Abstract
Background
The bone marrow (BM) is a major reservoir of resting memory T cells and long-lived plasma cells, capable of providing protection against recurrent infections. Whether the age-related accumulation of adipose tissue in the BM affects the functionality and maintenance of memory cells is not well understood.
Methods
For the first time, we compare human femur marrow adipose tissue (fMAT) and subcutaneous white adipose tissue of the thigh (tsWAT) obtained from the same donors. Therefore, we used microarrays for comparative global gene expression analysis, and employed assays to analyse parameters of adipocyte biology, inflammation and oxidative stress.
Findings
We show that fMAT adipocytes differ significantly from tsWAT adipocytes regarding specific gene expression profiles including inflammatory responses and adipogenesis/adipocyte phenotype. Concomitant with considerably lower levels of CD36, a membrane-associated protein important for long-chain fatty acid uptake that is used as maturation marker, fMAT adipocytes are smaller and contain less triglycerides. fMAT adipocytes secrete similar levels of adiponectin and leptin as tsWAT adipocytes, and express increased levels of pro-inflammatory molecules concomitant with an elevated generation of reactive oxygen species (ROS) and impaired function of plasma cells in the BM.
Interpretation
Our findings suggest that fMAT is a unique type of adipose tissue containing small adipocytes with lower CD36 protein and triglyceride levels than tsWAT but high adipokine secretion. Moreover, fMAT adipocytes secrete high levels of pro-inflammatory cytokines, contributing to inflammation and impairment of plasma cell function in the BM, suggesting that fMAT has more immune regulatory functions than tsWAT.
Keywords: Aging, Bone marrow adipocytes, CD36, Inflammation, ROS
Research in context.
Evidence before this study
Recent studies in mice suggest that MAT accumulation causes a loss of haematopoietic progenitor cells in vivo, age-related impairment of bone regeneration and bone healing, and dysfunction of bone marrow niches. MAT is restricted by the bone marrow microenvironment and is surrounded by haematopoietic and skeletal lineage cells. For this reason, MAT adipocytes may also provide support to surrounding cells and tissues e.g. protecting osteoblasts from lipotoxicity by ectopic lipid storage. Thus, MAT seems to have both detrimental and beneficial effects, and more insight is necessary to understand its impact on the maintenance of immunological memory within the bone marrow (BM). A gene expression profile comparing epididymal adipocytes from mice revealed lower expressions of adipogenic specific genes PPARγ, FABP4, and Plin1, and a higher level of the gene C/ebpß which is linked to early adipocyte differentiation. However, similarly to WAT, MAT acquires an inflammatory pattern, expressing greater levels of inflammatory response genes which are highly regulated by age in mice. Aside from the impact on lipid metabolism, adipokines may also affect the immune function and modulate T cells in mice.
Added value of this study
At the functional and molecular level fMAT significantly differs based on specific gene expression profiles including inflammatory response, redox regulation and adipogenesis/fatty acid metabolism from tsWAT. Higher expression of the effector/memory T cell survival factors IL7 and IL15 were found in fMAT compared to tsWAT adipocytes. The expression of the pro-inflammatory molecules TNFα and IL6, which contribute to the low-grade inflammatory background known as “inflamm-aging” observed in elderly persons, was also higher in fMAT. Reduced expression levels of the adipocyte-specific genes peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid binding protein 4 (FABP4), adiponectin (ADIPOQ) and fatty acid translocase (FAT/CD36) suggest that the BM is an immune regulatory organ which displays a unique type of adipose tissue affecting plasma cells that are essential for protective immunity in elderly people. No information is presently available whether fMAT adipocytes interact with plasma cells in the BM, whether such potential interactions are a specific feature of old age when adipocyte numbers in the BM are high and whether these interactions are detrimental or beneficial for the maintenance of immunological memory in old age in humans. The findings of this study lead to a better understanding of the function of MAT in order to find new ways to prevent loss of immune function with age and to ensure healthy aging. The performed experiments make an important contribution to the characteristic phenotype of MAT, to identify new cellular interactions, possible biomarkers of immunosenescence and targets for clinical research.
Implications of all the available evidence
Elderly people constitute one of the fastest growing fractions of a population throughout the world, leading to relevant demographic changes. With increasing age, elderly persons are more prone to age-related and chronic diseases e.g. obesity, osteoporosis, diabetes, Alzheimer's disease, cardiovascular diseases and cancer. Additionally, the function of the immune system declines in old age, leading to a high susceptibility to infectious diseases and a low efficiency of vaccinations in elderly persons. Global obesity represents a worldwide phenomenon in all age groups and is associated with several metabolic diseases including adipose tissue dysfunction in old age. Thus, accumulation and dysfunction of adipose tissue may be an essential contributor to the elevated risk of age-related diseases. Due to the fact that the number of elderly and obese persons increases worldwide, strategies to prevent/postpone immunosenescence are of high social, economic and industrial importance. For this reason it is necessary to fight against the degeneration of the immune system and investigate the impact of progressive adipose tissue throughout our body to ensure healthy aging and longevity.
Alt-text: Unlabelled Box
1. Introduction
Aging is associated with an increased susceptibility to opportunistic infections [1], an effect which is at least partially due to the aging of the immune system [2]. With aging, the immune system undergoes significant changes which are collectively termed “immunosenescence” and are characterized by a decrease in immune cell function. In particular, the involution of the thymus is an early consequence of age-related changes, which leads to a decreased output of new naïve T cells into the periphery. This process starts with puberty, and continues at a rate of 3% per year until middle age, by the age of 60 almost all functional thymus tissue has disappeared and is replaced by the expansion of nonthymopoietic perivascular space (adipocytes, peripheral lymphocytes, stroma) [3,4].
Within the bone marrow (BM), marrow adipose tissue (MAT) accumulates during aging and chronic diseases, and occupies the femoral cavity [[5], [6], [7]]. Thereby, functional haematopoietic tissue is replaced by BM fat [5,8]. Recent studies in mice suggest that MAT accumulation causes a loss of haematopoietic progenitor cells in vivo, age-related impairment of bone regeneration, bone healing, and dysfunction of BM niches [9,10]. However, others showed that human MAT adipocytes from the iliac crest play a supporting role on the proliferation of CD34+ haematopoietic stem cells [11]. Moreover, it was demonstrated that MAT adipocytes promote the regeneration of stem cells and haematopoiesis [12].Thus, MAT seems to have both detrimental and beneficial effects, and more insight is necessary to understand its impact on haematopoiesis in the BM.
Memory T helper (Th) cells and long-lived plasma cells can reside antigen-independently in the BM for decades [13]. The survival and maintenance of these cells is mediated by cytokine and chemokine producing stromal cells and myeloid cell types, forming specific areas known as BM niches. So far, whether the increase of adipose tissue in the BM affects the functionality and maintenance of memory immune cells is insufficiently described. The primary function of white adipose tissue (WAT) depots is to act as metabolic regulators, buffering nutrient availability and demand by storing excess calories in the form of triglycerides, and delivering free fatty acids during fasting [14]. WAT also acts as endocrine organ, producing and secreting cytokines known as adipokines [15], which have effects on various processes such as metabolism and immunity [[16], [17], [18]]. Adipokines exhibit either pro-inflammatory or anti-inflammatory characteristics and contribute to insulin resistance [17].
We designed a study to investigate functional properties of femur marrow adipose tissue (fMAT) in thigh bone in comparison to the adjacent fat depot of the thigh, one of the large subcutaneous WAT depots (tsWAT), which is protective against metabolic diseases [14]. We found that adipocytes of fMAT have a unique inflammatory gene expression profile showing increased levels of pro-inflammatory cytokines and ROS, which affect plasma cells. In contrast to classical tsWAT, a decreased expression of specific adipogenic marker genes was detected in fMAT adipocytes suggesting that MAT has an immune regulatory function rather than classical adipocyte features. Our findings support the hypothesis that fMAT adipocytes may contribute to inflamm-aging and have a negative impact on the maintenance of plasma cells in the BM.
