) | <0.001 |
| CKD‐EPI Scr‐CysC, mL/min/1.73 m2 | 50 (34–69) | 42 (29–62) | 58 (42–76) | <0.001 |
| CKD‐EPI CysC, mL/min/1.73 m2 | 47 (33–68) | 40 (28–59) | 58 (39–75) | <0.001 |
| Sodium, mEq/L | 138 (135–141) | 138 (135–141) | 139 (136–142) | 0.03 |
| NT‐proBNP, pg/mL | 3251 (1555–6799) | 3846 (1739–7961) | 2765 (1355–5645) | <0.001 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; CHF, chronic heart failure; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; CysC, cystatin C; DM, diabetes mellitus; HF, heart failure; Hg, hemoglobin; IQR, interquartile range; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease; SBP, systolic blood pressure; SCr, serum creatinine.
Data are expressed as mean ± SD, median (IQR), or n (%).
To test the hypothesis that simultaneous assessment of renal function and natriuretic peptides would improve risk stratification, CysC‐based CKD‐EPI equations and NT‐proBNP levels were added to a model containing clinical risk factors. Both CysC‐based CKD‐EPI equations and NT‐proBNP were analyzed as quantitative and categorical variables. For categorical analyses, patients were dichotomized to above/below median NT‐proBNP levels and above/below 60 mL/min/1.73 m2. Improvement in predictive accuracy was evaluated by calculating the net reclassification improvement (NRI) and integrated discrimination improvement (IDI), as described by Pencina et al16 and using the logistic regression method. The cumulative incidence of death from any cause or heart failure readmission was estimated according to the Kaplan‐Meier method, and the log‐rank statistic was used for comparisons. All P values <0.05 were accepted as statistically significant. Statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, IL).
Results
The study population consisted of 613 hospitalized patients with ADHF. The patients' baseline characteristics are summarized in Table 1. The median GRF was 50 mL/min/1.73 m2 (IQR, 34–69 mL/min/1.73 m2) and 47 mL/min/1.73 m2 (IQR, 33–68 mL/min/1.73 m2) using the CKD‐EPISCr‐CysC and CKD‐EPICysC equations, respectively. The median NT‐proBNP level was 3251 pg/mL (IQR, 1555–6799 pg/mL).
Over a median follow‐up of 365 days (IQR, 227–441 days), a total of 323 patients (0.65 %patient‐year) suffered adverse clinical events: 153 patients (0.31 %patient‐year) died and 247 patients (0.50 %patient‐year) were readmitted to hospital owing to heart failure decompensation. As shown in Table 1, subjects suffering events were more likely to be older and more likely to have diabetes mellitus, previous heart failure, and poorer functional status. Moreover, those patients who experienced adverse events had worse renal function parameters, lower hemoglobin and sodium concentrations, and higher levels of NT‐proBNP. They were also less frequently on β‐blockers at discharge.
Table 2 details the univariable and multivariable Cox regression analyses for adverse clinical events. After multivariate adjustment, CysC‐based CKD‐EPI equations and NT‐proBNP levels (as quantitative and categorical variables) were independent predictors of adverse outcomes. Similar results were obtained when each endpoint was analyzed separately (see Supporting Table 1 in the online version of this article). A significant interaction between CysC‐based CKD‐EPI equations and NT‐proBNP levels was observed regarding the risk of all clinical events (all P values <0.05). In addition, when GFR equations were entered in a multivariate adjustment as categorical variables based on the standard 4 categories of the National Kidney Foundation, there was an independently graded increase risk in the primary composite endpoint, and in its individual components, with decreasing values of all GFR equations (see Supporting Table 2 in the online version of this article).
Table 2.