2. Material and methods
2.1. Human subjects
Human bone from femur head biopsies of substantia spongiosa ossium and the surrounding subcutaneous fat tissue, which would otherwise have been discarded were harvested from healthy individuals during hip replacement surgery (Table S1–11). Human BM was obtained from systemically healthy individuals who did not receive immunomodulatory drugs or suffer from diseases known to influence the immune system, including autoimmune diseases and cancer. Informed consent for test and publication was given and documented from each patient. The average age of the donors that provided fMAT, tsWAT and BMMCs was 67 ± 11 years.
2.2. Histological H&E stains of formalin-fixed paraffin embedded tissue
fMAT and tsWAT sections were stained with Haematoxylin and Eosin. Paraffin-embedded 4-μm tissue sections were deparaffinised in xylene and re-hydrated in ethanol. The slides were stained for 8 min in Hemalaun, subsequently washed with tap water and then labelled with eosin for 45 s. After the afferent order of de-hydration the slices were embedded in Entellan and analysed with the Nikon ECLIPSE TE300 microscope. Decalcification, H&E staining and wash steps were done at room temperature. The calculation of the adipose tissue score was performed as follows: an area consisted of one to twenty field of view and at least three areas per donor. Images were made by 40-times magnification with a side length of 204 μm. The quantitative calculation of all images based on the adipose tissue score [arbitrary units 1–3] according to the tissue ratio was determined (Fig. S1c). To obtain the adipose tissue score the mean of all areas was calculated.
2.3. Whole mount immunofluorescence staining
fMAT and tsWAT pieces were stained by whole mount immunofluorescence (IF) staining and analysed using the confocal microscope Cell Voyager CV1000, Yokogawa (spinning-disc/50 μm pinholes). fMAT and tsWAT samples (5–15 mm diameter) were fixed in Lillie's neutral 4% formaldehyde for 2–3 h at room temperature with slow rotation. All further incubation periods were done with slow rotation. After blocking with blocking-buffer (PBS, 1% Triton X-100, 0.2% sodium azide, 5% FCS) for 24–48 h at 4 °C tissue samples were incubated with primary antibodies used for immunofluorescence staining (IF); goat anti-perilipin-1 (Abcam Cat#ab61682, RRID:AB_944751), and rabbit anti-human ATP Synthase beta (Invitrogen Cat#A21351, RRID:AB_221512) known as Complex V, in staining-buffer (PBS, 1% TritonX, 0.2% sodium azide, 5% FCS) for 48–72 h at 4 °C. Washing was done four times in wash-buffer (PBS, 1% TritonX, 0.2% sodium azide, 5% FCS) for at least 1 h at 4 °C. In between each washing step samples were washed four times with PBS for 10 min at 4 °C. A final fifth wash-step was performed overnight. Donkey anti-rabbit Alexa Fluor 488 (Abcam Cat#ab150105, RRID:AB_2732856), donkey anti-goat DyLight 549 (Abcam Cat#ab96936, RRID:AB_10679832) secondary antibodies and TO-PRO-3, a DNA nucleus dye, were diluted in staining-buffer and added for 12–24 h at 4 °C. Staining without primary antibodies resulted in no signal. Washing steps were repeated as previously described. Tissues were equilibrated in glycerol for at least 1 h and afterwards placed on a petri-dish with glass-bottom (CELLview Dish, Greiner Bio-One, Cat#:627861, Vienna, Austria) to view with confocal microscopy. First a map-overview was generated using the 10-times objective detecting nuclear staining. Images were made by 60-times magnification in z-stacks with a maximal step-high of 0.4 μm.
2.4. Adipocyte and bone marrow mononuclear cell isolation
Bone marrow biopsies were fragmented, washed once with complete RPMI medium (RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin; Invitrogen) and treated with purified collagenase (CLSPA, Worthington Biochemical; 20 U/ml in complete RPMI medium) for 1 h at 37 °C. After centrifugation for 10 min at 150 g with low break and before ficoll purification, adipocytes formed a floating layer on the top of the medium which was carefully transferred into a fresh 15 ml tube. BM biopsies were then centrifuged and BMMCs purified by density gradient centrifugation (Ficoll-Hypaque). Subcutaneous fat tissue was placed into a petri dish and reduced into small fragments of approximately 1 mm with a scissor. Small fragments were transferred into a 15 ml Falcon and filled with PBS + 2% FCS to 14 ml. For digestion 200 μl of collagenase (Worthington CLSPA #L5005273; Stock 1000 U/ml; Final concentration 200 U Collagenase) was added and incubated for 1 h at 37 °C. The digested suspension was passed through a 100 μM cell strainer and afterwards collected in a 15 ml tube. BM and subcutaneous adipocytes were treated the same. Both 15 ml tubes containing adipocytes were centrifuged at 150 g for 10 min and afterwards, adipocytes were isolated by flotation. Informed consent for test and publication was given and documented from each patient.
2.5. Positive selection and total RNA isolation for microarrays
To ensure high purity of adipocyte samples for microarray analysis a customized EasySep® protocol using the EasySep® purple magnet (ES) (Stemcell Technology, Cat#18000) was applied to remove residual leukocytes, erythrocytes, endothelia, and mesenchymal cells. Therefore, a customized cell separation kit containing antibodies against Glycophorin A (StemCell Technologies Cat#18352), CD31 (Thermo Fisher Scientific Cat# 13-0319-82, RRID:AB_466423), CD45 (StemCell Technologies Cat#18259) and CD105 (BioLegend Cat# 323214, RRID:AB_2293527) was used for the EasySep® positive selection procedure. Separation was done with EasySep, an immunomagnetic column-free method (Stemcell Technology). RNA was isolated from purified femur marrow and subcutaneous white adipose adipocytes using the RNeasy Lipid Tissue Mini Kit (Qiagen) including the reagent Qiazol according to the manufactures' protocol. Furthermore, isolated total mRNA of eight human female individuals with a RIN value >7 was used for microarray analysis which was done by Eurofins Genomics AROS from Denmark using Affymetrix® Human Gene ST Array Plate (chip type: HuGene 2.1). Biotinylated cRNA were prepared according to the standard Affymetrix protocol from 1 ng total RNA (Affymetrix® Human Gene ST Array Plate, Affymetrix WT Pico Amp Kit). GeneChips were scanned using the Gene Titan Microarray Scanner (Affimetrix GeneTitan Hyb. Module for WT array plates), Chip type: HuGene_2.1. Chip Lot#: 4310744.
2.6. Microarray GSEA-Gene expression analysis
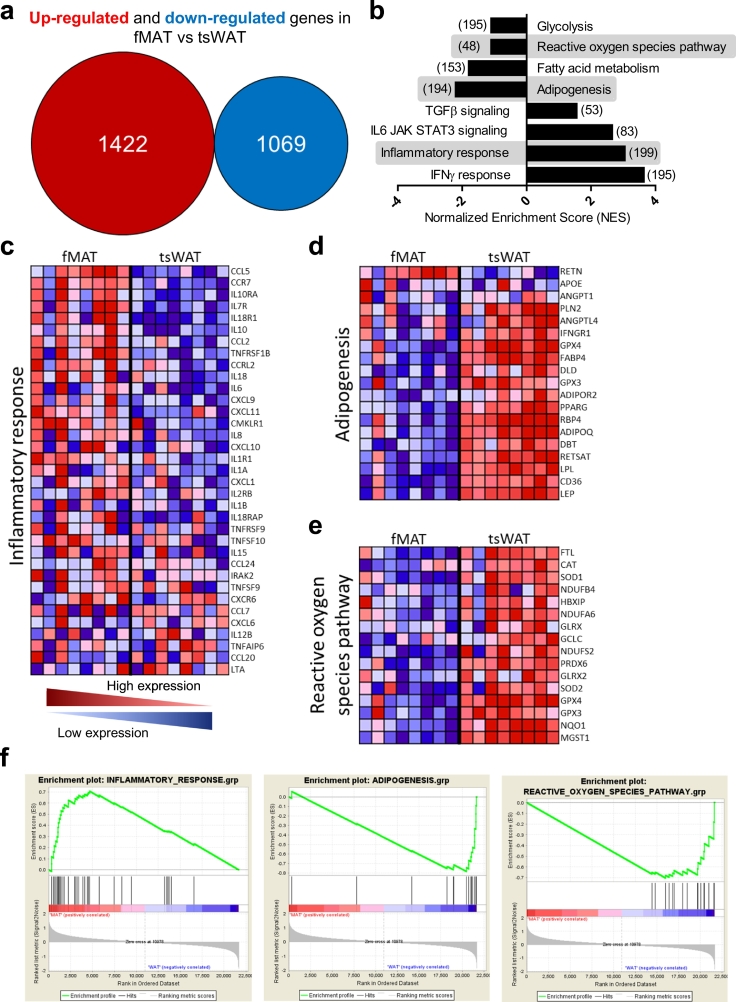
To obtain functional information on fMAT adipocytes, Gene Set Enrichment Analysis (GSEA) (Broad Institute, http://www.broadinstitute.org/gsea/index.jsp) was done as previously described [19], which identified gene sets that were statistically significant. GSEA was used to analyse data from fMAT and tsWAT obtained from eight female donors between the ages of 44 and 76 years (Donor No. 19, 20, 21, 22, 23, 24, 25, and 26; Table S3). The enrichment score (Fig. 2f) shows the degree to which a gene set is overrepresented at the top (positive correlated) or at the bottom (negative correlated) of the ranked list of genes concerning inflammatory response, adipogenesis and redox regulation. The centre of the plot indicates where the genes appear in a gene set. The ranked list metric shows positive values that correlate with the first phenotype (fMAT) and negative values that correlate with the second phenotype (tsWAT) at least 2-fold up- and down-regulated. The detailed information and the results of the microarray are accessible through GEO Series accession number GSE132411 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE132411).