Cox Regression Risk Analyses for Prediction of Death From Any Cause and/or HF Readmission
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR | P Value | HR | P Value | |
| CKD‐EPI SCr‐CysC per mL/min/1.73 m2 | 0.983 (0.978‐0.988) | <0.001 | 0.988 (0.983‐0.994) | <0.001 |
| CKD‐EPI SCr‐CysC <60 mL/min/1.73 m2 | 1.974 (1.541‐2.529) | <0.001 | 1.596 (1.232‐2.069) | <0.001 |
| CKD‐EPI CysC per mL/min/1.73 m2 | 0.983 (0.979‐0.988) | <0.001 | 0.987 (0.982‐0.992) | <0.001 |
| CKD‐EPI CysC <60 mL/min/1.73 m2 | 2.043 (1.579‐2.644) | <0.001 | 1.626 (1.241‐2.131) | <0.001 |
| NT‐proBNP per 100 pg/mL | 1.003 (1.002‐1.004) | <0.001 | 1.002 (1.001‐1.003) | <0.001 |
| NT‐proBNP >3251 pg/mL | 1.429 (1.147‐1.781) | 0.001 | 1.310 (1.046‐1.642) | 0.02 |
| NYHA class III–IV | 1.843 (1.476‐2.302) | <0.001 | 1.666 (1.328‐2.090) | <0.001 |
| DM | 1.417 (1.136‐1.768) | 0.002 | 1.327 (1.054‐1.671) | 0.02 |
| COPD | 1.295 (1.017‐1.650) | 0.04 | 1.358 (1.062‐1.738) | 0.02 |
| Sodium, per mEq/L | 0.969 (0.947‐0.992) | 0.008 | 0.973 (0.952‐0.995) | 0.02 |
| Hg, per g/dL | 0.905 (0.862‐0.950) | <0.001 | 0.947 (0.898‐0.999) | 0.05 |
| Prior HF | 1.977 (1.317‐2.970) | 0.001 | — | 0.08 |
| Heart rate, bpm | 0.998 (0.995‐1.002) | 0.40 | — | 0.78 |
| β‐Blockers | 0.814 (0.611‐1.085) | 0.16 | — | 0.65 |
| LVEF <50% | 0.965 (0.760‐1.224) | 0.77 | — | 0.53 |
| Male sex | 0.906 (0.728‐1.127) | 0.38 | — | 0.17 |
| Age, y | 1.026 (1.014‐1.039) | <0.001 | — | 0.14 |
Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; CysC, cystatin C; DM, diabetes mellitus; HF, heart failure; Hg, hemoglobin; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SCr, serum creatinine.
Adjusted for sex, age (y), heart rate (bpm), NYHA class III–IV, DM, prior HF, COPD, LVEF <50%, sodium (mEq/L), β‐blockers, Hg (g/dL), and NT‐proBNP (pg/mL). CysC‐CKD‐EPI equations were tested separately and multivariable HR and P value for other variables shown from the CKD‐EPI SCr‐CysC quantitative model.
Table 3 shows the improvement in model performance conferred by adding NT‐proBNP levels and CysC‐based CKD‐EPI equations (models 2–4) to a multivariable model adjusted for other risk factors (model 1). Moreover, the addition of the combined NT‐proBNP and CKD‐EPI equations resulted in improving the accuracy for adverse‐outcomes prediction, beyond the model based on other risk factors: NRI: 10.3% (7.5% events correctly reclassified, 2.8% no events correctly reclassified, P = 0.007) and IDI: 7% (P = 0.004) for the model including CKD‐EPISCr‐CysC equation; and NRI: 9.8% (5.6% events correctly reclassified, 4.2% no events correctly reclassified, P = 0.008) and IDI: 6% (P = 0.001) for the score including CKD‐EPICysC equation. (See Supporting Tables 3 and 4 in the online version of this article, which show the results of separate reclassification analyses for each of the study endpoints.)
Table 3.
Evaluating Added Predictive Ability of Adding CysC‐Based CKD‐EPI Equations to Other Risk Factors for Detection of Death From Any Cause and/or HF Readmission
| Model | NRI | P Value | % Events Correctly Reclassified | % No Events Correctly Reclassified | IDI | P Value |
|---|---|---|---|---|---|---|
| Model 1 (other risk factors) | — | — | — | — | — | — |
| Model 2: model 1 + NT‐proBNP (per pg/mL) | 9.4% | 0.022 | −1.2% | 10.6% | 15% | 0.003a |
| Model 3: model 2 + CKD‐EPI SCr‐CysC (per mL/min/1.73 m2) | 16.9% | <0.001a | 5.9% | 11.0% | 21% | <0.001a |
| 10.3% | 0.007b | 7.5% | 2.8% | 7% | 0.004b | |
| Model 4: model 2 + CKD‐EPI CysC (per mL/min/1.73 m2) | 17.5% | <0.001a | 4.4% | 13.1% | 22% | <0.00a |
| 9.8% | 0.008b | 5.6% | 4.2% | 6% | 0.001b | |
| 1.2% | 0.735c | 1.6% | −0.4% | 1.2% | 0.110c |
Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; CysC, cystatin C; DM, diabetes mellitus; HF, heart failure; Hg, hemoglobin; IDI, integrated discrimination improvement; LVEF, left ventricular ejection fraction; NRI, net reclassification improvement; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SCr, serum creatinine.