Fig. 2.
Identification of genes and pathways differentially expressed in fMAT versus tsWAT adipocytes. (a) Overall expression of up-regulated (red) and down-regulated (blue) genes in fMAT compared to tsWAT measured by GeneChip® HuGene 2.1 microarray. (b) Inversely up- and down-regulated pathways in fMAT vs tsWAT analysed with Gen Set Enrichment analysis. The number of genes of each gene set is shown within brackets. Highlighted enrichment gene sets show an FDR of <25%, p < .0001. (c - e) Heatmap of genes for selected pathways in fMAT and tsWAT. Heatmap colours indicate red, high; pink, moderate; light blue, low; and dark blue, lowest expression. Samples of eight donors pooled from at least three independent experiments. (c) Inflammatory response genes are shown. (d) Adipogenesis and fatty acid metabolism genes are shown. (e) Genes involved in the reactive oxygen species pathway are shown. (f) Corresponding enrichment plots of the pathways inflammatory response (left), adipogenesis (middle), and reactive oxygen species (right). The enrichment score (ES) shows the degree to which a gene set is overrepresented at the top (positive correlated) or at the bottom (negative correlated) of the ranked list of genes. The ranked list metric (SignalNoise) shows positive values that correlate with the first phenotype (fMAT) and negative values that correlate with the second phenotype (tsWAT) at least 2-fold up- and down-regulated. n = eight female individuals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.7. Adipogenic differentiation of human fMAT and tsWAT cells
Isolated adipocytes from fMAT and tsWAT were seeded in 6-well plates at a density of 10 × 106 cells, cultured for three days in RPMI medium (RPMI 1640 supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin; Invitrogen) in the presence or absence of adipocyte differentiation cocktail (ADM) composed of 0.2 μM Insulin, 0.25 μM Dexamethasone, 2.5% FCS, 10 μg/ml Apo-Transferrin and 0.5 mM IBMX (3-Isobutyl-1-methylxanthin). Adipocytes were collected, transferred to FACS tubes, stained, and analysed by flow cytometry.
2.8. ROS measurement
Adipocytes were treated for one day in complete RPMI at 37 °C in the presence or absence of 1 mM NAC, 10 μM vitamin C and 100 μM BSO (Sigma Aldrich). ROS levels were measured immediately after adipocyte isolation or after a 24 h treatment with antioxidants. The fluorescent dye dihydroethidium (Sigma-Aldrich) was used at a concentration of 20 μM in complete RPMI for 20 min at 37 °C.
2.9. Flow cytometry analysis
Using the EasySep magnet to exclude infiltrating cells, adipocytes were stained with antibodies against anti-CD31 to identify endothelial cells, anti-CD105 for mesenchymal cells, anti-CD24 for stroma cells, LipidTox for neutral lipid droplets, and ant-CD36 for the differentiation stage of adipocytes (Fig. S3a). To analyse the cell survival of isolated BMMCs, cells were transferred, centrifugation at 1500 g for 10 min and incubated with antibodies against anti-human CD27 (BD Biosciences Cat#562513, RRID:AB_11153497), CD38 (BD Biosciences Cat# 563251, RRID:AB_2738097), Annexin V (BD Biosciences Cat# 556419, RRID:AB_2665412), anti-human CD3 (BioLegend Cat# 300407, RRID:AB_314061), anti-CD14 (BioLegend Cat# 325618, RRID:AB_830691), anti-human CD138 (Miltenyi Biotec Cat# 130-117-544, RRID:AB_2751398), CD19 (BD Biosciences Cat# 557791, RRID:AB_396873), and 7AAD (Milteny Biotec) for 20 min at 37 °C. Annexin V was then added and stained for 15 min at room temperature. After incubation, BMMCs were washed, centrifuged, measured by a FACSCanto II (BD Biosciences) and resulting data were analysed using FlowJo software.
2.10. Triglyceride analysis by Image Stream
To assess the surface expression of CD36 (Miltenyi Biotec Cat# 130-110-740, RRID:AB_2657729), (BioLegend Cat# 336204, RRID:AB_1575025), perilipin-1 (Abcam Cat#ab61682, RRID:AB_944751) and the intracellular expression of LipidTox (ThermoFisher Cat#H34477), the same staining procedure as for FACSCanto II analysis was performed for ImageStream Mark II (MKII) Imaging Flow Cytometer system (Amnis). Cells were gated on aspect ratio vs. bright field area to include only single cells, using the gradient root-mean-square feature to include cells in focus. Apoptotic cells were excluded from the analysis based on morphology. Cells in focus were gated for the expression of CD36, perilipin-1, and LipidTox. Data were analysed using IDEAS v6.0340.0 software.
2.11. RNA extraction, cDNA synthesis and analysis of gene expression by quantitative RT-PCR
RNA was isolated from purified fMAT and tsWAT adipocytes using the RNeasy Plus Mini Kit (Qiagen). First-strand cDNA synthesis was performed using a Reverse Transcription system (Promega). Quantitative RT-PCR experiments were done using the LightCycler 480 System (Roche Diagnostics), 2× SYBR Green 1 Master (Roche Diagnostics), and 18S-RNA as housekeeping gene. Sequence-specific oligonucleotide primers were designed using Primer3 software and synthesized by MWG Biotech (Ebersberg, Germany).
2.12. Determination of cytokine and adipokine concentrations by ELISA
BM and subcutaneous adipocytes were seeded at a density of 1 × 106 cells, cultured for one day in complete RPMI medium (RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin; Invitrogen). After approximately 24 h supernatants were collected without disturbing the floating adipocyte layer and centrifuged for 10 min at 0.3 × 1000g. Supernatants were collected and aliquots were frozen at −20 °C. Adiponectin, leptin, TNFα, IL6, and IL8 were measured in the supernatants from fMAT and tsWAT adipocytes using ELISA kits (R&D Systems and BioLegend).
2.13. Enzyme-linked immunospot assay
The detection of antigen-producing plasma cells on the single cell level was done by ELISPOT assays as described in the manufacturer's instructions (Mabtech, Nacka Strand, Sweden). For the analysis of BM plasma cells of a certain specificity, ELISPOT multiscreen IP Filtration Plates (MAIPS4510; Millipore, Temecula, CA, USA) were coated with 5 μg/ml anti-human IgG antibody (Mabtech) overnight at 4 °C. Next, plates were washed with PBS and blocked with RPMI medium (RPMI 1640 supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin; Invitrogen) at least for 1 h at 37 °C, 20% O2, and 5% CO2. A total of 50,000 isolated BMMCs were cultured in triplicates with or without isolated fMAT and tsWAT adipocytes and supernatants of 1 × 106/ml fMAT and tsWAT adipocytes for 24 h with final concentrations as indicated. For fMAT and tsWAT adipocyte treatment with 1 mM N-acetylcysteine, 10 μM vitamin C and 100 μM BSO cells were preincubated for 24 h at 37 °C, 20% O2, and 5% CO2 before co-culturing them with BMMCs. Untreated BMMC's were used as a negative control. After the plates were washed with PBS, antigen-specific B cells were detected using firstly, 1 μg/ml biotinylated secondary antibody, and again after washing, using Streptavidin alkaline phosphatase (Mabtech) for 90 min at room temperature and the colorimetric substrate BCIP/NBT (Moss Inc., Pasadena, MA, USA). Spots were counted using a CTL reader (CTL, Bonn, Germany).
2.14. Statistical analysis
Normal distribution was calculated for all variables using the Spearman test. Wilcoxon matched pairs test and unpaired students t-test between tissues were calculated to determine statistical significance. For correlations between fMAT, BMI, and age, Spearman's rank correlation coefficient (rs) was calculated. Statistical analyses were determined using Microsoft®Excel®2013 (Microsoft Office Professional Plus 2013) or GraphPad Prism 5.0c software (GraphPad Software Inc.). The degrees of significance were indicated as: *p < .05, **p < .01, ***p < .001.
2.15. Study approval
All immunological studies involving human material from the Institute for Biomedical Aging Research were submitted and approved by the Ethics Committee of the Medical University Innsbruck. All participants gave their written informed consent in accordance with the Declaration of Helsinki.
3. Results
3.1. Adipocyte morphology differs between tissues
The role of adipose tissue in the BM microenvironment is largely unknown, and contrary to classical tsWAT depots, its morphology and functionality is insufficiently described [20,21]. Analysis of whole mount tissue stainings using the adipocyte marker perilipin-1 revealed that adipocytes in fMAT were significantly smaller than in tsWAT (Fig. S1a). The average diameters of adipocytes in tsWAT were nearly twofold (80μm2) compared to 45μm2 in fMAT (Fig. 1a and b). Using paraffin sections of fMAT, we quantified the amount of adipose tissue. With aging (Fig. 1c) and an elevated body mass index (BMI) (Fig. S1b), the amount of adipose tissue was increased in fMAT. fMAT was surrounded and strongly infiltrated by different immune cells, while tsWAT was hardly infiltrated (Fig. 1d). This suggests that fMAT may impact the BM microenvironment by the interaction and/or recruitment of immune cells.
Fig. 1.
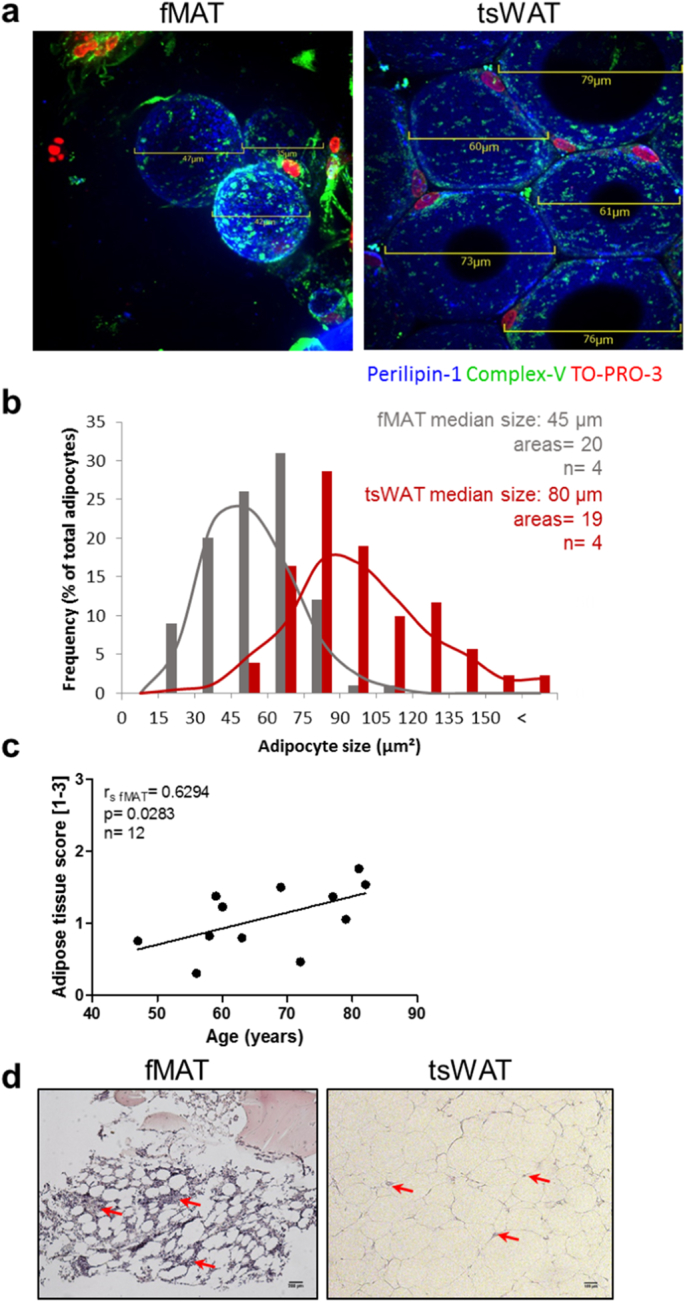
Comparison of human MAT of the femur (fMAT) with sWAT of the thighs (tsWAT). (a) and (b) Adipocyte size is smaller in fMAT than in tsWAT. (a) Representative merged IF images of whole mount stainings showing adipocytes labelled with perilipin-1 (blue). Nuclei were stained with To-Pro3 (red). Green fluorescence shows active mitochondria stained with ATP synthase β (ComplexV). Scale bars indicate the size of fMAT (left) and tsWAT (right) adipocytes. (b) Frequency of fMAT (gray) and tsWAT (red) adipocyte size of different donors using indicated scale bars of IF images. Adipocyte median size, number of areas and sample size (n) are indicated in the graph. Samples of four donors pooled from at least three independent experiments. (c) Human fMAT increases with age. Quantitative calculated adipose tissue score [arbitrary units 1–3] of fMAT is plotted against donor age. Each dot point represents one individual. Spearman coefficient (r fMAT), p value and sample size (n) are indicated in the graph. (d) Representative H&E stains of fMAT (left panel) and tsWAT (right panel) are shown. Red arrows indicate infiltrating cells within the tissues. Scale bars indicate 200 μm for fMAT and 100 μm for tsWAT. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Global gene expression highlights fMAT adipocytes as a unique type of fat cells
To identify functional properties of fMAT adipocytes in the BM microenvironment, we compared the global mRNA expression pattern in purified adipocytes from fMAT and tsWAT of the same donor of eight individuals by microarray (Fig. 2). 1422 genes were at least 2-fold higher in fMAT adipocytes than in tsWAT, whereas 1069 were down-regulated (Fig. 2a). Using GSEA, we assessed pathway analysis to examine up- and down-regulated hallmark gene sets (Fig. 2b). While TGFβ signalling, IL6 JAK STAT3 signalling, inflammatory response, and IFNγ response were enriched in fMAT, glycolysis, reactive oxygen species pathway, fatty acid metabolism, and adipogenesis were down-regulated. Inflammatory response, adipogenesis/fatty acid metabolism, and redox regulation were analysed in detail (Fig. 2c–e). CCL5, CCR7, IL10RA, CCL2, CCRL2, CXCL1, IL8, and IL10 were up-regulated in fMAT compared to tsWAT adipocytes (Fig. 2c). Furthermore, fMAT adipocytes expressed increased levels of IL6 and IL15, which are known to play a decisive role in maintaining memory T cells, and are associated with inflamm-aging [22]. Heatmap analysis of the adipogenic pathway demonstrated that expression of adiponectin (ADIPOQ), leptin (LEP), lipocalin (LPL), as well as adipocyte differentiation and transcription factors, CD36, fatty acid binding protein 4 (FABP4) and proliferator-activated receptor gamma (PPARγ), were at least 2-fold higher in tsWAT compared to fMAT adipocytes (Fig. 2d). Using GSEA, differential expression analysis of both fat tissues revealed several genes for white adipose tissue but additionally revealed potential new candidates to characterize fMAT (Fig. 2d). For instance, the adipose tissue-specific secretory factor, resistin was enriched in fMAT, which is otherwise known for endocrine function such as insulin resistance. Interestingly, super oxide dismutase 1 (SOD1), a major ROS scavenging enzyme, glutamate-cysteine ligase (GCLC), super oxide dismutase 2 (SOD2), and glutathione peroxidase 4 (GPX4) were elevated in tsWAT compared with fMAT (Fig. 2e). In addition, the positive and negative correlation of the inflammatory, adipogenic, and ROS gene set with fMAT and tsWAT is depicted in Fig. 2f. The gene expression profile suggests that fMAT is a unique type of adipose tissue and may have an immune regulatory function within the bone marrow.