Model 1 (other risk factors) included sex, age (y), heart rate (bpm), NYHA class III–IV, DM, prior HF, COPD, LVEF, sodium (mEq/L), β‐blockers, and Hg (g/dL).
As compared with Model 1.
As compared with Model 2.
As compared with Model 3.
Finally, Kaplan‐Meier survival analyses also showed the complementary prognosis value of CysC‐based CKD‐EPI equations and NT‐proBNP levels for the prediction of mortality and/or heart failure readmission. As detailed in Figures 1 and 2, the combination of GFR <60 mL/min/1.73 m2 and NT‐proBNP >3251 pg/mL was associated with the highest risk of adverse outcomes, and it remains unchanged after stratified for LVEF status (log rank test P < 0.001).
Figure 1.
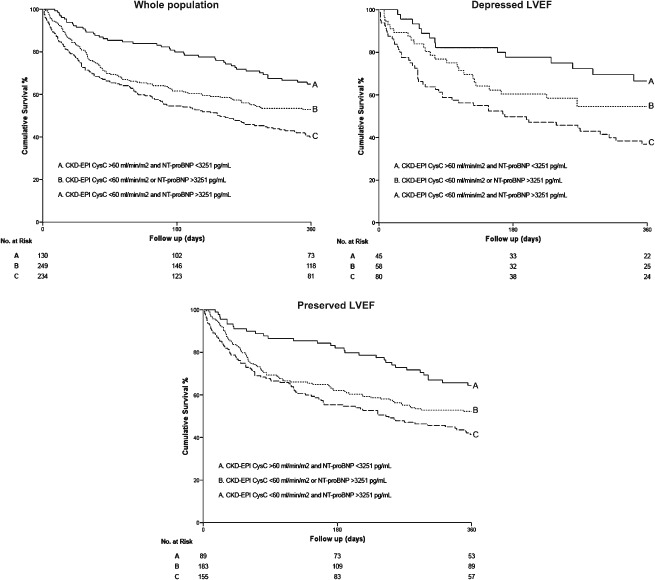
Kaplan‐Meier survival curves for death from any cause and heart failure readmission as a function of GFR estimated using CKD‐EPICysC equation and NT‐proBNP in the whole study population and in patients with depressed or preserved LVEF. Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 2.
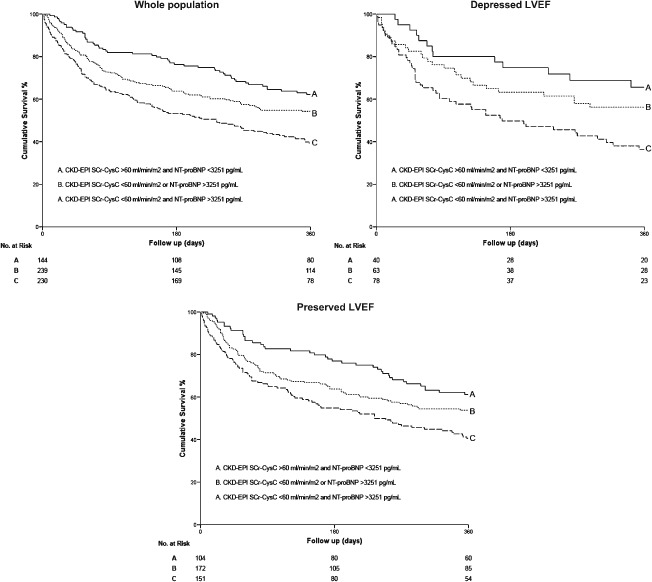
Kaplan‐Meier survival curves for death from any cause or heart failure readmission as a function of GFR calculated using CKD‐EPISCr‐CysC equation and NT‐proBNP in the whole study population and in patients with depressed or preserved LVEF. Abbreviations: CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; CysC, cystatin C; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SCr, serum creatinine.