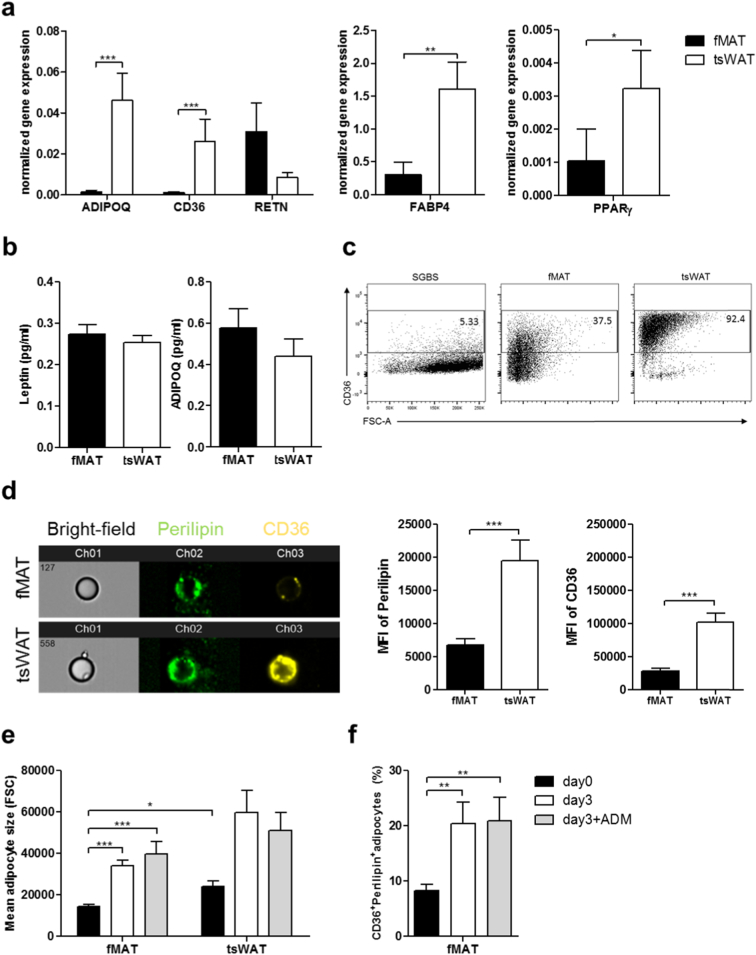
3.3. fMAT exhibits a less mature adipocyte phenotype
In accordance with the microarray data, adipogenic genes generally had a significantly higher expression in tsWAT than in fMAT adipocytes. Thus, adiponectin, CD36, FABP4 and PPARγ were enriched in subcutaneous compared to BM adipocytes. A conspicuous exception was the adipocyte-derived cytokine resistin, that is generally known to contribute to insulin resistance, inflammation and obesity [[23], [24], [25]] (Fig. 3a). Interestingly, in contrast to mRNA expression results, secretion of leptin and adiponectin varied strongly between the donors, with a tendency to be higher in fMAT adipocytes (Fig. 3b). CD36, which is crucial for long-chain fatty acid uptake into adipocytes [26], is used as a adipocyte maturation marker and correlates with the increase of the master transcription factor in adipogenesis, PPARγ, and major adipocyte markers like FABP4 [27,28]. Consequently, CD36 expression was low on the Simpson-Golabi-Behemil Syndrome (SGBS) [29] pre-adipocyte cell lineage, whereas nearly all tsWAT adipocytes were positive for CD36 (Fig. 3c). One third of fMAT adipocytes expressed CD36 indicating that only a small fraction represents mature adipocytes achieving a noteworthy long-chain fatty acid uptake (Fig. 3c). The intensity of CD36 on the surface correlates with the metabolic performance regarding fatty acid uptake, adipogenesis and triglyceride formation and, hence, the state of maturity. We therefore tested whether both adipose tissues vary in their stage of maturity and performed image-stream analysis (Fig. 3d). Increased perilipin-1 and CD36 expression were measured on tsWAT adipocytes (Fig. 3d). Beside other functions, CD36 labels human adipocyte progenitors with prominent intracellular adipogenic/triglyceride storage ability [27] and also adipocytes with high triglyceride formation [26]. Using image-stream analysis, we detected higher mean fluorescence intensity of CD36+ adipocytes in tsWAT compared to fMAT. LipidTox, a marker for neutral lipid droplets, was similarly expressed in fMAT and tsWAT (Fig. S2a and S2b). Consistently, the amount of CD36+LipidTox+ adipocytes was higher in tsWAT compared to fMAT (Fig. S2a, S2b and S2c). These data suggest an increased triglyceride storage ability of fMAT (Fig. S2). Next, we cultured fMAT adipocytes for three days in the presence or absence of adipocyte differentiation medium (ADM) (Fig. 3e and f). fMAT and tsWAT adipocytes increased in size after three days in culture (Fig. 3e). The number of CD36+LipidTox+ and CD36+Perilipin+ expressing fMAT adipocytes was elevated after three days in culture (in vitro) (Fig. 3e and f, Fig. S2d and S2e). These results suggest that fMAT adipocytes are restricted by the BM microenvironment thence are smaller in size. Considering the mean fluorescence intensity of CD36 and LipidTox, fMAT adipocytes are lipid metabolically less mature and in principle capable of increasing triglyceride uptake for further maturation if liberated from the BM microenvironment.
Fig. 3.
fMAT adipocytes exhibit a less mature adipocyte phenotype and have the ability to increase their triglyceride uptake and mature into larger adipocytes in vitro. (a) mRNA expression level of AIPOQ, CD36, RETN, FABP4, and PPARγ in fMAT and tsWAT adipocytes assessed by RT qPCR normalized to 18S-RNA. n = 20. (b) ELISA-based analysis of adipokines, leptin and adiponectin concentrations in supernatants of adipocytes from fMAT and tsWAT. n = 21. Average age of donors amounts 65 ± 13 years. (c) Counter-plots showing CD36+ expression among SGBS cells, fMAT and tsWAT adipocytes. Adipocytes have been gated from CD45- cells in order to exclude leukocytes. (d) Representative pictures of image stream analysis (left) and mean fluorescence intensities of perilipin-1 and CD36 cell surface expression (right) from fMAT and tsWAT adipocytes. n = 15. (e - f) fMAT as well as tsWAT adipocytes have the ability to increase their triglyceride uptake and to mature into larger adipocytes. Flow cytometry analysis showing (e) adipocyte size and (f) CD36+ and perilipin+ adipocytes from fMAT and tsWAT cultured with or without the adipogenic differentiation cocktail (ADM) containing Insulin, Dexamethasone, IBMX, Apo-Transferrin and FCS, as indicated. Unstimulated adipocytes were used as a control. n = 12. Data are represented as mean ± SEM. Samples of 12 to 21 donors pooled from at least three independent experiments. *p < .05, **p < .01, ***p < .001 calculated using two-tailed Wilcoxon matched pairs test.