Discussion
In the present study, we found both CysC‐based CKD‐EPI equations and NT‐proBNP to be independent predictors of worse outcomes in patients with ADHF. Notably, the combination of both parameters was superior for identifying patients at highest risk for adverse outcomes and added complementary prognostic information to each other, as well as to other established clinical risk factors. To the best of our knowledge, this is the first study to describe the complementary prognostic value of the CysC‐based CKD‐EPI equations and NT‐proBNP among patients with ADHF. These findings highlight the importance of the cardiorenal interaction in determining clinical outcomes in ADHF.
The interaction between heart and kidney disease has been an area of considerable interest in recent years. The term “cardiorenal syndrome” has been proposed to outline the interplay between cardiac and renal function, and in the setting of ADHF, acute kidney injury is referred to as cardiorenal syndrome type 1.17 Some degree of renal impairment is present in >50% of patients with ADHF, and moderate to severe impairment is observed in 30% of such patients.18, 19, 20, 21, 22 In the Acute Decompensated Heart Failure National Registry (ADHERE registry), a SCr >2 mg/dL was found in 21% of patients.23 Apart from this, renal dysfunction is one of the most important independent risk factors for morbidity, mortality, and length of hospitalization in patients with ADHF.24 Though renal dysfunction may occur consequent to more aggressive care (and in this setting is not typically associated with worse prognosis),25, 26 worsening renal function in the presence of severe congestion, reflected by an elevated NT‐proBNP, is more likely indicative of undertreatment and higher risk.27
Reliable identification of renal dysfunction is a key issue in identifying patients at risk of poor prognosis, although there is still some debate about which is the best way to evaluate kidney function in this setting. Glomerular filtration rate is considered the best overall index of kidney function. As the gold‐standard measurement of GFR using inulin or iothalamate clearance is not feasible in every patient, simpler methods have been developed to estimate GFR. The MDRD equation is commonly used and has widespread acceptance.28, 29, 30 However, this creatinine‐based equation is inaccurate, especially at higher GFR.31, 32, 33 A new CKD‐EPI equation was developed to increase accuracy, especially at higher GFR.5, 6, 7 A validation study showed that this new equation could reclassify more accurately and have less bias between estimated and measured GFR.5, 6, 7 Recently, data from a Korean registry confirmed a better performance of CKD‐EPI as compared with MDRD in clinical‐outcome prediction in ADHF.34 Moreover, estimated GFR based on serum CysC using CKD‐EPI equations has been recently published for use with standardized CysC and creatinine values.35 CysC, a cysteine protease that is produced by all nucleated cells and is freely filtered at the glomerulus and not secreted from the tubules, may offer some advantages over creatinine because it seems to be less influenced by non‐GFR determinants and probably detects renal damage earlier than creatinine.36, 37 We recently demonstrated in a population of ADHF patients that CysC‐based CKD‐EPI equations more accurately predicted the risk of mortality and/or heart failure hospitalization, especially in those patients with more preserved GFR.8
In this analysis we demonstrate that CysC‐based CKD‐EPI equations provide additional prognostic information to NT‐proBNP. Notably, although NT‐proBNP and CKD‐EPI equations were each significantly related to adverse events, the approach of combining the 2 measures led to our finding that the combination was a superior tool for identifying patients with ADHF at highest risk for poor outcomes. Interestingly, as detailed in Figures 1 and 2, the combination of GFR <60 mL/min/1.73 m2 and NT‐proBNP >3251 pg/mL was associated with the highest risk of adverse outcomes, and it remains unchanged after stratified for LVEF status. In contrast to our study, most prior analyses27, 38, 39, 40 exploring the interaction between renal‐function parameters and natriuretic peptides for prognostication in ADHF have used SCr and the MDRD equation. For example, in a subanalysis of 720 patients presenting with ADHF, both a GFR <60 mL/min/1.73 m2 using the MDRD equation and a NT‐proBNP level above the median (4647 pg/mL) predicted a poor outcome.27 Similar to our findings, these investigators identified that it was the combination of both that carried the greatest risk. Furthermore, the absence of either feature resulted in a short‐term outcome similar to that of patients with a GFR >60 mL/min/1.73 m2 and a NT‐proBNP level below the median. The superior prognostic impact of NT‐proBNP in patients with impaired renal function further supports the importance of this marker in those with CKD and contradicts the incorrect notion that NT‐proBNP cannot be applied in those with renal failure. We now expand on prior results by including the more accurate estimators of GFR (CysC‐based CKD‐EPI equations) in our combined risk‐stratification approach.
Study Limitations
This analysis does have some limitations. The lack of direct measure of GFR represents the main limitation, especially because the new estimated GFR equations have not been completely validated in the ADHF setting. In addition, given that patients involved in this study represent a quite elderly Caucasian population that does not account for a wide spectrum of patients with ADHF across the age continuum and African Americans patients, our findings should be replicated in different populations and extended to more diverse settings before making specific recommendations for use in clinical practice. Furthermore, we did not have serial renal‐function measurements during follow‐up or a separate group of patients with which to externally validate our results.
Conclusion
In patients with ADHF, the combination of NT‐proBNP with CysC‐based CKD‐EPI equations better predicts outcomes than either parameter alone and adds valuable complementary prognostic information to other established risk factors.
Supporting information
TableS1. Multivariable Cox regression risk analyses for prediction of each study endpoint.
TableS2. Cox Regression risk analyses for prediction of adverse events according to estimated GFR category.
TableS3. Evaluating added predictive ability of adding cystatin C based CKD‐EPI equations to other risk factors for detection of heart failure readmission.
TableS4. Evaluating added predictive ability of adding cystatin C based CKD‐EPI equations to other risk factors for detection of death from any cause.
Pedro J. Flores‐Blanco, MD, and Sergio Manzano‐Fernández, MD, PhD, contributed equally to this work.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. Ezekowitz J, McAlister FA, Humphries KH, et al; APPROACH Investigators . The association among renal insufficiency, pharmacotherapy, and outcomes in 6427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. [DOI] [PubMed] [Google Scholar]
- 2. Hillege HL, Nitsch D, Pfeffer MA, et al; Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators . Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. [DOI] [PubMed] [Google Scholar]
- 3. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators . Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. [DOI] [PubMed] [Google Scholar]
- 4. Abraham WT, Fonarow GC, Albert NM, et al; OPTIMIZE‐HF Investigators and Coordinators . Predictors of in‐hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF). J Am Coll Cardiol. 2008;52:347–356. [DOI] [PubMed] [Google Scholar]
- 5. Levey AS, Stevens LA, Schmid CH, et al; Chronic Kidney Disease Epidemiology Collaboration . A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155:408]. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manzano‐Fernández S, Flores‐Blanco PJ, Pérez‐Calvo JI, et al. Comparison of risk prediction with the CKD‐EPI and MDRD equations in acute decompensated heart failure. J Card Fail. 2013;19:583–591. [DOI] [PubMed] [Google Scholar]
- 9. Bruneau BG, Piazza LA, de Bold AJ. BNP gene expression is specifically modulated by stretch and ET‐1 in a new model of isolated rat atria. Am J Physiol. 1997;273(6 part 2):H2678–H2686. [DOI] [PubMed] [Google Scholar]
- 10. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators . Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- 11. Januzzi JL Jr, Camargo CA, Anwaruddin S, et al. The N‐terminal Pro‐BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. [DOI] [PubMed] [Google Scholar]
- 12. Hartmann F, Packer M, Coats AJ, et al. Prognostic impact of plasma N‐terminal pro‐brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–1786. [DOI] [PubMed] [Google Scholar]
- 13. Bettencourt P, Azevedo A, Pimenta J, et al. N‐terminal‐pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. [DOI] [PubMed] [Google Scholar]
- 14. Gustafsson F, Steensgaard‐Hansen F, Badskjaer J, et al. Diagnostic and prognostic performance of N‐terminal ProBNP in primary care patients with suspected heart failure. J Card Fail. 2005;11(5 suppl):S15–S20. [DOI] [PubMed] [Google Scholar]
- 15. Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. [DOI] [PubMed] [Google Scholar]
- 16. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 17. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 18. Akhter MW, Aronson D, Bitar F, et al. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure [published correction appears in Am J Cardiol. 2005;96:324]. Am J Cardiol. 2004;94:957–960. [DOI] [PubMed] [Google Scholar]