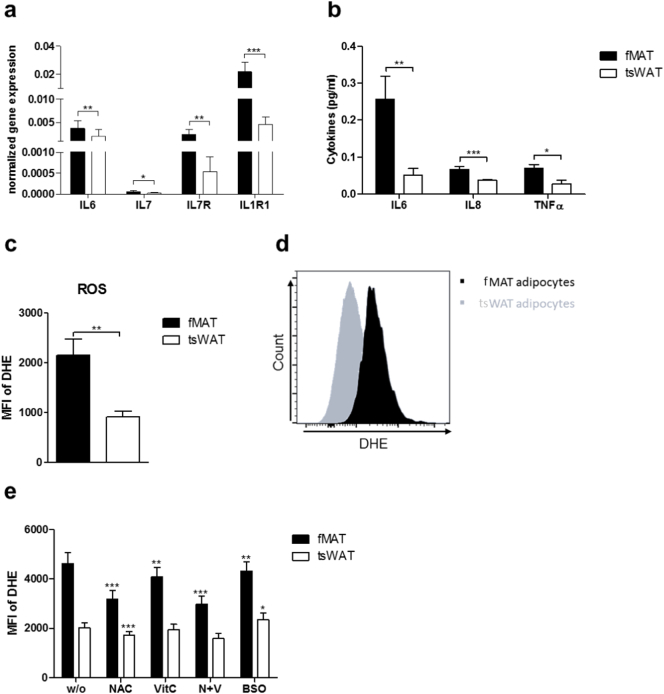
3.4. Elevated levels of pro-inflammatory molecules and ROS in fMAT adipocytes contribute to inflamm-aging
Cellular age-related oxidative stress is responsible for increased accumulation of ROS and linked to chronic inflammation, inflamm-aging, and mitochondrial dysfunction [30,31]. Recently, we demonstrated that elevated levels of IL15 and IL6 correlate with increased ROS levels in the BM of elderly persons [22]. Consistent with RNA-seq data, the mRNA expression level of the inflammatory genes IL6, IL7, IL7R, and IL1R1 was elevated in fMAT (Fig. 4a). Secretion of the pro-inflammatory cytokines IL6, IL8, and TNFα was increased in fMAT compared to tsWAT adipocytes measured by ELISA (Fig. 4b). Considering that ROS levels are increased in BM with aging, we hypothesized that fMAT adipocytes are accountable as a contributing factor to inflamm-aging [22]. Indeed, flow cytometry analysis exhibited higher ROS levels within fMAT than tsWAT adipocytes (Fig. 4c and d). Treating both tissues with ROS scavengers, N-acetylcysteine (NAC) and vitamin C, ROS levels were significantly reduced within fMAT compared to tsWAT (Fig. 4e). Consistent with an impaired redox regulation, the glutathione synthesis inhibitor L-buthionine sulfoximine (BSO) led to increased ROS levels in tsWAT compared to fMAT (Fig. 4e). This indicates that the redox regulation by glutathione was at least partially responsible for lower ROS in tsWAT. Together these data demonstrate that adipocytes in the BM are highly pro-inflammatory and exhibit increased ROS generation.
Fig. 4.
Elevated levels of pro-inflammatory cytokines and ROS within fMAT. (a) Gene expression analysis of IL6, IL7, the corresponding receptor IL7R, and IL1R1 by RT qPCR using mRNA of adipocytes obtained from fMAT and tsWAT. n = 23. (b) ELISA-based analysis of secreted pro-inflammatory cytokines IL6, IL8, and TNFα in supernatants of fMAT and tsWAT. n = 13–18. (c) Flow cytometry analysis and (d) representative histogram of dihydroethidium (DHE) stained fMAT and tsWAT adipocytes. n = 18. (e) fMAT and tsWAT adipocytes were preincubated with NAC, VitC, N + V, and BSO for 24 h and afterwards ROS levels were measured by flow cytometry. n = 15. Data are represented as mean ± SEM. Samples of 13 to 23 donors pooled from at least three independent experiments. *p < .05, **p < .01, ***p < .001 calculated using two-tailed Wilcoxon matched pairs test.
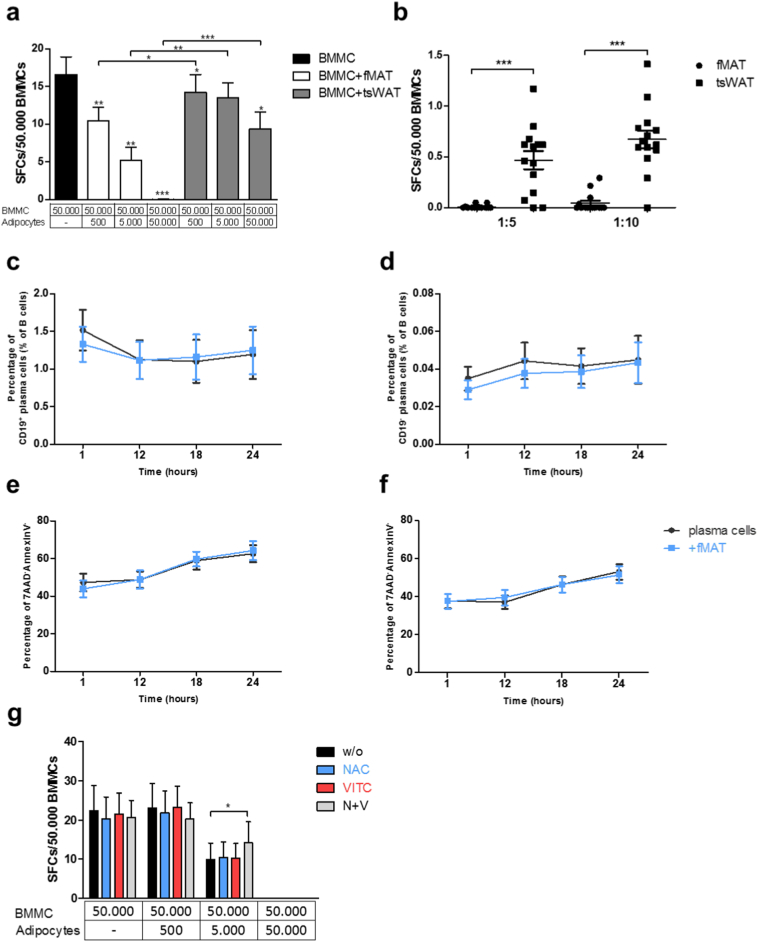
3.5. fMAT adipocytes inhibit IgG producing plasma cells
Advanced age and chronic systemic inflammation is accompanied by the expansion of memory B cells showing a senescent phenotype with impaired ability to differentiate into plasma cells. In this regard, plasma cell numbers decrease in the BM in old patients [32]. In the mid and late stages of life, a decline of B lymphocytes in the BM is associated with increased adipose tissue and adipocyte-derived factors in mice [33,34]. Whether fMAT adipocytes influence the human immune cell repertoire, in particular plasma cells in the BM microenvironment, has not yet been investigated. Therefore, we co-cultured BM plasma cells with fMAT and tsWAT adipocytes and analysed IgG secretion by ELISPOT. The number of antibody-producing bone marrow mononuclear cells (BMMCs) was drastically decreased in the presence of fMAT adipocytes (Fig. 5a). To determine whether cell-cell contact or soluble factors were responsible for inhibition of IgG secretion, we incubated BMMCs with supernatants of fMAT and tsWAT adipocytes (Fig. 5b). Decreased numbers of antibody-producing plasma cells were detected in supernatants of fMAT adipocytes. To identify whether plasma cells undergo apoptosis, we analysed plasma cell survival in the presence of adipocytes during a 24 h co-culture. CD19+ and CD19− plasma cells revealed a constant survival even after one day in culture together with fMAT adipocytes (Fig. 5c–f). Apoptosis was not responsible for the dramatic decrease of IgG secreting plasma cells, therefore we investigated the impact of ROS. The maintenance of antibody producing plasma cells was slightly improved by N-acetylcysteine and vitamin C in the presence of fMAT adipocytes (Fig. 5g). Thus, fMAT adipocytes influence BM plasma cells via the secretion of ROS. ROS scavengers at least partially reverse the negative effect of fMAT adipocytes on plasma cells.
Fig. 5.
fMAT adipocytes inhibit IgG secretion of plasma cells. (a) IgG producing plasma cells co-cultured with fMAT and tsWAT adipocytes and (b) different adipocyte supernatant concentrations, as indicated. n = 14. (c) Frequencies of CD19+ and (d) CD19− plasma cells over a time period of 24 h. n = 15. (e - f) Flow cytometry analysis of living CD19+ and CD19− plasma cells co-cultured with 1:10 fMAT adipocyte concentration as indicated. n = 15. (g) ELISPOT analysis measuring IgG+ spots derived from BMMCs co-cultured with treated fMAT adipocytes in the presence of ROS scavengers as indicated. Graphs showing IgG producing plasma cells from triplicate wells. n = six individuals. Co-cultures were performed with BMMCs, fMAT and tsWAT adipocytes obtained from the same donor. Data are represented as mean ± SEM. Samples of six to 15 donors pooled from at least three independent experiments. *p < .05, **p < .01, ***p < .001 calculated using two-tailed Wilcoxon matched pairs test. SFCs = spot forming cells.
4. Discussion
In the present work, we addressed the functional role of fMAT adipocytes on the BM microenvironment. It is well established that inflamm-aging causes a decline of B lymphocytes in the BM, associated with increased incidence of age-related diseases and decreased response to vaccinations [35]. Secretion of IgG by plasma cells was inhibited in the presence of fMAT adipocytes, yet was hardly influenced by tsWAT adipocytes, indicating that fMAT acts as a distinct immune regulatory tissue. The survival of CD19+ and CD19− plasma cells was unaffected by fMAT adipocytes and we therefore conclude that antibody secretion is abrogated. BM plasma cell survival is mediated by cytokines such as IL5, IL6, TNFα, CXCL12, and APRIL, depending on the BM niche [36]. Previous studies revealed that antibody production can be regulated without affecting plasma cell survival. In mice, mTOR inhibition leads to a profound decline of serum IgG antibodies from long lived plasma cells, while their frequencies were unaltered [37]. Furthermore, Tellier et al. could show secretion of IgG isotype was reduced in the BM of Blimp-1-deficient mice [38]. In summary, while the survival of plasma cells are unaffected, fMAT adipocytes are responsible for diminished antibody secretion that are essential for protective immunity in elderly people [39]. This effect may depend on the differential regulatory properties of fMAT compared to tsWAT. In the BM, fMAT adipocytes seem to play a deteriorative role in elderly people, contributing to inflammation levels and oxidative stress. Decreased SOD1 and GCLC expression in fMAT adipocytes may contribute to an impaired redox regulation and elevated levels of ROS. ROS secretion just partially explains the molecular differences between fMAT and tsWAT. Consequently, further mechanisms beyond ROS regulation are definitely involved. Our findings are consistent with recently published data, where increased ROS exhibited in SOD1−/− knockout mice was linked to increased IL6 and IL15 in the BM [22]. The evidence for the highly pro-inflammatory microenvironment induced by fMAT adipocyte accumulation is supported by elevated expression of resistin. Resistin is a specific secretory factor expressed by adipose tissue, which as previously indicated plays an immune regulatory role [24]. Resistin displays potent pro-inflammatory properties via the strong upregulation of IL6 and TNFα [40]. Furthermore, this adipokine correlates with markers of inflammation such as TNFα, IL1b, and IL6 in human peripheral blood mononuclear cells (PBMCs) [23,24,40].
Unlike WAT, which has the strong capability to increase in size, MAT is most likely restricted by the unique BM microenvironment and surrounding haematopoietic and skeletal lineage cells [21]. We showed that fMAT adipocytes are considerably smaller in size and contain less triglycerides compared to adipocytes of tsWAT. In our differential global gene expression analysis, a number of adipogenesis/adipocyte genes including the key adipogenic transcription factor PPARγ and adipocyte markers, such as FABP4, were lower expressed in fMAT adipocytes. These results are in accordance with studies in mice indicating a lower expression of PPARγ, FABP4 and perilipin-1 in bone marrow adipocytes compared to epididymal white adipocytes [41]. Importantly, one of the genes lower expressed in fMAT was Cluster of Differentiation 36 (CD36) that encodes for a membrane-associated protein necessary to facilitate the uptake of long-chain fatty acids across the plasma membrane of adipocytes [26]. Since long-chain fatty acids are the vast majority of the fatty acids taken up by adipocytes, CD36 is the predominant protein facilitating this process in adipocytes, by absorbing fatty acids, and stimulating adipogenesis and triglyceride storage. CD36 is a key player for adipogenic differentiation and triglyceride formation [26,42]. This is underscored by studies in mice suggesting that CD36 deficiency or downregulation impairs adipocyte differentiation in vitro and de novo fat pad formation in vivo [43]. We demonstrated that CD36 protein levels were lower in fMAT adipocytes relative to tsWAT adipocytes. Even more important, only one third of the human fMAT adipocytes stained positive for the CD36 protein, suggesting that fMAT adipocytes possess an impaired uptake of long-chain fatty acids. Thus, lower CD36 protein levels in fMAT adipocytes could mechanistically explain their smaller size and lower triglyceride content. Other studies identified CD36 as a maturation marker of adipocytes [27,28,44] and Gao et al., reported a fraction of CD36+ cells of human femoral WAT was significantly higher in depots associated with higher adipogenesis [27]. Along these lines the low CD36 protein levels found specifically in our study in fMAT adipocytes suggest that lipid metabolism in these cells is less mature (less well developed) than in tsWAT adipocytes. Interestingly, in the present study, we have shown that in vitro (in cell culture) without skeletal constrictions, fMAT adipocytes respond well to insulin stimuli, such as adipogenic differentiation cocktail, by increasing the levels of CD36, the stimulator of long-chain fatty acid uptake, as well as triglyceride formation. These findings suggest that fMAT adipocytes, when liberated from the BM microenvironment, possess high insulin sensitivity. Intriguingly, our ex vivo experiments revealed that human fMAT adipocytes secrete similar levels of the major adipokines, adiponectin and leptin, as tsWAT adipocytes. Moreover, fMAT adipocytes expressed high levels of pro-inflammatory cytokines. Based on these findings, we hypothesize that lipid metabolism, adipokine and pro-inflammatory cytokines secretion are separately regulated in the BM microenvironment; lipid metabolism is slowed down but simultaneously an adipocyte-associated secretory phenotype is activated, which leads to increased secretion of adipokines as well as pro-inflammatory cytokines. In compliance with these considerations, previous studies identified fMAT as an endocrine organ which can exert specific systemic effects throughout the body [45]. Although, little is known about leptin secretion from MAT adipocytes, our data that fMAT adipocytes secrete high levels of leptin, which are tendentially higher than the leptin level secreted by tsWAT adipocytes of the same donors, are consistent with an early study showing high secretion of leptin by in vitro cultured human MAT adipocytes [46]. In accordance with our data, previous work showed also that adiponectin is secreted from adipocytes isolated from human femurs [47,48]. Moreover, another study documented higher levels of secreted adiponectin in explants of human tibial MAT than WAT [49]. Thus, our study underlines the importance of fMAT as a source of leptin and adiponectin.
In summary, we compared fMAT adipocytes with adjacent tsWAT adipocytes from identical donors for the first time. Our data suggest that fMAT is distinct from tsWAT, having a reduced uptake of long-chain fatty acids and reduced lipid formation but a pronounced secretory phenotype with higher adipokine and adipocytokine secretion, and a pro-inflammatory and ROS generating role in the BM microenvironment in regards to antibody production decline. Future work will be necessary to extend our knowledge of the functional immune regulatory properties of fMAT adipocytes on BM niches to prevent inflamm-aging.
Acknowledgments/funding
We are grateful to Dr. Martin Wabitsch (Clinical University of Ulm) for providing SGBS cells. We thank Michael Keller for the excellent technical support. This work was supported by the FWF Austrian Science Fund funded PhD program HOROS (Host response on opportunistic infections, HOROS W-1253), the European Union's Seventh Framework Program (AITEC) [FP7/2007-2013] under Grant Agreement No 280873 ADITEC and the EU H2020 project “An integrated approach to dissect determinants, risk factors, and pathways of ageing of the immune system” (ImmunoAgeing, H2020-PHC-2014 grant agreement No: 633964). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
C.M. designed and performed the experiments, analysed the data, interpreted the results and wrote the manuscript. A. M. designed and performed the experiments. E. N., L. P., S. W., F. N, and G. F. performed experiments. A. E. helped with the adipocyte differentiation. B. J. helped with technical support and generated crucial tools for experiments. K. T. provided the human material and contributed to the study design. W. Z. designed experiments and edited the manuscript. B. G. L. designed the study, supervised the work and edited the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.07.023.