- 19. Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta‐analysis. J Am Coll Cardiol. 2006;47:1987–1996. [DOI] [PubMed] [Google Scholar]
- 20. Krumholz HM, Chen YT, Vaccarino V, et al. Correlates and impact on outcomes of worsening renal function in patients > or = 65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. [DOI] [PubMed] [Google Scholar]
- 21. Smith GL, Vaccarino V, Kosiborod M, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. [DOI] [PubMed] [Google Scholar]
- 22. Gottlieb SS, Abraham W, Butler J, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. [DOI] [PubMed] [Google Scholar]
- 23. Adams KF Jr, Fonarow GC, Emerman CL, et al; ADHERE Scientific Advisory Committee and Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100 000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 24. Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. [DOI] [PubMed] [Google Scholar]
- 25. Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network . Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Kimmenade RR, Januzzi JL Jr, Baggish AL, et al. Amino‐terminal pro‐brain natriuretic peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48:1621–1627. [DOI] [PubMed] [Google Scholar]
- 28. Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 29. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med. 2008;18:4]. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 30. Ruilope LM, Salvetti A, Jamerson K, et al. Renal function and intensive lowering of blood pressure in hypertensive participants of the Hypertension Optimal Treatment (HOT) study. J Am Soc Nephrol. 2001;12:218–225. [DOI] [PubMed] [Google Scholar]
- 31. Verhave JC, Gansevoort RT, Hillege HL, et al. Drawbacks of the use of indirect estimates of renal function to evaluate the effect of risk factors on renal function. J Am Soc Nephrol. 2004;15:1316–1322. [PubMed] [Google Scholar]
- 32. Stevens LA, Coresh J, Feldman HI, et al. Evaluation of the Modification of Diet in Renal Disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18:2749–2757. [DOI] [PubMed] [Google Scholar]
- 33. Rule AD, Rodeheffer RJ, Larson TS, et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population [published correction appears in Mayo Clin Proc. 2006;81:1639]. Mayo Clin Proc. 2006;81:1427–1434. [DOI] [PubMed] [Google Scholar]
- 34. Oh J, Kang SM, Hong N, et al; KorHF Registry . The CKD‐EPI is more accurate in clinical outcome prediction than MDRD equation in acute heart failure: data from the Korean Heart Failure (KorHF) Registry. Int J Cardiol. 2013;167:1084–1087. [DOI] [PubMed] [Google Scholar]
- 35. Inker LA, Schmid CH, Tighiouart H, et al; CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C [published corrections appear in N Engl J Med. 2012;367:681 and 2012;367:2060]. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. [DOI] [PubMed] [Google Scholar]
- 37. Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. [DOI] [PubMed] [Google Scholar]
- 38. McCullough PA, Duc P, Omland T, et al; Breathing Not Properly Multinational Study Investigators . B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly multinational study. Am J Kidney Dis. 2003;41:571–579. [DOI] [PubMed] [Google Scholar]
- 39. Anwaruddin S, Lloyd‐Jones DM, Baggish A, et al. Renal function, congestive heart failure and NT‐proBNP measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Am Coll Cardiol. 2006;47:91–97. [DOI] [PubMed] [Google Scholar]
- 40. Vanderheyden M, Bartunek J, Filippatos G, et al. Cardiovascular disease in patients with chronic renal impairment: role of natriuretic peptides. Congest Heart Fail. 2008;14(4 suppl 1):38–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1. Multivariable Cox regression risk analyses for prediction of each study endpoint.
TableS2. Cox Regression risk analyses for prediction of adverse events according to estimated GFR category.
TableS3. Evaluating added predictive ability of adding cystatin C based CKD‐EPI equations to other risk factors for detection of heart failure readmission.
TableS4. Evaluating added predictive ability of adding cystatin C based CKD‐EPI equations to other risk factors for detection of death from any cause.