Appendix A. Supplementary data
Supplemental Table S1-11.
Supplemental Fig. S1-S3.
References
- 1.Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boraschi D., Aguado M.T., Dutel C., Goronzy J., Louis J., Grubeck-Loebenstein B. The gracefully aging immune system. Sci Transl Med. 2013;5:185. doi: 10.1126/scitranslmed.3005624. [DOI] [PubMed] [Google Scholar]
- 3.Lynch H.E., Goldberg G.L., Chidgey A., Van den Brink, Marcel R.M., Boyd R. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George Andrew J.T., Ritter Mary A. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosen Clifford J., Ackert-Bicknell Cheryl, Pablo Rodriguez Juan, Pino Ana Maria. Marrow fat and the bone microenvironment developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moerman Elena J., Teng Kui, Lipschitz David A., Lecka-Czernik Beata. Aging activates adipogenic and suppresses osteogenic programsin mesenchymal marrow stroma/stem cells: the role of PPAR-γ2transcription factor and TGF-β/BMP signaling pathways. Aging Cell. 2004:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Justesen J., Stenderup K., Ebbesen E.N., Mosekilde L., Steiniche T., Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 8.Kricun M.E. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14:10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 9.Naveiras O., Nardi V., Wenzel P.L., Hauschka P.V., Fahey F., Daley G.Q. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosi T.H., Scialdone A., Graja A., Gohlke S., Jank A.-M., Bocian C. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poloni A., Maurizi G., Serrani F., Mancini S., Zingaretti M.C., Frontini A. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013;41:558. doi: 10.1016/j.exphem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Zhou B.O., Yu H., Yue R., Zhao Z., Rios J.J., Naveiras O. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19:891–903. doi: 10.1038/ncb3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokoyoda K., Zehentmeier S., Hegazy A.N., Albrecht I., Grun J.R., Lohning M. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Lee M.-J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Lago F., Gomez R., Gomez-Reino J.J., Dieguez C., Gualillo O. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009;34:500–510. doi: 10.1016/j.tibs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Procaccini C., de Rosa V., Galgani M., Carbone F., La Rocca C., Formisano L. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. doi: 10.3389/fimmu.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheller E.L., Doucette C.R., Learman B.S., Cawthorn W.P., Khandaker S., Schell B. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi: 10.1038/ncomms8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller E.L., Cawthorn W.P., Burr A.A., Horowitz M.C., MacDougald O.A. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016;27:392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pangrazzi L., Meryk A., Naismith E., Koziel R., Lair J., Krismer M. "Inflamm-aging" influences immune cell survival factors in human bone marrow. Eur J Immunol. 2016 doi: 10.1002/eji.201646570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherneva R.V., Georgiev O.B., Petrova D.S., Mondeshki T.L., Ruseva S.R., Cakova A.D. Resistin - the link between adipose tissue dysfunction and insulin resistance in patients with obstructive sleep apnea. J Diabetes Metab Disord. 2013;12:5. doi: 10.1186/2251-6581-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly M.P., Lehrke M., Wolfe M.L., Rohatgi A., Lazar M.A., Rader D.J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 25.Mitterberger M.C., Mattesich M., Klaver E., Lechner S., Engelhardt T., Larcher L. Adipokine profile and insulin sensitivity in formerly obese women subjected to bariatric surgery or diet-induced long-term caloric restriction. J Gerontol A Biol Sci Med Sci. 2010;65:915–923. doi: 10.1093/gerona/glq107. [DOI] [PubMed] [Google Scholar]
- 26.Glatz J.F.C., Luiken Joost J.F.P. From fat to FAT (CD36/SR-B2): understanding the regulation of cellular fatty acid uptake. Biochimie. 2017;136:21–26. doi: 10.1016/j.biochi.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Gao H., Volat F., Sandhow L., Galitzky J., Nguyen T., Esteve D. CD36 is a marker of human adipocyte progenitors with pronounced adipogenic and triglyceride accumulation potential. Stem Cells. 2017;35:1799–1814. doi: 10.1002/stem.2635. [DOI] [PubMed] [Google Scholar]
- 28.Durandt C., van Vollenstee F.A., Dessels C., Kallmeyer K., de Villiers D., Murdoch C. Novel flow cytometric approach for the detection of adipocyte subpopulations during adipogenesis. J Lipid Res. 2016;57:729–742. doi: 10.1194/jlr.D065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wabitsch M., Brenner R.E., Melzner I., Braun M., Moller P., Heinze E. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 30.Bauer M.E., La Fuente M.D. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech Ageing Dev. 2016;158:27–37. doi: 10.1016/j.mad.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Chougnet C.A., Thacker R.I., Shehata H.M., Hennies C.M., Lehn M.A., Lages C.S. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol. 2015;195:2624–2632. doi: 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pritz T., Lair J., Ban M., Keller M., Weinberger B., Krismer M. Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol. 2015;45:738–746. doi: 10.1002/eji.201444878. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy D.E., Knight K.L. Inhibition of B Lymphopoiesis by adipocytes and IL-1-producing myeloid-derived suppressor cells. J Immunol. 2015;195:2666–2674. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholz J.L., Diaz A., Riley R.L., Cancro M.P., Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bulati M., Caruso C., Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of B cell compartment are influenced by "inflamm-ageing". Ageing Res Rev. 2017;36:125–136. doi: 10.1016/j.arr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Cassese G., Arce S., Hauser A.E., Lehnert K., Moewes B., Mostarac M. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 37.Jones D.D., Gaudette B.T., Wilmore J.R., Chernova I., Bortnick A., Weiss B.M. mTOR has distinct functions in generating versus sustaining humoral immunity. J Clin Invest. 2016;126:4250–4261. doi: 10.1172/JCI86504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tellier J., Shi W., Minnich M., Liao Y., Crawford S., Smyth G.K. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol. 2016;17:323–330. doi: 10.1038/ni.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubeck-Loebenstein B., Berger P., Saurwein-Teissl M., Zisterer K., Wick G. No immunity for the elderly. Nat Med. 1998;4:870. doi: 10.1038/nm0898-870b. [DOI] [PubMed] [Google Scholar]
- 40.Bokarewa M., Nagaev I., Dahlberg L., Smith U., Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 41.Liu L.-F., Shen W.-J., Ueno M., Patel S., Kraemer F.B. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abumrad N.A., el-Maghrabi M.R., Amri E.Z., Lopez E., Grimaldi P.A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 43.Christiaens V., van Hul M., Lijnen H.R., Scroyen I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta. 2012;1820:949–956. doi: 10.1016/j.bbagen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Festy F., Hoareau L., Bes-Houtmann S., Pequin A.-M., Gonthier M.-P., Munstun A. Surface protein expression between human adipose tissue-derived stromal cells and mature adipocytes. Histochem Cell Biol. 2005;124:113–121. doi: 10.1007/s00418-005-0014-z. [DOI] [PubMed] [Google Scholar]
- 45.Scheller E.L., Burr A.A., MacDougald O.A., Cawthorn W.P. Inside out: bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016;5:251–269. doi: 10.1080/21623945.2016.1149269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laharrague P., Larrouy D., Fontanilles A.M., Truel N., Campfield A., Tenenbaum R. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. 1998;12:747–752. doi: 10.1096/fasebj.12.9.747. [DOI] [PubMed] [Google Scholar]
- 47.Uchihashi K., Aoki S., Shigematsu M., Kamochi N., Sonoda E., Soejima H. Organotypic culture of human bone marrow adipose tissue. Pathol Int. 2010;60:259–267. doi: 10.1111/j.1440-1827.2010.02511.x. [DOI] [PubMed] [Google Scholar]
- 48.Hozumi A., Osaki M., Sakamoto K., Goto H., Fukushima T., Baba H. Dexamethasone-induced plasminogen activator inhibitor-1 expression in human primary bone marrow adipocytes. Biomed Res. 2010;31:281–286. doi: 10.2220/biomedres.31.281. [DOI] [PubMed] [Google Scholar]
- 49.Cawthorn W.P., Scheller E.L., Learman B.S., Parlee S.D., Simon B.R., Mori H. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1-11.
Supplemental Fig. S1-S3.